Abstract
Haemodiafiltration (HDF) is the blood purification therapy of choice for those who want significant removal of uraemic solutes beyond the traditional range of small molecules. Combining diffusive and convective solute transport, a HDF treatment comprises the largest number of variables among blood purification therapies, and it is important to understand how they interact in order to optimize the therapy. This review discusses the parameters that determine the efficiency of HDF and how they can be controlled in the different forms of HDF and ‘HDF-like’ therapies practised today. The key to safe and effective HDF therapy is to have access to large volumes of high-quality fluids. Starting with ultrapure dialysis fluid, on-line preparation of a sterile, non-pyrogenic substitution solution can be made an integral part of the treatment, and we describe the necessary conditions for this. On-line HDF can provide the largest removal of the widest range of solutes among available dialysis therapies, and the potential clinical benefits of this are within practical reach for the increasing number of patients dialysed with high-flux membranes and ultrapure dialysis fluid.
Keywords: convection, haemodiafiltration, on-line fluid preparation, postdilution, predilution
Introduction
Data from two large, randomized, controlled studies, investigating whether the outcome of long-term dialysis can be improved by using high-flux rather than low-flux dialysis membranes, have shown significant benefits for certain high-risk patients and certain causes of death from the increased removal of larger solutes achieved with high-flux filters [1–4]. Extrapolating from these data leads to an assumption that if the removal of such solutes could be further increased, as in certain forms of haemodiafiltration (HDF), the benefit might be extended to more patients. In comparison with other dialysis modalities, HDF has also been shown to provide superior removal of some protein-bound uraemic solutes [5]. Although outcome evidence from randomized controlled trials is still missing, superior survival is reported from observational studies and clinical benefits have been documented in numerous studies, all for patients treated with HDF compared to conventional dialysis [6–8]. As a consequence, the interest in applying this form of blood purification is increasing worldwide. A recent survey among >6000 nephrology professionals showed that 80% consider dialysis with a high-flux membrane superior to using a low-flux membrane, and among them ∼50% prefer a convective therapy [9].
High-flux membranes are designed for high fluid flows and convective transport, and when applied in mainly diffusive modes with limited convection, such as haemodialysis (HD), their full potential is not utilized. When convective transport is consciously added to the therapy, as in HDF, the prescription needs to be carefully considered with regard to the different parameters to give the full benefit of the HDF therapy. In fact, a HDF treatment comprises the largest number of treatment variables among blood purification therapies, and it is important to understand how they interact with each other in order to optimize the therapy [10]. The interest in applying HDF therapy has resulted in a flora of modalities that differ mainly in the amount of convection applied, the way it can be controlled and the mode of fluid administration. To date, there is no overview of all these different modes of HDF. We therefore felt the need to review the basic parameters that determine the efficiency of HDF and discuss how they can be controlled in the forms of HDF practised today. The key to effective HDF therapy is to have access to high-quality fluids, and on-line preparation of sterile, non-pyrogenic substitution solution has met with regulatory obstacles in some countries and limited the adoption of therapy. We will discuss the safety of on-line fluid preparation with reference to the recently released ISO standard, an important document which for the first time, in a multinationally recognized standard, identifies and defines on-line ultrafiltration as a method to prepare a sterile solution [11].
Parameters controlling the efficiency of haemodiafiltration
Introduction
HDF is a form of blood purification that can be used for treatment of acute as well as chronic renal failure. HDF utilizes a combination of diffusive and convective solute transport through a highly permeable dialysis membrane. The diffusive transport requires the presence of dialysis fluid flowing through the dialyser counter-currently to blood. The convective transport requires ultrafiltration of fluid to an extent that exceeds the desired weight loss. Fluid balance is maintained by infusion of a replacement fluid, which can be administered before the filter (predilution), after the filter (postdilution) or inside the filter. The replacement fluid, also referred to as substitution fluid, is mixed with the blood and should therefore be sterile and non-pyrogenic with a composition similar to plasma water. This fluid can be provided in industrially prepared, autoclaved, plastic bags or can be generated as an integral part of the treatment, either externally through quality-controlled, stepwise ultrafiltration or internally through backfiltration of dialysate [10].
Diffusion [12]
In HDF, as well as in HD, the driving force for diffusive transport across the membrane is the difference in concentration between the blood and the dialysis fluid for each particular solute. The rate of diffusion depends on molecular size and resistance to flow. This resistance is mainly represented by the membrane, but the distance in the blood path, i.e. the diameter of the hollow fibre, also plays a role. Small molecules are favoured because the rate of diffusion is inversely proportional to the cubic root of the molecular weight. All characteristics of a dialyser for diffusive transport are expressed by K0A, the mass transfer area coefficient, showing clearance at infinite flow rates (K0) and incorporating the surface area (A).
To maximize diffusive transport in clinical practice, flow rates for blood and dialysis fluid should be high to maintain a large concentration gradient. The blood flow should be as high as the access conditions permit. An old rule of thumb says to aim for a 1:2 relationship between blood flow and dialysis fluid flow, but optimal conditions can be obtained with modern dialysers at somewhat lower dialysis fluid flow rates [13]. Among the three controlling parameters—blood flow rate, dialysis fluid flow rate and dialyzer surface area—the patient-related para- meter, i.e. blood flow, should be the determinant of the others.
Low-flux HD, frequently referred to as conventional HD, is a good example of a diffusive therapy. Low-flux membranes are characterized by high diffusive permeability, which means that small solutes move easily back and forth across the membrane along their respective concentration gradient. However, because neither transport of medium-sized and large solutes nor a high water flow can be achieved with low-flux membranes, the convective transport is reduced to negligible amounts. Increased efficiency, achieved through increased flow rates for blood and dialysis fluid and dialysers with a larger surface area, can be seen as increased removal of small solutes like urea and creatinine, while it has little effect on larger solutes such as ß2-microglobulin [1].
Convection [12]
Convective transport in dialysis consists of solutes passively following a fluid flow, ultrafiltration, across a highly permeable membrane (solvent drag). The hydraulic permeability of the membrane and the pressure gradient across the membrane, referred to as transmembrane pressure (TMP), determine the rate of ultrafiltration. The membrane permeability to solutes, sieving properties, is determined by the size of the pores in the membrane and sets the limit for which solutes can be dragged across the membrane by the fluid flow. The sieving coefficient (S) of a particular membrane for a specific solute, a value between 0 and 1, is the ratio between the solute concentration in the filtrate and the solute concentration in the blood, in the absence of adsorption. The sieving curve for a membrane shows how the sieving changes with increasing molecular weight, which is assumed to reflect the size (Figure 1). The shape of the curve where S falls from 1 to 0 indicates the distribution of pore sizes, and the molecular weight where S = 0.1 is referred to as the cut-off. Membranes with a sieving curve similar to that of the glomerular basement membrane, i.e. with a steeply falling profile and a cut-off just below the size of albumin, are ideal for HDF. Depending on the physico-chemical attraction between the blood components and the membrane polymer, the sieving properties may change at exposure to blood. It is therefore important to apply clinically relevant conditions when comparing the therapeutic utility of different membranes. For all solutes with S = 1, the rate of convective removal equals the ultrafiltration rate. If the ultrafiltration rate also equals the convective clearance from blood. The amount of solute removed depends on the concentration in the incoming fluid, i.e. the undiluted or diluted blood. For solutes with S < 1, the concentration needs to be multiplied with the relevant S value.
Fig. 1.
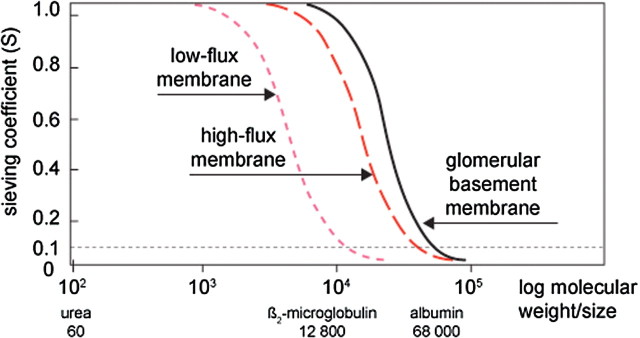
Sieving curves for low-flux and high-flux dialysis membranes and human glomerular basement membrane. The molecular size for which the sieving coefficient = 0.1 is the cut-off of the membrane.
Haemofiltration (HF) is a therapy in which solute removal relies entirely on convection. When applied in the traditional postdilution mode, using high blood flow rates and large ultrafiltration volumes, this therapy can provide adequate removal of small molecules and high removal of middle and large molecules [14]. When used in the predilution mode the clearance of small as well as middle molecules can be significantly increased, provided the infusion flow rate matches the blood flow rate. This has enabled clinical application in average size patients with normal blood flow capacity [15]. To increase the efficiency of HF, a higher ultrafiltration flow rate must be generated and this requires a higher blood flow rate and sometimes also a larger membrane surface area. The result will be seen as increased clearance of a wide range of solutes.
Interaction between diffusion and convection
In HDF, diffusion and convection occur simultaneously, but the effects are not simply additive because they interfere with each other. Diffusion reduces the concentration of small solutes, leaving less for convective removal, and convection reduces the blood flow and thus the driving force for diffusion. When on-line prepared fluid is used, convection also reduces the dialysis fluid flow as this fluid serves as the source of substitution fluid. Thus, convection has limited value for the clearance of small solutes, while it is of progressive importance for solutes of increasing molecular weight (Figure 2) [14]. Mathematical models suggest that 40–50% of the ultrafiltration rates represent the convective clearance that can be added to pure diffusion, when the transport processes are combined [16].
Fig. 2.
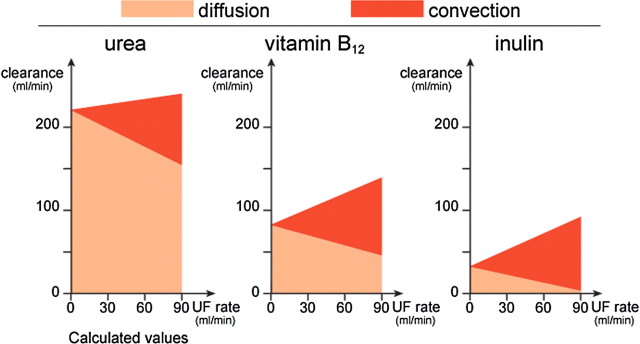
Clearance in postdilution HDF for urea (MW = 60), vitamin B12 (MW = 1 355) and inulin (MW = 5 000) illustrating the impact of increasing convection (dark areas) on diffusion (light areas) at a blood flow rate of 300 ml/min and increasing ultrafiltration rate. Reprinted from [14] with permission.
The balance between diffusion and convection can be adjusted through the controlling parameters. Convection is usually prioritized, and this is achieved by aiming for maximum ultrafiltration. A realistic filtration fraction without excessive build-up of pressure may be 50% of the plasma volume or 25–35% of the blood volume. However, finding the optimal UF rate requires an experienced nurse, and in reality, many HDF treatments are operated under suboptimal conditions to avoid TMP alarms and save nursing time [17]. A convenient approach is to let the machine control the UF rate by selecting and adjusting the TMP automatically. This has been found to result in higher filtration fraction and efficiency [18].
Dilution
Problems with low ultrafiltration volumes and high TMP values can be solved by diluting the blood before the filtration. However, both diffusion and convection are affected by the dilution, which means that ultrafiltration is facilitated at the cost of efficiency. Diluted blood means lower concentration of uraemic solutes which impacts diffusion as well as convection. The lower solute concentration means that the driving force for diffusive transport is reduced and the diluted ultrafiltrate contains less solutes. Treatment sessions with predilution HDF require approximately twice as much infusion fluid to provide a dose comparable to a postdilution HDF session under the same conditions of blood flow, filter size and treatment time [19]. With access to on-line prepared fluid, the volume requirement has neither practical nor economical limits, but there may be concerns about exposing patients to very large fluid volumes.
In postdilution modes, the volume of ultrafiltration is equal to that of convection and it gives a convenient measure of the dose. When the fluid is administered in the predilution mode, quantification becomes more difficult because the ultrafiltrate is now diluted. With externally added replacement fluid in the predilution mode, the ultrafiltration volume can be corrected for the dilution. The effective convection volume is calculated by multiplying the actual volume of diluted ultrafiltrate with the degree of dilution (Qb/(Qb+Qinf)). In all modes involving some form of uncontrolled dilution, whether as a combination of pre- and postdilution or administered inside the filter, the volume of ultrafiltrate is of little value for quantification, and the efficiency of the convective removal can only be assessed by standard clearance or removal measurements.
In special cases, predilution may be the only possibility to provide an adequate HDF treatment. One such instance is patients with very high haematocrit or blood composition that limits the filtration capacity. Another case is patients with low blood flow rates, e.g. paediatric patients for whom HDF has proven to be of significant benefit [20]. A compromise may be to use a limited amount of predilution, sufficient to avoid problems with filtration and elevated pressures, and combine it with postdilution, but finding the optimal distribution between the flow rates before and after the filter may be difficult. Automatic shifting between pre- and postdilution by the machine in response to pressure changes has been proposed by Pedrini and demonstrated to give a satisfactory result [21]. In spite of the drawbacks with predilution, it is frequently used to facilitate the management of the treatment, and users may be unaware of the loss of efficiency.
Conclusion
Comparing the clearance that can be obtained by the four modes of dialysis therapy—low-flux HD, high-flux HD, HDF and HF—shows that if we want significant removal of uraemic solutes in the middle and large molecular weight range, we need to maximize the convective transport by applying HDF or even HF [22]. If in addition we want to optimize the small solute removal, HDF is the choice. Starting from the blood flow that can be provided by the patient access, the ultrafiltration should be maximized with respect to blood composition and pressures. This can today be achieved with the support of biofeedback programmes in the dialysis equipment [18,21]. Operating in the postdilution mode is most effective, regarding the delivered dose as well as the quantification of it, and should be preferred whenever possible. Concerns about removing beneficial solutes and creating deficits by excess dialysis with highly efficient blood purification have been voiced, but the literature contains no evidence of this.
Different forms of haemodiafiltration
Introduction
The term ‘haemodiafiltration’ appeared first in the nephrology literature in 1975 to describe the treatment form that was later renamed ‘haemofiltration’ [23]. Today, HDF is used as a general term for treatment modes in which diffusion and convection are consciously combined. The original forms of HDF—classical HDF and on-line HDF—belong to this group and are additionally characterized by external infusion of controlled amounts of substitution solution (Figure 3). Some treatment modes set up as HD may also comprise considerable solute removal through convection. They may be referred to as HDF or HD, and they are characterized by uncontrolled ultrafiltration and uncontrolled generation of the substitution solution, the latter provided by backfiltration of dialysate inside the filter. Among such forms, we find push–pull HDF, double high-flux HDF and high-flux HD. When comparing the clinical outcome of different studies, it is important to realize that there may be wide differences among treatment modes referred to as HDF, and great similarities between some forms of HDF and some forms of HD.
Fig. 3.
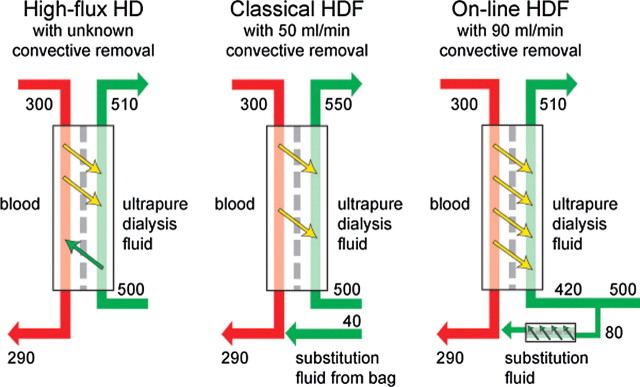
Flow diagrams for different forms of haemodiafiltration (HDF) at typical operating conditions (blood flow rate 300 ml/min, dialysis fluid flow rate 500 ml/min and weight loss 10 ml/min) showing possible convective removal.
Classical haemodiafiltration
This therapy was introduced in 1978 as an effective alternative to HD and HF [24]. Classical HDF is characterized by using external substitution fluid provided as a sterile solution, autoclaved in plastic bags (Figure 3). It can be performed on standard HD machines fitted with an extra pump and a weighing device for fluid balancing. For practical and economical reasons, the volume of the infusion solution is limited and 8–10 l of fluid is most commonly used. In the 1980s, the infusion solution contained lactate as the buffer source; however, with growing evidence of the benefits of bicarbonate as buffer in HD fluids, pharmaceutical preparations of bicarbonate-containing infusion solution for HDF started to appear and resulted in enhanced correction of acidosis [25]. Today, the use of classical HDF is limited to countries with special reimbursement for this treatment mode and physicians wanting specialized infusion solutions. An example of this is acetate-free biofiltration (AFB), mainly used in Italy, in which the dialysis fluid is buffer-free and an isotonic bicarbonate solution is infused in postdilution mode [26].
On-line haemodiafiltration
This therapy was first described in 1985, when Canaud reported his experience of using a prototype, multipurpose machine, which could perform HD, HDF and HF and could also prepare the substitution solution continuously during the treatment [27]. The principles of on-line preparation of the sterile, non-pyrogenic substitution solution are described below. With the development of easy-to-use equipment for on-line HDF, the use of this therapy has spread and it has largely replaced classical HDF. It is estimated that ∼10% of dialysis patients in Western Europe are treated with on-line HDF.
The limiting factors to further application have mainly been regulations and restrictions from authorities on the use of on-line prepared substitution fluid [28]. Continuous sterilization by ultrafiltration and immediate use, i.e. the essence of on-line preparation, is not considered in the Pharmacopoeia as a sterilization method [29,30]. Furthermore, the preparation of a drug (substitution solution) from a device (dialysis fluid) using another device (ultrafilter) is a process that falls outside this rulebook. Therefore, in the countries where on-line preparation has been accepted by the regulatory authorities, e.g. France, Sweden and the Netherlands, there are detailed instructions about conditions to be fulfilled and procedures for testing to be followed. In other countries, this has been left to the individual units and practitioners. However, the new International Standard for fluids for dialysis should pave the way for a more pragmatic, yet safe, view of how stepwise, controlled filtration can be used to prepare sterile, non-pyrogenic fluids [11].
When referring to on-line HDF as a concept, one emphasizes the fact that the substitution solution is prepared as an integral part of the treatment in practically unlimited volumes with individualized composition. To characterize the treatment further one should also state how the substitution solution is administered, because this may have major impact on the efficiency. While predilution is true dilution of the blood, postdilution is actually replacement of ultrafiltered volume. Mixed dilution is a combination of pre- and postdilution and so is mid-dilution, a method that requires a special type of filter where the fluid can be infused between two separate fibre bundles [31].
Haemodiafiltration with internal fluid substitution
The promising benefits of HDF therapy and the practical and economical problems connected with using infusion solution in bags, combined with the regulatory restrictions around on-line fluid preparation, have stimulated creative minds to seek other ways to apply the therapy. Common to these versions of HDF is that backfiltered dialysate is used as substitution solution and fluid balance is maintained by the volume control function of the HD machine. The amount of ultrafiltration usually cannot be controlled, but becomes a consequence of the treatment conditions.
Push-pull HDF.
Shinzato described push–pull HDF as infusion-free HDF already in 1982 [32]. He had created a system where alternating pressure pulses pull and push fluid across the membrane, resulting in fluid removal and fluid replacement taking place in rapid sequences inside the filter. The efficiency of this form of HDF shows that the added convective transport contributes to enhanced clearance of middle and large molecules, however, not to the extent that can be achieved with on-line HDF.
Double high-flux HDF.
This form of HDF was created in Los Angeles, and the clinical result using the super-efficient treatment was first reported in 1984 [33]. Two large filters in series are used and the pressure between them is adjusted so that fluid removal from blood takes place in the first filter and fluid replacement in the second. High blood flow rates, in excess of 500 ml/min, are required to achieve the desired effect. Diffusion occurs in both filters, but is greatly affected by the fluxes of fluid across the membrane. As in push–pull HDF, the substitution fluid consists of dialysate backfiltered across the membrane in the second dialyser. The clearances that can be achieved for a wide range of solutes with this form of HDF are impressive and reflect the large surface area of the filters and the high flow rates of blood and fluid.
High-flux dialysis.
The final form of infusion-free HDF is simply high-flux dialysis, i.e. HD in which a high-flux membrane is used and the weight loss is controlled by standard HD equipment (Figure 3). The hydraulic permeability of all high-flux filters (UF coefficient > 20 ml/h, mmHg, m2) invariably leads to ultrafiltration rates in excess of the desired fluid removal rate, and the volume control function of the dialysis machine automatically compensates for the excess by adjusting the fluid pressure and forcing fluid in the opposite direction. This is referred to as backfiltration and it takes place in all forms of high-flux dialysis, unless the desired weight loss is exceptionally large. As in the other forms of infusion-free HDF, the amount of ultrafiltration and thus convective transport cannot be controlled, but it can be affected through manipulation of the pressures over the filter. The more resistance the blood meets on its way through the hollow fibres, the higher is the inlet pressure, the larger the pressure drop and the greater the ultrafiltration. Thus, high blood flow rates, small fibre diameters and long filters will increase the fluid fluxes across the membrane inside the dialyser and thus the convection. The internal filtration in high-flux HD can amount to 30–40 ml/min under favourable conditions and the convective transport can thus be comparable to classic or low-volume HDF [34].
Comparing different forms of haemodiafiltration
Using pressure manipulations and backfiltration of dialysate may seem like a simple and elegant way to achieve additional convective clearance, but there are a number of caveats connected with this practice. The most serious concern is the microbiological quality of the fluid that is infused into patient blood. Compared to on-line preparation of sterile and non-pyrogenic substitution solution, a validated and highly controlled process, safe backfiltration relies on the dialysate being at least ultrapure (see below) and the dialysis membrane being able to function as a sterilizing filter. These requirements may be fulfilled in experienced clinics practising push–pull HDF and double high-flux dialysis, but are probably overlooked by the majority of clinics performing high-flux dialysis. Although few obvious clinical symptoms are directly associated with this practice, microinflammation manifested by elevated CRP levels may be a consequence [35]. Another risk connected with the high pre-filter pressures required to increase ultrafiltration in the arterial part of the filter is albumin leakage and mechanical damage to the blood cells [36].
Other drawbacks when performing high-flux HD in lieu of HDF relate to the efficiency of the therapy, which is obviously affected by the inability to determine the ultrafiltration. The ultrafiltration rate can neither be controlled nor measured, and the same applies to the convective removal. The efficiency is also reduced by the fact that dialysis fluid already exposed to uraemic blood is used for sustitution, rather than unused fluid. Although infused in the venous end of the filter, the quality cannot be compared to that of the substitution solution intended for the purpose. Finally, the efficiency suffers from competition between the three ongoing processes—diffusion, ultrafiltration and backfiltration—with flux in different directions taking place simultaneously across the same membrane surface. The dialyser now serves the purpose of fluid purification in addition to blood purification.
Conclusion
High-flux dialysis, which can be considered as a low-efficiency version of HDF, is today used for two-thirds of HD patients in the world [37], giving them the benefit of some convective clearance, but at the same time exposing them to the risk of bacterial products reaching the blood and inducing microinflammation. The convection volume in contemporary high-flux dialysis can be almost as high as in classical HDF, which is important to realize when discussing the outcome of different therapies. Compared to high-flux dialysis and classical HDF, on-line HDF can provide significantly larger convection volumes that can be reached without practical or economical constraints and with a high safety level for the patient. On-line HDF is thus the most efficient way of performing HDF. With access to on-line prepared fluids, the substitution solution can be added in pre-, mid-, mixed- or post-dilution mode. Diluting the blood before the filtration may facilitate the treatment, but it also dilutes the ultrafiltration and thus the efficiency.
Fluids for haemodiafiltration
Introduction
Dialysis patients are exposed to large volumes of fluid, only separated from their blood by a semipermeable membrane and sometimes even mixed with the blood. Standards and recommendations for the chemical quality of water and dialysis fluid should be followed whether the fluid is used for HD or HDF [38]. With respect to the microbiological quality, the fluids used in dialysis can be divided into three levels—standard, ultrapure and sterile—and all three are of relevance to HDF (Figure 4) [11]. The current maximum levels of microbiological contaminants, measured as colony-forming units (CFU) and endotoxin units (EU), in the different fluids for dialysis are shown below [11]. All values refer to fluids tested with sensitive methods, previously described and recommended [39] and now also confirmed by the new ISO standard [11].
Fig. 4.
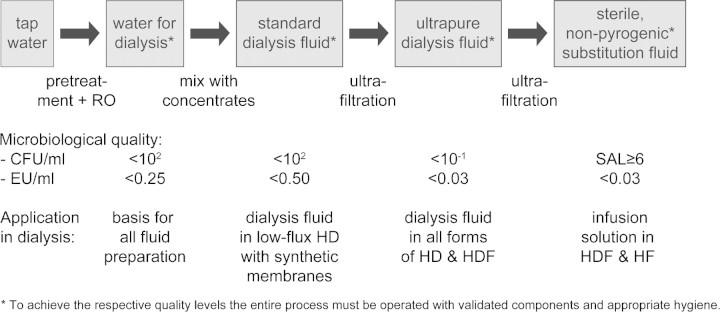
Process steps in the preparation of fluids for dialysis starting with tap water and resulting in sterile, non-pyrogenic substitution fluid for on-line, convective therapies. (RO = reverse osmosis, CFU = colony-forming units, EU = endotoxin units, SAL = sterility assurance level).
–Standard quality dialysis fluid should have a bacterial count <100 CFU/ml and an endotoxin level <0.50 EU/ml.
–Ultrapure quality dialysis fluid should have a bacterial count <0.1 CFU/ml and an endotoxin level <0.03 EU/ml.
–Sterile quality fluid cannot be defined by a certain bacterial content, because it can not be tested as such. The volume used for each application should be free from viable bacteria with a sterility assurance level (SAL) of 6 magnitudes, i.e. 1 000 000 times. It should also be non-pyrogenic, which is defined as an endotoxin level <0.03 EU/ml.
Standard quality dialysis fluid
Water for dialysis is prepared from tap water by passage through a series of filters to remove microparticles, organic and inorganic matter. Reverse osmosis is used as the final step and the result should be water that fulfils the recommended quality demands, chemically as well as microbiologically [11,38]. Standard quality water for dialysis (<100 CFU/ml and <0.25 EU/ml) can serve as the base for the preparation of all other fluids for dialysis (Figure 4). Should the treated water not meet this target, it should not be used for any form of dialysis. The microbiological quality of water can be safeguarded by one step of ultrafiltration before introduction into the dialysis machine and mixing with concentrates. In the preparation of dialysis fluid for standard HD, the microbiological quality of the water should not be allowed to deteriorate significantly, because the same upper limit for the bacterial content is valid for dialysis fluid as for water. In practice this means that the entire flow path, from the reverse osmosis equipment to the dialyser, should be frequently disinfected and the microbiological quality of the concentrates should be high. Special attention must be paid to the bicarbonate concentrate, which is prone to bacterial proliferation. The minimum requirement that can be placed on dialysis fluid today is to be of standard quality, but a reasonable recommendation is to use this fluid quality only in low-flux HD with synthetic membranes [40]. Backdiffusion of bacterial products has been documented with low-flux cellulosic membranes and backfiltration of dialysate into the blood may occur in all HD treatments with high-flux membranes [41]. Standard-quality dialysis fluid should therefore not be used in any form of HDF therapy, whether the substitution fluid is externally or internally infused [42].
Ultrapure dialysis fluid
One step of controlled ultrafiltration converts standard quality dialysis fluid into ultrapure dialysis fluid and it should take place as close to the dialyser inlet as possible to avoid further contamination of the fluid [43]. Most modern dialysis machines can be equipped with ultrafilters integrated in the fluid flow path. Ultrafilters work by retention through size exclusion as well as by adsorption through hydrophobic binding. They should be labelled for the purpose and validated to have a logarithmic reduction capacity for bacteria of at least 7 magnitudes and for endotoxin of 3–4 magnitudes. They should also have a declared resistance to multiple disinfection cycles [10]. The integrity of the membrane is guaranteed through pressure tests of individual filters in production [28]. Regular dialysers should not be used as ultrafilters, although they may appear to contain similar membranes, because polymer blends may lead to different adsorptive properties [44]. To date, there are no randomized controlled studies comparing the effect of ultrapure versus standard quality dialysis fluid on outcome. However, numerous clinical studies show significant improvement of inflammatory parameters when patients are switched from standard fluid to ultrapure fluid [45,46]. Based on these findings, the European Best Practice Guidelines as well as the guidelines from the Japanese Society for Dialysis Therapy recommend the use of ultrapure dialysis fluid for all forms of dialysis [47,48].
Sterile fluid, prepared in bags
Substitution fluid for convective therapies is regarded as a drug, or an infusion solution, by the European as well as the US Pharmacopoiea [29,30]. They both say that the fluid must be sterile and should contain <0.25 EU/ml. Considering the fluid volumes infused in modern versions of HDF, 4–6 l/h, the maximum recommended endotoxin exposure for healthy individuals (5 EU/kg body weight and hour) could be exceeded already at moderate infusion rates and body weights using this fluid [49]. It is therefore important that the substitution solution for HDF is of considerably higher microbiological quality than stipulated by the authorities.
Sterile fluid, on-line prepared
With access to ultrapure dialysis fluid, it takes one additional step of controlled ultrafiltration to reach a quality level that can be described as sterile and non-pyrogenic (Figure 4). To guarantee the end result, two basic conditions must be fulfilled: (1) the dialysis fluid must be ultrapure before the final filtration and (2) the final ultrafilter must have the capacity to function as a sterilizing filter. Each clinic practicing on-line HDF must have a validated process for their entire chain of fluid preparation from the incoming water to the final ultrafilter. A recent publication describes the important steps and the quality level achieved and illustrates the value of the quality control process [50]. When setting up such a process correct microbiological sampling must be frequently performed and the frequency can be reduced only when the result is satisfactory and reproducible [28].
Because sterility cannot be proven by testing, it is the quality of the fluid before the final filter and the functionality of this filter that together determine whether the final fluid can be referred to as sterile and non-pyrogenic. The safety philosophy among manufacturers of on-line systems differs on this issue. Some use a sterile, single-use filter for the final filtration step and regard this as vital, while others use a disinfected, multiple-use filter and refer to the final filtration step as redundant. This reflects a difference in the interpretation of the sterility concept by the respective manufacturers of equipment. The possible difference in fluid quality can be translated into a question of risk management. Still, the safety margin built into approved on-line systems, when managed according to the operating instructions, is several magnitudes and the experience from thousands of treatments has shown us that the procedure can be considered safe for the patient [50–52].
The literature, unfortunately, contains confusing terminology and statements regarding on-line prepared fluids. The substitution fluid, although universally regarded as sterile, is sometimes referred to as ‘ultrapure’. If it were ultrapure, it would mean infusion of up to 400–600 CFU/h directly into the blood, based on the current definition of ultrapure (<0.1 CFU/ml) and common versions of on-line HDF with 4–6 l infusion/h. Another misnomer occurs when ultrapure dialysis fluid is referred to as ‘sterile’. The background to this may be that no growth is found when small samples of the fluid are tested. An ultrapure fluid would produce at the most one bacterial colony on one agar plate for every 10 plates inoculated with 1 ml fluid each.
Backfiltered dialysate as a substitution solution
If the dialysis fluid is ultrapure and the dialyser membrane has the capability of a sterilizing filter, backfiltered dialysate should in theory become sterile. Under ideal conditions, this procedure would therefore be microbiologically safe. Although the use of ultrapure dialysis fluid is spreading and the most commonly used high-flux membranes provide reasonably good protection against a limited quantity of bacterial products, the risk of exposing the blood to microbiological products should not be underestimated [53]. Working with ultrapure dialysis fluid and making sure the respective high-flux membrane has been validated for endotoxin retention, in addition to limiting the volume of backfiltered fluid, all contribute to reduce the risk of inducing microinflammation.
Conclusion
In HDF, the fluid serves the purpose of dialysis fluid as well as substitution solution and the volumes required for efficient therapy are the largest among blood purification therapies. This places high and stringent demands on the chemical as well as the microbiological quality of the fluids. The preparation of high-quality fluids for dialysis, starting with tap water and finishing with ultrapure dialysis fluid or sterile substitution solution, should be viewed as an integrated process where each step in the chain fulfils a defined objective and is validated for this purpose (Figure 4). No step should be omitted, and each step must have a wide margin of operation to give maximum safety. The validation and quality assurance of all fluid preparation need to be considered in the light of the risk of exposing patient blood to bacterial products.
The large volumes of substitution fluid required for optimal HDF therapy necessitate the use of on-line prepared fluid, from a quality perspective as well as practically and economically. With access to ultrapure dialysis fluid, on-line preparation of sterile and non-pyrogenic fluid can be realized by one additional step of controlled ultrafiltration. The attention presently attached to the microbiological quality of dialysis fluid has led to an increasing number of clinics now operating with ultrapure dialysis fluid. Thus, the clinical benefits of high volume HDF are within reach for an increasing number of patients who are already dialysed with high-flux membranes and ultrapure dialysis fluid.
Conclusion: Haemodiafiltration-optimal efficiency and safety
To achieve significant removal of uraemic solutes in the middle and large molecular weight range, it is not sufficient to use a high-flux membrane in HD; we also need to add considerable amounts of convection by performing high volume HDF. Large convective volumes require large ultrafiltration volumes, which in turn require large volumes of high-quality substitution solution. On-line preparation of the substitution solution as an integral component of the treatment has eliminated the therapeutic as well as the practical and economic drawbacks of classical HDF and additionally brought the therapy to a safety level that exceeds any alternative form of HDF. On-line HDF has the potential to provide the largest dose of blood purification over the widest molecular weight range among blood purification therapies.
When HDF was first conceptualized, it was seen as a combination of the best of both HD and HF, i.e. a therapy providing high removal rates of small as well as medium-sized and large solutes [24]. However, considering the large number of variables of a HDF treatment and the technology available to us today, it is possible to manipulate the conditions beyond the original objective and miss some of the potential benefits. It is therefore important that users of HDF identify the most important outcome parameter for their patients and adjust the prescription accordingly. The target may be to reach an adequate dose of dialysis in a large patient, a hypercatabolic patient or in a patient with limited blood flow capacity. It may also be to provide extended removal of large solutes or it may just be to provide the most biocompatible and effective dialysis treatment available to us today—haemodiafiltration [54].
Conflict of interest statement. IL is employed by Gambro. PJB has received research grants and lecture fees from Fresenius and Gambro. The material has not been previously published in this format and is not considered for publication elsewhere.
References
- 1.Eknoyan G, Beck GJ, Cheung AK, et al. Effect of dose and membrane flux in maintenance hemodialysis. N Engl J Med. 2002;347:2010–2019. doi: 10.1056/NEJMoa021583. [DOI] [PubMed] [Google Scholar]
- 2.Cheung AK, Levin NW, Greene T, et al. Effects of high-flux hemodialysis on clinical outcomes: results of the HEMO study. J Am Soc Nephrol. 2003;14:3251–3263. doi: 10.1097/01.asn.0000096373.13406.94. [DOI] [PubMed] [Google Scholar]
- 3.Delmez JA, Yan B, Bailey J, et al. Cerebrovascular disease in maintenance hemodialysis patients: results of the HEMO study. Am J Kidney Dis. 2006;47:131–138. doi: 10.1053/j.ajkd.2005.09.031. [DOI] [PubMed] [Google Scholar]
- 4.Locatelli F, Martin-Malo A, Hannedouche T, et al. Effect of membrane permeability on survival of hemodialysis patients. J Am Soc Nephrol. 2009;20:645–654. doi: 10.1681/ASN.2008060590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meert N, Eloot S, Waterloos M-A, et al. Effective removal of protein-bound uraemic solutes by different convective strategies: a prospective trial. Nephrol Dial Transplant. 2009;24:562–570. doi: 10.1093/ndt/gfn522. [DOI] [PubMed] [Google Scholar]
- 6.Canaud B, Bragg-Gresham JL, Marshall MR, et al. Mortality risk for patients receiving hemodiafiltration versus hemodialysis: European results from the DOPPS. Kidney Int. 2006;69:2087–2093. doi: 10.1038/sj.ki.5000447. [DOI] [PubMed] [Google Scholar]
- 7.Jirka T, Cesare S, Di Benedetto A. Mortality risk for patients receiving hemodiafiltration versus hemodialysis. Kidney Int. 2006;70:1524. doi: 10.1038/sj.ki.5001759. [DOI] [PubMed] [Google Scholar]
- 8.Van Der Weerd NC, Penne EL, Van Den Dorpel MA, et al. Haemodiafiltration: promise for the future? Nephrol Dial Transplant. 2008;23:438–443. doi: 10.1093/ndt/gfm791. [DOI] [PubMed] [Google Scholar]
- 9.Ledebo I, Ronco C. The best dialysis therapy? Results from an international survey among nephrology professionals. NDT Plus. 2008;6:403–408. doi: 10.1093/ndtplus/sfn148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ledebo I. On-line hemodiafiltration: technique and therapy. Adv Renal Repl Therapy. 1999;6:195–208. doi: 10.1016/s1073-4449(99)70038-5. [DOI] [PubMed] [Google Scholar]
- 11.International Organization for Standardization Quality of dialysis fluid for haemodialysis and related therapies. 2009. ISO 11663.
- 12.Sargent JA, Gotch FA. Replacement of Renal Function by Dialysis. 4th edn. Dordrecht, The Netherlands: Kluwer; 1996. Principles and biophysics of dialysis; pp. 35–96. [Google Scholar]
- 13.Bhimani JP, Ouseph R, Ward RA. Reducing dialysate boundary layer resistance by increasing dialysate flow rate increases diffusive mass transfer of phosphorous but not urea nor beta2microglobulin in dialyzers with fiber undulations (abstract) J Am Soc Nephrol. 2008;19:460A. [Google Scholar]
- 14.Ledebo I. Principles and practice of hemofiltration and hemodiafiltration. Artif Organs. 1998;22:20–25. doi: 10.1046/j.1525-1594.1998.06072.x. [DOI] [PubMed] [Google Scholar]
- 15.Beerenhout CH, Luik AJ, Jeuken-Mertens SG, et al. Pre-dilution on-line hemofiltration vs low-flux haemodialysis: a randomized prospective study. Nephrol Dial Transplant. 2005;20:1155–1163. doi: 10.1093/ndt/gfh775. [DOI] [PubMed] [Google Scholar]
- 16.Jaffrin M. Convective mass transfer in hemodialysis. Artif Organs. 1995;19:1162–1171. doi: 10.1111/j.1525-1594.1995.tb02277.x. [DOI] [PubMed] [Google Scholar]
- 17.Penne EL, Van Der Weerd NC, Bots M, et al. Patient- and treatment-related determinants of convective volume in post-dilution haemodiafiltration in clinical practice. Nephrol Dial Transplant. 2009 doi: 10.1093/ndt/gfp265. doi:10.1093/ndt/gfp265. [DOI] [PubMed] [Google Scholar]
- 18.Joyeux V, Sijpkens Y, Haddj-Elmrabet A, et al. Optimized convective transport with automated pressure control in on-line postdilution hemodiafiltration. Int J Artif Organs. 2008;31:928–936. doi: 10.1177/039139880803101102. [DOI] [PubMed] [Google Scholar]
- 19.Colussi G, Frattini G. Quantitative analysis of convective dose in hemofiltration and hemodiafiltration: ‘predilution’ versus ‘postdilution’ reinfusion. Hemodial Int. 2007;11:76–85. doi: 10.1111/j.1542-4758.2007.00157.x. [DOI] [PubMed] [Google Scholar]
- 20.Fischbach M, Dheu C, Menouer S, Terzic J. In-center daily on-line hemodiafiltration: 4-year experience in children. Clin Nephrol. 2008;69:279–294. doi: 10.5414/cnp69279. [DOI] [PubMed] [Google Scholar]
- 21.Pedrini LA, De Christofaro V. On-line mixed hemodiafiltration with feedback for ultrafiltration control: effect on middle molecule removal. Kidney Int. 2003;64:1505–1513. doi: 10.1046/j.1523-1755.2003.00240.x. [DOI] [PubMed] [Google Scholar]
- 22.Leypoldt JK. Solute fluxes in different treatment modalities. Nephrol Dial Transplant. 2000;15(Suppl 1):3–9. doi: 10.1093/oxfordjournals.ndt.a027961. [DOI] [PubMed] [Google Scholar]
- 23.Henderson LW, Colton CK, Ford CA. Kinetics of hemodiafiltration: II. Clinical characterization of a new blood cleansing modality. J Clin Lab Med. 1975;85:372–391. [PubMed] [Google Scholar]
- 24.Leber HW, Wizemann V, Goubeaud G, et al. Simultaneous hemofiltration/ hemodialysis: an effective alternative to hemofiltration and conventional hemodialysis in the treatment of uremic patients. Clin Nephrol. 1978;9:115–121. [PubMed] [Google Scholar]
- 25.Biasiolo S, Feriani M, Chiaramonte S, et al. Different buffers for hemodiafiltration. A controlled study. Int J Artif Organs. 1989;12:25–30. [PubMed] [Google Scholar]
- 26.Galli G, Panzetta G. Acetate-free biofiltration (AFB): from theory to clinical results. Clin Nephrol. 1998;50:28–37. [PubMed] [Google Scholar]
- 27.Canaud B, N’Guyen QV, Lagarde C, et al. Clinical evaluation of a multipurpose dialysis system adequate for hemodialysis or for postdilution hemofiltration/ hemodiafiltration with on-line preparation of substitution fluid from dialysate. Contr Nephrol. 1985;46:184–186. doi: 10.1159/000410781. [DOI] [PubMed] [Google Scholar]
- 28.Ledebo I. On-line preparation of solutions for dialysis: practical aspects versus safety and regulations. J Am Soc Nephrol. 2002;13:S78–S83. [PubMed] [Google Scholar]
- 29.European Pharmacopoeia. 5th edition. 2005. Monograph.
- 30.2005. United States Pharmacopoeia, First supplement to USP 28-NF 23.
- 31.Krieter DH, Falkenhain S, Chalabi L, et al. Clinical cross-over comparison of mid-dilution hemodiafiltration using a novel dialyzer concept and post-dilution hemodiafiltration. Kidney Int. 2005;67:349–356. doi: 10.1111/j.1523-1755.2005.00088.x. [DOI] [PubMed] [Google Scholar]
- 32.Shinzato T, Sezaki R, Usuda M, et al. Infusion-free hemodiafiltration: simultaneous hemofiltration and dialysis with no need for infusion fluid. Artif Organs. 1982;6:453–456. doi: 10.1111/j.1525-1594.1982.tb04143.x. [DOI] [PubMed] [Google Scholar]
- 33.von Albertini B, Miller JH, Gardner PW, et al. High-flux hemodiafiltration: under six hours/week treatment. Trans Am Soc Artif Intern Organs. 1984;30:227–231. [PubMed] [Google Scholar]
- 34.Yamashita AC. New dialysis membrane for removal of middle molecule uremic toxins. Am J Kidney Dis. 2001;38(Suppl 1):217–219. doi: 10.1053/ajkd.2001.27450. [DOI] [PubMed] [Google Scholar]
- 35.Stenvinkel P, Alvestrand A. Inflammation in end-stage renal disease: sources, consequences and therapy. Semin Dial. 2002;15:329–337. doi: 10.1046/j.1525-139x.2002.00083.x. [DOI] [PubMed] [Google Scholar]
- 36.Santoro A, Canova C, Manzini E, et al. Protein loss in on-line hemofiltration. Blood Purif. 2994;22:261–268. doi: 10.1159/000078495. [DOI] [PubMed] [Google Scholar]
- 37.Grassmann A, Gioberge S, Moeller S, et al. End-stage renal disease: global demographics in 2005 and observed trends. Artif Organs. 2006;30:895–897. doi: 10.1111/j.1525-1594.2006.00321.x. [DOI] [PubMed] [Google Scholar]
- 38.Ward RA. Worldwide water standards for hemodialysis. Hemodial Int. 2007;11:S18–S25. [Google Scholar]
- 39.Ledebo I, Nystrand R. Defining the microbiological quality of dialysis fluid. Artif Organs. 1999;23:37–43. doi: 10.1046/j.1525-1594.1999.06275.x. [DOI] [PubMed] [Google Scholar]
- 40.Schindler R, Ertl T, Beck W, et al. Reduced cytokine induction and removal of complement products with synthetic hemodialysis membranes. Blood Purif. 2006;24:203–211. doi: 10.1159/000090520. [DOI] [PubMed] [Google Scholar]
- 41.Pereira BJ, Snodgrass BR, Hogan PJ, et al. Diffusive and convective transfer of cytokine-inducing bacterial products across hemodialysis membranes. Kidney Int. 1995;47:603–610. doi: 10.1038/ki.1995.76. [DOI] [PubMed] [Google Scholar]
- 42.Panichi V, De Pietro S, Andreini B, et al. Cytokine production in haemodiafiltration: a multicentre study. Nephrol Dial Transplant. 1998;13:1737–1744. doi: 10.1093/ndt/13.7.1737. [DOI] [PubMed] [Google Scholar]
- 43.Ledebo I. Ultrapure dialysis fluid—direct and indirect benefits in dialysis therapy. Blood Purif. 2004;22(Suppl 2):20–25. doi: 10.1159/000081869. [DOI] [PubMed] [Google Scholar]
- 44.Bommer J, Becker KP, Urbaschek R. Potential transfer of endotoxin across high-flux polysulfone membranes. J Am Soc Nephrol. 1996;7:883–888. doi: 10.1681/ASN.V76883. [DOI] [PubMed] [Google Scholar]
- 45.Masakane I. Review: clinical usefulness of ultrapure dialysate—recent evidence and perspectives. Ther Apher Dial. 2006;10:348–354. doi: 10.1111/j.1744-9987.2006.00388.x. [DOI] [PubMed] [Google Scholar]
- 46.Ledebo I. Ultrapure dialysis fluid—how pure is it and do we need it? Nephrol Dial Transplant. 2007;22:20–23. doi: 10.1093/ndt/gfl574. [DOI] [PubMed] [Google Scholar]
- 47.European Best Practice Guidelines for Haemodialysis (Part 1), SECTION IV Dialysis fluid purity. Nephrol Dial Transplant. 2002;17(Suppl 7):45–62. [PubMed] [Google Scholar]
- 48.Kawanishi H, Akiba T, Masakane I, et al. Standards on microbiological management of fluids for hemodialysis and related therapies by the Japanese society for dialysis therapy 2008. Ther Apher Dial. 2009;13:161–166. doi: 10.1111/j.1744-9987.2009.00674.x. [DOI] [PubMed] [Google Scholar]
- 49.Ledebo I. Fluid in bags for hemodiafiltration. Contrib Nephrol. 2007;158:87–93. doi: 10.1159/000107238. [DOI] [PubMed] [Google Scholar]
- 50.Penne EL, Visser L, Van Den Dorpel A, et al. Microbiological quality and quality control of purified water and ultrapure dialysis fluids for online hemodiafiltration in routine clinical practice. Kidney Int. 2009;76:665–672. doi: 10.1038/ki.2009.245. [DOI] [PubMed] [Google Scholar]
- 51.Vaslaki L, Karátson A, Vörös P, et al. Can sterile and pyrogen-free on-line substitution fluid be routinely delivered? A multicentric study on the microbiological safety of on-line haemodiafiltration. Nephrol Dial Transplant. 2000;15(Suppl 1):74–78. doi: 10.1093/oxfordjournals.ndt.a027968. [DOI] [PubMed] [Google Scholar]
- 52.Guth H-J, Gruska S, Kraatz G. On-line production of ultrapure substitution fluid reduces TNF-alpha- and IL-6 release in patients on hemodiafiltration therapy. Int J Artif Organs. 2003;26:181–187. doi: 10.1177/039139880302600301. [DOI] [PubMed] [Google Scholar]
- 53.Teehan GS, Guo D, Perianayagam MC, et al. Reprocessed (high-flux) Polyflux dialyzers resist transmembrane endotoxin passage and attenuate inflammatory markers. Blood Purif. 2004;22:329–337. doi: 10.1159/000078926. [DOI] [PubMed] [Google Scholar]
- 54.Ledebo I. Does convective dialysis therapy applied daily approach renal blood purification? Kidney Int. 2001;59(Suppl 78):286–291. doi: 10.1046/j.1523-1755.2001.59780286.x. [DOI] [PubMed] [Google Scholar]


