SUMMARY
The amygdala processes and directs inputs and outputs that are key to fear behavior. However, whether it directly senses fear-evoking stimuli is unknown. Because the amygdala expresses acid sensing ion channel-1a (ASIC1a), and ASIC1a is required for normal fear responses, we hypothesized that the amygdala might detect a reduced pH. We found that inhaled CO2 reduced brain pH and evoked fear behavior in mice. Eliminating or inhibiting ASIC1a markedly impaired this activity, and localized ASIC1a expression in the amygdala rescued the CO2- induced fear deficit of ASIC1a-null animals. Buffering pH attenuated fear behavior, whereas directly reducing pH with amygdala microinjections reproduced the effect of CO2. These data identify the amygdala as an important chemosensor that detects hypercarbia and acidosis and initiates behavioral responses. They also give a molecular explanation for how rising CO2 concentrations elicit intense fear and provide a foundation for dissecting the bases of anxiety and panic disorders.
INTRODUCTION
Fear is an evolutionarily conserved process that ensures survival by initiating avoidance or escape from predators and harmful situations (Ohman and Mineka, 2001). In humans, abnormal fear and anxiety are hallmarks of many psychiatric diseases; panic disorder and post-traumatic stress disorder are two extreme examples. Previous studies have revealed much about the complex behavioral neurobiology that underlies fear in mammals, including both conditioned (or acquired) and unconditioned (or innate) fear (Gorman et al., 2000; Kim and Jung, 2006; Maren, 2008; Phelps and LeDoux, 2005; Rosen, 2004). Acquired fear involves the learned association of a conditioned stimulus, such as a specific environment, with an unconditioned stimulus such as a foot shock. An example is the contextual fear conditioning protocol often utilized in mice (Kim and Jung, 2006; Maren, 2008). In contrast, innate fear does not require prior conditioning; examples are a fear of snakes in humans and the innate fear of fox feces odor (trimethylthiazoline) in mice (Ohman and Mineka, 2001; Rosen, 2004).
For both innate and acquired fear behavior, the amygdala, including lateral, basal and central nuclei, plays a critical role (Adolphs, 2002; Anglada-Figueroa and Quirk, 2005; Bechara et al., 1995; Goosens and Maren, 2001; Kim and Jung, 2006; Maren, 2008; Phelps and LeDoux, 2005; Rabinak and Maren, 2008; Rosen, 2004). The lateral amygdala accepts input arising from sensory systems including sensory cortices and thalamus, from executive cortices, and hippocampus. The basal amygdala communicates with both lateral and central nuclei of the amygdala and also contributes to fear behavior. Much of the output from the amygdala flows from the central nucleus of the amygdala, which is critical for signaling areas producing physiological and behavioral manifestations of fear. The amygdala is also important in learned fear, forming associations between conditioned and unconditioned stimuli. Thus, the amygdala is central for processing and directing the inputs and outputs that are key to fear behavior. However, the amygdala itself is not known to be a sensor of fear evoking stimuli.
In previous studies, we discovered that the acid sensing ion channel-1a (ASIC1a) is particularly abundant in the amygdala and other fear circuit structures (Coryell et al., 2007; Wemmie et al., 2003), and it is required for normal responses in tests of both conditioned and unconditioned fear behavior (Coryell et al., 2007; Coryell et al., 2008; Wemmie, 2004; Wemmie et al., 2003). ASIC1a is located in dendrites and on dendritic spines, as well as on neuronal cell bodies (Wemmie et al., 2002; Zha et al., 2009; Zha et al., 2006). ASICs are subunits that associate as trimers (Jasti et al., 2007) to form channels permeable to Na+ and Ca2+ (Waldmann et al., 1997; Xiong et al., 2004; Yermolaieva et al., 2004). In the CNS, the predominantly expressed ASIC subunits are ASIC1a, -2a, and -2b, which assemble into homo- and hetero-trimeric complexes (Benson et al., 2002; Jasti et al., 2007; Lingueglia et al., 1997). ASICs are activated in vitro when extracellular pH falls, and although acidic pH modifies the activity of many receptors and proteins, few besides ASICs are activated by extracellular acidosis, and few are as exquisitely pH-sensitive (Diochot et al., 2007; Krishtal, 2003; Wemmie et al., 2006). Disrupting the ASIC1a gene (ACCN2) in mice eliminates currents evoked by pH as low as 5.0 in central neurons (Askwith et al., 2004; Wemmie et al., 2002). Thus, ASIC1a is critical for acid-evoked currents in CNS neurons.
The contribution of both the amygdala and ASIC1a to fear behavior raised questions of how ASIC1a in the amygdala is activated, especially in vivo. We hypothesized that a reduced pH might induce fear behavior by activating ASIC1a, thereby allowing the amygdala to function as a chemosensor deep within the fear circuit. To explore this issue, we used CO2 inhalation for three main reasons. First, physiologic effects of CO2 in mammals typically result from a reduced pH (Zandbergen et al., 1989), which might activate ASIC1a channels. Brain pH rapidly falls when the enzyme carbonic anhydrase catalyzes the conversion of CO2 and water to carbonic acid (Nattie, 1998). Second, it is well known that rising CO2 concentrations can elicit intense fear (Papp et al., 1993). Third, the use of CO2 as a probe has important clinical implications for psychiatric disorders.
Nearly a century ago, it was discovered that CO2 inhalation can trigger panic attacks, and patients with panic disorder are particularly susceptible (Drury, 1918). Many subsequent clinical studies fostered hope that understanding CO2 sensitivity would provide unique insight into the neurobiology underlying anxiety disorders and lead to better treatments (Dratcu, 2000; Gorman et al., 2000; Rassovsky and Kushner, 2003; Smoller et al., 1996). Several theories have sought to explain how CO2 inhalation produces fear and panic (Drury, 1918; Gorman et al., 2000; Klein, 1993; Sanderson et al., 1989). Early on, cardiovascular sensitivity was suspected (Drury, 1918). Others suggested that CO2 sensitivity in panic might be due to a psychological vulnerability to losing control (Sanderson et al., 1989). A role for brainstem ventilatory chemosensors and hyperventilation have also been speculated (Gorman et al., 2000; Sullivan et al., 2003) and the “false-suffocation-alarm theory” proposes that rising CO2 heralds impending suffocation and that this alarm is falsely triggered in patients with panic disorder (Klein, 1993). However, the molecular identity and neuroanatomical location of the putative CO2 alarm have remained uncertain (Dratcu, 2000; Gorman et al., 2000; Griez and Schruers, 1998; Klein, 1993).
With this background, we hypothesized that inhaling CO2 would reduce brain pH and that the acidosis would trigger ASIC channels in the amygdala to elicit fear. While CO2 is known to stimulate chemosensors in the periphery and brainstem, if CO2 and hence acidosis were to directly activate the amygdala, it would suggest that the amygdala is an important chemosensory structure (Brannan et al., 2001; Gorman et al., 2000).
RESULTS
Loss or inhibition of ASIC1a impairs CO2-induced fear behavior
We developed four paradigms to test CO2-triggered fear in mice: 1) CO2-evoked freezing, 2) CO2 effects in the open-field test 3) CO2 aversion, and 4) CO2-potentiated context fear conditioning.
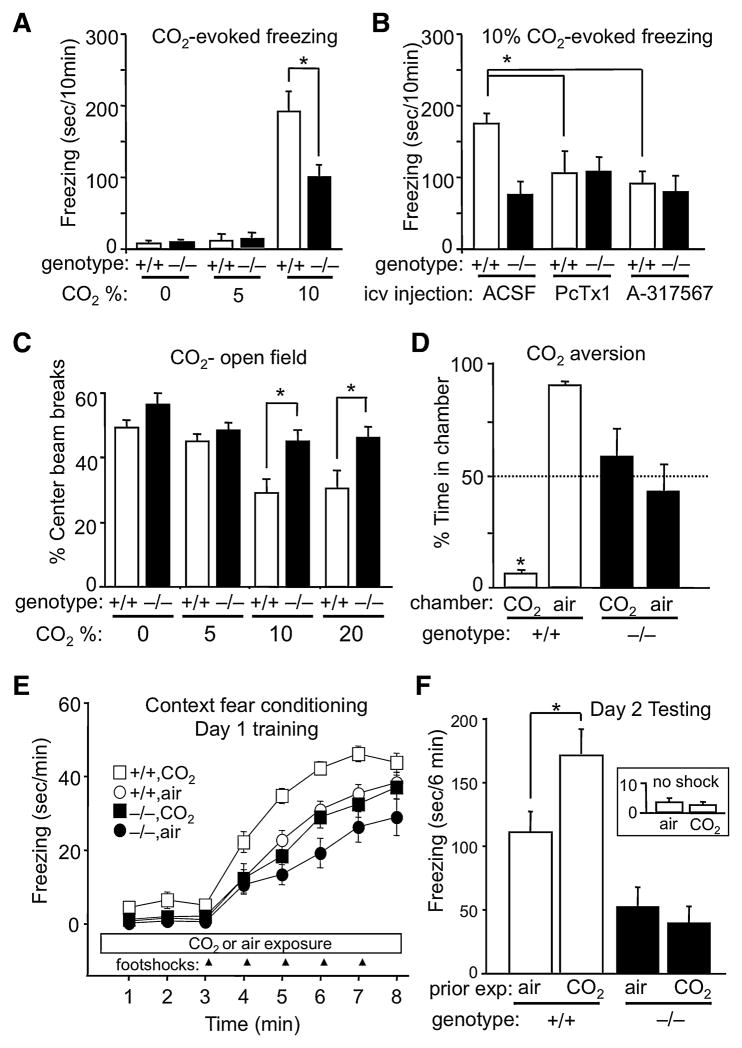
To assess the response to CO2, we assayed freezing, which is used as a correlate of fear and panic in mice (Mongeluzi et al., 2003). In humans with panic disorder, breathing 5% CO2 evokes panic attacks (Rassovsky and Kushner, 2003) but it rarely evokes panic in control subjects. Consistent with this, 5% CO2 produced little or no freezing in mice (Fig. 1A). In humans who do not have panic disorder, breathing higher CO2 concentrations is more likely to evoke panic (Rassovsky and Kushner, 2003). Likewise, breathing 10% CO2 triggered significant freezing in control mice (Fig. 1A). However, disrupting the ASIC1a gene substantially blunted the response. Moreover, acutely inhibiting ASIC1a with either psalmotoxin (PcTx1) (Diochot et al., 2007) or A-317567 (Dube et al., 2005) also reduced CO2-evoked freezing in wild-type mice (Fig. 1B). Neither inhibitor reduced freezing in ASIC1a−/− animals, suggesting that their effects were ASIC1a-specific. Thus, CO2 produced fear-like freezing behavior that depended on ASIC1a.
Figure 1.
ASIC1a disruption attenuates CO2 effects in multiple behavioral models of fear. (A) CO2 evoked prominent fear-like freezing behavior compared to air. ASIC1a−/− mice were significantly impaired relative to wild-type controls. ANOVA revealed a significant CO2 by genotype interaction (F(2, 35) = 4.62, p < 0.05). Planned contrast testing revealed a significant attenuation of freezing in the ASIC1a−/− mice in response to 10% CO2 (*p < 0.001), but not in air or 5% CO2 (p > 0.05). From left to right, groups contained 5, 6, 7, 6, 7 and 10 mice. (B) ASIC1a antagonists PcTx1 and A-317567 reduced 10% CO2-evoked freezing in wild-type mice but had no effect on CO2-evoked freezing in the ASIC1a−/− mice. ANOVA revealed significant effect of group (F(5, 43) = 3.87, p < 0.01). Significant pairwise comparisons are indicated (*p < 0.05; n = 7–10 mice per group). (C) CO2 increases anxiety-like behavior in the open field test. ANOVA revealed significant main effects of CO2 (F(3, 64) = 8.57, p < 0.001) and genotype (F(1, 64) = 15.95, p < 0.001), but not a CO2 by genotype interaction (F(3, 64) = 1.38, p > 0.05). Planned contrast comparisons revealed significant differences between ASIC1a+/+ and ASIC1a−/− mice at the higher CO2 concentrations (* p < 0.01; n = 7–10 mice per group). This difference was not due to differences in general locomotor activity; we previously showed that ASIC1a disruption does not alter total locomotor activity at baseline (Coryell et al., 2007). Similarly, in these studies during CO2 exposure we found no effect of ASIC1a disruption on total beam breaks (F < 1). (D) ASIC1a+/+ mice avoided the CO2 chamber, whereas ASIC1a−/− CO2 did not. Consequently, the ASIC1a+/+ spent significantly less time on the CO2 side than the ASIC1a−/− mice (* p = 0.004, Wilcoxon rank sum test; n = 7 and 9 per group). (E) CO2 enhanced freezing in ASIC1a+/+ mice during context fear conditioning. Analysis of total freezing during training revealed a significant difference between the groups (F(3, 62) = 13.94, p < 0.001, ANOVA). Planned contrast comparisons revealed a significant effect of CO2 in ASIC1a+/+ mice (p < 0.001), but not in ASIC1a−/− mice (p > 0.05). (F) Previous CO2 exposure potentiated context-evoked fear memory in ASIC1a+/+ mice, but not in ASIC1a−/− mice. Significant overall differences were detected between the groups (F(3, 62) = 7.99, p < 0.001, ANOVA). Planned contrast testing revealed a significant effect of CO2 in ASIC1a+/+ mice (* p < 0.05), but not in ASIC1a−/− mice (p > 0.05).
The tendency of mice to avoid the center of an open field is used as a measure of their fear and anxiety of open spaces. In wild-type mice, breathing CO2 concentrations greater than 5% reduced the amount of center activity in an open field (Fig. 1C). However this effect was much attenuated in ASIC1a−/− mice.
We examined the aversive effect of CO2 on behavior by allowing mice to move between two chambers, one with 15% CO2 and one with <2% CO2. During a 10 min assay, ASIC1a+/+ mice only briefly sampled the CO2 chamber, spending >90% of the time in the air chamber (Fig. 1D). In contrast, ASIC1a−/− mice spent similar amounts of time in both chambers. Failure to avoid CO2 did not generalize to other aversive stimuli as both genotypes avoided moving air infused into one of the chambers; ASIC1a+/+ mice spent 69 ± 7% and ASIC1a−/− mice 82 ± 4% of time in the non-air-infused chamber (n = 7 per group; p > 0.15; Wilcoxon rank sum).
Context fear conditioning is often used to measure fear and anxiety, and our earlier work showed that disrupting or inhibiting ASIC1a impaired fear conditioning (Coryell et al., 2007; Coryell et al., 2008; Wemmie, 2004). During training with a series of footshocks, wild-type mice froze (Fig. 1E), as previously described (Coryell et al., 2008). However, when training was conducted in the presence of 10% CO2, mice began to freeze before receiving footshocks and froze even more once the footshocks were delivered. The following day, we returned the mice to the original context (minus footshocks and minus CO2) and found that wild-type mice trained in CO2 showed more freezing than those not exposed to CO2 (Fig. 1F). In contrast, CO2 had no significant effect on context fear conditioning in ASIC1a−/− mice. To learn whether CO2 itself might have served as an unconditioned stimulus, we used wild-type mice and delivered CO2 or air but no footshocks on the training day. On the testing day, neither group froze in response to the context (Fig. 1F, inset). Thus, CO2 by itself did not act as an unconditioned stimulus, but it enhanced fear memory when coupled with footshocks.
Thus, results in four different models suggest that CO2 evokes fear-like responses in wild-type mice and that those behaviors depend on ASIC1a.
The ventilatory response to CO2 is intact in ASIC1a−/− mice
It seemed unlikely that genotype-dependent disparities in the arterial pressure of CO2 (PaCO2) would have accounted for differences in fear behavior in ASIC1a+/+ and ASIC1a−/− mice. Consistent with that assumption, breathing 10% CO2 induced a similar elevation of PaCO2 in both genotypes (Fig. 2A).
Figure 2.
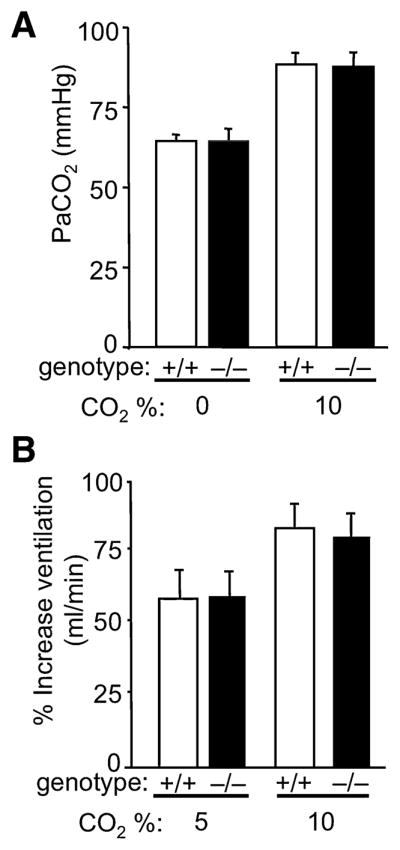
CO2 inhalation increases arterial CO2 partial pressure (PaCO2) and stimulates ventilation normally in the ASIC1a−/− mice. (A) PaCO2 did not differ between ASIC1a+/+ and ASIC1a−/− mice. ANOVA revealed significant main effect of CO2 (F(1,8) = 50.2, p < 0.001; n = 3 per group), but not genotype (F < 1) and no interaction (F < 1). (B) CO2-evoked increases in minute ventilation relative to baseline did not differ between ASIC1a+/+ and ASIC1a−/− mice (F(1, 46) = 0.065, p = 0.80, ANOVA; from left to right groups comprised of 17, 17, 12, and 4 mice).
Because hypercarbic acidosis is well known to increase ventilation, we also measured the ventilatory response to CO2 inhalation. Wild-type and ASIC1a−/− mice showed a similar response to hypercarbia (Fig. 2B). These data suggest that ASIC1a does not affect the chemosensory system controlling ventilation.
Breathing CO2 reduces amygdala pH
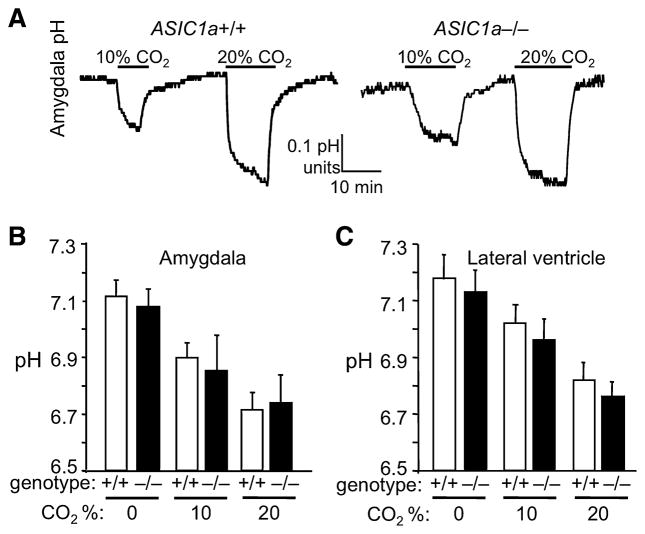
We then turned our attention to the amygdala because of the possible association between the amygdala and panic (Gorman et al., 2000), and because of the robust ASIC1a expression in the basolateral amygdala (Coryell et al., 2007). We measured pH in the basolateral amygdala of anesthetized mice and found that breathing CO2 reduced pH of both genotypes similarly (Fig. 3A,B). We recorded similar reductions in the lateral ventricle (Fig. 3C). Baseline pH in the mice breathing air was less than the expected range (pH ~7.29–7.39) (Mayhan et al., 1988; Mitchell et al., 1965), likely due to respiratory suppression during anesthesia. Supporting this, PaCO2 in the anesthetized mice was elevated (65 ± 3 mmHg) (Fig. 2A) compared to levels (39 – 45 mm Hg) reported for awake or mechanically ventilated animals (Mayhan et al., 1988; Mitchell et al., 1965).
Figure 3.
CO2 inhalation reduces amygdala pH. (A) Examples of pH recordings in amygdala showing effects of CO2 inhalation. (B) Mean pH response in basolateral amygdala to CO2 inhalation was normal in ASIC1a−/− mice. ANOVA revealed a significant effect of CO2 (F (2, 15) = 7.8, p < 0.01), but no effect of genotype (F(1, 15) = 0.2, p = 0.66) and no interaction (F< 1, n = 3–4 mice per group). (C) Lateral ventricle pH response to CO2 inhalation. ANOVA revealed a significant effect of CO2 (F (2, 30) = 12.0, p < 0.001), but no effect of genotype (F(1, 30) = 1.2, p = 0.3) and no interaction (F< 1, n = 6 mice per group).
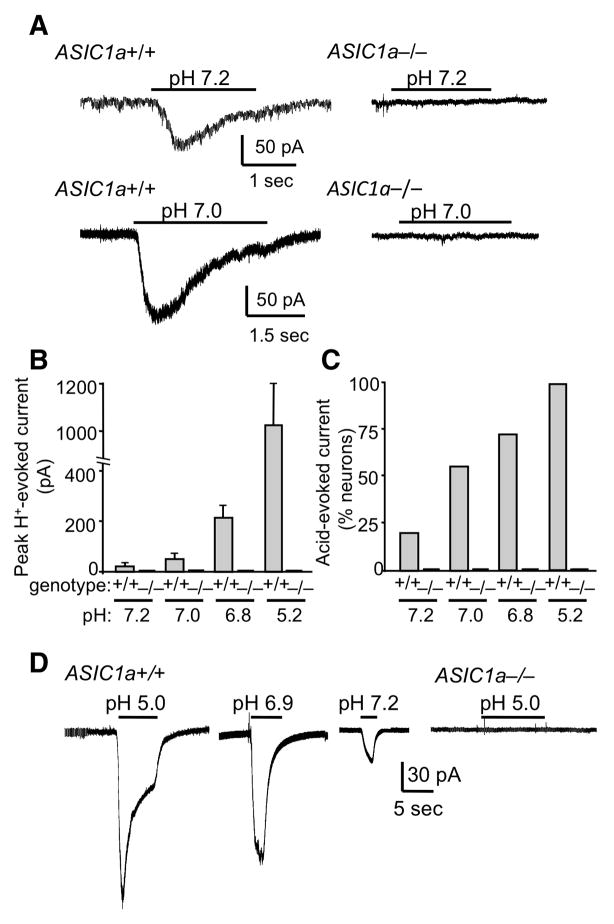
To determine if these changes were sufficient to activate ASICs, we measured current in cultured amygdala neurons. In wild-type neurons, lowering pH to 7.2 induced current in 21% of cells, and further reductions generated current in a greater percentage of neurons and evoked larger currents (Fig. 4A–C). The acidosis elicited no current in amygdala neurons from ASIC1a−/− mice, consistent with data from neurons of other brain areas (Askwith et al., 2004; Wemmie et al., 2002; Xiong et al., 2004). We also detected acid-evoked currents in amygdala neurons from ASIC+/+, but not ASIC−/− brain slices (Fig. 4D). These data are consistent with our studies of cultured neurons and earlier studies in brain slices from other brain regions (Chen et al., 2009; Pidoplichko and Dani, 2006; Zha et al., 2006).
Figure 4.
Acid-evoked currents in amygdala neurons are mediated by ASIC1a. (A) Representative examples of responses to pH 7.2 and 7.0 in cultured amygdala neurons from ASIC1a+/+ and ASIC1a−/− mice recorded by whole-cell voltage clamp. (B) Mean peak current evoked by increasingly acidic pH levels in cultured amygdala neurons from ASIC1a+/+ and ASIC1a−/− mice. From left to right, ASIC1a+/+ groups included 14, 17, 19, and 9 neurons. ASIC1a−/− groups each included 7 neurons. (C) As pH was lowered, a greater percentage of amygdala neurons from ASIC1a+/+ mice exhibited acid-evoked current. None of the ASIC1a−/− neurons showed acid-evoked current. (D) Examples of acid-evoked currents in basolateral amygdala neurons in acute slices from ASIC1a+/+ and ASIC1a−/− mice. The number of cells exhibiting acid-evoked currents versus the total number tested at each pH value were as follows: (ASIC1a+/+: pH 5 = 16/21, pH 6.9 = 6/9, pH 7.2 = 2/5), (ASIC1−/−: pH 5 = 0/9, pH 6.9 = 0/7, pH 7.2 = 0/4).
Administering HCO3− attenuates the CO2-induced pH reduction and fear behavior
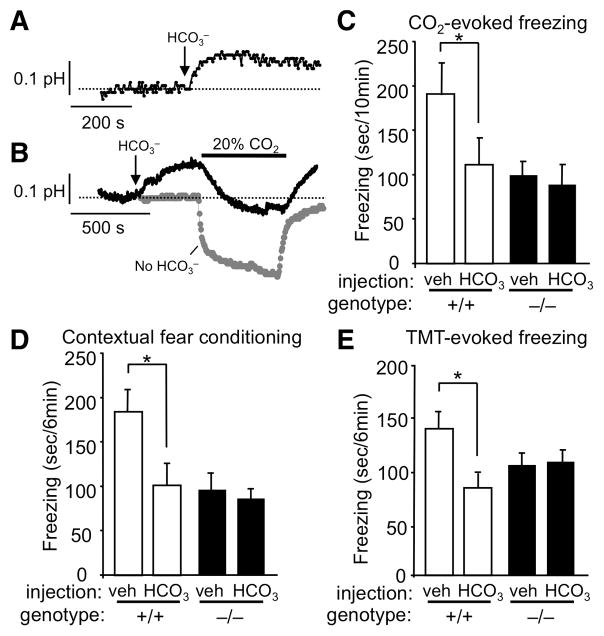
Because reducing amygdala pH was sufficient to trigger fear through ASIC1a, we reasoned that minimizing the pH reduction induced by CO2 inhalation would attenuate the fear response. To counteract hypercarbic acidosis, we administered HCO3− systemically and found that it raised amygdala pH (Fig. 5A). Subsequent CO2 breathing lowered pH, but not to values as low as observed in control mice (Fig. 5B).
Figure 5.
Raising brain pH with HCO3− blocks CO2–evoked freezing, fear conditioning, and predator odor-evoked fear. (A) Representative tracing of amygdala pH response to HCO3− injection (2 mM/kg, ip). (B) Effect of HCO3− injection on pH response to CO2 inhalation (black trace) versus no HCO3− injection (gray trace). (C) HCO3− reduced CO2-evoked freezing in ASIC1a+/+ mice, but not in ASIC1a−/− mice. ANOVA revealed significant differences between groups (F(3, 30) = 3.92, p < 0.05). Planned contrast testing revealed a significant effect of HCO3− in ASIC1a+/+ mice (*p < 0.05), but not in ASIC1a−/− mice (p = 0.8). From left to right, group sizes were 8, 7, 10, and 9 mice. (D) HCO3− injection prior to fear conditioning inhibited the contextually conditioned fear response in ASIC1a+/+ mice but not in ASIC1a−/− mice. ANOVA revealed significant differences between groups (F(3, 30) = 5.91, p < 0.01). Planned contrast testing found a significant effect of HCO3− in ASIC1a+/+ mice (*p < 0.05), but not in ASIC1a−/− mice (p = 0.9). From left to right, group sizes were 14, 6, 7, and 7 mice. (E) HCO3− injection prior to predator odor exposure reduced freezing in ASIC1a+/+ mice but not in ASIC1a−/− mice. ANOVA revealed significant differences between groups (F(3, 26) = 3.46, p < 0.05). Planned contrast testing found a significant effect of HCO3− in ASIC1a+/+ mice (*p < 0.01), but not in ASIC1a−/− mice (p = 0.9). From left to right, group sizes were 5, 7, 10, and 8 mice.
We therefore pretreated mice with HCO3− and tested CO2-evoked fear behavior. HCO3− attenuated freezing in ASIC1a+/+ mice breathing CO2 (Fig. 5C). In contrast, administering HCO3− had no effect in ASIC1a−/− mice. These data further indicate that a reduced pH initiates the fear-related response to CO2 inhalation.
HCO3− impairs ASIC1a-dependent effects on conditioned and unconditioned fear behavior
In earlier studies, we found that ASIC1a was required for normal responses in tests of acquired and innate fear (Coryell et al., 2007; Coryell et al., 2008; Wemmie, 2004; Wemmie et al., 2003). Our current results raised the question of whether HCO3− administration might also interfere with those fear-related behaviors. We injected mice with HCO3− and within 15 min, while brain pH was still elevated, we trained them in a contextual fear conditioning task. The following day, after brain pH had normalized, we tested conditioned fear memory. HCO3− reduced conditioned freezing in ASIC1a+/+ mice, but not in ASIC1a−/− mice (Fig. 5D). These results indicate that elevating brain pH interfered with the ability of ASIC1a to promote fear learning
We also tested the effects of HCO3− on unconditioned fear. Alkalinizing brain pH by administering HCO3− reduced freezing evoked by the predator odor TMT in ASIC1a+/+, but not ASIC1a−/− mice (Fig. 5E). The ability of HCO3− to reduce conditioned and unconditioned fear in wild-type mice and the absence of this effect in ASIC1a-null mice, suggest that ASIC1a activation in vivo is pH-dependent.
Reducing pH in the amygdala elicits fear behavior
Our data indicate that CO2 reduces amygdala pH, that the reduction is sufficient to activate amygdala ASIC currents, and that ASIC1a is required for CO2-induced fear behavior. However, breathing CO2 likely reduces pH throughout the brain, as indicated by the reduced pH in the ventricles (Fig. 3C). Moreover, although ASIC1a expression is abundant in the amygdala, it is also widely distributed in the CNS. Therefore, we asked if pH reductions limited to the amygdala would produce fear behavior.
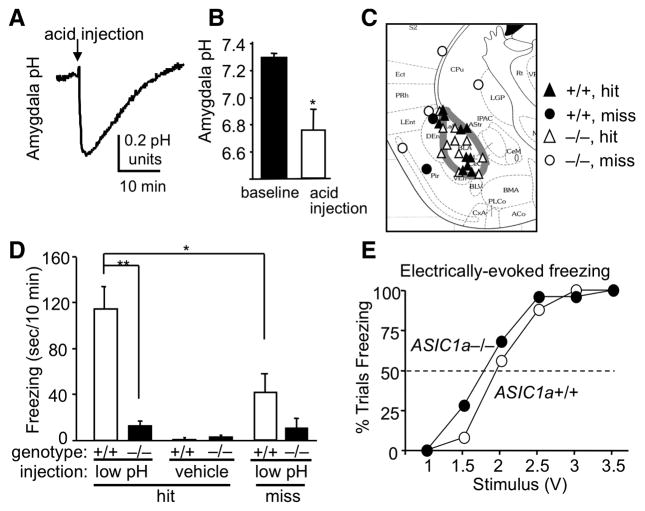
We microinjected acidified ACSF into the amygdala, and with a pH sensor positioned <1 mm from the injection site showed that pH fell to ~6.8 (Fig. 6A,B). We detected no acidosis with the sensor ~3 mm from the injector tip, indicating a localized pH reduction. Microinjections of acidic ACSF into the amygdala produced robust freezing in wild-type mice (Fig. 6C,D). There was no freezing when we microinjected neutral pH ACSF, and injections that missed the amygdala produced much less freezing (Fig. 6C,D). In contrast, acidic microinjections induced very little freezing in ASIC1a null mice. This freezing deficit did not carry over to other stimuli since both genotypes developed similar freezing when we electrically stimulated the basolateral amygdala (Fig. 6E). These data suggest that amygdala acidosis evokes freezing behavior and that ASIC1a plays a critical role.
Figure 6.
Amygdala acidosis evokes ASIC1a-dependent freezing behavior. (A) Injecting acidic ACSF into the amygdala of an anaesthetized mouse lowered amygdala pH by several tenths of a pH unit. (B) Acid injection lowered pH to 6.8 or below. (C) Acidic injections that hit the basolateral amygdala (gray boundary) versus those that missed. (D) Acidic injections that hit the amygdala in awake and freely moving mice triggered robust freezing in ASIC1a+/+ mice and very little freezing in ASIC1a−/− mice. ANOVA revealed a significant pH by genotype interaction (F(1, 28) = 12.8, p < 0.001). In the low-pH-injected mice, planned contrast testing revealed significantly greater freezing in ASIC1a+/+ hit mice compared to ASIC1a−/− hit mice (**p < 0.001) and compared to ASIC1a+/+ miss mice (*p < 0.05) (group sizes from left to right were 11, 10, 4, 7, 2, and 4 mice). (E) Electrically stimulating the left amygdala with a bipolar electrode evokes similar rate of freezing responses in ASIC1a+/+ and ASIC1a−/− mice. Shown are percentages of trials per 5 attempts that produced freezing at each voltage (n = 5 mice per group; ASIC1a+/+ ED50 = 1.99 V, 95% CI = 1.64–2.27; ASIC1a−/− ED50 = 1.81 V, 95% CI = 1.44 – 2.09).
ASIC1a in the amygdala is sufficient for CO2-evoked fear behavior
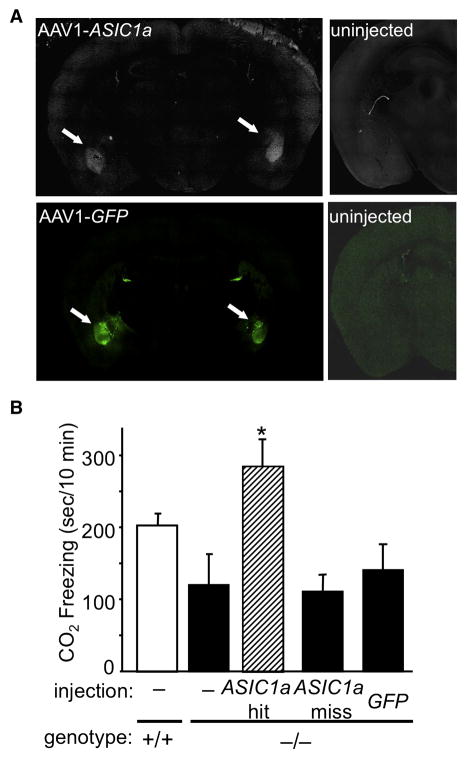
These findings raised the question of whether ASIC1a expression restricted to the amygdala was sufficient for CO2-evoked fear behavior. To answer this question, we injected an adeno-associated virus encoding ASIC1a into the amygdala of ASIC1a−/− mice (Fig. 7A,B), as we have previously described (Coryell et al., 2008). This injection involved lateral and basal nuclei of the amygdala, as did the acid injection described above. We found that expressing ASIC1a in the basolateral amygdala restored CO2-evoked freezing to levels comparable to or higher than those in ASIC1a+/+ mice (Fig. 7B). However, injections that missed the amygdala or expression of GFP had minimal effects on CO2-evoked freezing.
Figure 7.
Restoring ASIC1a expression bilaterally in the basolateral amygdala of ASIC1a−/− mice rescues CO2-evoked freezing. (A) Example of AAV-mediated ASIC1a expression and eGFP expression in bilateral amygdala of ASIC1a−/− mouse. (B) AAV-ASIC1a injections that hit the basolateral amygdala (BLA) bilaterally (ASIC1a-hit) increased CO2-evoked freezing. One-way ANOVA revealed significant differences between the groups (F(4, 42) = 4.85, p < 0.01), and planned contrast tests revealed a significant increase in CO2-evoked freezing in the ASIC1a-hit group relative to other ASIC1a−/− groups (*p < 0.001). From left to right groups sizes were 14, 8, 5, 12, and 8.
DISCUSSION
Earlier studies demonstrated the critical contribution of the amygdala to the fear circuit and fear-related behavior (Adolphs, 2002; Kim and Jung, 2006; Maren, 2008; Phelps and LeDoux, 2005; Rosen, 2004). Our results now indicate that the amygdala does more than mediate the fear response, it also has an important chemosensory role. ASIC1a channels in the amygdala detect a reduced pH arising from an increased CO2 or from direct injection of acid. That CO2 initiates a fear response is particularly intriguing because rising CO2 forewarns suffocation, a terrifying situation that demands sensitive detection and action to ensure survival. Thus, it is interesting that evolution positioned a sensor for hypercarbic acidosis in the amygdala, a structure that stimulates the sympathetic nervous system for fight-or-flight and links to other brain regions involved in the response to threat. Thus, the amygdala both senses a threat, posed by CO2, and initiates a response.
The amygdala also plays a primary role in forming memories of events that induce strong emotions (Kim and Jung, 2006; Maren, 2008). It is key to learning the association between conditioned and unconditioned, aversive, fear-evoking stimuli (Kim and Jung, 2006; Maren, 2008). Our discovery that CO2 enhanced context fear-conditioning reveals a previously unrecognized relationship between CO2 and fear memory. Based on our earlier findings that ASIC1a is located post-synaptically (Schnizler et al., 2009; Wemmie et al., 2004; Wemmie et al., 2003; Wemmie et al., 2002; Zha et al., 2009; Zha et al., 2006) and that ASIC1a is required for normal synaptic plasticity in the hippocampus (Wemmie et al., 2002; Zha et al., 2006), we speculate that CO2 may have potentiated synaptic plasticity in the amygdala by activating ASIC1a.
ASIC1a loss and inhibition did more than impair CO2-induced fear behavior; it also attenuated conditioned and unconditioned fear behavior (Fig. 1E,1F,5D,5E) (Coryell et al., 2007; Coryell et al., 2008; Wemmie, 2004; Wemmie et al., 2003). In addition, ASIC1a−/− mice tended (not statistically significant) to show less anxiety/fear (enter the center more frequently) than wild-type mice, and the difference between genotypes was accentuated by CO2 (Fig. 1C). These results raised the possibility that ASIC1a action in those assays was also pH-dependent. Indeed, supporting that possibility, we found that HCO3− administration inhibited contextual fear conditioning and TMT-evoked freezing. Thus, we speculate that when fear-evoking stimuli activate the amygdala, its pH may fall. For example, synaptic vesicles release protons (DeVries, 2001; Palmer et al., 2003), and intense neural activity is known to lower pH (Kaila and Chesler, 1998). An ability to measure these pH changes in an awake, behaving animal might be informative, but also challenging. Supporting an important functional role for pH, studies in C. elegans recently demonstrated that protons act as a direct transmitter from intestinal to muscle cells (Beg et al., 2008). Based on all these considerations, we speculate that local pH fluctuations and ASIC activation might also be important in brain regions outside the amygdala, and might explain the requirement of ASIC1a for normal long term potentiation (Wemmie et al., 2002).
While our data emphasize the importance of ASIC1a for the amygdala’s response to CO2, they also suggest that there must be additional pH sensors in the brain. For example, the ventilatory response to CO2 inhalation remained intact in ASIC1a−/− mice, suggesting that respiratory control centers utilize different molecular mechanisms to detect acidosis. In addition, disrupting the ASIC1a gene did not completely eliminate all the behavioral responses to breathing CO2. It seems unlikely that ASIC2a or -2b are responsible, since they are much less pH sensitive than ASIC1a, and loss of ASIC1a eliminates currents evoked by pH values as low as 5.0 (Wemmie et al., 2002). Other pH-sensitive receptors might include channels such as pH-sensitive TRP channels (Huang et al., 2006), K+ channels (Trapp et al., 2008), or other pH-sensitive proteins.
It has long been known that breathing CO2 triggers panic attacks in patients with panic disorder (Drury, 1918; Klein, 1993; Papp et al., 1993; Sanderson et al., 1989), and that these patients show an increased sensitivity to CO2 inhalation (Papp et al., 1993; Rassovsky and Kushner, 2003). Dysregulated brain pH has also been implicated in panic disorder (Friedman, 2006; Maddock, 2001). In addition, patients with increasing hypercarbia due to respiratory failure become extremely anxious (Smoller et al., 1996). Our studies suggest a molecular and anatomical foundation for further dissecting CO2-triggered anxiety and panic in humans. They also suggest that new therapeutic strategies for panic and anxiety might target changes in brain pH or ASIC channels.
METHODS
Mice
ASIC1a−/− mice were described previously (Wemmie et al., 2002). Briefly, the neomycin resistance gene replaced the first translated exon in the ASIC1a-encoding gene (ACCN2), eliminating expression of the ASIC1a protein. ASIC1a−/− and ASIC1a+/+ mice were maintained in a congenic C57BL/6 background. Experimental groups were matched for age (ranging from 9–18 weeks) and gender. Mice were kept on a standard 12-hr light-dark cycle and received standard chow (LM-485; Teklab, Madison, Wisconsin) and water ad libitum. Animal care met National Institutes of Health standards and the University of Iowa Animal Care and Use Committee approved all procedures.
CO2 evoked freezing, PcTx1 and A-317567
Freezing behavior was defined as the absence of motion except for respiration and was assessed by placing mice in a Plexiglas chamber (20.3 cm wide × −20.3 cm deep × 16.5 cm high), similar to that used previously to test predator-odor-evoked freezing (Coryell et al., 2007). CO2 diffused through a large valve in the ceiling of the chamber to create a steady-state concentration of 5 or 10% CO2. This setup avoided blowing CO2 or air, which can be aversive to mice. Behavior was videotaped and scored by a trained observer blinded to genotype and condition. To administer the ASIC1a antagonists, intracerebroventricular (ICV) guide cannulae were implanted into the left lateral ventricle of anesthetized mice (relative to bregma: anteroposterior −0.3 mm, lateral −1.0 mm, ventral −3.2 mm). Three to five days later, 5 μL of PcTx1-containing venom (9 ng/μL) (SpiderPharm, Phoenix, AZ), A-317567 (0.9 mM from 10 mM stock suspended in 5% DMSO – kindly provided by Drs. Alan Light and Jon Rainier), or ACSF alone (in mM: NaCl 124, KCl 3, NaH2PO4 1.2, MgSO4 1.2, CaCl2 2, NaHCO3 26) were injected over ten seconds with a 10 μL-Hamilton syringe connected to a 30-gauge injector. CO2-evoked freezing was assessed 2 hr or 30 min after injecting PcTx1 and A-317567 respectively.
Open-field
Mice were placed in an open-field (40.6 cm wide × 40.6 cm deep × 36.8 cm high, 55 lux) (San Diego Instruments). The open field was covered with a clear Plexiglas lid and pre–filled with compressed air or CO2 concentrations similar to those that trigger panic in humans, 5 to 20% (in air). Behavior in the open-field was assessed for 20 min. Center activity was defined as percent beam breaks in the center (30.5 × 30.5 cm) relative to total beam breaks.
CO2–aversion assay
Mice were placed in a clear Plexiglas chamber with two sides (20.3 cm wide × 20.3 cm deep × 14.0 cm high) connected by a swinging door (4.3 cm × 4.6 cm) that allowed mice to cross freely, but limited gas mixing between the two sides. To further prevent gas mixing the lid of each side was left ajar to provide a low resistance outflow. CO2 (20% in 21% O2, balanced with N2) was administered to one side (10 L/min), whereas compressed air was administered at the same rate to the opposite side leading to steady state-concentrations of 15% CO2 and 2% CO2, respectively. Prior to gas infusion, mice were allowed to explore the chamber until they learned to cross freely between the two sides at least 4 times. The side receiving CO2 was randomly alternated between trials. Ten-minute trials were videotaped and a trained observer blinded to genotype scored the time each mouse spent in the two sides of the chamber. Aversion to compressed blowing air was similarly assessed by administering compressed air to one side (flow rate 15 L/min) and leaving the other side undisturbed.
Context fear conditioning and predator odor evoked fear
Mice were placed in a near infrared fear conditioning chamber (Med Associates, St. Albuns, VT) and allowed to habituate for 3 min. Mice received five footshocks (1 sec, 0.75 mA) with an inter-shock interval of 1 min. Control groups received no footshocks. −10% CO2 or air (9 L/min) was infused into the chamber during conditioning. Mice were then returned to the home cage. Context-evoked freezing was tested 24 hours later by placing the mice back into the conditioning chamber for 5 min (minus CO2 and minus footshocks). Freezing was defined as an absence of movement other than respiration and was scored with VideoFreeze software (Med Associates), which correlated closely with manual scoring (Coryell et al., 2008). HCO3− (2 mM/Kg, ip) was injected 15 min prior to fear conditioning or TMT exposure. Contextual fear conditioning was performed as above except the each footshock was preceded by a 20 sec tone. As before, conditioned freezing response to the context was assessed 24 hrs after training. TMT-evoked freezing was assessed as previously described (Coryell et al., 2007).
Whole-body plethysmography and arterial partial pressure CO2 (PaCO2) measurements
Respiration was assessed in a plethysmograph chamber (Kent Scientific, Litchfield, CT). Mice were habituated to the chamber for one hr. Ventilatory patterns were then recorded while perfusing the chamber with compressed air, 5% CO2, or 10% CO2 (in air) for ten min. During stable breathing (no extraneous body movement), the average peak-to-peak ventilatory rate (VR) was calculated. Tidal volume (VT) was determined by measuring area under curve (AUC) for 20 expiratory events in at least two epochs of stable breathing. The AUC was then transformed to volume using a calibration injection of a known volume. Minute ventilation was calculated by multiplying VR by VT. The CO2–evoked increase in minute ventilation versus air was then determined. PaCO2 measurements were obtained from 100 μl arterial blood from a right femoral artery line in mice anesthetized with ketamine and xylazine breathing compressed air or 10% CO2 (21% O2) for 5 min with an ABL-5 blood gas analyzer (Radiometer Copenhagen).
Amygdala neurons, culture, slices, and whole-cell voltage clamp
Cultured amygdala neurons were obtained from newborn pups as described (Askwith et al., 2004; Wemmie et al., 2003). The amygdala was removed and digested (0.25% trypsin (Sigma) in phosphate buffered saline) for 10 min at 37°C. Tissue was triturated through Pasteur pipettes of decreasing diameter in MEM medium (Gibco, USA), with 10% horse serum (Invitrogen, USA), insulin (Sigma, USA), and gentamycin (Invitrogen, USA). Cells were transferred to Petri dishes (35 mm dia.) and adhered to coverslips (12 mm dia.) coated with poly-L-lysine and laminin (Sigma, USA), and incubated in 5% CO2 (in air) at 37°C. To inhibit glial proliferation, 10 μM cytosine-A-D-arabinofuranoside (Sigma, USA) was added at 72–96 hrs. Medium was changed every 24 hr. Neurons were cultured for 14–18 days and studied by whole cell voltage-clamp without selection for neuron size or morphology. Amygdala slices (300 μm) were obtained from 18–22 day old mouse brains sectioned with a Vibratome in ice-cold aCSF, then maintained at room temp for 2 hr in aCSF (in mM: 126 NaCl, 5 KCl, 1.6 CaCl2, 1.8 MgSO4, 10 D-glucose, 27.7 NaHCO3, 1.25 NaH2PO4, 95% O2/5% CO2) prior to whole-cell voltage-clamp recording. Recordings were obtained at 20–23°C using an Axopatch 200B amplifier and Clampex 9.2 (Axon Instruments) sampled at 50 μs interval and filtered at 5 kHz. Membrane voltage was maintained at −70 mV. Recording pipettes (3–7 MΩ) were pulled from capillary glass with a micropipette puller (Sutter instruments), polished (MF830, Narishige, Japan), and contained (in mM): 5 NaCl, 90 Kgluconate, 15 KCl, 1 MgCl2, 10 EGTA, 25 HEPES, and 3 Na2ATP, adjusted topH 7.25 with TMAOH. Bath solutions contained for culture (in mM): 145 NaCl, 5.4 KCl, 2 CaCl2, 1 MgCl2, 10 HEPES, 10 MES, 10 glucose, pH was adjusted with TMA·OH. For slices, the bath solution contained (in mM: 125 NaCl, 2.5 KCl, 2 CaCl2, 1 MgCl2, 25 D-glucose, 25 NaHCO3, 1.25 NaH2PO4, 95% 02/5% CO2, room temp). Extracellular solutions were changed with a rapid solution changer (SF77B, Warner Instruments).
Measuring brain pH during CO2 inhalation, acid and bicarbonate injection
A fiber optic pH sensor (pHOptica, WPI; detection range pH 5–9) was placed in the left lateral ventricle (relative to bregma in mm: anteroposterior −0.3, lateral +1.0, ventral −3.2) or the basolateral amygdala (anteroposterior −1.2, lateral +3.6; ventral −5.2 from skull surface) of ASIC1a+/+ and ASIC1a−/− mice anesthetized with ketamine and xylazine. Brain pH was measured in response to compressed air or CO2 (in 21% O2/69% N2). To measure the amygdala pH during acid injection, a 30-gauge stainless steel injector was glued to the pH sensor and positioned ~1mm from the sensor. The pH sensor and injector were inserted into the mouse amygdala. pH was recorded at baseline and in response to injecting 1 μL of ACSF (pH 7.3 and pH 3.0). ACSF pH was lowered to 3 with HCl. Injecting HCO3− (2 mM/kg, i.p.) raised brain pH for ~ 1 hr, as described by others (Schuchmann et al., 2006).
Freezing response to amygdala acidosis
A 26-gauge guide cannula was implanted into the left basolateral amygdala (relative to bregma in mm anteroposterior −1.2, lateral +3.6; ventral −4.2 from skull surface). Mice recovered from the surgery for at least 3 days. A 10 μL-Hamilton syringe connected to a 30-gauge injector was inserted 1 mm past the cannula tip to inject ACSF (1 μL pH 3 or pH 7.3) over 5 sec. The behavioral response was videotaped and freezing was scored for 10 min by a trained observer blinded to genotype and condition. The injection sites were mapped post-mortem by sectioning the brain (7 μM coronal) and performing cresyl violet staining (in 100 ml: 0.5 g Cresyl, 20 ml of 100% EtOH, 1.5 ml glacial acetic acid, pH to 3.5–3.7).
Amygdala stimulation and electrocorticography (ECoG) recordings
Custom made bipolar tungsten stimulating electrodes (0.2 mm diameter, 0.5 mm gap, 450 kΩ impedance) were stereotactically implanted into the left amygdala under ketamine/xylazine anesthesia (relative to bregma in mm: anteroposterior −1.2, lateral +3.6, ventral −4.2 from brain surface). Three stainless steel screws (3.2 mm, Stoelting) were implanted above the right frontal lobe, right parietal lobe, and cerebellum. The first two screws served as epidural recording electrodes and the latter as a reference/ground electrode (coordinates from bregma in mm, frontal electrode: anteroposterior +2.0, lateral −1.5; parietal electrode: anteroposterior −2.0 mm, lateral −1.5 mm; cerebellar electrode: anteroposterior −6.0, lateral 0). The amygdala was stimulated 5 times at each voltage with a GRASS SD9 stimulator (5 sec, 100 Hz, 1 – 3.5 V in 0.5 V steps). Freezing was scored at the absence of movement except for respiration. Amygdala ECoG recordings were obtained using a TDT MEDUSA preamplifier and base-station (sampling rate 2034.5 Hz, filtered at 1 Hz and 3000 Hz) and recorded with TDT OpenX software and analyzed with MATLAB R2007b. ECoG tracings were examined for evidence of epileptic spike activity and after discharges; none were observed during this experiment.
Adeno-associated virus (AAV) vectors
Vectors were produced by the University of Iowa Gene Transfer Vector Core and injected as described previously (Coryell et al., 2008). The vectors were AAV2/1 chimeric viruses with AAV1 capsids and AAV2 ITRs and contained a CMV promoter driving an ASIC1a or an enhanced green fluorescent protein (eGFP) cDNA. To facilitate the screening of AAV-ASIC1a transduced brain regions we inserted an internal ribosomal re-entry site (IRES) followed by eGFP, downstream from ASIC1a in the AAV-ASIC1a vector; however, this vector produced no detectable eGFP expression (not shown). Therefore, in the AAV-ASIC1a group, we co-injected AAV-eGFP as described (Coryell et al., 2008). Mice recovered for 14 days before behavioral analysis. Targeting was confirmed postmortem as described (Coryell et al., 2008). Correctly targeted injections (hits) were defined as having fluorescence above background within bilateral BLA. Off target injections (misses) were defined as having fluorescence above background in the temporal lobe, but not in both BLA.
Statistical analysis
Values are expressed as mean ± s.e.m. Two-way analysis of variance (ANOVA) was used to test for interaction effects. One-way ANOVA was also used to assess overall differences in experiments involving more than two groups. Planned contrast testing within the context of the full ANOVA was used to test relationships hypothesized a priori between groups. Wilcoxon rank sum test was used assess for significant differences in CO2 aversion. ED50 values were determined by Probit analysis. P-values (2-tailed) less than 0.05 were considered significant.
Acknowledgments
We thank Sam Hartman, Huiyu Gong, and Runping Wang for assistance. This work was supported by Young Investigator Awards from ADAA and NARSAD, VA Merit Award, 1R01MH085724-01 (to J.A.W) and by the University of Iowa Interdisciplinary Training Program in Pain Research NINDS T32NS045549 (to AEZ). MJW is an Investigator of the Howard Hughes Medical Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adolphs R. Neural systems for recognizing emotion. Curr Opin Neurobiol. 2002;12:169–177. doi: 10.1016/s0959-4388(02)00301-x. [DOI] [PubMed] [Google Scholar]
- Anglada-Figueroa D, Quirk GJ. Lesions of the basal amygdala block expression of conditioned fear but not extinction. J Neurosci. 2005;25:9680–9685. doi: 10.1523/JNEUROSCI.2600-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Askwith CC, Wemmie JA, Price MP, Rokhlina T, Welsh MJ. ASIC2 modulates ASIC1 H+-activated currents in hippocampal neurons. J Biol Chem. 2004;279:18296–18305. doi: 10.1074/jbc.M312145200. [DOI] [PubMed] [Google Scholar]
- Bechara A, Tranel D, Damasio H, Adolphs R, Rockland C, Damasio AR. Double dissociation of conditioning and declarative knowledge relative to the amygdala and hippocampus in humans. Science. 1995;269:1115–1118. doi: 10.1126/science.7652558. [DOI] [PubMed] [Google Scholar]
- Beg AA, Ernstrom GG, Nix P, Davis MW, Jorgensen EM. Protons act as a transmitter for muscle contraction in C elegans. Cell. 2008;132:149–160. doi: 10.1016/j.cell.2007.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson CJ, Xie J, Wemmie JA, Price MP, Henss JM, Welsh MJ, Snyder PM. Heteromultimerics of DEG/ENaC subunits form H+-gated channels in mouse sensory neurons. Proc Natl Acad Sci U S A. 2002;99:2338–2343. doi: 10.1073/pnas.032678399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brannan S, Liotti M, Egan G, Shade R, Madden L, Robillard R, Abplanalp B, Stofer K, Denton D, Fox PT. Neuroimaging of cerebral activations and deactivations associated with hypercapnia and hunger for air. Proc Natl Acad Sci U S A. 2001;98:2029–2034. doi: 10.1073/pnas.98.4.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CH, Hsu YT, Chen CC, Huang RC. Acid-sensing ion channels in neurones of the rat suprachiasmatic nucleus. J Physiol. 2009;587:1727–1737. doi: 10.1113/jphysiol.2008.166918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coryell M, Ziemann AE, Westmoreland PJ, Haenfler JM, Kurjakovic Z, Zha XM, Price M, Schnizler MK, Wemmie J. Targeting ASIC1a reduces innate fear and alters neuronal activity in the fear circuit. Biol Psychiatry. 2007;62:1140–1148. doi: 10.1016/j.biopsych.2007.05.008. [DOI] [PubMed] [Google Scholar]
- Coryell MW, Wunsch AM, Haenfler JM, Allen JE, McBride JL, Davidson BL, Wemmie JA. Restoring Acid-sensing ion channel-1a in the amygdala of knockout mice rescues fear memory but not unconditioned fear responses. J Neurosci. 2008;28:13738–13741. doi: 10.1523/JNEUROSCI.3907-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVries SH. Exocytosed protons feedback to suppress the Ca(2+) current in mammalian cone photoreceptors. Neuron. 2001;32:1107–1117. doi: 10.1016/s0896-6273(01)00535-9. [DOI] [PubMed] [Google Scholar]
- Diochot S, Salinas M, Baron A, Escoubas P, Lazdunski M. Peptides inhibitors of acid-sensing ion channels. Toxicon. 2007;49:271–284. doi: 10.1016/j.toxicon.2006.09.026. [DOI] [PubMed] [Google Scholar]
- Dratcu L. Panic, hyperventilation and perpetuation of anxiety. Prog Neuro-Psychopharmacol & Biol Psychiat. 2000;24:1069–1089. doi: 10.1016/s0278-5846(00)00130-5. [DOI] [PubMed] [Google Scholar]
- Drury AN. The percentage of carbon dioxide in the alveolar air, and the tolerance to accumulating carbon dioxide in case of so-called “irritable heart”. Heart. 1918;7:165–173. [Google Scholar]
- Dube GR, Lehto SG, Breese NM, Baker SJ, Wang X, Matulenko MA, Honore P, Stewart AO, Moreland RB, Brioni JD. Electrophysiological and in vivo characterization of A-317567, a novel blocker of acid sensing ion channels. Pain. 2005;117:88–96. doi: 10.1016/j.pain.2005.05.021. [DOI] [PubMed] [Google Scholar]
- Friedman MJ. Posttraumatic stress disorder among military returnees from Afghanistan and Iraq. Am J Psychiatry. 2006;163:586–593. doi: 10.1176/ajp.2006.163.4.586. [DOI] [PubMed] [Google Scholar]
- Goosens KA, Maren S. Contextual and auditory fear conditioning are mediated by the lateral, basal, and central amygdaloid nuclei in rats. Learn Mem. 2001;8:148–155. doi: 10.1101/lm.37601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman JM, Kent JM, Sullivan GM, Coplan JD. Neuroanatomical hypothesis of panic disorder, revised. Am J Psychiatry. 2000;157:493–505. doi: 10.1176/appi.ajp.157.4.493. [DOI] [PubMed] [Google Scholar]
- Griez E, Schruers K. Experimental pathophysiology of panic. J Psychosom Res. 1998;45:493–503. doi: 10.1016/s0022-3999(98)00027-0. [DOI] [PubMed] [Google Scholar]
- Huang AL, Chen X, Hoon MA, Chandrashekar J, Guo W, Trankner D, Ryba NJ, Zuker CS. The cells and logic for mammalian sour taste detection. Nature. 2006;442:934–938. doi: 10.1038/nature05084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasti J, Furukawa H, Gonzales EB, Gouaux E. Structure of acid-sensing ion channel 1 at 1.9 A resolution and low pH. Nature. 2007;449:316–323. doi: 10.1038/nature06163. [DOI] [PubMed] [Google Scholar]
- Kaila K, Chesler M. Activity-evoked changes in extracellular pH. In: Kaila K, Ransom BR, editors. pH and Brain Function. Wiley-Liss, Inc; 1998. p. 309. [Google Scholar]
- Kim JJ, Jung MW. Neural circuits and mechanisms involved in Pavlovian fear conditioning: a critical review. Neurosci Biobehav Rev. 2006;30:188–202. doi: 10.1016/j.neubiorev.2005.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein DF. False suffocation alarms, spontaneous panics, and related conditions. An integrative hypothesis. Arch Gen Psychiatry. 1993;50:306–317. doi: 10.1001/archpsyc.1993.01820160076009. [DOI] [PubMed] [Google Scholar]
- Krishtal O. The ASICs: signaling molecules? Modulators? Trends Neurosci. 2003;26:477–483. doi: 10.1016/S0166-2236(03)00210-8. [DOI] [PubMed] [Google Scholar]
- Lingueglia E, de Weille JR, Bassilana F, Heurteaux C, Sakai H, Waldmann R, Lazdunski M. A modulatory subunit of acid sensing ion channels in brain and dorsal root ganglion cells. J Biol Chem. 1997;272:29778–29783. doi: 10.1074/jbc.272.47.29778. [DOI] [PubMed] [Google Scholar]
- Maddock RJ. The lactic acid response to alkalosis in panic disorder: an integrative review. J Neuropsychiatry Clin Neurosci. 2001;13:22–34. doi: 10.1176/jnp.13.1.22. [DOI] [PubMed] [Google Scholar]
- Maren S. Pavlovian fear conditioning as a behavioral assay for hippocampus and amygdala function: cautions and caveats. Eur J Neurosci. 2008;28:1661–1666. doi: 10.1111/j.1460-9568.2008.06485.x. [DOI] [PubMed] [Google Scholar]
- Mayhan WG, Faraci FM, Heistad DD. Effects of vasodilatation and acidosis on the blood-brain barrier. Microvasc Res. 1988;35:179–192. doi: 10.1016/0026-2862(88)90061-1. [DOI] [PubMed] [Google Scholar]
- Mitchell RA, Carman CT, Severinghaus JW, Richardson BW, Singer MM, Shnider S. Stability of cerebrospinal fluid pH in chronic acid-base disturbances in blood. J Appl Physiol. 1965;20:443–452. doi: 10.1152/jappl.1965.20.3.443. [DOI] [PubMed] [Google Scholar]
- Mongeluzi DL, Rosellini RA, Ley R, Caldarone BJ, Stock HS. The conditioning of dyspneic suffocation fear. Effects of carbon dioxide concentration on behavioral freezing and analgesia. Behav Modif. 2003;27:620–636. doi: 10.1177/0145445503256316. [DOI] [PubMed] [Google Scholar]
- Nattie E. Central chemoreceptors, pH, and respiratory control. In: Kaila K, Ransom BR, editors. pH and Brain Function. Wiley-Liss, Inc; 1998. pp. 535–560. [Google Scholar]
- Ohman A, Mineka S. Fears, phobias, and preparedness: toward an evolved module of fear and fear learning. Psychol Rev. 2001;108:483–522. doi: 10.1037/0033-295x.108.3.483. [DOI] [PubMed] [Google Scholar]
- Palmer MJ, Hull C, Vigh J, von Gersdorff H. Synaptic cleft acidification and modulation of short-term depression by exocytosed protons in retinal bipolar cells. J Neurosci. 2003;23:11332–11341. doi: 10.1523/JNEUROSCI.23-36-11332.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papp LA, Klein DF, Gorman JM. Carbon dioxide hypersensitivity, hyperventilation, and panic disorder. Am J Psychiatry. 1993;150:1149–1157. doi: 10.1176/ajp.150.8.1149. [DOI] [PubMed] [Google Scholar]
- Phelps EA, LeDoux JE. Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron. 2005;48:175–187. doi: 10.1016/j.neuron.2005.09.025. [DOI] [PubMed] [Google Scholar]
- Pidoplichko VI, Dani JA. Acid-sensitive ionic channels in midbrain dopamine neurons are sensitive to ammonium, which may contribute to hyperammonemia damage. Proc Natl Acad Sci U S A. 2006;103:11376–11380. doi: 10.1073/pnas.0600768103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinak CA, Maren S. Associative structure of fear memory after basolateral amygdala lesions in rats. Behav Neurosci. 2008;122:1284–1294. doi: 10.1037/a0012903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rassovsky Y, Kushner MG. Carbon dioxide in the study of panic disorder: issues of definition, methodology, and outcome. J Anx Disord. 2003;17:1–32. doi: 10.1016/s0887-6185(02)00181-0. [DOI] [PubMed] [Google Scholar]
- Rosen JB. The neurobiology of conditioned and unconditioned fear: a neurobehavioral system analysis of the amygdala. Behav Cogn Neurosci Rev. 2004;3:23–41. doi: 10.1177/1534582304265945. [DOI] [PubMed] [Google Scholar]
- Sanderson WC, Rapee RM, Barlow DH. The influence of an illusion of control on panic attacks induced via inhalation of 5.5% carbon dioxide-enriched air. Arch Gen Psychiatry. 1989;46:157–162. doi: 10.1001/archpsyc.1989.01810020059010. [DOI] [PubMed] [Google Scholar]
- Schnizler MK, Schnizler K, Zha X, Hall DD, Wemmie JA, Hell JW, Welsh MJ. The Cytoskeletal Protein α-Actinin Regulates Acid-sensing Ion Channel 1a Through a C-Terminal Interaction. J Biol Chem. 2009;284:2697–2705. doi: 10.1074/jbc.M805110200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuchmann S, Schmitz D, Rivera C, Vanhatalo S, Salmen B, Mackie K, Sipila ST, Voipio J, Kaila K. Experimental febrile seizures are precipitated by a hyperthermia-induced respiratory alkalosis. Nat Med. 2006;12:817–823. doi: 10.1038/nm1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smoller JW, Pollack MH, Otto MW, Rosenbaum JF, Kradin RL. Panic anxiety, dyspnea, and respiratory disease. Theoretical and clinical considerations. Am J Respir Crit Care Med. 1996;154:6–17. doi: 10.1164/ajrccm.154.1.8680700. [DOI] [PubMed] [Google Scholar]
- Sullivan GM, Apergis J, Gorman JM, LeDoux JE. Rodent doxapram model of panic: behavioral effects and c-Fos immunoreactivity in the amygdala. Biol Psychiatry. 2003;53:863–870. doi: 10.1016/s0006-3223(02)01733-x. [DOI] [PubMed] [Google Scholar]
- Trapp S, Aller MI, Wisden W, Gourine AV. A role for TASK-1 (KCNK3) channels in the chemosensory control of breathing. J Neurosci. 2008;28:8844–8850. doi: 10.1523/JNEUROSCI.1810-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldmann R, Champigny G, Bassilana F, Heurteaux C, Lazdunski M. A proton-gated cation channel involved in acid-sensing. Nature. 1997;386:173–177. doi: 10.1038/386173a0. [DOI] [PubMed] [Google Scholar]
- Wemmie J, Coryell M, Askwith C, Lamani E, Leonard S, Sigmund C, Welsh M. Overexpression of acid-sensing ion channel 1a in transgenic mice increases fear-related behavior. Proc Natl Acad Sci U S A. 2004;101:3621–3626. doi: 10.1073/pnas.0308753101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wemmie JA, Askwith CC, Lamani E, Cassell MD, Freeman JHJ, Welsh MJ. Acid-sensing ion channel 1 is localized in brain regions with high synaptic density and contributes to fear conditioning. J Neurosci. 2003;23:5496–5502. doi: 10.1523/JNEUROSCI.23-13-05496.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wemmie JA, Chen J, Askwith CC, Hruska-Hageman AM, Price MP, Nolan BC, Yoder PG, Lamani E, Hoshi T, Freeman JHJ, et al. The acid-activated ion channel ASIC contributes to synaptic plasticity, learning, and memory. Neuron. 2002;34:463–477. doi: 10.1016/s0896-6273(02)00661-x. [DOI] [PubMed] [Google Scholar]
- Wemmie JA, Price MP, Welsh MJ. Acid-sensing ion channels: advances, questions and therapeutic opportunities. Trends Neurosci. 2006;29:578–586. doi: 10.1016/j.tins.2006.06.014. [DOI] [PubMed] [Google Scholar]
- Xiong ZG, Zhu XM, Chu XP, Minami M, Hey J, Wei WL, MacDonald JF, Wemmie JA, Price MP, Welsh MJ, et al. Neuroprotection in ischemia: blocking calcium-permeable acid-sensing ion channels. Cell. 2004;118:687–698. doi: 10.1016/j.cell.2004.08.026. [DOI] [PubMed] [Google Scholar]
- Yermolaieva O, Leonard AS, Schnizler MK, Abboud FM, Welsh MJ. Extracellular acidosis increases neuronal cell calcium by activating acid-sensing ion channel 1a. Proc Natl Acad Sci U S A. 2004;101:6752–6757. doi: 10.1073/pnas.0308636100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zandbergen J, Pols H, De Loof C, Lousberg H, Griez E. Effect of hypercapnia and other disturbances in the acid-base-balance on panic disorder. Hillside J Clin Psychiatry. 1989;11:185–197. [PubMed] [Google Scholar]
- Zha XM, Costa V, Harding AM, Reznikov L, Benson CJ, Welsh MJ. ASIC2 subunits target acid-sensing ion channels to the synapse via an association with PSD-95. J Neurosci. 2009;29:8438–8446. doi: 10.1523/JNEUROSCI.1284-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zha XM, Wemmie JA, Welsh MJ. ASIC1a is a postsynaptic proton receptor that influences the density of dendritic spines. Proc Natl Acad Sci USA. 2006;103:16556–16561. doi: 10.1073/pnas.0608018103. [DOI] [PMC free article] [PubMed] [Google Scholar]