Abstract
Brain arteriovenous malformations (BAVMs) are a rare but potentially devastating hemorrhagic disease. Transforming growth factor-β signaling is required for proper vessel development, and defective transforming growth factor-β superfamily signaling has been implicated in BAVM pathogenesis. We hypothesized that expression of the transforming growth factor-β activating integrin, αvβ8, is reduced in BAVMs and that decreased β8 expression leads to defective neoangiogenesis. We determined that β8 protein expression in perivascular astrocytes was reduced in human BAVM lesional tissue compared with controls and that the angiogenic response to focal vascular endothelial growth factor stimulation in adult mouse brains with local Cre-mediated deletion of itgb8 and smad4 led to vascular dysplasia in newly formed blood vessels. In addition, common genetic variants in ITGB8 were associated with BAVM susceptibility, and ITGB8 genotypes associated with increased risk of BAVMs correlated with decreased β8 immunostaining in BAVM tissue. These three lines of evidence from human studies and a mouse model suggest that reduced expression of integrin β8 may be involved in the pathogenesis of sporadic BAVMs.
Brain arteriovenous malformations (BAVMs) are rare vascular lesions characterized by an interanastomosing mass of morphologically abnormal arteries and veins surrounded by vascularized gliotic tissue, and are potentially lethal if they rupture.1 The pathogenesis of BAVMs remains uncertain and it is unknown whether BAVMs occur de novo during adult life or represent congenital defects that evolve in the postnatal brain.1 Descriptive studies have provided some insights, but cannot address the early mechanistic steps involved in BAVM development since they use BAVM tissue from adult patients, essentially the only patient material that is available. These descriptive studies suggest that aberrant angiogenesis in the setting of an altered cellular microenvironment are associated with the BAVM phenotype.2,3,4,5 On the other hand, most animal models examine genes required for normal brain vascular development, but the phenotypes do not recapitulate the features of mature BAVMs.1 Examples of such gene products include transcription factors (1D1/1D3), cell-surface molecules and their ligands such as Notch-4,6 Neuropilin-1,7 integrins,8,9,10,11 and various receptors and signaling mediators of the bone morphogenic protein (BMP)/transforming growth factor-β (TGF-β) superfamily.12
The BMP/TGF-β superfamily is of particular interest in AVM pathogenesis since mutations in multiple TGF-β superfamily signaling mediators are found in patients with hereditary hemorrhagic telangiectasia, a disease associated with AVMs in multiple organs, including the brain.1 The canonical BMP/TGF-β signaling pathway involves the binding of TGF-β to a type II receptor (ie, TGF-β receptor-2), which recruits and phosphorylates a type I receptor (ie, activin-like kinase-1, ALK-1, or ALK-5).13 The interaction of TGF-β with its signaling effectors can be modulated by other cell surface TGF-β co-receptors such as endoglin (ENG).13 Type I receptors then initiate phosphorylation of intracellular receptor regulated SMADs (ie, SMAD-1/5/8 or SMAD-2/3), which then bind to SMAD4 and form a heterodimeric complex that then translocates to the cell nucleus, binds to SMAD response elements located in many promoters, and modulates gene transcription.13
Mutations in ENG, ALK1, or rarely, SMAD4 are found in hereditary hemorrhagic telangiectasia patients.1 The exact roles of individual BMP/TGF-β superfamily ligands and receptors in BAVM pathogenesis remain controversial and a very active area of investigation.12,14,15 However, it is widely accepted based on the above and on genetic deletion experiments in mice that autocrine and paracrine TGF-β signaling, in general, is crucial for normal vascular development.16
TGF-β has three isoforms in mammals, which are ubiquitously expressed but almost completely sequestered in a latent form referred to as the small latent complex by the non-covalent association of the propeptide of TGF-β, known as latency-associated peptide (LAP), with the active TGF-β peptide.17 Thus, a critical step in regulation of TGF-β function is its activation. We have previously identified a mechanism of TGF-β activation in astrocytes, whereby the integrin αvβ8 binds to an integrin recognition (RGD) sequence present in LAP-β1 and -β3, and through a metalloproteolytic mechanism involving the transmembrane protease MT1-MMP mediates the activation and paracrine release of TGF-β.18,19
The integrin αv subunit pairs with 5 different β subunits (β1, β3, β5, β6, and β8) of which several (αvβ3, αvβ5) are thought to play major roles in angiogenesis and differentiation.20,21 However, genetic deletion of the αv- subunit associated integrins has only shown a crucial role for the αvβ8 integrin in developmental vasculogenesis.8,11 Thus, knockout of the integrin αv or β8 subunits result in a nearly identical lethal phenotype involving defective vasculogenesis during early development, and in later development, defective brain vessel formation resulting in lethal perinatal brain hemorrhage.8,11 The brain vessels of either αv or β8 deficient embryos show defective anastomotic connections and increased endothelial cell proliferation resulting in “glomeruloid” vascular malformations, which are often associated with hemorrhage.8,11 Ultrastructural and immunocytochemical examination of either αv-null or β8-null embryos reveals a primary defect of “end-feet” association of perivascular astrocytes with endothelial cells, with no defect in the periendothelial pericytes.9,10 Conditional deletion of the αv or β8 subunit in glial or neuroepithelial cells (which give rise to neurons and glia) shows a similar, albeit less severe, developmental and perinatal brain hemorrhagic phenotype as the integrin αv and β8 knockout mice.9,10 However, all of these conditional knockout mice survive into adulthood and then through an unknown mechanism repair and normalize their cerebral vasculature.9,10 Interestingly, conditional knockout of either the αv- or β8- subunits in vascular endothelium results in no phenotype, indicating that the primary function of αvβ8 resides on perivascular astrocytes.9,10
The integrin αvβ8 is expressed by astrocytic foot processes surrounding cerebral blood vessels in adult mice and rats, and through binding to LAP is the major mechanism used by astrocytes, in vitro, to activate TGF-β.18,22 Paracrine release of active TGF-β by astrocytic integrin αvβ8 mediates endothelial differentiation in co-culture models.23 Furthermore, when TGF-β1 knockin mice with a mutation of the integrin binding site (RGD to RGE) of LAP-β1 are crossed into a TGF-β3 null background, the brain vascular phenotype is identical to the αv and β8 integrin subunit knockout mice.24 Furthermore, mice with combined deficits in the αvβ6 and αvβ8 integrins recapitulate the phenotypes of TGFβ1 and TGFβ3-null mice.25 Taken together, these data suggest that integrin αvβ8-mediated activation of TGF-β1 and TGF-β3 plays a critical role in normal brain vascular development.
We hypothesized that reduced integrin αvβ8-mediated activation of TGF-β had the potential to alter the neoangiogenic program in the adult brain, therefore having the potential to play a role in the expansion and evolution of BAVMs. Thus, we used a model of vascular endothelial growth factor (VEGF)-induced neoangiogenesis in the adult mouse brain and found that local Cre-mediated deletion of integrin β8 (itgb8) caused reduced TGF-β activation/signaling and aberrant neoangiogesis. In human BAVM tissues we found reduced β8 expression, which correlated with genetic variation in the ITGB8 locus. Together these findings suggest that reduced αvβ8-mediated activation of TGF-β may play a role throughout the pathogenic sequence of BAVM development.
Materials and Methods
Tissue Specimens, Cell Lines and Reagents
Informed consent was obtained from all surgical participants as part of an approved ongoing research protocol by the University of California San Francisco Committee on Human Research, in full accordance with the declaration of Helsinki principles. A total of 38 paraffin-embedded BAVM resected tissue, 10 temporal lobe resections for epilepsy, and 5 autopsy brain samples, where death resulted from non–central nervous system causes, were evaluated. Of the 38 BAVM samples, 34 had sufficient tissue for β8 evaluation, and 28 of these had blood samples available for ITGB8 genotyping. BAVM patient characteristics are given in Supplemental Table T1 (http://ajp.amjpathol.org). Temporal lobe biopsies were obtained from the University of California San Francisco Brain Tumor Research Center Registry. Autopsy material was obtained from the University of California San Francisco autopsy service. TMLC TGF-β reporter cells (gift of John Munger, NYU Medical Center, New York City, NY) were maintained and used as previously described.19 All chemicals were from Sigma (St. Louis, MO) unless otherwise specified.
Immunohistochemistry and Immunoblotting
Immunohistochemistry of human tissues was performed using polyclonal goat anti-β8 with corresponding immunogenic peptide (G-19, Santa Cruz Biotechnology, Santa Cruz, CA) or monoclonal mouse anti-β3 (clone BV4, Santa Cruz Biotechnology), and of mouse tissues using anti-Cre, anti-pSMAD2, anti-pSMAD1/5/8 (Cell Signaling, Beverly, MA) essentially as previously described.26 Samples were assessed by a pathologist (S.L.N.) or an independent investigator (S.M.C.) who were blinded to the clinical diagnosis, genotype data and experimental group. Tissue staining intensity for β8 was graded on a scale of 0–3, with 0 being absent staining; grade 1, faint diffuse punctate staining of the neuropil; grade 2, diffuse punctate staining of the neuropil with astrocytic cell body staining; and grade 3, diffuse punctate staining of the neuropil with astrocytic cell body staining and perivascular astrocytic cell process staining, the previously described staining pattern for β8 in neural tissue.22 pSMAD staining was assessed by localizing the needle tract in the basal ganglia using Cre immunolocalization in serial sections. Sections adjacent to the injection site were stained for pSMAD2 (Cell Signaling), and pSMAD1/5/8 (Cell Signaling), and digital images from fields (n = 5) surrounding the injection site or the corresponding contralateral noninjected site were assessed for nuclear staining. Grading was on a scale of 0–2 with 0 = absent/light, 1 = indeterminate, and 2 = definite positive dense nuclear staining.
Western blotting was performed essentially as previously described.23 Briefly, the injection site in the basal ganglia of C57BL/6J mice with loxP sites engineered to flank Exon 4 of itgb8 were stereotactically injected with adenoviruses (Ad) expressing either green fluorescent protein (GFP) or a Cre-GFP fusion protein were localized by green fluorescence using an inverted phase microscope. Basal ganglia from Ad-Cre-GFP, Ad-GFP or the noninjected basal ganglia were lysed in 1% TX-100, 50 mmol/L Tris, pH 7.4, 150 mmol/L NaCl, 1 mmol/L EDTA, with a protease inhibitor cocktail (Calbiochem, San Diego, CA), 1 mmol/L phenylmethylsulfonyl fluoride, 5 mmol/L NaF and 1 mmol/L sodium orthovanadate. Twenty mg of each sample were resolved by 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis and immunoblotted proteins were detected using rabbit anti-pSMAD 2 (Cell Signaling). Blots were reprobed with rabbit anti-SMAD 2/3 (Cell Signaling).
Mice and Polymerase Chain Reaction (PCR) Genotyping
C57BL/6J mice with loxP sites engineered to flank Exon 4 of itgb8 and mice with loxP sites flanking exon 8 of smad4 (gift of Chiu-Xia Deng, Mammalian Genetics GDDB, NIDDK) have been described.10,27 Mice were maintained to be homozygous for the “floxed” itgb8 (itgb8 fl/fl) and smad4 (smad4 fl/fl) alleles and males 2–3 months of age were used for experiments. PCR genotyping of mice was performed as described using published primer sequences.10,27
Viral Vectors
Adenoviral-Cre (Ad-Cre), Ad-Cre-GFP, or control Ad-GFP vectors were purchased (Vector Biolabs, Philadelphia, PA; University of Iowa, Gene Transfect Vector Core, Iowa City, IA). Adenoviral-associated virus (AAV) vectors, AAV-VEGF and AAV-lacZ, have been previously described.28,29
We chose to use an adenoviral vector to deliver Cre recombinase since protein production following transduction peaks earlier than with AAV.30 Therefore, we co-injected Ad-Cre with AAV-VEGF to favor Cre-mediated itgb8 deletion before the peak of VEGF stimulation. We chose to look at a 3-week time point since capillary density increases at that time point following AAV-VEGF injection.28
Stereotactic Injection of Vectors into the Mouse Brain
AAV-VEGF or AAV-LacZ was co-injected with Ad-Cre, Ad-Cre-GFP or Ad-GFP to determine the angiogenic response to VEGF with local deletion of itgb8 (n = 6) or smad4 (n = 6). As an additional control, AAV-VEGF was co-injected with AAV-LacZ (n = 6). Following anesthesia, animals underwent stereotactic injection into the right basal ganglia, as previously described.28 Phosphate-buffered saline , 2 μl, or adenoviral vector stocks diluted in phosphate-buffered saline, 4 × 107, 2 × 107, 4 × 107 pfu, were used for dose-response experiments, or 2 μl of adenoviral vector stocks (2 × 107 pfu) combined with AAV vectors (2 × 109 genome copies) were used for neoangiogenesis assays.
Quantitative Assessment of Vessel Morphology
The brains were embedded in Tissue-Tek O.C.T. (Sakura Finetek, USA). Coronal sections (20 μm thick) were cut using a cryostat (Leica, CM1900, Germany). Frozen sections of brains injected with Ad-GFP were observed directly without staining to observe the distribution of the virus. GFP was observed 1 week after injection and was absent three weeks after injection. For quantitative assessment of vessel morphology, 20-μm-thick frozen coronal sections were fixed with 100% ethanol at 20°C for 20 minutes, then incubated with fluoresceinlycopersicin esculentum lectin (Vector Laboratories, Burlingame, CA) 2 g/ml at 4°C overnight.
This procedure produced specific staining of the blood vessels as no autofluorescence was noted in vessels in unstained sections (data not shown). Two brain coronal sections from each mouse were chosen, 0.5 mm anterior and 0.5 mm posterior to the needle track. Capillaries contained within digital images representing six 10X objective fields in the three areas adjacent to injection sites (left, right, and bottom within approximately 0.5 mm from the needle track) were counted using NIH Image 1.63 software and reported as mean vessel counts/field. Dysplastic capillaries appear tortuous and enlarged.31 Thus, to create an objective measurement of vessel dysplasia, we used capillary diameter (>10 μm) as a variable. The Dysplasia Index was calculated as the number of dysplastic capillaries per 200 capillaries examined. CD45 (BD PharMingen) immunostaining was performed on frozen sections of smad4 fl/fl mice after vessel quantification, as previously described.23 Digital images of multiple fields were taken using the 20X objective, and CD45+ leukocytes were counted and reported as leukocytes (±SEM)/3 fields.
Assessment of Conditional Deletion of Itgb8 and Smad4
Genomic and total RNA was isolated from the Ad-Cre or control vector stereotactically injected basal ganglia, or contralateral noninjected basal ganglia of mice (n = 3) using commercial kits (Qiagen, Valencia, CA). PCR of genomic DNA or SYBR green PCR (qPCR) was performed as previously described.10,26 Briefly, the recombined itgb8 fl/fl or smad4 locus was determined using PCR. A forward primer flanking the upstream 5′ loxP site in intron 3 (5′-GTGGTTAAGAGCACCGATTG-3′) was paired with a reverse primer flanking the 3′ loxP site (5′-CACTTTAGTATGCTAATGATGG-3′) to give a 340-bp product for the recombined and 2093-bp product for the non-recombined itgb8 floxed locus. Similarly, primers flanking exon 8 of smad4 were used to characterize conditional deletion (200bp) of smad4 (genoF: 5′-ACAGGTTTCAGTTCAGGTGC-3′; smad4 genoR: 5′-CTGCTTCCTGACTGCAAATG-3′).32 Primers for qPCR were: itgb8 F-5′-GATGTGTGTGCTGGGCATG-3′, R-5′-GAGGATTGGTTCCCGTTTGC-3′. Results were normalized to gapdh, as previously described.26 Primers used for gapdh were forward, 5′-CCAAGTATGATGACATCAAGAAGGTGG-3′; reverse, 5′-CTGTTGCTGTAGCCATATTCATTGTCA-3′. For some experiments, astrocytes from neonatal itgb8 fl/fl mice were harvested and cultured essentially, as previously described.18 Astrocytes in six-well dishes were infected with Ad-Cre or Ad-LacZ (3 × 107 pfu/ml). After 72 hours, the astrocytes were detached and assessed for TGF-β activation using TMLC cells in the presence or absence of a TGF-β neutralizing antibody (1D11, ATCC), as previously described.18
BAVM Cases and Healthy Controls
BAVM cases were recruited at University of California San Francisco or at Kaiser Permanente Medical Care Plan of Northern California33 and classified using standardized guidelines. Controls were normal, healthy volunteers from the same clinical catchment area with no chronic disease or medication and without significant past medical history.34 All subjects provided written, informed consent and blood or saliva specimens for genetic studies. A subset of 194 BAVM patients and 127 healthy controls who all self-reported as Caucasian were included in the analysis.
Genotyping, SNP Selection, and Sequencing
The human integrin β8 gene (ITGB8) is highly conserved and approximately 76 kbp long. There are no known validated common polymorphic variants in the ITGB8 promoter region or exon 1 (dbSNP build 126), but the strong linkage disequilibrium (LD) pattern across the 5′ end of the gene suggested that any undiscovered promoter variants could be captured by haplotype tagging. We selected five haplotype-tag SNPs (minor allele frequency >5%) for a 10-kb region encompassing the promoter, exon 1, and intron 1 of ITGB8 using the Tagger algorithm35 implemented in Haploview36 with pairwise selection and r2 > 0.8. SNPs were genotyped by template-directed primer extension with fluorescence polarization detection.37,38
We sequenced a 952-bp amplicon encompassing the proximal promoter area, including two regions of high evolutionary conservation, which overlapped part of the 5′ untranslated region. Approximately 900 bp of this amplicon (chr7:20,336,600–20,337,500) was analyzed for sequence variation in a panel of 24 AVM patients carrying the AVM risk genotypes (rs10486391 AA and rs11982847 TT) identified in the haplotype tagging association study described above.
Statistical Analysis
Genotyping results were checked for adherence to Hardy-Weinberg equilibrium using χ2 goodness-of-fit tests. Individual SNPs were tested for association with BAVM using logistic regression analysis to obtain odds ratios (OR) and 95% confidence intervals (CI). Haplotype frequencies were inferred using the expectation-maximization algorithm, and association testing was performed using WHAP software.39 Both a global likelihood ratio test of association comparing the overall haplotype distribution between BAVM cases and controls, and haplotype-specific tests of association comparing each haplotype versus all other haplotypes were performed. Degrees of freedom are equal to number of haplotypes tested minus 1 and significance was set at α < 0.05. Only common haplotypes with a minor allele frequency greater than 5% were considered for analysis.
Unpaired t-tests were used to compare mean β8 immunohistochemical staining between high-risk versus low-risk (reference) genotype groups, means of vascular density and dysplasia index of mouse brains between AAV-VEGF/Ad-Cre or AAV-VEGF/AAV-lacZ versus AAV-lacZ/Ad-Cre injected groups, and means of pSMAD2 and pSMAD1/5/8 immunostaining. A two-tailed α < 0.05 was considered statistically significant. Linear regression analysis with β8 immunohistochemical staining as the dependent variable and clinical characteristics and SNP genotype as independent variables was performed to adjust for the effect of multiple predictors. Data in figures are presented as mean plus SEM.
Results
Reduced Expression of the Integrin β8 Subunit in Human BAVMs
Integrin β8 immunoreactivity was detected in three patterns in both BAVM and control brain samples: diffuse punctate staining in the neuropil, cell body staining of neurons and astrocytes, and delicate cytoplasmic cell processes surrounding blood vessels. We have previously extensively characterized the β8 staining pattern in the adult mouse and rat brain.22 In rodent brain, like human brain, we previously found that the pattern of immunoperoxidase staining was diffuse in the neuropil and localized in the cell body and cell processes. In these previous studies we used human fetal brain, adult mouse and rat brain, and used subcellular fractionation, immunofluorescence and/or immunoelectron microscopy to confirm that β8 staining was localized in dendrites and the tips of glial processes apposed to neurites and surrounding blood vessels.18,22 Presently, we confirmed by immunofluorescence that approximately 50% of the anti-β8 immunostaining pattern in the neuropil and all of the perivascular staining was localized in glial processes as determined by co-localization with glial fibrillary acidic protein (See supplemental Figure S1 at http://ajp.amjpathol.org). In general, β8 staining of the BAVM samples showed marked decrease in the perivascular staining surrounding the abnormal vessels (Figure 1, A–L). In comparison, the β3 integrin subunit was either not expressed or faintly expressed in the neuropil surrounding blood vessels, similar to the absent staining seen in the no primary antibody control samples (Figure 1). When BAVM samples (n = 34) were compared with temporal lobe control samples or to autopsy brain samples from the frontal cortex, insular cortex, hippocampus or cerebellum (n = 15), there was a significant decrease in overall β8 staining (P = 0.002, Figure 2). There was little to no expression of β3 in the neuropil of normal brain consistent with other reports.40 No increase in β3 staining was seen between control and BAVM samples (Figure 2). The β8 staining intensity did not show statistically significant differences based on the anatomical location of the control samples (data not shown).
Figure 1.
Integrin subunit β8 and β3 immunostaining in BAVMs relative to control brain samples. β8 immunohistochemical localization (A, D, G, J) was compared with β3 (B, E, H, K) and no primary antibody control staining (C, F, I, L). Shown are representative staining patterns seen in the normal insular cortex (A–C), temporal lobe from a patient with epilepsy (D–F) or from two separate BAVMs (G–L). A and D: Photomicrographs demonstrate the presence of perivascular β8 staining adjacent to small cerebral vessels (V) or in G and J, absence of staining in the perivascular cell processes surrounding the walls of large BAVM vessels (VW) or small perinidal BAVM vessels (v) vessels. Scale bar = 50 μm in A–C, G–L) and 25 μm in D–F. Shown in A and D are photomicrographs of small vessels with perivascular staining tracing the outer wall of the vessel (white arrows), which is absent from BAVM samples (G and J, black arrows). Diffuse β8 immunostaining of the neuropil is shown in both control (A and D) and BAVM (G and J) samples. β8 neuropil staining tended to be lighter in BAVM samples. Little or no staining is observed in samples stained with anti-β3 (B, E, H, K) or no primary antibody controls (C, F, I, L).
Figure 2.
Integrin β8 and not β3 subunit immunostaining is reduced in BAVM samples relative to controls. Tissue staining intensity from BAVM (n = 34) and control (n = 15) samples was graded on a scale of 0–3 with diffuse neuropil stain being grade 1, neuropil + cell body staining, grade 2, and neuropil + cell body + perivascular staining grade 3. Shown are bar graphs (±SEM); **P = 0.002.
Requirement of the Integrin β8-Mediated Activation of TGF-β for Normal Vascular Differentiation During Cerebral Neoangiogenesis in the Adult Mouse Brain
As a test of the biological relevance of αvβ8 expression by cerebral astrocytes in cerebral vascular formation, we conditionally deleted itgb8 in the murine brain using stereotactic injection of Adenoviral-Cre (Ad-Cre). Dose response curves revealed that the 4 × 107 pfu dose of Ad-Cre, which was the maximum dose allowed (based on a maximal 2-μl injection volume,41 and on adenoviral stock titers) produced efficient recombination of the itgb8 genomic locus (See Supplemental Figure S2 at http://ajp.amjpathol.org). However, at half the dose (2 × 107), which was the maximal viral dose allowed when the Ad-Cre was co-injected with AAV-VEGF, recombination was still seen, albeit slightly less efficiently (see Supplemental Figure S2A at http://ajp.amjpathol.org). This dose of adenovirus produced strong GFP expression in an area approximately 1 mm surrounding the needle tip (see Supplemental Figure S2, B and C, at http://ajp.amjpathol.org). Evidence of successful itgb8 conditional deletion was demonstrated by the appearance of a PCR amplicon of the exact expected size from the injection site in the brains of itgb8 fl/fl mice (n = 3) injected with Ad-Cre (Figure 3A). This PCR amplicon was specific as it was not seen in the noninjected contralateral control tissue or from mice injected with control virus (Figure 3A). The extent to which Ad-Cre injection reduced β8 expression was determined using quantitative PCR (qPCR). Ad-Cre injection resulted in a 58% reduction in β8 copy number from the entire basal ganglia when compared with the contralateral noninjected basal ganglia (Figure 3B). The 58% reduction in β8 mRNA most likely represents more complete reduction of β8 expression in focal areas since the Ad-Cre virus did not diffuse evenly throughout the entire basal ganglia (see Supplemental Figure S2, B and C at http://ajp.amjpathol.org). Significant differences in β8 copy numbers were not seen between the control adenoviral injected and noninjected brain tissue (Figure 3B).
Figure 3.
Adenoviral Cre-mediated deletion of itgb8 in mouse brain. Adenoviral Cre (Ad-Cre) or adenoviral associated virus-LacZ (AAV-LacZ) was stereotactically injected into the basal ganglia of adult male C57BL/6J mice with loxP sites flanking exon 4 of the murine integrin β8 (itgb8) gene. A: The recombined locus was detected by PCR of genomic DNA isolated from the injected (lesional) site or the noninjected (contralateral) site, 3 weeks after injection, using primers designed to flank the upstream and downstream loxP sites. An amplicon of the expected size (340 bp) was detected only in the Ad-Cre injected mouse brains (n = 3) and not in the contralateral hemisphere or in the brains of mice injected with AAV-LacZ. B: qPCR was used to determine the efficacy of Cre-mediated recombination of the itgb8 locus. Total RNA was isolated from the basal ganglia of the ipsilateral injected (lesional) or contralateral noninjected sites and SYBR green PCR was performed. Shown is mean transcript copy number relative to GAPDH. *Ad-Cre lesional versus Ad-Cre contralateral; Ad-Cre versus AAV-LacZ lesional, P < 0.05.
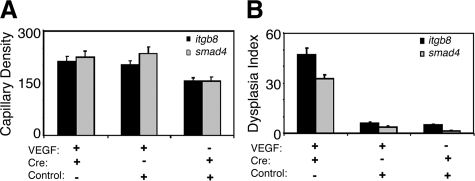
Three weeks after vector-injection, the capillary density was higher in the brains co-injected with AAV-VEGF/Ad-Cre (212 ± 14/10X field) or AAV-VEGF/AAV-lacZ (201 ± 13/10X field) than in the brains co-injected with AAV-lacZ/Ad-Cre (154 ± 11/10X field, P < 0.05, Figures 4 and 5A). Increased numbers of enlarged, dysplastic capillaries were observed in the brain co-injected with AAV-VEGF/Ad-Cre (47 ± 4/200 capillaries, Figures 4 and 5B). Few dysplastic capillaries were observed in AAV-VEGF/AAV-lacZ (6 ± 0.7/200 capillaries) or AAV-lacZ/Ad-Cre co-injected brains (5 ± 0.7/200 capillaries, P < 0.05, Figures 4 and 5B).
Figure 4.
Morphological alterations in VEGF-induced new blood vessels resulting from local deletion of itgb8 or smad4. Digital images display lectin staining of blood vessels (green) around stereotactic injection sites. The insets show enlarged images of capillary formations. Shown in the upper panels are itgb8 fl/fl and on the lower panels smad4 fl/fl mice. The insets show enlarged images of capillaries in AdCre and AAV-VEGF injected brain and normal capillaries in the AAV-VEGF or AdCre plus control vector injected brain. The co-injection strategy with AAV-VEGF, Ad-Cre, or control adenovirus is indicated. Scale bar = 100 μm.
Figure 5.
Vascular morphology but not vascular density is affected in VEGF-induced new blood vessel formation resulting from local deletion of itgb8 or smad4. Bar graphs show capillary density (mean vessel counts/field) (A) and the dysplasia index (number of enlarged, irregular capillaries for every 200 capillaries examined) (B) 3 weeks following viral transduction in the basal ganglia of itgb8 fl/fl or smad4 fl/fl mice. Shown is SEM; *P < 0.05.
We next compared the brain vascular phenotypes of focal VEGF stimulation in mice with conditional deletion of itgb8 to mice with conditional deletion of smad4. Ad-Cre-GFP mediated deletion produced efficient recombination of the floxed smad4 locus (data not shown). Three weeks after vector-injection, there were more capillaries in the smad4 fl/fl brains co-injected with AAV-VEGF/Ad-Cre-GFP (224 ± 16/10X field) or AAV-VEGF/Ad-GFP (234 ± 18/10X field) than in the brains co-injected with AAV-lacZ/Ad-Cre-GFP (155 ± 11/10X field, P < 0.05, Figures 4 and 5). Increased numbers of enlarged, dysplastic capillaries were observed in the brain co-injected with AAV-VEGF/Ad-Cre-GFP (32 ± 2.4/200 capillaries, Figures 4 and 5). Only a few dysplastic capillaries were observed in AAV-VEGF/Ad-GFP (4 ± 0.6/200 capillaries) or AAV-lacZ/AdCre-GFP co-injected brains (1 ± 0.5/200 capillaries, P < 0.05, Figures 4 and 5). No hemorrhage or edema was noted in any of the itgb8 fl/fl or smad4 fl/fl groups.
Ad-Cre infection of cultured mouse itgb8 fl/fl astrocytes resulted in an almost complete abrogation of activation of TGF-β using a TGF-β bioassay (Figure 6A). The partial deletion of β8 by stereotactic injection of Ad-Cre in itgb8 fl/fl mouse basal ganglia resulted in a small but significant decrease in pSMAD2 immunostaining in the sites of injection compared with the contralateral noninjected hemispheres (Figure 6, B–D). This decrease was specific to the canonical pSMAD2/3/4 TGF-β signaling pathway since no difference in overall staining was observed using antibodies to the BMP TGF-β signaling effectors pSMAD1/5/8 (Figure 6B), and as determined by immunoblotting, correlated with a 50% decrease in phosphorylation of Smad-2 (pSmad-2) in basal ganglia injected with Ad-Cre compared with Ad-GFP while no changes in total Smad-2/3 levels were seen (Figure 6, E and F).
Figure 6.
Adenoviral-Cre mediated deletion of itgb8 results in abrogation of TGF-β activation, in vitro and in vivo. A: Neonatal astrocytes were cultured from itgb8 fl/fl mice (n = 8) and infected with Ad-Cre or Ad-LacZ. After 72 hours the astrocytes were harvested and co-cultured with TGF-β reporter cells (TMLC) in the presence or absence of a pan-TGF-β isoform neutralizing antibody (1D11). Shown are arbitrary luciferase units relative to the 1D11 control; ***P = 0.0003 B–D: Ad-Cre was injected into the basal ganglia of 6 weeks old itgb8 fl/fl mice (n = 2) and after 7 days the brains were harvested, fixed, immunohistochemically stained with anti-pSmad-2 or anti-pSmad-1/5/8 and the area surrounding the needle tip or the corresponding contralateral side were digitally imaged. Microscopic fields (n = 64) from digital images were blindly assessed for nuclear staining. Shown are bar graphs showing quantification of nuclear staining intensity (0–2 scale). Shown are representative fields showing staining in the area of the needle tract (C) compared with the noninjected side (D). Scale bar = 100 μm; *P = 0.031. E: Lysates from the from the basal ganglia of itgb8 fl/fl mice injected with Ad-Cre or Ad-GFP (or the contralateral noninjected basal ganglia) were immunoblotted using anti-pSmad-2 (top panel) or anti-Smad-2/3 (bottom panel). Shown is a representative experiment of 4 with similar results. F: Densitometric analysis of pSmad-2 from Ad-Cre and Ad-GFP injected basal ganglia relative to noninjected basal ganglia. Shown in SEM; *P < 0.05.
Because adenoviral transduction can incite an inflammatory reaction that can influence the angiogenic response,42 we compared the presence of leukocytes using anti-CD45 immunostaining. We found no significant differences in leukocyte counts between groups [AAV-VEGF/Ad-Cre-GFP (8.0 ± 0.3/10X field); AAV-VEGF/Ad-GFP (8.6 ± 0.4/10X field), AAV-lacZ/Ad-Cre-GFP (6.3 ± 0.5/10X field)]. We conclude that αvβ8-mediated activation of TGF-β is required for normal vessel differentiation during VEGF-induced neoangiogenesis, but is not required for neoangiogenesis, per se.
Genetic Variants of the Human Integrin β8 (ITGB8) Gene Are Associated with Increased Risk of BAVMs
We genotyped five common haplotype-tagging SNPs located in intron 1 of ITGB8 in a panel of 194 BAVM cases and 127 healthy controls, all of self-reported Caucasian ancestry. Genotype frequencies for ITGB8 SNPs were in Hardy-Weinberg equilibrium among controls. Figure 7A shows the LD plot and marker order of ITGB8 SNPs from 5′ to 3′ end (left to right). Two ITGB8 SNPs were significantly associated with BAVM with subjects homozygous for the major allele at increased risk: rs10486391 (AA versus AG+GG; OR = 1.96, 95% CI = 1.21–3.17) and rs11982847 (TT versus AT+AA; OR = 1.84, 95% CI = 1.16–2.93). These SNPs remained associated after adjusting for age and gender (Figure 7B).
Figure 7.
Genetic variation in ITGB8 is associated with BAVM risk. A: LD plot of ITGB8 5′ region in Caucasian HapMap samples. In the upper panel, the genomic location on chromosome 7 of ITGB8 (NM 002214) exon 1 (gray shaded box) and intron 1 (black line) is shown. In the lower panel is the LD plot where shading represents pairwise LD between SNPs in terms of r2: black shading = 1 (perfect correlation), white shading = 0 (no correlation), shades of gray = 0 < r2 < 1. SNPs identified by Haploview residing in the ITGB8 5′ flanking region, Exon 1 or intron 1 are numbered 1 through 15 and their location indicated by lines extending to the upper panel. Note that SNPs 2 and 3 are not shown since they are monomorphic (ie, minor allele frequency is 0%). Thus, no data exists between SNP1 and 4 and the 5′ boundary of the 4.2-kb LD block remains undefined. ITGB8 haplotype-tagging SNPs were selected using the Tagger algorithm with pairwise selection, minor allele frequency >5%, and r2 > 0.8; genotyped SNPs are indicated by large bold font. B: OR and 95% CI for ITGB8 haplotype-tagging SNPs in 194 BAVM cases and 127 healthy controls, all of Caucasian ancestry. Vertical dotted line indicates an OR = 1 (no association). Two SNPs (rs10486391 and rs11982847) were associated with BAVM with 95% CI excluding 1.0.
Next, we performed haplotype analyses to refine the association signal, as combinations (haplotypes) of the genotyped markers can be used to infer association signals from SNPs not genotyped that reside in the same haplotype block. Overall, nine common haplotypes were predicted with frequencies between 2 to 38%, accounting for 98% of all possible haplotypes present in our data. A global test of association comparing the overall haplotype distribution between cases and control was borderline significant (likelihood ratio test = 15.1, degrees of freedom = 8, P = 0.057).
The two most common haplotypes were associated with increased risk (GTCCA, OR = 1.49, P = 0.02) and decreased risk (CACCG, OR = 0.64, P = 0.01) of BAVM. These results were consistent with the individual SNP analysis (Figure 7B), where the high-risk alleles of both rs11982847 (T) and rs10486391 (A) were located on the at-risk haplotype, GTCCA. However, two other haplotypes tested also contained the high-risk alleles (CTCCA and CTACA), but were not associated with BAVM (P > 0.4), suggesting that the actual functional variant may be another SNP residing on the GTCCA (but not on CTCCA or CTACA) haplotype, perhaps in the ITGB8 promoter region.
The ITGB8 promoter has not yet been defined. To identify possible ITGB8 promoter polymorphisms in BAVM patients, we sequenced approximately 900 bp (chr7:20,336,600–20,337,500) covering the 5′ flanking region of ITGB8 in 24 BAVM patients carrying the risk genotypes (rs10486391 AA and rs11982847 TT). Only two singleton SNPs were identified, one each in two BAVM patients (rs62456081 A>G chr7:20336748 and a novel T>C SNP at ch7:20,337,317). Neither SNP lies in a conserved transcription factor binding site. Hence, sequencing of the putative proximal promoter did not reveal any additional candidate SNPs.
Correlation of β8 Staining with ITGB8 Genotype
For the most significantly associated ITGB8 SNP, rs10486391, we identified BAVM patients in whom both blood and tissue samples were available (n = 28) and correlated genotypes to β8 protein immunostaining in BAVM tissue. Figure 8 shows that tissue samples from BAVM patients with the at-risk rs10486391 AA genotype (n = 11) had significantly lower mean β8 immunostaining levels compared with AG or GG genotypes (n = 17), respectively (0.73 ± 0.65 vs. 1.47 ± 0.80; P = 0.016). Similar results were observed for the at-risk rs11982847 TT genotype (n = 14) vs. AT or AA genotype (n = 14) groups (0.79 ± 0.58 vs. 1.57 ± 0.85; P = 0.008).
Figure 8.
Decreased integrin β8 immunostaining correlates with ITGB8 genotypes associated with increased risk of BAVMs. Tissue staining intensity was graded on a scale of 0–3. AA (n = 11), AG (n = 12), GG (n = 5). *P = 0.016, AA versus AG+GG.
There was no association between β8 staining and clinical characteristics (See Supplemental Table T1 at http://ajp.amjpathol.org), except for patients with lesions in eloquent regions (P = 0.039). In multivariable regression analysis, both eloquent location (P = 0.047) and ITGB8 rs10486391 AA genotype (P = 0.040) were independently associated with ∼60% reduction in mean β8 staining. Exploratory analysis could not explain the association with eloquent location, except for a nonsignificant trend for lesions with any cortical involvement to also have lower β8 staining (β = −0.21, 95% CI = −1.28, 0.87, P = 0.70). Otherwise, there was no apparent association with anatomical location of lesions.
Discussion
This study suggests that decreased expression of the integrin β8 subunit in BAVMs contributes to the dysplastic vascular phenotype through decreased TGF-β activation. Furthermore, our data suggest that genetic variation is associated with varying levels of expression of the integrin β8 gene. While a critical role for β8 has been defined in proper brain vessel differentiation during development, this study is the first to address the function of integrin β8 in vascular differentiation during neoangiogenesis in the postnatal brain.
Conditional deletion of αv or β8 in glial cells or glial progenitors lead to disorganization of perivascular astrocytes and failure to properly form contacts between astrocyte end-feet and the vascular endothelium.9,10 Thus, reduced expression of β8 in perivascular astrocytes might contribute to BAVM pathogenesis through alterations in paracrine astrocyte-glial interactions. The major and perhaps the only biologically relevant ligand for αvβ8 is the LAP of TGF-β.19 In fact, in vitro and genetic models indicate that integrin αvβ8-mediated activation of TGF-β accounts for the majority of TGF-β1 and TGF-β3 activated in the brain during development and postnatal life.18,19,24,25,43,44 We have previously found in a three-dimensional co-culture model of human astrocytes with a murine brain endothelial cell line that astrocytic αvβ8 was a crucial mechanism of activating TGF-β, which functioned as a paracrine factor in inhibiting endothelial migration and promoting endothelial differentiation.18 Thus, we anticipated that during neoangiogenesis in the adult brain that perturbation of β8 function would result in defects in vascular differentiation.
Genetic deletion of itgb8 in cultured itgb8 fl/fl astrocytes resulted in nearly complete abrogation of TGF-β activation and subsequent SMAD-dependent signaling. However, in vivo, only a small decrease (12%) in TGF-β signaling, as determined by pSmad-2 immunostaining was seen. This small decrease may reflect the reduced transduction efficiency of Ad-Cre in the in vivo compared with in vitro setting. On the other hand, the small decrease in pSmad-2 immunostaining corresponded with a much larger decrease of pSmad-2 (50%) signal intensity by immunoblotting. The differences in magnitude between differences seen with pSmad-2 immunostaining and immunoblotting are likely due to the improved ability of immunoblotting to discriminate between nonspecific signals. Overall, these data verify that reduced expression of β8 in the postnatal brain cause reduced TGF-β signaling.
The partial deletion of itgb8 and partial abrogation of TGF-β signaling, in vivo, was sufficient to result in dilated and tortuous capillaries in response to focal VEGF stimulation. The role of vascular dilation in BAVM pathogenesis has been suggested by ultrastructural studies of early cutaneous lesions in hereditary hemorrhagic telangiectasia patients. In that study, arteriolar dilation was an early feature that preceded the formation of direct arteriovenular communication, suggesting that AVMs develop along a stepwise progression of morphological abnormalities.45 Thus, we hypothesized that β8 deficiency resulted in loss of a critical astrocytic regulatory mechanism that controls early vascular differentiation and via TGF-β activation coordinates signaling events required for proper vascular morphology. Indeed, we confirmed that focal VEGF stimulation in mice with conditional deletion of smad4, the essential downstream TGF-β signaling mediator implicated in a subset of hereditary hemorrhagic telangiectasia patients,5 produced a very similar vascular phenotype as seen in the mice with conditional deletion of β8. Signaling inputs from both the TGF-β and activin/BMP pathways converge on SMAD-4, via phosphorylation of SMAD-2/3 and SMAD-1/5/8, respectively13 and the relative contributions of TGF-β and BMPs in vascular morphogenesis have been a subject of active debate.12,14 We found that deletion of β8 in mouse brain was associated with a decrease in pSmad-2 and not pSmad-1/5/8. Therefore, our data suggest the vascular abnormalities resulting from β8 deficiency in our model are due to failure of TGF-β activation and not due to BMPs, which is expected since BMPs lack the canonical RGD binding sequence required for β8 ligand binding.19
Genetic variation is one plausible mechanism that could account for alterations in β8 expression contributing to BAVM pathogenesis. This hypothesis is supported by our findings that common polymorphisms located in the 5′ region of ITGB8 are associated with increased risk of BAVM. The high-risk genotypes for two BAVM-associated SNPs (rs10486391 and rs11982847, both located in intron 1) were also significantly correlated with lower β8 staining, in BAVM tissue, as predicted. Overall results from the haplotype analysis were consistent with the individual SNP analysis. However, two haplotypes tested also contained the high-risk alleles, but were not associated with BAVM (P > 0.4), suggesting that the actual functional variant may be another SNP residing on the at-risk haplotype, ie, an unknown SNP in the LD block, eg, in the ITGB8 promoter region. Sequencing of the putative proximal promoter area identified two singleton SNPs (each present in one AVM patient), neither of which are likely to be functional. Therefore, complete resequencing of the region in LD with the two associated SNPs (at least 4 kb, and potentially up to 10 kb, given the uncertainty of where the LD block 5′ boundary is located, Figure 7A) will be necessary to evaluate all variants in the region and to identify the functional variants explaining the reduced β8 expression levels seen in BAVM tissue. Interestingly, rs11982847, located in intron 1, is in a long, highly conserved mammalian intronic sequence, which may contain important regulatory elements.
Our dysplasia model (VEGF overexpression in the setting of reduced TGF-β activation and signaling) represents a model of defective neoangiogenesis since it falls short of producing bona fide BAVMs. Thus, we do not have direct causal evidence that links reduced β8 expression and BAVMs. The potential importance of our model is that it may provide insights into the events that lead to enlargement of congenital BAVMs in adult life, or to de novo BAVM formation in the postnatal, mature brain. Almost all models of brain vascular abnormalities examine developmental brain vasculogenesis and not neoangiogenesis in the adult brain. This is not a trivial difference since the cellular differentiation, cellular scaffold, and panoply of molecular mediators are very distinct in the developing and mature brain. In our model, partial deletion of itgb8 or smad4 causes vascular dysplasia; in human mature BAVM tissues there is reduced β8 expression, which correlates with genetic variation in the ITGB8 locus. These data suggest that reduced αvβ8 expression, while not sufficient to alone cause BAVMs, has the potential to play a pathogenic role when combined with other causal factors to produce the wide range of pathological phenotypes of BAVMs.
Acknowledgments
We thank Annie Poon, Pirro Hysi, Shantel Weinsheimer, Brad Dispensa, and Voltaire Gungab for technical or manuscript assistance.
Footnotes
Address reprint requests to Stephen L. Nishimura, M.D., Department of Pathology, 1001 Potrero Avenue, Bldg. 3, Rm. 211, San Francisco, CA 94110. E-mail: stephen.nishimura@ucsf.edu.
Supported by National Institutes of Health grants HL63993 and NS44655 (to S.N.), NS27713 (to W.L.Y.), and NS44155 (to W.L.Y. and G.Y.Y.).
H.S. and H.K. contributed equally to this work.
Supplemental material for this article can be found on http://ajp.amjpathol.org.
References
- Young WL, Kwok PY, Pawlikowska L, Lawton MT, Kim H, Hysi PG, Marchuk DA. Arteriovenous malformation. J Neurosurg. 2007;106:731–733. doi: 10.3171/jns.2007.106.4.731. [DOI] [PubMed] [Google Scholar]
- Chen Y, Fan Y, Poon KY, Achrol AS, Lawton MT, Zhu Y, McCulloch CE, Hashimoto T, Lee C, Barbaro NM, Bollen AW, Yang GY, Young WL. MMP-9 expression is associated with leukocytic but not endothelial markers in brain arteriovenous malformations. Front Biosci. 2006;11:3121–3128. doi: 10.2741/2037. [DOI] [PubMed] [Google Scholar]
- Kim H, Pawlikowska L, Chen Y, Su H, Yang GY, Young WL. Brain arteriovenous malformation biology relevant to hemorrhage and implication for therapeutic development. Stroke. 2009;40:S95–S97. doi: 10.1161/STROKEAHA.108.533216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Pawlikowska L, Young WL: Molecular and genetic aspects of brain vascular malformations. Edited by Mohr JP, Wolf PA, Grotta JC, Moskowitz MA, Mayberg M, von Kummer R. Philadelphia, Churchill Livingstone Elsevier (in press) [Google Scholar]
- Young WL, Yang GY. Are there genetic influences on sporadic brain arteriovenous malformations? Stroke. 2004;35:2740–2745. doi: 10.1161/01.STR.0000145054.35083.32. [DOI] [PubMed] [Google Scholar]
- Murphy PA, Lu G, Shiah S, Bollen AW, Wang RA. Endothelial Notch signaling is upregulated in human brain arteriovenous malformations and a mouse model of the disease. Lab Invest: 2009;89:971–982. doi: 10.1038/labinvest.2009.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada Y, Oike Y, Ogawa H, Ito Y, Fujisawa H, Suda T, Takakura N. Neuropilin-1 on hematopoietic cells as a source of vascular development. Blood. 2003;101:1801–1809. doi: 10.1182/blood-2002-01-0119. [DOI] [PubMed] [Google Scholar]
- Bader BL, Rayburn H, Crowley D, Hynes RO. Extensive vasculogenesis: angiogenesis, and organogenesis precede lethality in mice lacking all αv integrins. Cell. 1998;95:507–519. doi: 10.1016/s0092-8674(00)81618-9. [DOI] [PubMed] [Google Scholar]
- McCarty JH, Lacy-Hulbert A, Charest A, Bronson RT, Crowley D, Housman D, Savill J, Roes J, Hynes RO. Selective ablation of αv integrins in the central nervous system leads to cerebral hemorrhage, seizures, axonal degeneration and premature death. Development. 2005;132:165–176. doi: 10.1242/dev.01551. [DOI] [PubMed] [Google Scholar]
- Proctor JM, Zang K, Wang D, Wang R, Reichardt LF. Vascular development of the brain requires beta8 integrin expression in the neuroepithelium. J Neurosci. 2005;25:9940–9948. doi: 10.1523/JNEUROSCI.3467-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Motejlek K, Wang D, Zang K, Schmidt A, Reichardt LF. β8 integrins are required for vascular morphogenesis in mouse embryos. Development. 2002;129:2891–2903. doi: 10.1242/dev.129.12.2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho RL, Itoh F, Goumans MJ, Lebrin F, Kato M, Takahashi S, Ema M, Itoh S, van Rooijen M, Bertolino P, Ten Dijke P, Mummery CL. Compensatory signalling induced in the yolk sac vasculature by deletion of TGF-β receptors in mice. J Cell Sci. 2007;120:4269–4277. doi: 10.1242/jcs.013169. [DOI] [PubMed] [Google Scholar]
- Goumans MJ, Liu Z, ten Dijke P. TGF-β signaling in vascular biology and dysfunction. Cell Res. 2009;19:116–127. doi: 10.1038/cr.2008.326. [DOI] [PubMed] [Google Scholar]
- Park SO, Lee YJ, Seki T, Hong KH, Fliess N, Jiang Z, Park A, Wu X, Kaartinen V, Roman BL, Oh SP. ALK5- and TGFBR2-independent role of ALK1 in the pathogenesis of hereditary hemorrhagic telangiectasia type 2. Blood. 2008;111:633–642. doi: 10.1182/blood-2007-08-107359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang LT, Li WY, Kaartinen V. Tissue-specific expression of Cre recombinase from the Tgfb3 locus. Genesis. 2008;46:112–118. doi: 10.1002/dvg.20372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho RL, Jonker L, Goumans MJ, Larsson J, Bouwman P, Karlsson S, Dijke PT, Arthur HM, Mummery CL. Defective paracrine signalling by TGF-β in yolk sac vasculature of endoglin mutant mice: a paradigm for hereditary haemorrhagic telangiectasia. Development. 2004;131:6237–6247. doi: 10.1242/dev.01529. [DOI] [PubMed] [Google Scholar]
- Annes JP, Munger JS, Rifkin DB. Making sense of latent TGF-β activation. J Cell Sci. 2003;116:217–224. doi: 10.1242/jcs.00229. [DOI] [PubMed] [Google Scholar]
- Cambier S, Gline S, Mu D, Collins R, Araya J, Dolganov G, Einheber S, Boudreau N, Nishimura SL. Integrin αvβ8-mediated activation of transforming growth factor-beta by perivascular astrocytes: an angiogenic control switch. Am J Pathol. 2005;166:1883–1894. doi: 10.1016/s0002-9440(10)62497-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu D, Cambier S, Fjellbirkeland L, Baron JL, Munger JS, Kawakatsu H, Sheppard D, Broaddus VC, Nishimura SL. The integrin αvβ8 mediates epithelial homeostasis through MT1-MMP-dependent activation of TGF-β1. J Cell Biol. 2002;157:493–507. doi: 10.1083/jcb.200109100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks PC, Clark RA, Cheresh DA. Requirement of vascular integrin αvβ3 for angiogenesis. Science. 1994;264:569–571. doi: 10.1126/science.7512751. [DOI] [PubMed] [Google Scholar]
- Friedlander M, Brooks PC, Shaffer RW, Kincaid CM, Varner JA, Cheresh DA. Definition of two angiogenic pathways by distinct alpha v integrins. Science. 1995;270:1500–1502. doi: 10.1126/science.270.5241.1500. [DOI] [PubMed] [Google Scholar]
- Nishimura SL, Boylen KP, Einheber S, Milner TA, Ramos DM, Pytela R. Synaptic and glial localization of the integrin αvβ8 in mouse and rat brain. Brain Research. 1998;791:271–282. doi: 10.1016/s0006-8993(98)00118-8. [DOI] [PubMed] [Google Scholar]
- Cambier S, Mu DZ, O'Connell D, Boylen K, Travis W, Liu WH, Broaddus VC, Nishimura SL. A role for the integrin αvβ8 in the negative regulation of epithelial cell growth. Cancer Res. 2000;60:7084–7093. [PubMed] [Google Scholar]
- Mu Z, Yang Z, Yu D, Zhao Z, Munger JS. TGF-β1 and TGF-β3 are partially redundant effectors in brain vascular morphogenesis. Mech Dev. 2008;125:508–516. doi: 10.1016/j.mod.2008.01.003. [DOI] [PubMed] [Google Scholar]
- Aluwihare P, Mu Z, Zhao Z, Yu D, Weinreb PH, Horan GS, Violette SM, Munger JS. Mice with combined deficits of αvβ6 and αvβ8 integrins reproduce the phenotypes of TGFβ1 and TGFβ3-null mice. J Cell Sci. 2009;15:227–232. doi: 10.1242/jcs.035246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araya J, Cambier S, Markovics JA, Wolters P, Jablons D, Hill A, Finkbeiner W, Jones K, Broaddus VC, Sheppard D, Barzcak A, Xiao Y, Erle DJ, Nishimura SL. Squamous metaplasia amplifies pathologic epithelial-mesenchymal interactions in COPD patients. J Clin Invest. 2007;117:3551–3562. doi: 10.1172/JCI32526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Li C, Herrera PL, Deng CX. Generation of Smad4/Dpc4 conditional knockout mice. Genesis. 2002;32:80–81. doi: 10.1002/gene.10029. [DOI] [PubMed] [Google Scholar]
- Shen F, Su H, Liu W, Kan YW, Young WL, Yang GY. Recombinant adeno-associated viral vector encoding human VEGF165 induces neomicrovessel formation in the adult mouse brain. Front Biosci. 2006;11:3190–3198. doi: 10.2741/2042. [DOI] [PubMed] [Google Scholar]
- Su H, Lu R, Kan YW. Adeno-associated viral vector-mediated vascular endothelial growth factor gene transfer induces neovascular formation in ischemic heart. Proc Natl Acad Sci USA: 2000;97:13801–13806. doi: 10.1073/pnas.250488097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu D, Sullivan CC, Weitzman MD, Du L, Wolf PL, Jamieson SW, Thistlethwaite PA. Direct comparison of efficiency and stability of gene transfer into the mammalian heart using adeno-associated virus versus adenovirus vectors. J Thorac Cardiovasc Surg. 2003;126:671–679. doi: 10.1016/s0022-5223(03)00082-5. [DOI] [PubMed] [Google Scholar]
- Sato S, Kodama N, Sasaki T, Matsumoto M, Ishikawa T. Perinidal dilated capillary networks in cerebral arteriovenous malformations. Neurosurgery. 2004;54:163–168; discussion 168–170. doi: 10.1227/01.neu.0000097518.57741.be. [DOI] [PubMed] [Google Scholar]
- Kim BG, Li C, Qiao W, Mamura M, Kasprzak B, Anver M, Wolfraim L, Hong S, Mushinski E, Potter M, Kim SJ, Fu XY, Deng C, Letterio JJ. Smad4 signalling in T cells is required for suppression of gastrointestinal cancer. Nature. 2006;441:1015–1019. doi: 10.1038/nature04846. [DOI] [PubMed] [Google Scholar]
- Kim H, Sidney S, McCulloch CE, Poon KY, Singh V, Johnston SC, Ko NU, Achrol AS, Lawton MT, Higashida RT, Young WL. Racial/Ethnic differences in longitudinal risk of intracranial hemorrhage in brain arteriovenous malformation patients. Stroke. 2007;38:2430–2437. doi: 10.1161/STROKEAHA.107.485573. [DOI] [PubMed] [Google Scholar]
- Shu Y, Brown C, Castro RA, Shi RJ, Lin ET, Owen RP, Sheardown SA, Yue L, Burchard EG, Brett CM, Giacomini KM. Effect of genetic variation in the organic cation transporter 1. OCT1, on metformin pharmacokinetics. Clin Pharmacol Ther. 2008;83:273–280. doi: 10.1038/sj.clpt.6100275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bakker PI, Yelensky R, Pe'er I, Gabriel SB, Daly MJ, Altshuler D. Efficiency and power in genetic association studies. Nat Genet. 2005;37:1217–1223. doi: 10.1038/ng1669. [DOI] [PubMed] [Google Scholar]
- Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21:263–265. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- Hsu TM, Kwok PY. Homogeneous primer extension assay with fluorescence polarization detection. Methods Mol Biol. 2003;212:177–187. doi: 10.1385/1-59259-327-5:177. [DOI] [PubMed] [Google Scholar]
- Pawlikowska L, Tran MN, Achrol AS, McCulloch CE, Ha C, Lind DL, Hashimoto T, Zaroff J, Lawton MT, Marchuk DA, Kwok PY, Young WL. Polymorphisms in genes involved in inflammatory and angiogenic pathways and the risk of hemorrhagic presentation of brain arteriovenous malformations. Stroke. 2004;35:2294–2300. doi: 10.1161/01.STR.0000141932.44613.b1. [DOI] [PubMed] [Google Scholar]
- Purcell S, Daly MJ, Sham PC. WHAP: haplotype-based association analysis. Bioinformatics. 2007;23:255–256. doi: 10.1093/bioinformatics/btl580. [DOI] [PubMed] [Google Scholar]
- Gingras MC, Roussel E, Bruner JM, Branch CD, Moser RP. Comparison of cell adhesion molecule expression between glioblastoma multiforme and autologous normal brain tissue. J Neuroimmunol. 1995;57:143–153. doi: 10.1016/0165-5728(94)00178-q. [DOI] [PubMed] [Google Scholar]
- Lee CZ, Xue Z, Zhu Y, Yang GY, Young WL. Matrix metalloproteinase-9 inhibition attenuates vascular endothelial growth factor-induced intracerebral hemorrhage. Stroke. 2007;38:2563–2568. doi: 10.1161/STROKEAHA.106.481515. [DOI] [PubMed] [Google Scholar]
- Acsadi G, Jani A, Massie B, Simoneau M, Holland P, Blaschuk K, Karpati G. A differential efficiency of adenovirus-mediated in vivo gene transfer into skeletal muscle cells of different maturity. Hum Mol Genet. 1994;3:579–584. doi: 10.1093/hmg/3.4.579. [DOI] [PubMed] [Google Scholar]
- Travis MA, Reizis B, Melton AC, Masteller E, Tang Q, Proctor JM, Wang Y, Bernstein X, Huang X, Reichardt LF, Bluestone JA, Sheppard D. Loss of integrin αvβ8 on dendritic cells causes autoimmunity and colitis in mice. Nature. 2007;449:361–365. doi: 10.1038/nature06110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Mu Z, Dabovic B, Jurukovski V, Yu D, Sung J, Xiong X, Munger JS. Absence of integrin-mediated TGF-β1 activation in vivo recapitulates the phenotype of TGF-β1-null mice. J Cell Biol. 2007;176:787–793. doi: 10.1083/jcb.200611044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braverman IM, Keh A, Jacobson BS. Ultrastructure and three-dimensional organization of the telangiectases of hereditary hemorrhagic telangiectasia. J Invest Dermatol. 1990;95:422–427. doi: 10.1111/1523-1747.ep12555569. [DOI] [PubMed] [Google Scholar]