Abstract
Complex interactions between environmental and biological factors influence the susceptibility of Culex pipiens quinquefasciatus to St. Louis encephalitis virus and could affect the epidemiology of virus transmission. Similar interactions could have epidemiologic implications for other vector-virus systems. We conducted an experiment to examine four such factors in combination: mosquito age, extrinsic incubation temperature (EIT), virus dose, and colony. The proportion of mosquitoes with body infections or disseminated infections varied between colonies, and was dependant on age, EIT, and dose. We also show that the probability of a body or leg infection interacted in complex ways between colonies, ages, EITs, and doses. The complex interactive effects of environmental and biological factors must be taken into account for studies of vector competence and epidemiology, especially when laboratory studies are used to generalize to natural transmission dynamics where the extent of variation is largely unknown.
Introduction
Mosquito biology and the environment can play important roles in mosquito vector competence. The effect of environmental factors such as extrinsic incubation temperature (EIT) and virus dose on vector competence for arboviruses is widely appreciated and has been studied and reviewed for a number of insect-virus systems. 1–6 However, although environmental factors influence mosquito vector competence for arboviruses, there has been little work to determine how various environmental and biological factors may interact with one another. For example, several studies generally show a positive relationship between EIT and vector competence in mosquitoes. 1,2 Higher virus doses overcame barriers to dissemination for Culex tarsalis infected with Western equine encephalitis virus (WEEV),3,4 Cx. pipiens pipiens and Cx. p. quinquefasciatus with St. Louis encephalitis virus (SLEV),5 and Cx. nigripalpus and Cx. p. quinquefasciatus with West Nile virus (WNV).6
Biological factors such as mosquito age and mosquito strain, as well as environmental factors such as EIT and dose may have indirect effects on arbovirus infection and transmission insofar as vectors must survive long enough to blood feed on an infectious host, complete the extrinsic incubation period (EIP) required for virus dissemination, oviposit, and subsequently feed on another host. The importance of mosquito age on arbovirus transmission was shown by the observation that young Aedes aegypti have a higher survival probability compared with older mosquitoes, and would thereby be more likely to complete the EIP required for virus transmission.7 However, there may also be a direct effect of vector age on viral infection and dissemination rates. Decreased abdomen infection rates were found in Cx. tritaeniorhynchus fed WNV at 12 days post-emergence, compared with mosquitoes fed at four and eight days post-emergence.8 However, there were no differences in transmission of Japanese encephalitis virus by Cx. tritaeniorhynchus that were infected at 10 or 24 days post-emergence.9
A positive relationship was observed between EIT and dose with WNV infection and dissemination rates in Cx. p. quinquefasciatus.10 However, the relationship between infection and dissemination changed depending on the EIT, but not the dose. This inconsistency and also the limited information on the impact of mosquito age on vector competence was the basis for the current investigation. Most laboratory studies have focused on the effects of single environmental factors with little attention to how different factors may interact with each other and how such interactions may change because of biological factors such as mosquito age or mosquito strain.
St. Louis encephalitis virus (family Flaviviridae, genus Flavivirus) is maintained in an enzootic cycle involving ornithophilic mosquitoes and susceptible birds. This virus threatens humans whenever large numbers of infectious mosquitoes exhibiting opportunistic feeding coincide with amplification hosts and human populations. 11–13 The range of SLEV extends from southern Canada to Argentina; however, most human cases have occurred in the central and eastern portion of the United States.14,15 The primary vectors of SLEV in North America have been identified as Cx. tarsalis, Cx. p. pipiens, Cx. p. quinquefasciatus, and Cx. nigripalpus.16–18 Culex p. quinquefasciatus feeds on avian and mammalian hosts,19–21 is a competent vector of SLEV in the laboratory,22,23 and has been found in nature infected with SLEV.18,24
Vector competence in the present study was characterized using four epidemiologically important phenotypes: susceptibility to infection, disseminated infection, virus body titer, and leg titer. These phenotypes are aspects of vector competence because viruses must cause midgut infections and overcome barriers to dissemination. Female mosquitoes that have no body infection show a midgut infection barrier (MIB), and females having an infected body with no dissemination to the legs show a midgut escape barrier (MEB).25 Basic knowledge of individual properties of the MIB and MEB under different conditions, as well as the relationship between the MIB and MEB is needed to develop mechanistic hypotheses and design further studies of vector competence. We use virus titers in the body and leg as a quantitative measure of the ability of the viruses to replicate in mosquito tissues. Although other investigators have shown a positive relationship between disseminated infection and transmission for Cx. p. pipiens and WNV,26,27 further studies are needed to directly address the relationship between dissemination out of the midgut and salivary gland infection with regard to actual vector transmission under varying environmental and biological conditions.
The intra-population and inter-population variability in interactions between environmental and biological factors is virtually unknown. These interactions must be characterized and the responsible mechanisms ultimately elucidated to understand the impact of vector competence on the epidemiology of arbovirus cycles. Results from the current study are expected to inform mechanistic studies of vector competence by elucidating some of the factors and complexity between factors and the effect on vector competence. We explore the complexity of environmental and biological effects on vector competence for arboviruses using Cx. p. quinquefasciatus and SLEV.
Materials and Methods
Mosquitoes
Culex p. quinquefasciatus from two colonies were used for experiments. The first colony was established in 1995 from a collection from Alachua County in north central Florida (generation > F40) and will be referred to as the 1995 colony. The second colony was established in 2007 from a collection of 32 egg rafts from Indian River County in east central Florida (generation F3) and will be referred to as the 2007 colony. Mosquitoes were reared at 28°C and maintained under a 14:10 (light:dark) cycle. Rearing conditions were stan dardized to generate similar sized individuals. For each colony, three egg rafts were placed in each of approximately 15 enameled pans (24 cm × 36 cm × 5 cm) containing approximately 700 mL water. Larvae were fed daily with a slurry (20 mg/mL) of Brewer's yeast and liver powder. Pupae were transferred to 500 mL plastic cups containing approximately 250 mL of water, and male and female adults were allowed to emerge and mate in square cages (33 cm3) and provided 20% sucrose ad libitum. Plastic cups containing pupae were removed from adult emergence cages approximately 24 hours after the first respective adult female emergence so that females did not differ in age by more than one day. Twenty four hours prior to experiments, sucrose was removed, adult females were transferred to one-liter cardboard cages with mesh screening, and water was provided ad libitum.
Sample size
Large sample sizes are often needed to differentiate between treatment groups in vector competence tests.28 Consequently, we conducted a priori power analyses to determine sample sizes needed to detect significant differences in mosquito infection and dissemination rates among treatments (GPower, http://www.psycho.uni-duesseldorf.de/aap/projects/gpower/).29 Our power analyses were based on chi-square contingency analyses that compared infection and dissemination rates between treatment groups. A priori analyses use Cohen's effect size w, a measure of the size of the difference between the null (groups were different) and alternative (groups were not different) hypotheses.30 Cohen's w is often characterized as small (w = 0.1), medium (w = 0.3), and large (w = 0.5), and we calculated the sample sizes necessary to detect different levels of effect size with 99% power. To detect a small to medium (w = 0.2) effect of age, EIT, dose, and colony (P = 0.05, degrees of freedom [df] = 3), a total sample size of at least 589 mosquitoes would be required to test infection, and a total of 262 mosquitoes would be required if only a medium (w = 0.3) effect size was expected. Assuming good feeding success and survival, our planned sample sizes of 50–100 mosquitoes per group (1,200– 2,400 total/colony) would thus enable detection of small to medium effect sizes in infection rates. Sample sizes (and therefore power to detect small differences between groups) for dissemination rates were expected to be lower because we did not expect 100% infection rates.
Blood meal preparation
Previous results showed that fresh preparation of low-titered SLEV is an effective method for infecting mosquitoes by artificial feeding systems.31 Consequently, a T-75 cm2 flask of confluent African green monkey (Vero) cells was inoculated with 0.15 mL of SLEV stock previously determined by plaque assay to have 6.0 logs plaque-forming units (PFU)/mL. Then, 12 mL of Medium 199 (with Earle's salts, 10% fetal bovine serum, penicillin/streptomycin, and mycostatin) was added to the flask and incubated at 35°C in an atmosphere of 5% CO2. At 72 hours post-inoculation, the supernatant was mixed with citrated bovine blood to create blood meals of low and high dose. Because of a lower feeding success rate of the 2007 colony, a second set of flasks was inoculated with SLEV 24 hours after the first flask so that another feeding could be attempted the day after the initial feeding. Two 0.1-mL samples of the blood meal were each added to separate tubes containing 0.9 mL BA-1 diluent32 and stored at −80°C until tested to determine blood meal titer.
Mosquito infection
The TBH28 strain of SLEV (passaged three times in Vero cells) was isolated from a human in Florida in 1962. More than 200 female mosquitoes were offered a blood meal for each colony, age group, dose, and EIT to achieve a minimum of 50 mosquitoes per treatment that fed and survived the EIP. Mosquitoes from the 1995 colony at four (young), seven (middle age), and ten (old) days post-emergence were allowed to feed for 30 minutes on membrane feeders33 containing infectious citrated bovine blood (Hemostat, Dixon, CA) warmed (35°C) for 10 minutes. Mosquitoes from the 2007 colony at three, seven, and eleven days post-emergence were allowed to feed on cotton pledgets containing infectious citrated bovine blood with a final concentration of 5.0% sucrose, warmed (35°C) for 10 minutes. Because of the low feeding rate of the 2007 colony, for which reason pledgets were used, a second feeding was provided 24 hours later at four, eight, and twelve days post-emergence. Thus, mosquitoes ages three to four, seven to eight, and eleven to twelve days post-emergence will respectively be referred to as young, middle age, and old. We observed an approximately 50% and 85% feeding rate in the pledget-fed 2007 colony and membrane-fed 1995 colony, respectively.
Subsequent to feeding, mosquitoes were immobilized with cold, and five freshly fed fully engorged mosquitoes from each treatment group were frozen at −80°C until tested to determine the quantity of virus actually imbibed. The remaining fully engorged specimens were transferred to one-liter cardboard cages with mesh screening and maintained in incubators for 13 days post-infection at either 25°C (low EIT) or 28°C (high EIT) and provided 20% sucrose ad libitum. These EITs were chosen based on temperatures observed in Florida during estimated SLEV amplification (25°C) and early transmission (28°C) phases.10,34
Blood meal and mosquito processing
We used an EIP of 13 days as the time interval for Cx. p. quinquefasciatus to become infected and disseminated after a blood meal at either 25°C or 28°C. This EIP was determined to be adequate for observing variation in vector susceptibility between groups based on prior reports of vector competence in Culex species.35,36 Surviving mosquitoes were removed from each cage after 13 days, killed by freezing, and their legs were removed with forceps. To prevent contamination, separate forceps were used in handling bodies and legs, and forceps were soaked in 70% ethanol and flamed between processing of each mosquito. Mosquito bodies and legs were triturated separately in 0.9 mL BA-1 diluent and stored at −80°C until two 4.5-mm zinc-plated beads (BB-caliber air gun shot) were added to each sample, which was homogenized at 25 Hz for 3 minutes (TissueLyser; Qiagen, Valencia, CA) and centrifuged at 4°C and 3,148 × g for 4 minutes.
Virus assays
Viral RNA was extracted from 0.25 mL of homogenate and eluted in 0.05 mL of buffer with the MagNA Pure LC System and Total Nucleic Acid Isolation Kit (Roche, Mannheim, Germany). The amount of viral RNA in each sample was determined using a LightCycler® 480 system (Roche) and Superscript III One-Step Quantitative reverse transcription–polymerase chain reaction (RT-PCR) kit (Invitrogen, Carlsbad, CA) for quantitative real-time TaqMan RT-PCR32,37 using methods described elsewhere.10 Standard curves38 were based on 10-fold serial dilutions of known SLEV titers determined by plaque assay, except that the second overlay containing neutral red was added 120 hours after the first overlay.39
Virus found in the body but not in the legs represented a non-disseminated infection limited to the midgut. Virus in the body and legs was considered a disseminated infection.40
Statistical analysis
Box plots were used to test viral titers for normality. The lack of normality was verified with Kolmogorov-Smirnov tests.41 Viral titers in freshly fed mosquitoes, as well as body and leg titers at the end of the EIP were log-transformed [log (x + 1)] prior to analysis of variance (ANOVA) with the generalized linear model (GLM) procedure in SAS.41 An ANOVA was carried out to examine virus titers from whole bodies of freshly fed mosquitoes for all possible fixed effects (i.e., age, dose, colony) and interactions. The EIT was not included in this analysis of freshly fed mosquitoes because samples were acquired immediately after feeding. Separate ANOVA tests were conducted for body and leg titers of virus-positive mosquitoes at the end of the EIP and included all possible fixed effects (i.e., age, dose, EIT, colony) and interactions. Individual mosquitoes were treated as experimental units in these analyses. If significance (P < 0.05) was observed in an ANOVA, then a Duncan multiple comparison procedure41 was used to determine which means were significantly different. Because means comparisons in SAS cannot be conducted for interactions, each treatment group was coded differently with a dummy variable. A single-factor ANOVA with means comparisons was conducted on the dummy variable for each body part separately. This produces identical results for the overall model and enables identification of treatment means that differ.
We conducted two separate analyses of the probability of different body parts (i.e., bodies and legs) becoming infected with SLEV. For ease of comparison with other studies, we analyzed the infection and dissemination rates with respect to mosquito age only in different colony, EIT, and dose treatments. Pearson chi-square tests were used to evaluate independence in contingency table analyses and determine significant differences (P < 0.05) in rates of infection or dissemination between mosquito age groups41 within each colony. The infection rate was the percentage of all mosquitoes tested having infected bodies. The dissemination rate was the percentage of mosquitoes with infected bodies that also had infected legs. Our null hypothesis was that infection and dissemination rates were equal for different mosquito ages.
We could not directly compare infection and dissemination rates and the effect of the treatments on the difference between them because of the lack of independence between the infection phenotype of bodies and legs. To fully examine the effect of the environmental and biological factors on the probability that a body or leg is infected, we used a generalized linear mixed model (GLMM) (PROC GLIMMIX with the logit link function in SAS).41 Through iterative model fitting, we used this analysis to determine significant differences (P < 0.05) in the likelihood of virus occurrence in mosquito bodies or legs between age, EIT, dose, and colony treatments. Here, individual mosquitoes were treated as experimental units and data were coded for a binary distribution for presence or absence of virus infection. Body or leg samples having no virus infection were coded as 0 and samples with virus infection were coded as 1.
Our large sample size enabled us to include both fixed (colony, age, EIT, dose) and random (individual mosquito) effects in this mixed model. Infection by body part was the response variable for each individual mosquito because each individual was measured twice. If there was insufficient power to examine all possible interactions, interactions were dropped in a hierarchical fashion: five-way, then four-way interactions. If some interactions at a given level could be considered, but not all, we dropped those that were of less biological interest, retaining those that previous studies suggested were of primary interest. Our null hypothesis was that the occurrence of virus was equivalent for body part, mosquito age, EIT, dose, and colony. Mixed model population estimates of the logit (log of the odds ratio, i.e., probability of infection/probability of no infection) were calculated using the LS-means statement in SAS and estimates for some treatment effects on colonies and type of body part infection were graphed for illustrative purposes.41 Higher logit estimates on graphs show a higher probability of virus occurrence in one treatment group compared with other treatment groups under consideration. Because the reverse was also true, lower logit estimates show a lower probability of occurrence in one group versus another group.
Results
Virus titer of blood meals and freshly fed mosquitoes
Mosquitoes were fed blood meals containing (mean ± SE) 5.1 ± 0.1 logs PFU of SLEV/mL (high dose) or 4.5 ± 0.03 logs PFU of SLEV/mL (low dose) and titers of five freshly fed fully engorged specimens were determined (Table 1). There was a significant effect of age, dose, and colony on the amount of virus imbibed by freshly fed mosquitoes (Table 2). The interaction terms were all non-significant, indicating that the colonies and age groups responded similarly to the initial dose (Table 2). However, our small sample sizes of five mosquitoes per group may have limited the power of this analysis and thereby the ability to detect significant interactions. The means comparisons (Table 1) indicate differences between treatment groups for freshly fed mosquitoes. Although there were relatively few significant differences in the means comparisons, we observed a tendency for young mosquitoes to have higher viral titers, compared with middle age and old mosquitoes (Tables 1 and 2). We also found a pattern of higher viral titers in mosquitoes fed the high dose compared with the low dose and in the 1995 colony compared with the 2007 colony (Tables 1 and 2). The highest titers were observed in young mosquitoes in the 1995 colony fed the high dose and lowest titers were found in old mosquitoes in the 2007 colony fed the low dose (Table 1).
Table 1.
Mean ± SE titers (log plaque-forming units of SLEV/mL) of whole bodies of freshly fed Culex pipiens quinquefasciatus fed blood meals containing a low or high dose of SLEV at different ages*
| Dose† | |||
|---|---|---|---|
| Mosquito age | No. tested | Low | High |
| 1995 Colony | |||
| Young | 5 | 3.6 ± 0.1abc | 3.8 ± 0.1a |
| Middle age | 5 | 3.4 ± 0.1bcd | 3.7 ± 0.04ab |
| Old | 5 | 3.3 ± 0.1cde | 3.5 ± 0.1bc |
| 2007 Colony | |||
| Young | 5 | 3.3 ± 0.1cde | 3.6 ± 0.1abc |
| Middle age | 5 | 3.2 ± 0.1de | 3.6 ± 0.1abc |
| Old | 5 | 3.1 ± 0.2e | 3.5 ± 0.1bc |
SLEV = St. Louis encephalitis virus.
Treatment groups with the same letter are not significantly different by means comparisons.
Table 2.
Analysis of variance of effects of age, dose, and colony on SLEV body titers of freshly fed Culex pipiens quinquefasciatus*
| Effect | F | Degrees of freedom, numerator, denominator |
P |
|---|---|---|---|
| Age | 3.75 | 2, 48 | 0.031 |
| Dose | 37.02 | 1, 48 | < 0.0001 |
| Colony | 12.45 | 1, 48 | 0.0009 |
| Age × dose | 0.07 | 2, 48 | 0.932 |
| Dose × colony | 1.70 | 1, 48 | 0.199 |
| Age × colony | 1.17 | 2, 48 | 0.318 |
| Age × dose × colony | 0.21 | 2, 48 | 0.811 |
SLEV = St. Louis encephalitis virus.
Multi-factorial ANOVA for effects of environmental and biological factors on body and leg titers
The means of body and leg titers for each treatment group, along with infection rate and dissemination rate, are shown in Table 3 (25°C) and Table 4 (28°C). Results of ANOVA for environmental and biological effects on body and leg titers for virus-positive mosquitoes are shown in Table 5. All main effects were significant for body titer, indicating that age, dose, EIT, and colony each influenced this phenotype. Most of the two-way and three-way interactions were also significant, showing a large degree of complexity in the effects of the environment on body titer. The means comparisons (Tables 3 and 4) indicate differences between treatment groups, although the complexity of the interactions makes it difficult to observe patterns. There were significant differences in body titer between different ages of mosquitoes at 28°C, but not at 25°C, and the differences between ages were dependant on the dose and colony. The significant differences in body titer at different EITs were dependant on the dose and colony, with higher titers at 28°C more often in the 1995 colony and the low dose. Between doses, the significant dose × colony interaction indicates that the colonies responded differently to the two doses. This was apparent in the consistently higher titers after a high dose in the 2007 colony, but not in the 1995 colony. The two-way interaction for age × dose was not significant, but the three-way age × dose × colony interaction was significant. This finding indicates that the colonies responded differently to the age × dose interaction, with the 1995 colony having more differences between age classes but the 2007 colony more differences between doses. The single four-way interaction was non-significant, indicating that the colony-age group treatments responded similarly to the environmental dose × EIT interaction.
Table 3.
Mean ± SE titers (log plaque-forming units of SLEV/mL), infection rates, and dissemination rates for Culex pipiens quinquefasciatus fed blood meals containing a low or high dose at different ages and tested after a 13-day extrinsic incubation period at 25°C*
| Mosquito age | No. tested | No. infected (%) | No. disseminated (%) | Body titer† | Leg titer† | |
|---|---|---|---|---|---|---|
| 1995 colony, low dose | ||||||
| Young | 101 | 94 (93) | 2 (2) | 3.1 ± 0.1ghi | 2.6 ± 1.0ab | |
| Middle age | 90 | 46 (51) | 5 (11) | 3.1 ± 0.2ghi | 2.8 ± 0.5ab | |
| Old | 101 | 36 (36) | 3 (8) | 2.7 ± 0.1ijk | 2.5 ± 0.5ab | |
| 1995 colony, high dose | ||||||
| Young | 50 | 47 (94) | 6 (13) | 3.2 ± 0.1efgh | 2.7 ± 0.3ab | |
| Middle age | 50 | 48 (96) | 4 (8) | 3.1 ± 0.1fgh | 3.1 ± 0.2a | |
| Old | 39 | 20 (51) | 2 (10) | 2.7 ± 0.2hij | 2.8 ± 0.1ab | |
| 2007 colony, low dose | ||||||
| Young | 71 | 10 (14) | 1 (1) | 2.2 ± 0.4kl | 1.0bc | |
| Middle age | 56 | 10 (18) | 1 (1) | 2.1 ± 0.5l | 2.4ab | |
| Old | 91 | 22 (24) | 2 (9) | 2.5 ± 0.2jkl | 2.6 ± 0.1ab | |
| 2007 colony, high dose | ||||||
| Young | 76 | 56 (74) | 15 (27) | 3.6 ± 0.1cdef | 2.3 ± 0.2abc | |
| Middle age | 49 | 34 (69) | 5 (15) | 3.7 ± 0.1cdef | 2.6 ± 0.4ab | |
| Old | 61 | 38 (62) | 4 (11) | 3.3 ± 0.1defgh | 1.6 ± 0.2bc | |
SLEV = St. Louis encephalitis virus.
Treatment groups with the same letter in the same column are not significantly different by means comparisons across Tables 3 and 4.
Table 4.
Mean ± SE titers (log plaque-forming units of SLEV/mL), infection rates, and dissemination rates for Culex pipiens quinquefasciatus fed blood meals containing a low or high dose at different ages and tested after a 13-day extrinsic incubation period at 28°C*
| Mosquito age | No. tested | No. infected (%) | No. disseminated (%) | Body titer† | Leg titer† |
|---|---|---|---|---|---|
| 1995 colony, low dose | |||||
| Young | 101 | 101 (100) | 68 (67) | 4.5 ± 0.1a | 2.8 ± 0.1ab |
| Middle age | 82 | 46 (56) | 8 (17) | 3.5 ± 0.2cdefg | 2.5 ± 0.2ab |
| Old | 100 | 72 (72) | 18 (25) | 3.8 ± 0.1cd | 2.7 ± 0.1ab |
| 1995 colony, high dose | |||||
| Young | 52 | 52 (100) | 43 (83) | 4.3 ± 0.04ab | 3.0 ± 0.1ab |
| Middle age | 50 | 48 (96) | 12 (25) | 3.9 ± 0.1bc | 2.4 ± 0.1abc |
| Old | 54 | 40 (74) | 12 (30) | 3.7 ± 0.1cde | 2.9 ± 0.2ab |
| 2007 colony, low dose | |||||
| Young | 81 | 14 (17) | 1 (7) | 2.9 ± 0.4l | 3.2a |
| Middle age | 58 | 9 (16) | 1 (11) | 2.5 ± 0.4kl | 2.5ab |
| Old | 54 | 15 (28) | 4 (27) | 3.1 ± 0.3ghi | 2.7 ± 0.1ab |
| 2007 colony, high dose | |||||
| Young | 80 | 77 (96) | 46 (60) | 3.9 ± 0.1bc | 2.3 ± 0.1abc |
| Middle age | 57 | 54 (95) | 31 (57) | 3.8 ± 0.1cd | 2.5 ± 0.1ab |
| Old | 68 | 59 (87) | 24 (41) | 3.8 ± 0.1cd | 2.2 ± 0.1abc |
SLEV = St. Louis encephalitis virus.
Treatment groups with the same letter in the same column are not significantly different by means comparisons across Tables 3 and 4.
Table 5.
Results of analysis of variance effects of age, dose, EIT, and colony on SLEV body and leg titer for Culex pipiens quinquefasciatus after a 13-day extrinsic incubation period*
| Effect | Body titer of SLEV per infected mosquito | Leg titer of SLEV per infected mosquito | ||||
|---|---|---|---|---|---|---|
| F | Degrees of freedom, numerator, denominator |
P | F | Degrees of freedom, numerator, denominator |
P | |
| Age | 14.28 | 2, 1036 | < 0.0001 | 1.83 | 2, 299 | 0.161 |
| Dose | 36.03 | 1, 1036 | < 0.0001 | 4.92 | 1, 299 | 0.027 |
| EIT | 167.88 | 1, 1036 | < 0.0001 | 1.24 | 1, 299 | 0.266 |
| Colony | 35.69 | 1, 1036 | < 0.0001 | 18.47 | 1, 299 | < 0.0001 |
| Age × dose | 2.51 | 2, 1036 | 0.082 | 0.24 | 2, 299 | 0.786 |
| Age × EIT | 5.27 | 2, 1036 | 0.005 | 1.62 | 2, 299 | 0.200 |
| Age × colony | 7.88 | 2, 1036 | 0.0004 | 4.84 | 2, 299 | 0.009 |
| Dose × EIT | 6.83 | 1, 1036 | 0.009 | 0 | 1, 299 | 0.979 |
| Dose × colony | 105.85 | 1, 1036 | < 0.0001 | 1.26 | 1, 299 | 0.263 |
| EIT × colony | 36.70 | 1, 1036 | < 0.0001 | 0.18 | 1, 299 | 0.674 |
| Colony × dose × EIT | 0.50 | 1, 1036 | 0.481 | 0.58 | 1, 299 | 0.447 |
| Age × dose × EIT | 2.63 | 2, 1036 | 0.072 | 0.97 | 2, 299 | 0.381 |
| Age × dose × colony | 5.42 | 2, 1036 | 0.005 | 1.01 | 2, 299 | 0.364 |
| Age × EIT × colony | 2.39 | 2, 1036 | 0.092 | 0.38 | 2, 299 | 0.683 |
| Age × dose × EIT × colony | 1.42 | 2, 1036 | 0.242 | 1.84 | 2, 299 | 0.161 |
EIT = extrinsic incubation temperature; SLEV = St. Louis encephalitis virus.
The mean leg titer of SLEV per infected mosquito was significantly different between doses and between colonies, but leg titers did not differ between ages and between EITs (Table 5). All two-way interactions were non-significant, except for age × colony, showing that leg titer in colonies responded similarly to changes in environmental factors of dose and EIT, but responded differently to changes in the biological factor of age. Most treatment groups were similar in leg titer, with no apparent pattern to the significant differences in the means comparisons. Young mosquitoes from the 2007 colony fed the low dose and held at 28°C, and middle age 1995 colony mosquitoes fed the high dose and held at 25°C showed significantly higher leg titers than two treatment groups from the 2007 colony at 25°C (old, high dose and young, low dose). For leg titer, all three-way and four-way interactions were not significant, showing that colonies and ages respond similarly to environmental factors. The lower sample sizes for leg titers resulted in reduced power, which may have affected our ability to detect significant high order interactions.
Effects of mosquito age on infection and dissemination rates
The 1995 colony showed significantly different infection rates between ages at SLEV doses and EITs, with highest rates generally in the young and middle age cohorts (25°C: low dose χ2 = 74.11, df = 2, P < 0.0001; high dose χ2 = 37.61, df = 2, P < 0.0001; 28°C: low dose χ2 = 52.41, df = 2, P < 0.0001; high dose: χ2 = 22.47, df = 2, P < 0.0001) (Tables 3 and 4). The dissemination rates for the 1995 colony at either dose of SLEV were also significantly different between ages at 28°C, but not at 25°C (28°C: low dose χ2 = 46.23, df = 2, P < 0.0001; high dose χ2 = 40.45, df = 2, P < 0.0001; 25°C: low dose χ2 = 5.00, df = 2, P = 0.082; high dose χ2 = 0.54, df = 2, P = 0.777) (Tables 3 and 4). At 28°C, infection and dissemination rates were highest in the young mosquito cohorts.
In contrast to the 1995 colony, the SLEV infection or dissemination rates of the 2007 colony mosquitoes did not differ between ages of mosquitoes, regardless of environmental conditions. Infection rates for the 2007 colony mosquitoes exposed to either dose of SLEV were not significantly different between ages at either EIT (25°C: low dose χ2 = 2.71, df = 2, P = 0.258; high dose χ2 = 2.06, df = 2, P = 0.358; 28°C: low dose χ2 = 3.17, df = 2, P = 0.205; high dose: χ2 = 5.37, df = 2, P = 0.068) (Tables 3 and 4). Dissemination rates between mosquitoes of different ages exposed to either dose of SLEV were also not significantly different at either EIT (25°C: low dose χ2 = 0.010, df = 2, P = 0.995; high dose χ2 = 4.43, df = 2, P = 0.109; 28°C: low dose χ2 = 3.17, df = 2, P = 0.205; high dose χ2 = 5.42, df = 2, P = 0.067) (Tables 3 and 4).
Multi-factorial GLMM for effects of environmental and biological factors on probability of infection
A GLMM examining the effects of biological and environmental factors on the probability of infection is shown in Table 6. Our sample size provided the power to examine single-factor effects, as well as effects of two-way, three-way, and some four-way interactions. However, even with large sample sizes, there was insufficient power to consider all possible interactions between the five main effects. The five-way interaction was dropped from the model, and only the age × dose × colony × EIT and age × dose × EIT × body part four-way interactions were retained. The age × dose × colony × EIT interaction was left in the model because we were interested in the relationship between all main effects, regardless of body part. The age × dose × EIT × body part interaction was also chosen because we were interested if the body parts differed in their response to age and environmental factors.
Table 6.
Results of generalized linear mixed model for the effects of age, dose, EIT, and colony on presence or absence of SLEV body or leg infection in Culex pipiens quinquefasciatus after a 13-day extrinsic incubation period*
| Type III tests of fixed effects | |||
|---|---|---|---|
| Effect | F | Degrees of freedom, numerator, denominator |
P |
| Age | 26.80 | 2, 3367 | < 0.0001 |
| Dose | 135.38 | 1, 3367 | < 0.0001 |
| EIT | 124.58 | 1, 3367 | < 0.0001 |
| Colony | 49.69 | 1, 3367 | < 0.0001 |
| Body part | 373.70 | 1, 3367 | < 0.0001 |
| Age × dose | 14.19 | 2, 3367 | < 0.0001 |
| Age × EIT | 8.67 | 2, 3367 | 0.0002 |
| Age × colony | 32.88 | 2, 3367 | < 0.0001 |
| Age × body part | 5.36 | 2, 3367 | 0.005 |
| Dose × EIT | 13.07 | 1, 3367 | 0.0003 |
| Dose × colony | 35.22 | 1, 3367 | < 0.0001 |
| Dose × body part | 4.39 | 1, 3367 | 0.036 |
| EIT × colony | 11.46 | 1, 3367 | 0.001 |
| EIT × body part | 0.61 | 1, 3367 | 0.435 |
| Colony × body part | 20.13 | 1, 3367 | < 0.0001 |
| Colony × dose × EIT | 3.71 | 1, 3367 | 0.054 |
| Age × dose × EIT | 0.94 | 2, 3367 | 0.389 |
| Age × dose × colony | 3.83 | 2, 3367 | 0.022 |
| Age × dose × body part | 1.52 | 2, 3367 | 0.218 |
| Age × EIT × colony | 7.63 | 2, 3367 | 0.001 |
| Age × EIT × body part | 1.29 | 2, 3367 | 0.274 |
| Age × colony × body part | 1.80 | 2, 3367 | 0.165 |
| Dose × EIT × body part | 0.02 | 1, 3367 | 0.879 |
| Dose × colony × body part | 0.93 | 1, 3367 | 0.335 |
| EIT × colony × body part | 0.31 | 1, 3367 | 0.576 |
| Age × dose × EIT × colony | 0.99 | 2, 3367 | 0.371 |
| Age × dose × EIT × body part | 0.47 | 2, 3367 | 0.623 |
SLEV = St. Louis encephalitis virus.
Mosquito age, dose, EIT, colony, and body part all show significant effects on the probability of infection (Table 6). All two-way interactions were significant, except EIT × body part, which indicated that the probability of a body or leg infection responds differently to changes in age, dose, and colony (Table 6), but not to the two EITs. Figure 1 illustrates the interaction between body part and age, which shows that the probability of both body and leg infection differs between mosquito ages, and that the shapes of the curves are different. This finding is more apparent in the model-derived estimates than in the treatment group means, which demonstrate how interactions in complex data sets may be difficult to identify or interpret. The three-way interactions age × dose × colony and age × EIT × colony were significant, indicating that age groups within the two colonies respond differently to both dose and EIT. Figure 2 gives an example of these complex interactions, and shows that the slopes of the response to the two doses varies more in the 1995 colony than the 2007 colony. The 2007 colony shows higher infection rates corresponding to higher doses at each age (Figure 2), and the 1995 colony shows a stronger effect of dose in the middle age mosquitoes, with less of an effect in young mosquitoes and little or no effect in old mosquitoes (Figure 2). The remaining three-way or four-way interactions included in the model were not significant, indicating that the probability of infection in bodies and legs responded similarly to the combination of age and dose factors.
Figure 1.

Logit estimates from generalized linear mixed model showing the relationship between the occurrence of body or leg infection between different ages of mosquitoes (age × body part; F = 5.36, degrees of freedom = 2, 3367, P = 0.005). The estimated mean probability of infection (model-based estimate, see text for details) and the mean probability of infection (calculated from the data for the appropriate treatment groups combined) are plotted for different body parts (circle = body; triangle = leg). The probability of body infection differs between ages, but leg infections are more consistent between ages.
Figure 2.
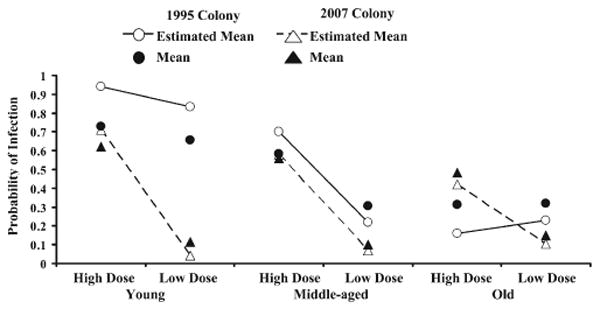
Logit estimates from generalized linear mixed model showing the relationship between the occurrence of body and leg infection between different ages, doses, and colonies (age × dose × colony; F = 3.83, degrees of freedom = 2, 3367, P = 0.022). The probability of infection and the estimated mean probability of infection are plotted for different colonies (circle = 1995 colony; triangle = 2007 colony). Colonies show different infection responses caused by age and dose. Means and estimated means are as in Figure 1.
Discussion
Several general conclusions can be made for Cx. p. quinquefasciatus vector competence for SLEV, based on the current study. First, mosquito age can affect Cx. p. quinquefasciatus vector competence for SLEV. Second, the effects of mosquito age, however, are influenced and can be dependent on the strain of mosquito, initial virus dose, and EIT in complex interactive ways. Third, in some mosquito strains, infection in the body and in legs (a measure of virus dissemination out of the midgut to other tissues) responds differently to environmental and biological factors. Fourth, an EIT difference of only 3°C had a significant effect on SLEV vector competence. There were differences in infection and dissemination for biological factors of age and colony, and differences for environmental factors of dose and EIT. The significant and complex interactions between the effects of these environmental and biological factors show that caution is necessary for studies of vector competence and epidemiology. The ability to generalize observations to other populations in nature, under environmental and biological conditions in the field, is problematic and difficult.
The membrane and cotton pledget methods used to feed the 1995 and 2007 colony females, respectively, may have resulted in some of the differences between the two colonies in the amount of virus actually imbibed. However, the differences in virus intake may have resulted from genetic differences between the colonies and cannot be fully attributed to feeding method although others have reported lower volumes of blood meals imbibed by pledget-feed versus membrane-fed insects.42 The lack of a significant colony × dose interaction in freshly fed mosquitoes indicates that mosquitoes from the two colonies responded similarly to the two doses in the amount of virus imbibed. Similarly, the lack of a significant age × colony interaction indicates that the age classes had similar patterns of virus ingestion between the two colonies. Thus, although mosquitoes from the two colonies may have imbibed different amounts of virus, this is not likely to have strongly influenced the effects of age, dose, and EIT on infection and dissemination. Our major observation of complex interactions between different environmental and biological factors was observed for each colony, regardless of the differences in titers imbibed.
The colonies in our study showed several differences in their SLEV susceptibility, demonstrating the well known effect of mosquito strain variation on vector competence that could be caused by geographic differences and/or colonization effects.39,43 This phenomenon has been explored in several vector-virus systems, e.g., Ae. aegypti and yellow fever virus,43 Culex pipiens and Rift Valley fever virus,39 with trends showing increased infection rates with generation in colony for Ae. aegypti and decreased infection rates with generation in colony for Cx. pipiens. No studies have been conducted for Cx. p. quinquefasciatus and SLEV. However, our findings that the two colonies used respond to environmental and biological factors differently with different complex interactions in their effects on vector competence show the difficulty in interpreting observations using any mosquito population, be it natural or colony.
Mosquito age is clearly an important factor that can influence vector competence. Not only may the age of mosquitoes play a role in infection and dissemination, the influence of age changes depending on dose, EIT, and strain of mosquito. For example, in the 1995 colony, young mosquitoes had higher infection rates than old mosquitoes at both doses and both EITs, although young mosquitoes showed higher dissemination rates only at the higher EIT. Age was not a factor influencing infection and dissemination in the 2007 colony. Our results show that mosquito age can be a significant factor with influence on vector competence in only some populations. Mosquito age has dynamic properties in the field, indicating that vector competence of field populations likely also changes.
At every age and dose, mosquitoes from the 1995 colony held at 25°C had higher infection rates than the 2007 colony. This finding suggests that the 1995 colony was the more susceptible colony to infection at 25°C. However, with only a 3°C difference, the colonies showed similar infection rates at the high dose and 28°C, suggesting that under the most permissive conditions used, the colonies have a similar MIB. In our studies, the presence of an infection in the body indicates the virus overcame the MIB, and infection in the legs (dissemination out of the midgut) indicates the virus overcame the MEB. Overcoming the MIB and MEB, as well as the salivary infection and escape barriers, are necessary for transmission. Differences in the MIB or MEB phenotypes depending on environmental (here, EIT or dose) or biological (here, colony or age) factors are critical components of the vector competence of a mosquito population and the risk of virus transmission. The colonies were different in dissemination at low dose and high EIT where the 1995 colony showed higher dissemination rates, showing that the dissemination (MEB) phenotype in this colony responds differently to the environment than the infection (MIB) phenotype. The observations that the MEB can change depending on the mosquito strain and conditions, shows that virus dissemination to other tissues outside of the midgut is population-specific and condition-specific. Therefore, the same response to the environment cannot be assumed for every population.
Mechanisms affecting the MEB and mechanisms affecting viral growth in mosquito tissues have been reported for Cx. tarsalis and WEEV, and there are likely effects caused by the virus dose that the mosquito imbibes.3,4 The EIT also affects the ability of a virus to traverse the MEB for Cx. tarsalis infected with WNV, SLEV, and WEEV and for Cx. pipiens infected with WNV.44–46 In the current study, SLEV body and leg titers in each colony differed in their responses to the environment. At the low dose, body and leg titers were generally higher in 1995 colony females at all ages and EITs, compared with the 2007 colony. However at the high dose, the pattern differed by EIT. At 25°C, the 2007 colony females had higher body titers but lower leg titers than the 1995 colony females for all age classes. At 28°C and high dose, the 1995 colony generally had higher titers. This finding demonstrates that SLEV replication in the midgut was more permissive in the 2007 colony only at 25°C, but replication in other tissues was generally more permissive in the 1995 colony. Mosquito age influenced body titer in females only from the 1995 colony. In the 1995 colony, EIT was more important than dose because at 28°C and after the EIP, the young females had higher body titer than middle age or old females, regardless of initial virus dose. The differences between the two colonies are likely caused by differences in midgut barriers under the conditions used in our tests. Although mosquito age did not have a significant effect on leg titer, the significant age × colony interaction showed that leg titer in colonies responded differently to age. For each colony, the lack of a significant effect of age or EIT and the few significant differences between treatment means on leg titer suggests an upper limit to SLEV replication in leg tissues. This finding indicates a limit to virus replication in mosquito tissues once the virus surpasses the MEB for this virus-insect system under the conditions of our tests.4,10
Our large sample sizes provided sufficient power to analyze the data with a generalized linear mixed model for binary data with fixed effects and two-way and three-way interactions.41,47 A random effect was also included to take into account the repeated measurements on one individual.41,47 The probability of infection in different body parts of the same mosquito was examined in conjunction with age, dose, EIT, and colony. The environmental and biological factors we tested affected the probability of infection in mosquito bodies and legs. Mosquito age, dose, and colony showed significant interactions with body part, indicating that the probability of a body or leg infection was influenced by these factors in complex ways such that the difference in the probability of infection between the body (overcoming the MIB) and leg (overcoming the MEB) was not the same between ages, doses, and colonies. The observation of no interaction between EIT and body part is also interesting because this observation shows that there was a more consistent and predictable relationship between body and leg infection at the EITs we used that is not affected by our dose, age, or colony treatments. Such a consistency with no interactions under diverse environmental and biological conditions suggests a common mechanism for the effect of EIT on the MIB and MEB for SLEV. Conversely, the MIB and MEB could react to the environment in similar ways even under different control mechanisms.
It is not possible to differentiate between these hypotheses with these data, and the details of the mechanisms controlling the MIB and MEB will be needed to address these alternatives. If such a relationship proves common in other populations, then infection rate (MIB) could potentially be used to predict dissemination rate (MEB) across different EITs. However, the significant interactions between body part and other treatment effects indicate that environmental and biological factors may affect the relationship between infection and dissemination rates. Because we have shown that there are biological factors in the form of mosquito strain/population effects resulting in different responses caused by environmental conditions, it is difficult to generalize these predictions beyond our two colonies. Again, caution is warranted and future work would be needed to assess the responses of other populations.
Other studies have emphasized the importance of large sample sizes to increase the ability to assess treatment group variation.10,28 Infection rate sample sizes for experiments using mosquitoes of different ages, EITs, and fed different SLEV doses (n = 650 for the 1995 colony and n = 398 for the 2007 colony) were within the sample size estimations from a priori power analyses for detecting small to medium effect sizes with 99% power. Because of low dissemination rates, these sample sizes (n = 183 for the 1995 colony and n = 135 for the 2007 colony) could detect a medium effect size (w = 0.3) with 99% power. It is possible that the larger sample sizes in the 1995 colony compared with 2007 colony provided greater precision to determine statistical significance. However, as indicated earlier, the power analyses indicate that our tests were appropriate for detection of small to medium effect sizes. Large sample sizes also enabled investigation of interactions between factors in the ANOVA and GLMM analyses, although we were still limited in our ability to consider four-way and five-way interactions. The potential for such complex interactions should be considered during experimental design and will require large sample sizes to adequately address.
The findings of the present study indicate that differences in vector susceptibility to infection may result from spatiotemporally variable environmental and biological factors. Because arbovirus transmission cycles are sustained by vector populations, knowledge of how these factors interact has public health relevance. The current study shows that virus dose, EIT, mosquito age, and colony are all important factors influencing the vector competence of Cx. p. quinquefasciatus and that the effects of each factor are influenced by the other factors in complex ways. Thus, it is not surprising that there have been mixed reports of the effects of each factor when tested one at a time in different species, in different populations, and with different viruses. The complex interactions observed here caused by environmental and biological factors highlight the importance of studies that address both intrinsic and extrinsic factors influencing differential vector competence between different populations of a mosquito species. Such studies need to examine not only multiple factors in combination, but several levels of each factor with large sample sizes. Multiple experiments may be required to examine multiple factors because these large-scale studies can be logistically challenging. Knowledge of these interactions is important for determining how environmental and biological factors drive epidemiologic cycles.
Acknowledgments
We thank C. Mores in providing BioSafety Laboratory 3 containment space and technical advice on portions of this project; H. Robinson and K. Greene for laboratory assistance; P. Lounibos, J. Day, and two anonymous reviewers for critically reviewing earlier versions of the manuscript; D. Bustamante and L. Young for advice on statistical analyses; and D. Shroyer (Indian River County Mosquito Control, Vero Beach, FL) for providing material used to establish the 2007 mosquito colony.
Financial support: This study was supported by the National Institute of Health (grant AI-49326) to Cynthia C. Lord and Walter J. Tabachnick. Kendra Pesko was supported by a University of Florida Graduate Alumni Fellowship.
Contributor Information
Stephanie L. Richards, Institute of Food and Agricultural Sciences, Florida Medical Entomology Laboratory, University of Florida, 200 9th Street Southeast, Vero Beach, FL 32962, slrichar@ufl.edu.
Cynthia C. Lord, Institute of Food and Agricultural Sciences, Florida Medical Entomology Laboratory, University of Florida, 200 9th Street Southeast, Vero Beach, FL 32962, clord@ufl.edu
Kendra Pesko, School of Medicine, Pathology MSC08 4560, University of New Mexico, Albuquerque, NM 87131, KPesko@salud.unm.edu.
Walter J. Tabachnick, Institute of Food and Agricultural Sciences, Florida Medical Entomology Laboratory, University of Florida, 200 9th Street Southeast, Vero Beach, FL 32962, wjt@ufl.edu
References
- 1.Hardy JL, Houk EJ, Kramer LD, Reeves WC. Intrinsic factors affecting vector competence of mosquitoes for arboviruses. Annu Rev Entomol. 1983;28:229–262. doi: 10.1146/annurev.en.28.010183.001305. [DOI] [PubMed] [Google Scholar]
- 2.Mellor PS. Replication of arboviruses in insect vectors. J Comp Pathol. 2000;123:231–247. doi: 10.1053/jcpa.2000.0434. [DOI] [PubMed] [Google Scholar]
- 3.Kramer LD, Hardy JL, Presser SB, Houk EJ. Dissemination barriers for western equine encephalomyelitis virus in Culex tarsalis infected after ingestion of low viral doses. Am J Trop Med Hyg. 1981;30:190–197. doi: 10.4269/ajtmh.1981.30.190. [DOI] [PubMed] [Google Scholar]
- 4.Mahmood F, Chiles RE, Fang Y, Green EN, Reisen WK. Effects of time after infection, mosquito genotype, and infectious viral dose on the dynamics of Culex tarsalis vector competence for western equine encephalomyelitis virus. J Am Mosq Control Assoc. 2006;22:272–281. doi: 10.2987/8756-971X(2006)22[272:EOTAIM]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 5.Chamberlain RW, Sudia WD, Gillett JD. St. Louis encephalitis virus in mosquitoes. Am J Hyg. 1959;70:221–236. doi: 10.1093/oxfordjournals.aje.a120072. [DOI] [PubMed] [Google Scholar]
- 6.Sardelis MR, Turell MJ, Dohm DJ, O'Guinn ML. Vector competence of selected North American culex and Coquillettidia mosquitoes for West Nile virus. Infect Dis. 2001;7:1018–1022. doi: 10.3201/eid0706.010617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Styer L, Carey J, Wang J, Scott T. Mosquitoes do senesce: departure from the paradigm of constant mortality. Am J Trop Med Hyg. 2007;76:111–117. [PMC free article] [PubMed] [Google Scholar]
- 8.Baqar S, Hayes CG, Ahmed T. The effect of larval rearing conditions and adult age on the susceptibility of Culex tritaeniorhynchus to infection with West Nile virus. Mosq News. 1980;40:165–170. [Google Scholar]
- 9.Takahashi M. The effects of environmental and physiological conditions of Culex tritaeniorhynchus on the pattern of transmission of Japanese encephalitis virus. J Med Entomol. 1976;13:275–284. doi: 10.1093/jmedent/13.3.275. [DOI] [PubMed] [Google Scholar]
- 10.Richards SL, Mores CN, Lord CC, Tabachnick WJ. Impact of extrinsic incubation temperature and virus exposure on vector competence of Culex pipiens quinquefasciatus (Diptera: Culicidae) for WNV. Vector Borne Zoonotic Dis. 2007;7:629–636. doi: 10.1089/vbz.2007.0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Day JF, Stark LM. Avian serology in a St. Louis encephalitis epicenter before, during, and after a widespread epidemic in south Florida, USA. J Med Entomol. 1999;36:614–624. doi: 10.1093/jmedent/36.5.614. [DOI] [PubMed] [Google Scholar]
- 12.Day JF, Stark LM. Frequency of Saint Louis encephalitis virus in humans from Florida, USA: 1990–1999. J Med Entomol. 2000;37:626–633. doi: 10.1603/0022-2585-37.4.626. [DOI] [PubMed] [Google Scholar]
- 13.Godsey MS, Blackmore MS, Panella NA, Burkhalter K, Gottfried K, Halsey LA, Rutledge CR, Langevin SA, Gates R, Lamonte KM, Lambert A, Lanciotti RS, Blackmore CG, Loyless T, Stark L, Oliveri R, Conti L, Komar N. West Nile virus epizootiology in the southeastern United States, 2001. Vector Borne Zoonotic Dis. 2005;5:82–89. doi: 10.1089/vbz.2005.5.82. [DOI] [PubMed] [Google Scholar]
- 14.Monath TP. Epidemiology. In: Monath TP, editor. St Louis Encephalitis. Washington, DC: American Public Health Association; 1980. [Google Scholar]
- 15.Diaz LA, Viviana R, Almirón WR, Farías A, Vázquez A, Sanchez-Seco MP, Javier A, Spinsanti L, Konigheim B, Visintin A, García J, Morales MA, Tenorio A, Contigiani M. Genotype III St. Louis encephalitis virus outbreak 2005. Infect Dis. 2006;12:486. doi: 10.3201/eid1211.060486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Monath TP, Tsai TF. St. Louis encephalitis: lessons from the last decade. Am J Trop Med Hyg. 1987;37:40S–59S. doi: 10.4269/ajtmh.1987.37.40s. [DOI] [PubMed] [Google Scholar]
- 17.McCaig LF, Janowski HT, Gunn RA, Tsai TF. Epidemiologic aspects of a St. Louis encephalitis epidemic in Fort Walton Beach, Florida in 1980. Am J Trop Med Hyg. 1994;50:387–391. doi: 10.4269/ajtmh.1994.50.387. [DOI] [PubMed] [Google Scholar]
- 18.Sudia WD, Coleman PH, Chamberlain RW, Wiseman JS, Work TH. St. Louis encephalitis vector studies in Houston, Texas, 1964. J Med Entomol. 1967;4:32–36. doi: 10.1093/jmedent/4.1.32. [DOI] [PubMed] [Google Scholar]
- 19.Irby WS, Apperson CS. Hosts of mosquitoes in the coastal plain of North Carolina. J Med Entomol. 1988;25:85–93. doi: 10.1093/jmedent/25.2.85. [DOI] [PubMed] [Google Scholar]
- 20.Niebylski ML, Meek CL. Blood-feeding of Culex mosquitoes in an urban environment. J Am Mosq Control Assoc. 1992;8:173–177. [PubMed] [Google Scholar]
- 21.Molaei G, Andreadis TG, Armstrong PM, Bueno R, Jr, Dennett JA, Real SV, Sargent C, Bala A, Randle Y, Guzman H, Travassos da Rosa A, Wuithiranyagool T, Tesh RB. Host feeding pattern of Culex quinquefasciatus (Diptera: Culicidae) and its role in transmission of West Nile virus in Harris County, Texas. Am J Trop Med Hyg. 2007;77:73–81. [PubMed] [Google Scholar]
- 22.Chamberlain RW, Gogel RH, Sudia WD. Experimental vector studies with strains of St. Louis encephalitis virus isolated from mosquitoes during the 1964 epidemics. J Med Entomol. 1966;3:268–270. doi: 10.1093/jmedent/3.3-4.268. [DOI] [PubMed] [Google Scholar]
- 23.Reisen WK, Fang Y, Martinez VM. Avian host and mosquito (Diptera: Culicidae) vector competence determine the efficiency of West Nile and St. Louis encephalitis virus transmission. J Med Entomol. 2005;42:367–375. doi: 10.1093/jmedent/42.3.367. [DOI] [PubMed] [Google Scholar]
- 24.Lillibridge KM, Parsons R, Randle Y, Travassos Da Rosa A, Guzman H, Siirin M, Wuithiranyagool T, Hailey C, Higgs S, Bala A, Pascua R, Meyer T, Vanlandingham D, Tesh R. The 2002 introduction of West Nile virus into Harris County, Texas, an area historically endemic for St. Louis encephalitis. Am J Trop Med Hyg. 2004;70:676–681. [PubMed] [Google Scholar]
- 25.Bosio CF, Fulton RE, Salasek ML, Beaty BJ, Black WC. Quantitative trait loci that control vector competence for dengue-2 virus in the mosquito Aedes aegypti. Genetics. 2000;156:687–698. doi: 10.1093/genetics/156.2.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Turell MJ, O'Guinn M, Oliver J. Potential for New York mosquitoes to transmit West Nile virus. Am J Trop Med Hyg. 2000;62:413–414. doi: 10.4269/ajtmh.2000.62.413. [DOI] [PubMed] [Google Scholar]
- 27.Turell MJ, Sardelis M, Dohm D, O'Guinn M. Potential North American vectors of West Nile virus. Ann NY Acad Sci. 2001;952:317–324. doi: 10.1111/j.1749-6632.2001.tb02707.x. [DOI] [PubMed] [Google Scholar]
- 28.Lord CC, Rutledge CR, Tabachnick WJ. Relationships between host viremia and vector susceptibility for arboviruses. J Med Entomol. 2006;43:623–630. doi: 10.1603/0022-2585(2006)43[623:rbhvav]2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39:175–191. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- 30.Cohen J. A power primer. Psychol Bull. 1992;111:155–159. doi: 10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- 31.Richards SL, Pesko KP, Alto BW, Mores CN. Reduced infection in mosquitoes exposed to previously frozen flaviviruses. Virus Res. 2007;129:224–227. doi: 10.1016/j.virusres.2007.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lanciotti RS, Kerst AJ, Nasci RS, Godsey MS, Mitchell CJ, Savage HM, Komar N, Panella NA, Allen BC, Volpe KE, Davis BS, Roehrig JT. Rapid detection of West Nile virus from human clinical specimens, field-collected mosquitoes, and avian samples by a TaqMan reverse transcriptase-PCR assay. J Clin Microbiol. 2000;38:4066–4071. doi: 10.1128/jcm.38.11.4066-4071.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alto BW, Lounibos LP, Juliano SA. Age-dependent blood-feeding of Aedes aegypti and Aedes albopictus on artificial and living hosts. J Am Mosq Control Assoc. 2003;19:347–352. [PubMed] [Google Scholar]
- 34.Day JF, Curtis G. Annual emergence patterns of Culex nigripalpus females before, during, and after a widespread St. Louis encephalitis epidemic in south Florida. J Am Mosq Control Assoc. 1993;9:249–253. [PubMed] [Google Scholar]
- 35.Goddard LB, Roth AE, Reisen WK, Scott TW. Vector competence of California mosquitoes for West Nile virus. Infect Dis. 2002;8:1385–1391. doi: 10.3201/eid0812.020536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Anderson JF, Main AJ, Delroux K, Fikrig E. Extrinsic incubation periods for horizontal and vertical transmission of West Nile virus by Culex pipiens pipiens. J Med Entomol. 2008;45:445–451. doi: 10.1603/0022-2585(2008)45[445:eipfha]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 37.Lanciotti RS, Kerst AJ. Nucleic acid sequence-based amplification assays for rapid detection of West Nile and St. Louis encephalitis viruses. J Clin Microbiol. 2001;39:4506–4513. doi: 10.1128/JCM.39.12.4506-4513.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bustin SA. Absolute quantification of mRNA using real-time reverse transcription polymerase chain reaction assays. J Mol Endocrinol. 2000;25:169–193. doi: 10.1677/jme.0.0250169. [DOI] [PubMed] [Google Scholar]
- 39.Gargan TP, Bailey CL, Higbee GA, Gad A, Said S. The effect of laboratory colonization on the vector-pathogen interactions of Egyptian Culex pipiens and Rift Valley fever virus. Am J Trop Med Hyg. 1983;32:1154–1163. doi: 10.4269/ajtmh.1983.32.1154. [DOI] [PubMed] [Google Scholar]
- 40.Turell MJ, Gargan TP, Bailey CL. Replication and dissemination of Rift Valley fever virus in Culex pipiens. Am J Trop Med Hyg. 1984;33:176–181. doi: 10.4269/ajtmh.1984.33.176. [DOI] [PubMed] [Google Scholar]
- 41.SAS. SAS/STAT User's Guide for Personal Computers Computer Program, Version 8.0. Cary, NC: SAS; 2002. [Google Scholar]
- 42.Venter GJ, Paweska JT, Lunt H, Mellor PS, Carpenter S. An alternative method of blood-feeding Culicoides imicola and other haematophagous Culicoides species for vector competence studies. Vet Parasitol. 2005;131:331–335. doi: 10.1016/j.vetpar.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 43.Lorenz L, Beaty BJ, Aitken THG, Wallis GP, Tabachnick WJ. The effect of colonization upon Aedes aegypti susceptibility to oral infection with yellow fever virus. Am J Trop Med Hyg. 1984;33:690–694. doi: 10.4269/ajtmh.1984.33.690. [DOI] [PubMed] [Google Scholar]
- 44.Reisen WK, Meyer RP, Presser SB, Hardy JL. Effect of temperature on the transmission of western equine encephalomyelitis and St. Louis encephalitis viruses by Culex tarsalis (Diptera: Culicidae) J Med Entomol. 1993;30:151–160. doi: 10.1093/jmedent/30.1.151. [DOI] [PubMed] [Google Scholar]
- 45.Dohm DJ, O'Guinn ML, Turell MJ. Effect of environmental temperature on the ability of Culex pipiens (Diptera: Culicidae) to transmit West Nile virus. J Med Entomol. 2002;39:221–225. doi: 10.1603/0022-2585-39.1.221. [DOI] [PubMed] [Google Scholar]
- 46.Reisen WK, Fang Y, Martinez VM. Effects of temperature on the transmission of West Nile virus by Culex tarsalis (Diptera: Culicidae) J Med Entomol. 2006;43:309–317. doi: 10.1603/0022-2585(2006)043[0309:EOTOTT]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 47.Bolker BM, Brooks ME, Clark CJ, Geange SW, Poulsen JR, Stevens HH, White JS. Generalized linear mixed models: a practical guide for ecology and evolution. Trends Ecol Evol. 2008;24:127–135. doi: 10.1016/j.tree.2008.10.008. [DOI] [PubMed] [Google Scholar]


