Abstract
Successful neonatal immunization of humans has proven difficult. We have evaluated CpG-containing oligonucleotides as an adjuvant for immunization of young mice (1–14 days old) against hepatitis B virus surface antigen. The protein-alum-CpG formulation, like the DNA vaccine, produced seroconversion of the majority of mice immunized at 3 or 7 days of age, compared with 0–10% with the protein-alum or protein-CpG formulations. All animals, from neonates to adults, immunized with the protein-alum vaccine exhibited strong T helper (Th)2-like responses [predominantly IgG1, weak or absent cytotoxic T lymphocytes (CTL)]. Th2-type responses also were induced in young mice with protein-CpG (in 1-, 3-, and 7-day-old mice) and protein-alum-CpG (in 1- and 3-day-old mice) but immunization carried out at older ages gave mixed Th1/Th2 (Th0) responses. DNA vaccines gave Th0-like responses when administered at 1 and 7 days of age and Th1-like (predominantly IgG2a and CTL) responses with 14-day-old or adult mice. Surprisingly, the protein-alum-CpG formulation was better than the DNA vaccine for percentage of seroconversion, speed of appearance, and peak titer of the antibody response, as well as prevalence and strength of CTL. These findings may have important implications for immunization of human infants.
Newborns are at risk for exposure to many infectious diseases, yet vaccination generally is not carried out until 2–3 months of age, owing to the immaturity of the neonatal immune system (1). In particular, B cell responses are weak and preferentially generate IgM/IgG1 antibody isotypes, and cytotoxic T lymphocyte (CTL) responses are poor (see ref. 2). In addition, maternally derived antibodies can interfere with the vaccine (3–6). Young mice are useful models to test immunization strategies for newborn humans since their response to protein antigens has similar limitations (7).
Although it has been thought that immunization early in life would induce immunological tolerance (8–11), humoral responses have been induced in newborn mice against a variety of antigens (12–14). This recently has been shown to depend on an appropriate dose of antigen (in this case, live virus) for the number of T cells (13) and on antigen being presented in the context of a “danger signal” that induces expression of the necessary costimulatory molecules (12).
DNA vaccines can also effectively immunize young mice, including those born to immune mothers (15–22). This is likely because of (i) prolonged antigen expression (23) that persists until the immune system is more mature, (ii) intracellular synthesis of antigen, allowing efficient and appropriate antigen presentation (24–27), and/or (iii) immunostimulatory CpG motifs within the plasmid that provide a “danger signal” (28–30). CpG motifs have many effects on the innate immune system, including the direct stimulation of B cells to divide and secrete more antibodies and of macrophages and dendritic cells to secrete T helper (Th)1-type cytokines that enhance the generation of antigen-specific responses and increased expression of costimulatory molecules (31, 32). CpG motifs within DNA vaccines appear to be essential to their function (33, 34).
CpG-containing oligodeoxynucleotides (CpG ODN) can also induce, in adult mice, potent Th1-like humoral and cell-mediated immune responses against a variety of antigens (29, 35–39), and these effects synergize with those of alum (35). In the present study we evaluate CpG ODN, alone or with alum, to induce immune responses against the hepatitis B virus (HBV) surface antigen (HBsAg) in very young mice. We compare it to alum, which has worked poorly when used with other antigens in early life (7), and with a DNA vaccine, such as those demonstrated to work in young mice (15–22). HBsAg is a strong antigen for which clear, protective surrogate end points are known, and a significant proportion of infants of HBV chronic carrier mothers become infected despite early immunization with current vaccines (40, 41).
MATERIALS AND METHODS
Vaccines.
Protein vaccines contained recombinant surface protein of HBV (S-protein, ay subtype, produced in yeast; Genzyme), hereafter referred to as HBsAg, at a final concentration of 0.05 and 0.02 mg/ml for pups and adults, respectively. HBsAg was combined with alum (protein-alum; 25 μg Al3+/μg protein), 10 μg CpG ODN (protein-CpG; 10 μg CpG ODN 1826 = TCCATGACGTTCCTGACGTT), or alum plus CpG ODN (protein-alum-CpG) as adjuvants, as described previously (35).
The DNA vaccine, which encoded S (ay) under the control of a cytomegaloviral (CMV) promoter (pCMV-S) (42, 43), was purified on Qiagen DNA purification columns, redissolved in 0.15 M NaCl at 0.5 and 0.2 mg/ml for pups and adults, respectively, and stored at or below −20°C.
Immunization of Young and Adult Mice.
BALB/c mice (Charles River Laboratories) bred onsite were checked daily for births. Immunization was carried out on groups of 5–35 pups aged <1, 3, 7, or 14 days (larger numbers when immune responses were rare or absent), or 10–15 adults (6- to 8-week-old females, Charles River Laboratories). Pups were weaned and sexes were separated at 3 weeks of age.
All priming immunizations consisted of i.m. injection of either protein (1 μg HBsAg ± adjuvants) or DNA (10 μg pCMV-S) vaccines. I.m. injection was used for HBsAg-based vaccines since this is how they are administered to humans, and for DNA vaccines because we have found the i.m. route to be the best among 14 different routes in mice (H.L.D., unpublished data). Pups received bilateral injections of 10 μl vaccine solution into the thigh muscles (44). Adult mice were immunized with 50 μl into the left tibialis anterior muscle (35, 45). All mice were bled at 4, 6, 8, 10, and 12 weeks postimmunization by retroorbital puncture. In some instances, mice were “challenged” 12 weeks postimmunization with 1 μg HBsAg (no adjuvant), then bled 3, 7, 14, and 28 days later.
Evaluation of Immune Responses to HBsAg.
Antibodies specific to HBsAg (total IgG, IgG1, and IgG2a) were quantified by ELISA on individual samples (35). Endpoint titers were defined as the highest plasma dilution that resulted in an absorbance value (OD450) two times greater than that of nonimmune plasma, with a cut-off value of 0.05, and seroconversion was defined as a dilution titer ≥100. The relationship between endpoint titers and those in milli-international units/ml (mIU/ml), as defined by the World Health Organization, was determined to be very close to 1:1 by comparing a panel of mouse plasma against human-derived standards (Monolisa Anti-HBs “Standards,” Sanofi Diagnostics Pasteur, Montreal, Canada) using a non-species-specific conjugate (Monolisa Anti-HBs Detection Kit, Sanofi Diagnostics Pasteur). A titer of 10 mIU/ml is considered protective against HBV infection in humans (46, 47). For determination of IgG2a/IgG1 ratios, antibody-negative sera were assigned a value of one-half the lowest dilution assessed; in most cases this was a value of 5.
CTL responses were evaluated 2 or 12 weeks postimmunization. Mice were given in vivo restimulation (1 μg HBsAg) 3 days before sacrifice, and recovered splenocytes were given 5 days of ex vivo restimulation with a congenic HBsAg-expressing cell line. These same cells served as target cells in the chromium release CTL assay, which was carried out as described previously (48). Control mice received no priming immunization but only HBsAg 3 days before sacrifice.
Statistical Analysis.
Antibody titers against HBsAg (anti-HBs) were expressed as group geometric means ± SEM of individual animal values, which were the average of duplicate or triplicate assays. The significance of differences between values was determined by Student’s t test (for two groups) or one-factor ANOVA followed by Tukey’s multiple-range testing (for three or more groups) on logarithmic-transformed data, with P > 0.05 being considered not significant (instat, Graphpad Software, San Diego).
RESULTS
Seroconversion.
DNA was the only immunogenic vaccine in 1-day-old mice, resulting in anti-HBs (titer ≥100) in 53% of mice by 12 weeks postimmunization (Fig. 1). In 3-day-old mice, the rate of seroconversion was still zero for protein-CpG, but was about 10% higher than at 1 day for each of the DNA and protein-alum groups. In contrast, there was a dramatic improvement in the immunogenicity of protein-alum-CpG in 3-day-old mice (75%), and this reached 100% by 7 days. By this time, seroconversion rates were improved for the other three vaccines, with antibodies appearing for the first time in protein-CpG-immunized mice (11%). All vaccines were immunogenic in 100% of 14-day-old or adult (not shown) mice.
Figure 1.
Percentage of seroconversion for BALB/c mice immunized in early life using either HBsAg with adjuvant(s) or an HBsAg-expressing DNA vaccine. HBsAg (1 μg) was combined with either 25 μg Al3+ (open bars), 10 μg CpG ODN (striped bars), or both alum and CpG ODN (shaded bars). The DNA vaccine (10 μg) encoded HBsAg under the control of a cytomegalovirus promoter (pCMV-S) (solid bars). Mice were immunized at <1, 3, 7, or 14 days after birth, and plasma taken at 12 weeks postimmunization was assayed for anti-HBs by endpoint-dilution ELISA assay. The fraction of mice exhibiting an anti-HBs endpoint-dilution titer >100 for any treatment is listed above the bar for that treatment.
Mice immunized at 1 or 3 days that failed to seroconvert (titer <10) were “challenged” at 12 weeks with HBsAg without adjuvant, and all mice developed anti-HBs antibodies (not shown). This indicates that administration to young mice of doses of antigen appropriate to induce immune responses does not induce permanent tolerance, limiting further vaccine responses. In most mice (26/37), the kinetics and magnitude of the response were typical of a primary response in adult mice (i.e., seroconversion not detected until 14–28 days and titers generally <1,000 by 28 days). However, in some animals (11/37) there was a clear anamnestic response with more rapid appearance of higher titers of anti-HBs than in adult controls, indicating that a humoral response had, in fact, been primed (seroconversion first detected by 7 days and titers usually much more than 1,000 by 14 and 28 days). Comparison of vaccine groups showed the highest rate of anamnestic response (67%) with protein-alum-CpG (6/9), half as many (33%) with protein-alum (3/9) or DNA (2/6), and none with protein-CpG.
Kinetics and Magnitude of Anti-HBs Response.
The rate of appearance of antibodies was about 2 weeks slower in very young than in 14-day-old and adult mice for all vaccine formulations (Fig. 2). This may result from a later initiation and/or a longer period for antibodies to develop, both of which are compatible with the immaturity of the young immune system.
Figure 2.
Kinetics and strength of anti-HBs humoral responses in BALB/c mice that responded to immunization with either HBsAg protein with alum and/or CpG ODN as adjuvant(s) or an HBsAg-expressing DNA vaccine. Mice were immunized at <1 day (•), 3 days (○), 7 days (■), or 14 days (□) after birth, or as adults (▴). Each point represents the group geometric mean for all seroconverted animals (endpoint titer >100) within that group. Titers of anti-HBs antibodies (total IgG) are expressed as the highest plasma dilution that resulted in an absorbance value (OD450) two times greater than that of nonimmune sera, with a cut-off value of 0.05.
Peak titers varied with the vaccine formulation and age of immunization. With all protein-based vaccines, peak titers were proportional to age. In contrast, peak titers were similar for all ages with the DNA vaccine (albeit more slowly developing in younger mice), suggesting that antigen expression from the DNA vaccine likely continued until the immune system was capable of full adult-like responses (i.e., >2 weeks). Highest geometric mean titers were attained with protein-alum-CpG vaccine when compared with other vaccine formulations, and this was significant at 3 and 14 days and in adults (P < 0.05) (Fig. 2).
Antibody Isotype Profiles.
Different IgG antibody isotypes were used to evaluate the type of Th response, with a predominance of IgG2a or IgG1 antibodies indicating a Th1- or Th2-like response, respectively. At all ages, protein-alum gave clear Th2 responses with virtually no IgG2a. In contrast, both protein-CpG and protein-alum-CpG induced a Th2 response at the earliest age at which responses could be induced (7 and 1 days, respectively), but a mixed Th1/Th2 (Th0) response in older mice, including adults. Finally, DNA vaccines gave Th0 responses in mice even as young as 1 day old and, by 14 days, gave the adult-like Th1 response (Table 1). It is notable that in CpG groups with Th0 responses, mice had either predominantly IgG1 or IgG2a isotypes of anti-HBs, but those with the more Th1-like responses had very high IgG2a-to-IgG1 ratios, such that the group mean was also high.
Table 1.
Anti-HBs antibody isotypes at 10–12 weeks after immunization of young or adult BALB/c mice with DNA or protein vaccines
| Formulation | Day | Mice with IgG isotypes | Major anti-HBs isotype*
|
Mean IgG2a/IgG1 ratio† | Th response‡ | |
|---|---|---|---|---|---|---|
| IgG1 | IgG2a | |||||
| Protein-alum | 1 | 1/10 | 1 | 0 | 0.25 | Th2 |
| 3 | 3/21 | 3 | 0 | 0.02 | Th2 | |
| 7 | 3/9 | 3 | 0 | 0.08 | Th2 | |
| 14 | 5/5 | 5 | 0 | 0.04 | Th2 | |
| Adult | 14/14 | 14 | 0 | 0.17 | Th2 | |
| Protein-CpG | 1 | 0/35 | No response | |||
| 3 | 0/14 | No response | ||||
| 7 | 4/18 | 4 | 0 | 0.16 | Th2 | |
| 14 | 6/6 | 3 | 3 | 5.01 | Th0 | |
| Adult | 14/14 | 5 | 9 | 3.23 | Th0 | |
| Protein- | 1 | 1/24 | 1 | 0 | 0.01 | Th2 |
| alum-CpG | 3 | 5/8 | 4 | 1 | 1.50 | Th0 |
| 7 | 6/6 | 3 | 3 | 47.33 | Th0 | |
| 14 | 6/6 | 1 | 5 | 6.29 | Th0 | |
| Adult | 13/13 | 7 | 6 | 4.23 | Th0 | |
| DNA | 1 | 6/15 | 2 | 4 | 2.16 | Th0 |
| 3 | 7/14 | 3 | 4 | 5.72 | Th0 | |
| 7 | 5/10 | 2 | 3 | 3.20 | Th0 | |
| 14 | 7/7 | 1 | 6 | 12.72 | Th1 | |
| Adult | 8/8 | 2 | 6 | 4.82 | Th1 | |
Groups of mice were immunized with either 10 μg pCMV-S, or 1 μg HBsAg + 25 μg Al3+ (as Al2O3; protein-alum) or 10 μg CpG ODN (protein-CpG), or alum plus CpG ODN (protein-alum-CpG), on the day after birth indicated (or as 6- to 8-week-old adults). Ten to 12 weeks postimmunization, plasma from individual mice was assayed for IgG1- and IgG2a-specific anti-HBs antibodies. The number of mice with detectable IgG isotype responses (endpoint titer >100) as a fraction of the number of mice assessed (this was sometimes less than those with total IgG), as well the number of those with predominantly IgG1 or IgG2a response, is indicated.
Plasma from individual mice was assayed in duplicate for IgG1- and IgG2a-specific anti-HBs antibodies. The mean IgG2a/IgG1 ratio is indicated. This was derived from all animals with detectable responses for at least one isotype and where only one isotype was detected; the other was assigned a value of one-half the lowest dilution assessed (usually 5).
The IgG2a/IgG1 ratio was used as an indicator of either a predominantly Th1 (IgG2a ≫ IgG1), predominantly Th2 (IgG1 ≪ IgG2a), or a mixed Th1/Th2 (Th0, IgG1 ≈ IgG2a) response (Th0, 0.5 to 2.0). Groups where some animals were more Th1 and other animals were more Th2 were also classified as Th0.
Cell-Mediated Immunity.
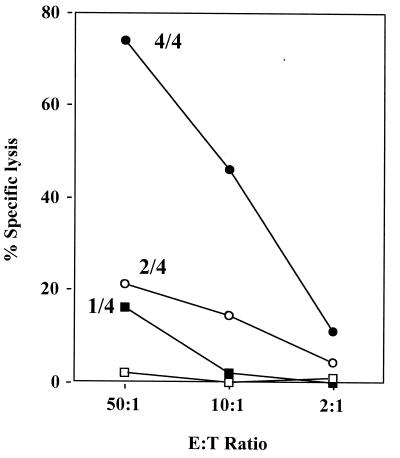
CTL induction is another element of cell-mediated responses that is suboptimal in early life. Potent CTL were detected in all four mice immunized with protein-alum-CpG at 7 days of age, with 74% specific lysis at an effector-to-target cell ratio (E/T) of 50:1 (Fig. 3). In contrast, only weak CTL (≈20% lysis at 50:1) were detected in one of four protein-alum treated mice and two of four DNA-immunized mice. However, CTL activity evaluated 12 weeks after immunization was similar for DNA and protein-alum-CpG vaccines (not shown). This agrees with an earlier report of slowly developing CTL after DNA-based immunization of young mice (21). No CTL were induced within 7 days by protein alone, indicating that in vivo protein restimulation could not account for CTL detected. Surprisingly, CTL were detected (12 weeks later) in some mice immunized as young as 1 or 3 days of age not only with the DNA vaccine (maximal lysis of 40–60% at E/T of 1:100 in three of nine mice) but also with protein-alum-CpG (25–55% in two of six) and protein-alum (30–45% in 3 of 17) vaccines. To our knowledge this is the youngest age reported for induction of CTL in mice with a protein vaccine.
Figure 3.
CTL activity in splenocytes removed from BALB/c mice 2 weeks after immunization of 7-day-old mice with protein-alum-CpG (•), protein-alum (■), protein-CpG (□), or a DNA vaccine (○). All mice were given in vivo restimulation (1 μg HBsAg without adjuvant) 3 days before removal of the spleen. Each point represents the mean of values obtained from CTL-positive mice that were each assayed in triplicate. Fraction of CTL-positive mice is indicated for each line. E/Ts are indicated on the horizontal axis. The vertical axis shows HBsAg-specific lysis as a percentage of the total possible lysis.
DISCUSSION
It is generally accepted that it is more difficult to successfully immunize newborns than adults because of insufficient number and/or maturity of immune cells (see ref. 2). Thus, vaccination of newborn humans has been limited to specific situations such as bacillus Calmette–Guérin, poliomyelitis, or hepatitis B. Although direct comparison of immune maturation between mice and humans is difficult, the immune system is less mature in newborn mice than in humans. For example, tolerance can be induced during the first week of life in mice, whereas true immunological tolerance cannot be induced in humans after birth. As such, newborn mice are a good model to test the limits of early-life immunization. After birth, the maturation process is significantly more rapid in mice (3–4 weeks) than humans (12–24 weeks). Analysis of the progressive acquisition of immune responses to protein and polysaccharide antigens suggests that the stage of immune maturation of 7-day-old mice best approximates human newborns (C.-A.S., unpublished data). In the present study, we have evaluated CpG DNA for its ability to induce antigen-specific humoral and cell-mediated immune responses in mice at various stages of their immune maturation.
Seroconversion in a significant proportion (>40%) of mice was detected with immunization at <1 day for the DNA vaccine, 3 days for protein-alum-CpG, and not until 14 days for protein-alum and protein-CpG. A similar hierarchy of responsiveness was observed for CTL responses. We interpret these findings to indicate that the murine immune system cannot optimally respond to antigen until 7 days of age (12, 49). Successful immunization at 7 days or younger could be attributed to factors that (i) ensured antigen remained available to the immune system until it was mature enough to respond and (ii) provided sufficient stimulation to antigen-presenting cells (APC). Among the protein vaccines, successful immunization on or before 7 days only occurred with the formulation containing both alum, which delays antigen clearance, and CpG ODN, which enhances antigen presentation (50). The importance of the depot effect of alum is consistent with the finding that protein-CpG is superior to protein-alum in adult mice (35), but not in mice up to 14 days of age. Furthermore, the earlier success with protein-alum-CpG than with protein-alum (where presumably the depot effects would be equal) indicates the importance of immunostimulatory effects of CpG motifs in young mice. With DNA vaccines, the early success can be attributed to both contained CpG and prolonged antigen expression, but efficient antigen presentation likely plays a role as well. An indication of this is that responses induced in young mice by 10 μg of DNA vaccine, where only nanogram quantities of antigen are expressed and not all CpG motifs are optimal, are almost as good as those with 1 μg protein and 10 μg CpG ODN (with alum).
In young mice, the protein-alum-CpG vaccine generally induced stronger and/or faster antibody and CTL (assayed 2 weeks postimmunization) responses than the other vaccine approaches. However, differences were partly kinetic since CTL responses detected 12 weeks after immunization were similar to those from protein-alum-CpG and DNA. Possibly the greater number of CpG motifs with CpG ODN than plasmid DNA may induce CTL more rapidly at young ages. The ability of DNA vaccines to induce CTL in adults has been linked to CpG motifs in the plasmid backbone (34, 51). Remarkably, CTL were induced in some mice immunized with protein-alum-CpG and DNA at less than 1 day of age. Previous studies have shown induction of CTL in young mice by DNA vaccines (18, 21), but our report demonstrates this with a protein vaccine. Furthermore, we report CTL responses in splenocytes recovered from mice as young as 3 weeks of age. It should be noted that while CpG-alum is an effective adjuvant combination for inducing CTL against HBsAg in young mice, this antigen self-assembles into virus-like particles, and CTL may not be induced in the case of other, less structured forms of proteins.
Consistent with a less mature immune system, responses in young animals differed both quantitatively and qualitatively from those in adults. Quantitative differences included a slower rate of appearance of antibodies and lower peak titers, except for DNA vaccines where all ages had similar peak titers, in agreement with other reports (17, 52, 53). The relatively high titers at young ages with the DNA vaccine probably are related to the longevity of antigen expression and efficient antigen presentation. CTL, when induced, were equally strong in young and adult mice.
Qualitative differences related to Th1/Th2 profiles. The Th2 bias of the immature immune system is thought to be a result of antigen presentation by APC with inadequate costimulation, different functioning of T cells upon antigen stimulation, lower efficiency of neonatal APC, and fewer T cells and APC (17, 19, 52–54). As such, antigen load, antigen persistence, and “danger signals” that induce costimulatory activity can have an influence (12, 55). For example, protein vaccines that contain excess antigen for the relatively few professional APC (i.e., dendritic cells) in the immature immune system can result in antigen presentation by unactivated B cells that induces Th2-like responses. The Th1 influence of CpG cannot be detected with the protein-CpG vaccine until the immune system is fairly mature (i.e., 14 days). Despite the fact that alum typically reinforces the Th2 bias, the CpG in the protein-alum-CpG vaccine can induce a more Th1-like response in mice as young as 3 days, presumably because it capitalizes on the depot effect of alum to retain antigen until the immune system matures. DNA vaccines appear to overcome the strong Th2 bias of the immature immune system (even in neonates), and this is likely because of a combination of their CpG content, expression of small amounts of antigen (less non-APC presentation), and efficient presentation of that antigen by virtue of direct transfection of APC. Further research is required to elucidate the exact mechanisms contributing to the Th-related responses observed after immunization of young mice.
CTL are associated with Th1-type antibody responses in adults, and this has also been noted in early life. However, in some mice immunized at 7 days with protein-alum, which elicits predominantly IgG1 (Th2) antibodies, we did see CTL. This agrees with previous observations of coexistence of CTL and Th2 responses in young mice (49, 56). It is possible that the Th1 cytokine threshold for induction of CTL in young mice is lower than that for isotype switching to IgG2a antibodies, although further studies will be required to confirm this. It is noteworthy that very high titers of antibody can accompany a Th1-like response, contrary to the notion that Th1 responses are better suited for cellular immune responses than for antibody responses.
Mice failing to seroconvert after early-life vaccination showed one of two responses after adulthood challenge with HBsAg without adjuvant. Some had anti-HBs titers similar to those of adult controls that were administered a single dose of HBsAg, indicating that they had remained naïve. Others had higher titers than adult controls, indicating that they had been primed. None had titers lower than adult controls, indicating that permanent tolerance had not been induced. There has been concern that DNA vaccines might induce tolerance since antigen is expressed for a prolonged period. Despite a single report of DNA vaccine-induced tolerance in young mice (57), our results corroborate a growing body of evidence showing that even neonatal (i.e., 1-day-old) mice can be immunized successfully using DNA (13, 15–22). We have not detected any adverse effects with CpG ODN in very young mice (present study), in infant nonhuman primates or in high-dose toxicity studies in adult rats and rabbits (H.L.D., unpublished data).
The findings of the present study have important clinical relevance as there is a clear need for vaccines that are effective in newborns and infants, and particularly those that can induce cell-mediated immunity, which is crucial for the prevention or control of many diseases commonly afflicting neonates. DNA vaccines have been shown to induce good cell-mediated immunity, but safety concerns may delay their use for prophylactic immunization of babies and young children. We show that a protein-alum-CpG formulation induces more Th1-like immune responses (Th0-Th1) in young mice and is superior to a DNA vaccine for kinetics and strength of both humoral and cell-mediated immune responses. Although best results were obtained in combination with alum, CpG ODN alone could be used as an adjuvant with vaccines for which it is not possible to use alum (i.e., live attenuated vaccines such as measles virus and measles recombinant canarypox vaccines; ref. 58). CpG ODN could allow vaccine administration at a younger age with increased efficacy. Also, the possibility to induce Th1 responses may help reduce the incidence of Th2-related diseases such as asthma, which, in developed nations, has been linked to the reduced incidence of bacterial infections (59).
Cell-mediated responses are not necessary for prophylactic vaccination against HBV; however, they may be beneficial to certain babies born to chronic carrier mothers. As many as 80–95% of infants born to mothers positive for both HBsAg and HBeAg (hepatitis B e antigen) become chronic carriers with a greatly increased risk of developing cirrhosis and hepatocellular carcinoma (41, 60–63). Even with active vaccination and passive immunization using anti-HBs IgG, 5–10% of such infants (16% in one study) still become chronically infected (40, 41). These failures are possibly cases with transplacental rather than perinatal infection, and they may benefit from a vaccine that induces Th1-like responses (64). Evidence that CTL and, in particular, the Th1 cytokines they secrete may play an important role in preventing or overcoming the chronic carrier state have been provided by numerous studies by Chisari (see ref. 65).
It remains to be seen whether the promising results obtained in mice will hold true in humans. It is encouraging, however, that two chimpanzees immunized with a DNA vaccine on the day of birth were protected from subsequent challenge with hepatitis B (66).
Acknowledgments
We are most grateful to Amanda Boyd and Lu Zhang for their extensive technical assistance and also wish to thank Paul Payette for helpful discussions. This work was supported by CpG ImmunoPharmaceuticals, Inc., and an operating grant from Medical Research Council (Canada) to H.L.D., who was also the recipient of an Ontario Ministry of Health Career Scientist Award. C.-A.S. was supported by the Swiss National Research Foundation (SCORE A), and A.M.K. was supported by the Department of Veteran Affairs and the National Institutes of Health.
ABBREVIATIONS
- Th
T helper
- ODN
oligodeoxynucleotides
- HBV
hepatitis B virus
- CTL
cytotoxic T lymphocytes
- HBsAg
HBV surface antigen
- E/T
effector-to-target ratio
- APC
antigen-presenting cells
References
- 1.Hunt D W, Huppertz H I, Jiang H J, Petty R E. Blood. 1994;84:4333–4343. [PubMed] [Google Scholar]
- 2.Kovarik J, Siegrist C A. Immunol Today. 1998;19:150–152. doi: 10.1016/s0167-5699(97)01230-9. [DOI] [PubMed] [Google Scholar]
- 3.Albrecht P, Ennis F A, Saltzman E J, Krugman S. J Pediatr. 1977;91:715–718. doi: 10.1016/s0022-3476(77)81021-4. [DOI] [PubMed] [Google Scholar]
- 4.Bona C, Bot A. The Immunologist. 1997;5:5–9. [Google Scholar]
- 5.Murphy B R, Olmsted R A, Collins P L, Chanock R M, Prince G A. J Virol. 1988;62:3907–3910. doi: 10.1128/jvi.62.10.3907-3910.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Van Maanen C, Bruin G, de Boer-Liutzje E. Vet Q. 1992;14:13–17. doi: 10.1080/01652176.1992.9694319. [DOI] [PubMed] [Google Scholar]
- 7.Barrios C, Brandt C, Berney M, Lambert P H, Siegrist C A. Eur J Immunol. 1996;26:2666–2670. doi: 10.1002/eji.1830261118. [DOI] [PubMed] [Google Scholar]
- 8.Billingham R, Brent L, Medawar P. Proc R Soc London (Biol) 1956;239:44–45. [Google Scholar]
- 9.Holan V, Chutna J, Hasek M. Nature (London) 1978;274:895–897. doi: 10.1038/274895a0. [DOI] [PubMed] [Google Scholar]
- 10.Owen R D. Fed Proc. 1958;16:581–591. [PubMed] [Google Scholar]
- 11.Streilein J W. Transplantation. 1991;52:1–10. doi: 10.1097/00007890-199107000-00001. [DOI] [PubMed] [Google Scholar]
- 12.Ridge J P, Fuchs E J, Matzinger P. Science. 1996;271:1723–1726. doi: 10.1126/science.271.5256.1723. [DOI] [PubMed] [Google Scholar]
- 13.Sarzotti M, Robbins D S, Hoffman P M. Science. 1996;271:1726–1728. doi: 10.1126/science.271.5256.1726. [DOI] [PubMed] [Google Scholar]
- 14.Forsthuber T, Yip H C, Lehmann P V. Science. 1996;271:1728–1730. doi: 10.1126/science.271.5256.1728. [DOI] [PubMed] [Google Scholar]
- 15.Bagarazzi M L, Boyer J D, Javadian M A, Chattergoon M, Dang K, Kim G, Shah J, Wang B, Weiner D B. J Med Primatol. 1997;26:27–33. doi: 10.1111/j.1600-0684.1997.tb00316.x. [DOI] [PubMed] [Google Scholar]
- 16.Bot A, Bot S, Garcia-Sastre A, Bona C. Viral Immunol. 1996;9:207–210. doi: 10.1089/vim.1996.9.207. [DOI] [PubMed] [Google Scholar]
- 17.Bot A, Antohi S, Garcia-Sastre A, Bona C. Int Immunol. 1997;9:1641–1650. doi: 10.1093/intimm/9.11.1641. [DOI] [PubMed] [Google Scholar]
- 18.Hassett D E, Zhang J, Whitton J L. J Virol. 1997;71:7881–7888. doi: 10.1128/jvi.71.10.7881-7888.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Manickan E, Yu Z, Rouse B T. J Clin Invest. 1997;100:2371–2375. doi: 10.1172/JCI119777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Monteil M, Le Potier M F, Cariolet R, Houdayer C, Eliot M. J Gen Virol. 1997;78:3303–3310. doi: 10.1099/0022-1317-78-12-3303. [DOI] [PubMed] [Google Scholar]
- 21.Sarzotti M, Dean T A, Remington M P, Ly C D, Furth P A, Robbins D S. Vaccine. 1997;15:795–797. doi: 10.1016/s0264-410x(96)00250-2. [DOI] [PubMed] [Google Scholar]
- 22.Wang Y, Xiang Z, Pasquini S, Ertl H C J. Virology. 1997;228:278–284. doi: 10.1006/viro.1996.8384. [DOI] [PubMed] [Google Scholar]
- 23.Davis H L, Millan C L B, Watkins S C. Gene Ther. 1997;4:181–188. doi: 10.1038/sj.gt.3300380. [DOI] [PubMed] [Google Scholar]
- 24.Ulmer J B, Deck R R, DeWitt C M, Donnelly J J, Liu M A. Immunology. 1996;89:59–67. doi: 10.1046/j.1365-2567.1996.d01-718.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Doe B, Selby S, Barnett J, Baenziger J, Walker C M. Proc Natl Acad Sci USA. 1996;93:8578–8583. doi: 10.1073/pnas.93.16.8578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iwasaki A, Torres C A T, Ohashi P, Robinson H L, Barber B H. J Immunol. 1997;159:11–14. [PubMed] [Google Scholar]
- 27.Corr M, Lee D J, Carson D A, Tighe H. J Exp Med. 1996;184:1555–1560. doi: 10.1084/jem.184.4.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krieg A M, Yi A-K, Schorr J, Davis H L. Trends Immunol. 1997;6:23–26. [Google Scholar]
- 29.Roman M, Martinorozco E, Goodman S, Nguyen M D, Sato Y, Ronaghy A, Kornbluth R S, Richman D D, Carson D A, Raz E. Nat Med. 1997;3:849–854. doi: 10.1038/nm0897-849. [DOI] [PubMed] [Google Scholar]
- 30.Pisetsky D. J Immunol. 1996;156:421–423. [PubMed] [Google Scholar]
- 31.Krieg A M, Yi A-K, Matson S, Waldschmidt T J, Bishop G A, Teasdale R, Koretzky G A, Klinman D M. Nature (London) 1995;374:546–549. doi: 10.1038/374546a0. [DOI] [PubMed] [Google Scholar]
- 32.Ballas Z K, Rasmussen W L, Krieg A M. J Immunol. 1996;157:1840–1845. [PubMed] [Google Scholar]
- 33.Klinman D M, Yamshchikov G, Ishigatsubo Y. J Immunol. 1997;158:3635–3639. [PubMed] [Google Scholar]
- 34.Sato Y, Roman M, Tighe H, Lee D, Corr M, Nguyen M-D, Silverman G J, Lotz M, Carson D A, Raz E. Science. 1996;273:352–354. doi: 10.1126/science.273.5273.352. [DOI] [PubMed] [Google Scholar]
- 35.Davis H L, Weeratna R, Waldschmidt T J, Tygrett L, Schorr J, Krieg A M. J Immunol. 1998;160:870–876. [PubMed] [Google Scholar]
- 36.Moldoveanu Z, Love-Homan L, Huang W Q, Krieg A M. Vaccine. 1998;16:1216–1224. doi: 10.1016/s0264-410x(98)80122-9. [DOI] [PubMed] [Google Scholar]
- 37.Chu R S, Targoni O S, Krieg A M, Lehmann P V, Harding C V. J Exp Med. 1997;186:1623–1631. doi: 10.1084/jem.186.10.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lipford G B, Bauer M, Blank C, Reiter R, Wagner H, Heeg K. Eur J Immunol. 1997;27:2340–2344. doi: 10.1002/eji.1830270931. [DOI] [PubMed] [Google Scholar]
- 39.Weiner G J, Liu H-M, Wooldridge J E, Dahle C E, Krieg A M. Proc Natl Acad Sci USA. 1997;94:10833–10837. doi: 10.1073/pnas.94.20.10833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee C Y, Beasley R P, Hwang L Y. Lancet. 1989;2:860–861. doi: 10.1016/s0140-6736(89)93018-3. [DOI] [PubMed] [Google Scholar]
- 41.Chen D-S, Hsu H-M, Bennett C L, Pajeau T S, Blumberg B, Chang P, Nishioka K, Huang A, Sung J-L. Cancer Causes Control. 1996;7:305–311. doi: 10.1007/BF00052935. [DOI] [PubMed] [Google Scholar]
- 42.Davis H L, Michel M-L, Whalen R G. Hum Mol Genet. 1993;2:1847–1851. doi: 10.1093/hmg/2.11.1847. [DOI] [PubMed] [Google Scholar]
- 43.Michel M L, Davis H L, Schleef M, Mancini M, Tiollais P, Whalen R G. Proc Natl Acad Sci USA. 1995;92:5307–5311. doi: 10.1073/pnas.92.12.5307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Millan, C. L. B. & Davis, H. L. (1998) Methods Mol. Med., in press.
- 45.Davis H L, Mancini M, Michel M-L, Whalen R G. Vaccine. 1996;14:910–915. doi: 10.1016/0264-410x(95)00255-y. [DOI] [PubMed] [Google Scholar]
- 46.Centers for Disease Control. Morb Mortal Wkly Rep. 1987;36:353–360. [Google Scholar]
- 47.Calandra G B, West D J. In: Hepatitis B Vaccines in Clinical Practice. Ellis R W, editor. New York: Dekker; 1993. pp. 1–16. [Google Scholar]
- 48.McCluskie M J, Davis H L. J Immunol. 1998;161:4463–4466. [PubMed] [Google Scholar]
- 49.Bot, A., Antohi, S. & Bona, C. (1997) Front. Biosci. 173–188. [DOI] [PubMed]
- 50.Gupta R K, Rost B E, Relyveld E, Siber G R. Vaccine Design: The Subunit and Adjuvant Approach. New York: Plenum; 1995. pp. 229–248. [Google Scholar]
- 51.Klinman D M, Yi A, Beaucage S L, Conover J, Krieg A M. Proc Natl Acad Sci USA. 1996;93:2879–2883. doi: 10.1073/pnas.93.7.2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Siegrist C A, Lambert P H. Springer Semin Immunopathol. 1997;19:233–243. doi: 10.1007/BF00870271. [DOI] [PubMed] [Google Scholar]
- 53.Siegrist C-A. Vaccine. 1997;15:798–800. doi: 10.1016/s0264-410x(96)00253-8. [DOI] [PubMed] [Google Scholar]
- 54.Burstein H J, Shea C M, Abbas A K. J Immunol. 1992;148:3687–3691. [PubMed] [Google Scholar]
- 55.Martinez X, Brandt C, Saddallah F, Tougne C, Barrios C, Wild F, Dougan G, Lambert P H, Siegrist C A. Proc Natl Acad Sci USA. 1997;94:8726–8731. doi: 10.1073/pnas.94.16.8726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Siegrist C A, Saddallah F, Tougne C, Martinez X, Kovarik J, Lambert P H. Vaccine. 1998;16:1473–1478. doi: 10.1016/s0264-410x(98)00111-x. [DOI] [PubMed] [Google Scholar]
- 57.Mor G, Yamshchikov G, Sedegah M, Takeno M, Wang R, Houghten R A, Hoffman S, Klinman D M. J Clin Invest. 1996;98:2700–2705. doi: 10.1172/JCI119094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kovarik, J., Bozzotti, P., Love-Homan, L., Pihlgren, M., Davis, H. L., Lambert, P.-H., Krieg, A. M. & Siegrist, C.-A. (1998) J. Immunol., in press. [PubMed]
- 59.Cookson W O C M, Moffatt M F. Science. 1997;275:41–42. doi: 10.1126/science.275.5296.41. [DOI] [PubMed] [Google Scholar]
- 60.Goldfarb J, Baley J, Medendorp S V, Seto D, Garcia H, Toy P, Watson B, Gooch M W, III, Krause D. Pediatr Infect Dis J. 1994;13:18–22. doi: 10.1097/00006454-199401000-00005. [DOI] [PubMed] [Google Scholar]
- 61.Esteban R. Vaccine. 1995;13:S35–S36. doi: 10.1016/0264-410x(95)80045-f. [DOI] [PubMed] [Google Scholar]
- 62.Grob P. Vaccine. 1995;13:S14–S15. doi: 10.1016/0264-410x(95)80039-g. [DOI] [PubMed] [Google Scholar]
- 63.McMahon B J, Alward W L M, Hale D B, Heyward W L, Bender T R, Francis D P, Maynard J E. J Infect Dis. 1985;151:599–603. doi: 10.1093/infdis/151.4.599. [DOI] [PubMed] [Google Scholar]
- 64.Schirmbeck R, Melber K, Kuhröber A, Janowicz Z A, Reimann J. J Immunol. 1994;152:1110–1119. [PubMed] [Google Scholar]
- 65.Chisari F V. J Clin Invest. 1997;99:1472–1477. doi: 10.1172/JCI119308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Prince A M, Whalen R, Brotman B. Vaccine. 1997;15:916–919. doi: 10.1016/s0264-410x(96)00248-4. [DOI] [PubMed] [Google Scholar]