Abstract
HIV infection is associated with an increased risk of thrombosis; and as antiretroviral therapy has increased the lifespan of HIV-infected patients, their risk for cardiovascular events is expected to increase. A large clinical study found recently that all-cause mortality for HIV+ patients was related to plasma levels of interleukin-6 and to D-dimer products of fibrinolysis. We provide evidence that this elevated risk for coagulation may be related to increased proportions of monocytes expressing cell surface tissue factor (TF, thromboplastin) in persons with HIV infection. Monocyte TF expression could be induced in vitro by lipopolysaccharide and flagellin, but not by interleukin-6. Monocyte expression of TF was correlated with HIV levels in plasma, with indices of immune activation, and with plasma levels of soluble CD14, a marker of in vivo lipopolysaccharide exposure. TF levels also correlated with plasma levels of D-dimers, reflective of in vivo clot formation and fibrinolysis. Thus, drivers of immune activation in HIV disease, such as HIV replication, and potentially, microbial translocation, may activate clotting cascades and contribute to thrombus formation and cardiovascular morbidities in HIV infection.
Introduction
HIV infection is associated with an increased risk of both arterial and venous thrombosis.1–6 As the efficacy of highly active antiretroviral therapy (HAART) improves and patients with HIV infection live longer, cardiovascular disease is increasing in prevalence in this population.7–11 Recently, the Strategies for Management of Anti-Retroviral Therapy (SMART) study compared the efficacy of CD4+ T cell–guided interruption of HAART with continuous therapy and found that overall mortality, including deaths related to cardiovascular events, was significantly higher in the patient group randomized to receive intermittent therapy.12 Independent predictors of mortality in the entire study included plasma levels of the proinflammatory cytokine interleukin-6 (IL-6) and D-dimer products of fibrinolysis.13
We and others have found increased levels of microbial products in the plasma of HIV-infected persons.14–16 High levels of bacterial lipopolysaccharide (LPS) and bacterial DNA were correlated directly with indices of immune activation and inversely with CD4+ T-cell restoration after administration of HAART.14,16 Moreover, in vitro exposure to Toll-like receptor (TLR) ligands drove T-cell activation and turnover.17,18 Taken together, these data suggest that heightened systemic translocation of microbial products from the damaged gut might drive immune activation and contribute to the pathogenesis of immune deficiency in chronic HIV infection.
Certain microbial TLR ligands, such as LPS, can also increase surface expression of the procoagulant, tissue factor (TF, also known as thromboplastin) on circulating blood monocytes.19 TF expression on monocytes promotes coagulation, as demonstrated by the catastrophic thrombosis that can occur after infusion of TF+ monocytes in rabbit and canine models.20
Here, we confirmed the effects of the bacterial TLR ligands LPS and flagellin on induction of monocyte TF expression in vitro. This effect was not mediated through induction of IL-6, as direct addition of this cytokine tended to decrease slightly monocyte TF expression.
We also found dramatically higher frequencies of monocytes expressing TF in fresh blood samples from HIV-infected persons than in samples from uninfected controls. The frequency of TF+ monocytes correlated significantly with markers of immune activation that are known predictors of disease progression in HIV infection. TF expression correlated both with plasma levels of HIV RNA and with soluble CD14 (sCD14), the LPS receptor that is released by monocytes after LPS stimulation in vivo.14,21 Finally, the in vivo biologic activity of monocyte TF expression was suggested by a correlation with plasma levels of D-dimers. These findings suggest that a variety of microbial products, including those derived from HIV itself, and perhaps bacterial products translocated from the damaged gut in chronic HIV infection, may contribute to a heightened risk for clotting and cardiovascular disease in HIV infection by increasing cell surface expression of the procoagulant, TF.
Methods
Cell preparation and incubation
These studies were performed with the approval of the Institutional Review Board at Case Western Reserve University/University Hospitals of Cleveland. After informed consent was obtained in accordance with the Declaration of Helsinki, blood from 19 healthy donors and 60 HIV-infected patients was drawn into either heparin- or ethylenediaminetetraacetic acid–coated tubes. Peripheral blood mononuclear cells were isolated over a Ficoll-Hypaque cushion. Whole blood (20 μL) was incubated in 15-mL Falcon tubes for 3 hours with individual TLR ligands or cytokines. LPSs (from Escherichia coli) and flagellin (from Salmonella typhimurium) were purchased from Invivogen. IL-6 was purchased from R&D Systems.
Flow cytometry
Cell surface molecule expression was monitored by staining cells with the following fluorochrome-labeled antibodies: anti-TF fluorescein isocyanate (American Diagnostica), anti-CD14 peridinin chlorophyll protein, anti-HLA-DR fluorescein isocyanate, anti-CD38 phycoerytherin, anti-CD8 allophycocyanin-cy7, anti-CD4 Pacific Blue, anti-CD3 allophycocyanin, and appropriate isotype control monoclonal antibodies (BD Biosciences PharMingen). Whole-blood samples were incubated for 15 minutes on ice with FACS Lyse buffer (BD Biosciences) and then washed in wash buffer (phosphate-buffered saline with 1% bovine serum albumin and 0.1% sodium azide). Cells were then stained for 15 minutes in the dark on ice and then washed in wash buffer, fixed in 1% formaldehyde, and analyzed using an LSRII flow cytometer (BD Biosciences). FACSDiva (Version 6.1.1, BD Biosciences) and Prism 5.0 (GraphPad) software were used to organize and analyze the data. Cell types were identified by size, granularity, and positive expression of surface markers; CD14 for monocytes, CD3 and either CD4 or CD8 for T cells.
Measurement of TF activity in plasma
Fresh whole-blood samples were collected in trisodium citrate–containing tubes and were centrifuged for 10 minutes at 1000g. The plasma was then removed and frozen at −80°C until thawed once and analyzed in batch. Levels of TF activity were measured by the Actichrome TF kit (American Diagnostica).
Measurement of D-dimers and sCD14 in plasma
Fresh whole-blood samples collected in ethylenediaminetetraacetic acid–containing tubes and were centrifuged for 10 minutes at 495g, and plasma was then removed and frozen at −80°C until thawed once and then analyzed in batch. Levels of D-dimers were measured using the Asserachrom D-DI immunoassay (Diagnostica Stago). sCD14 levels were measured using the Quantikine sCD14 kit (R&D Systems).
Statistical methods
We used conventional measures of central tendency and spread to describe the data. We tested variables of interest for normality using both the Kolmogorov-Smirnoff test with the Lilliefors correction and the Shapiro-Wilk test, and rejected the hypothesis of normality if either test was significant. We tested pairwise comparisons of independent groups with the Mann-Whitney U test, and comparisons of more than 2 groups with the Kruskal-Wallis H test. We used the Wilcoxon signed-ranks test and Friedman test to compare pairs and more than 2 groups of related observations, respectively. To correct for multiple pairwise comparisons within a given dimension (eg, comparisons of TF expression in response to various stimuli to unstimulated TF expression), we used the Holm-Bonferroni correction. We tested associations between pairs of continuous variables with Spearman rank correlation. We processed the analyses using SPSS Version 16.02 (SPSS Inc) and Stata MP Version 10.1 (Stata Corp). All tests are 2-sided. A P value of .05 or less was considered significant, with the exception of tests within a family of multiple comparisons.
Results
Exposure of human monocytes to bacterial LPS and flagellin, but not to IL-6, increases surface expression of the procoagulant TF
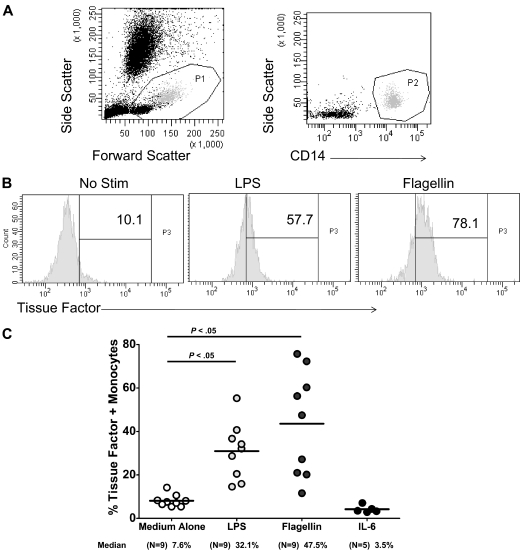
Freshly obtained whole-blood samples obtained from HIV-uninfected controls were cultured for 3 hours at 37°C, either unstimulated, or in the presence of individual TLR ligands or cytokines. Cell surface expression of TF was measured by flow cytometry on CD14+ monocytes (Figure 1). Both expression levels and proportions of monocytes expressing TF were increased significantly in samples exposed to the TLR-4 ligand LPS (n = 9) and to the TLR 5 ligand flagellin (n = 9). These effects were not probably mediated through IL-6 induction as samples exposed to IL-6 (n = 6) tended to decrease TF expression.
Figure 1.
Exposure of human monocytes to TLR ligands, but not to IL-6, increases surface expression of the procoagulant TF. Whole blood was obtained from HIV-uninfected subjects and was exposed to LPS (50 ng/mL), flagellin (10 μg/mL), or IL-6 (30 μg/mL) for 3 hours. Surface expression of TF was measured on CD14+ monocytes by flow cytometry. (A) Gating strategy. (B) Representative histograms. (C) Summary data.
Monocyte surface expression of TF is increased in persons with HIV infection
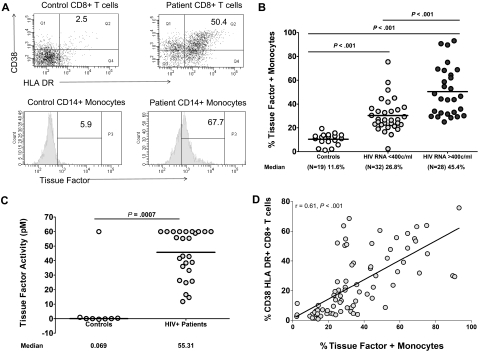
As we had found earlier that HIV-infected persons have high plasma levels of microbial products, and having demonstrated that certain microbial TLR ligands could increase monocyte surface expression of TF in vitro, we next designed a series of experiments to ascertain if sustained exposure to circulating microbial products, such as is seen in chronic HIV infection, might result in heightened monocyte expression of TF in vivo. To address this, we first examined the expression of TF on monocytes freshly obtained from persons with HIV infection and from uninfected controls. The uninfected population was composed of 11 males and 8 females with a median age of 37 years. The HIV-infected population was divided into 2 groups based on levels of plasma viremia. Of the 28 patients with plasma viremia greater than 400 copies/mL of HIV RNA, there were 17 males, 8 females, and the sex of 3 is unknown. Of the identified patients the median age was 42 years, and the median time since HIV+ diagnosis was 7.5 years, with 13 of these patients on HAART. Among the 32 patients with fewer than 400 copies/mL of HIV RNA, 21 were male, 9 were female, and the sex of 2 patients was not known. The median age of the 30 patients was 44 years; the median time since HIV+ diagnosis was 9.5 years. At the time of blood draw, 3 patients had concurrent infections (2 from the controlled viremia group, cellulitis and bronchitis, and 1 from the uncontrolled viremia group with an upper respiratory infection). Among the uninfected controls, the median [interquartile range] percentage of monocytes expressing surface TF (11% [8.5%-13.9%], n = 19) was significantly lower than the median percentages of TF+ monocytes in both the viremic (HIV RNA > 400 copies/mL) and aviremic (HIV RNA < 400 copies/mL) HIV-infected patients (45.4% [32%-66.2%], n = 28; and 26.8% [22.7%-35.8%], n = 32, respectively, P < .001 for each comparison, Figure 2A-B). Levels of bioactive TF were measured in plasma samples from a subset of our patients (n = 28) and controls (n = 8); patient samples had much higher levels of TF than did samples of controls (median levels of plasma TF were 55pM and < 2pM, respectively, P < .001; Figure 2C).
Figure 2.
The proportion of monocytes expressing TF and plasma levels of bioactive TF are increased in HIV infection, and monocyte TF correlates with markers of immune activation. Whole-blood samples obtained from 60 HIV-infected donors and 19 healthy controls were examined for expression of TF on gated CD14+ monocytes and for markers of immune activation (HLA-DR and CD38) on gated CD8+ T cells by flow cytometry. (A) Representative histograms and dot plots. (B) Summary data among healthy controls, patients with controlled (HIV RNA < 400 copies/mL), and uncontrolled (HIV RNA > 400 copies/mL) viremia. (C) Bioactive TF was significantly higher in plasma samples from HIV-infected patients (n = 28) than in samples from uninfected controls (n = 8, P < .001). (D) Correlation between the proportion of TF+ monocytes and the proportion of CD8+ T cells that express activation markers (r = 0.61, P < .001).
Monocyte TF expression correlates directly with markers of immune activation
Immune activation is a recognized predictor of HIV disease progression, and we had demonstrated earlier that indices of immune activation (specifically expression of CD38 and/or HLA-DR on T cells) correlate strongly with levels of microbial products (LPS and bacterial DNA) in plasma.14,16 We therefore examined the relationship between coexpression of CD38 and HLA-DR on CD8+ T cells, and expression of TF on circulating monocytes. In samples from HIV-infected patients, these markers of immune activation correlated strongly with TF expression (r = 0.61, P < .001; Figure 2D).
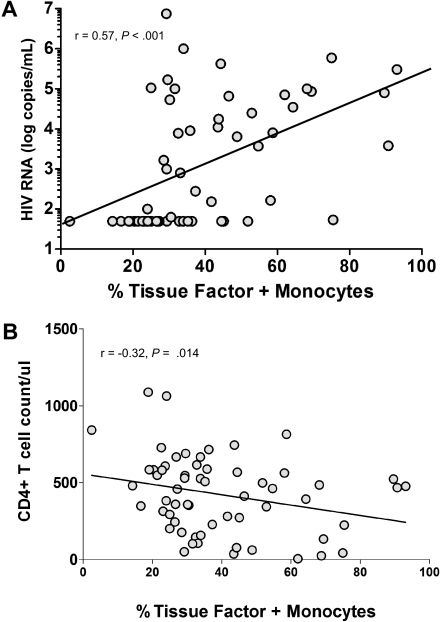
Indices of immune activation in HIV infection are linked both to the magnitude of HIV replication22,23 and to levels of microbial products in plasma.14,16 Because we found a significant relationship between monocyte surface TF expression and indices of immune activation, and because we found the highest TF expression on monocytes of persons with uncontrolled viremia (Figure 2B), we next asked if TF expression was related to plasma HIV RNA levels or with the stage of disease as reflected in CD4+ T-cell counts. Among all HIV-infected subjects, there was a relationship between the magnitude of viremia and the percentage of monocytes expressing TF (r = 0.57, P < .001; Figure 3A); this relationship disappeared when only persons with detectable viremia were examined (r = 0.08, P = .68). There was also a weak negative correlation between monocyte TF expression and CD4+ T-cell count among all HIV+ subjects (r = −0.32, P = .014, Figure 3B).
Figure 3.
TF expression is correlated with the magnitude of viremia and is weakly correlated with CD4+ T-cell counts. (A) Correlation between the proportion of TF+ monocytes and plasma levels of HIV RNA in patients (r = 0.57, P < .001). (B) Correlation between the proportion of TF+ monocytes and circulating CD4+ T-cell numbers in patients (r = −0.32, P = .014).
Circulating CD14, a marker of microbial translocation, correlates directly with the proportion of monocytes expressing TF in HIV infection
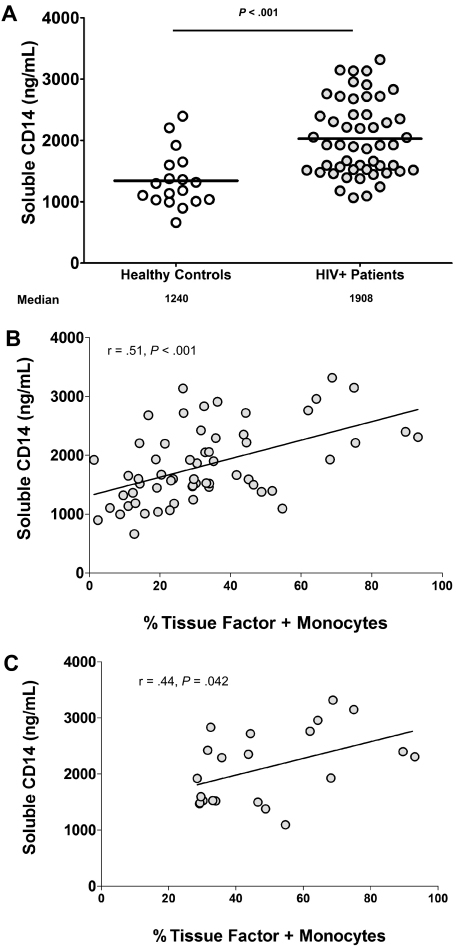
To ascertain whether circulating bacterial products might play a role in the monocyte expression of TF, we measured plasma levels of sCD14, an LPS coreceptor that is shed by monocytes and macrophages after LPS binding in vivo.14,21 sCD14 levels were significantly higher in the plasma of HIV-infected persons (median [interquartile range] = 1908 [1514-2396] ng/mL, n = 46) than in the plasma of uninfected controls (1240 [1029-1598] ng/mL, n = 18, P < .001, Figure 4A). The proportion of monocytes expressing TF and levels of plasma sCD14 were directly related when data from all subjects were examined (r = 0.51, P < .001, Figure 4B). In addition, among patients with uncontrolled HIV replication wherein plasma HIV RNA levels did not correlate with TF expression, the proportions of monocytes expressing detectable TF correlated directly with levels of sCD14 (r = 0.44, P = .042, n = 22, Figure 4C). Thus, in HIV-infected persons with uncontrolled viremia, monocyte expression of TF does not correlate with the magnitude of HIV replication but does correlate with a marker of in vivo binding of bacterial LPS to its cellular receptors.
Figure 4.
Plasma levels of the LPS coreceptor, CD14, are increased in HIV infection and correlate with the proportion of TF+ monocytes in patients with detectable viremia. Plasma was collected from whole-blood samples provided by HIV-infected (N = 46) and -uninfected donors (N = 18), and levels of sCD14 were measured. (A) sCD14 levels are higher in plasmas of HIV+ patients than in plasmas of healthy controls (P < .005). (B) Plasma levels of sCD14 correlate with the proportion of TF+ monocytes among all subjects (r = 0.51, P < .001). (C) Plasma levels of sCD14 correlate with the proportion of TF+ monocytes among patients with detectable viremia (r = 0.44, P = .042, n = 22).
TF expression correlates directly with markers of fibrinolysis
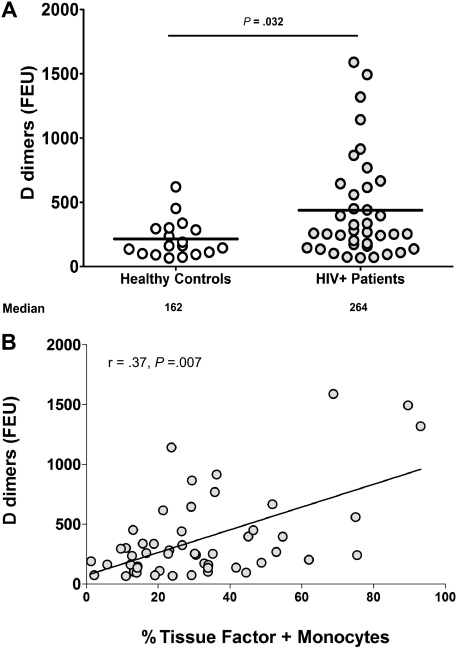
D-dimers are found in plasma as markers of clot formation and breakdown. To ascertain if increased TF expression resulted in in vivo activation of clotting factors and resultant fibrinolysis, we measured plasma levels of D-dimers. Concentrations of D-dimers were significantly higher in the plasma of HIV-infected patients (median [interquartile range] = 264 [160-615] fibrinogen equivalent units, n = 38) than in plasmas of healthy controls (162 [101-295] fibrinogen equivalent units, n = 18, P = .032, Figure 5A). In all subjects, there was a significant positive correlation between plasma levels of D-dimers and the proportion of monocytes expressing TF (r = 0.37, P = .007, Figure 5B). This association persisted in the HIV-infected group, although in this subgroup it was only marginally significant. (r = 0.31, P = .06, n = 38). Thus, increased TF expression on circulating monocytes was related to in vivo evidence of activation of clotting factors and fibrinolysis.
Figure 5.
Plasma D-dimer levels are increased in HIV infection and correlate with the proportion of monocytes expressing TF. Plasma was collected from whole-blood samples provided by HIV-infected (n = 38) and -uninfected donors (n = 18), and levels of D-dimers were measured. (A) Levels of D-dimers are higher in plasma samples from HIV-infected patients than in samples from HIV-uninfected controls (P = .032). (B) Plasma levels of D-dimers correlate with the proportion of TF+ monocytes in all samples tested (r = 0.37, P = .007).
Discussion
HIV infection has been associated in some, but not all, studies with an increased risk of cardiovascular disease; potential mechanisms for this are, however, not fully understood.1,3–6 As current HAART strategies are prolonging life and as the HIV-infected population ages, an increasing occurrence of cardiovascular complications is nonetheless anticipated. The SMART study findings linking cardiovascular and all-cause mortalities to indices of inflammation and coagulation13 allowed us to explore a model that could link our recent demonstration of heightened microbial translocation in chronic HIV infection14–16 to inflammation and coagulation and, hence, to cardiovascular risk. Here, we have found that monocytes analyzed directly ex vivo from HIV-infected persons more often express detectable TF on their cell surfaces than do monocytes from uninfected controls. As others also have shown, monocyte expression of TF is induced by exposure to bacterial TLR ligands that activate monocytes directly.24,25 Although we did not attempt to measure increased TF activity on monocytes directly, we are able to demonstrate that plasma samples from HIV-infected patients contain higher levels of bioactive TF than do samples from controls. Moreover, the relevance of increased TF expression in HIV infection is underscored by the high levels of D-dimers in plasmas and the correlation between TF expression and D-dimer levels. Thus, high levels of TF probably contribute to an increased coagulation tendency in chronic HIV infection.
TF initiates the coagulation cascade in vivo by binding factor VII, cleaving it to its active form, factor VIIa, and stabilizing it in an open conformation, increasing its catalytic activity as a serine protease.26 Factor VIIa can then activate factor X to factor Xa, which, in the presence of its cofactor, factor Va, activates thrombin. Thrombin feeds back on its own generation and cleaves fibrinogen to fibrin to form a stable clot.27,28
TF is expressed on a variety of surfaces, including those of platelets, damaged endothelium, and monocytes, and there is a clear linkage between cellular TF expression and clotting.29,30 TF may help to drive the coagulopathy seen in infectious inflammatory processes, such as sepsis, as administration of monoclonal antibodies that block TF can reduce mortality of endotoxemia in animal models.31,32 We propose that monocytes, activated directly by microbial products translocated through the damaged gut,14 or through indirect mechanisms that may involve activation of TLRs 7/8 by HIV RNAs,18 increase surface expression of TF, and thereby contribute to an increased tendency to coagulation, especially within atheromatous plaques that typically are enriched with activated monocytes and macrophages.30 Increased monocyte expression of TF has been linked to myocardial infarction as well as to both chronic-stable and unstable angina.33 Thus, increased expression of TF on peripheral blood monocytes is a potential indicator of cardiovascular disease risk and may contribute directly to clot formation and thrombosis in vivo.19,34–36
There is increasing concern that poorly controlled HIV infection may increase risks for cardiovascular morbidity.9,10 Patients who underwent antiretroviral treatment interruptions in the SMART study had an increased risk of cardiovascular and other mortalities compared with outcomes in patients receiving continuous antiretroviral therapy.13 These risks were predicted both by increased levels of the proinflammatory cytokine, IL-6, and by plasma levels of D-dimers, suggesting that inflammation and coagulation were contributors.
Here, we link these observations in a model wherein stage of disease, as measured by levels of HIV replication, immune activation, and CD4+ T-cell counts predict monocyte TF expression. The increased levels of inflammation, immune activation, and expression of TF may be driven, at least in part, by HIV replication, as well as by translocation of microbial products from the damaged gut, resulting in an increased tendency for coagulation. We have shown earlier that persons with uncontrolled HIV infection have elevated plasma levels of bacterial products, such as LPS and bacterial DNAs, and that plasma levels of these TLR ligands can be lowered (but not normalized) by effective control of viral replication.14,16 In the present work, we find that plasma levels of HIV RNA correlate with monocyte TF expression in examining all patients, but not when persons with uncontrolled viremia are examined separately. Although we did not measure directly the levels of LPS in the plasma of our HIV-1–infected patients, a marker of microbial translocation (ie, the soluble LPS receptor CD14), seems to correlate better with monocyte expression of TF than does plasma viremia. CD14 is shed from monocytes after LPS binding and previous work by our group and by others has shown that plasma LPS levels and sCD14 levels are directly related.14,21 Thus, our data are compatible with a model wherein systemic exposure to translocated bacterial products, related to the stage of disease, increases monocyte expression of TF. The modest correlation with sCD14 levels is not surprising as we have also found increased levels of bacterial peptidoglycan (J.M.B., unpublished data, April 2007) and bacterial DNAs in plasmas of HIV-infected persons that would not be reflected in sCD14 levels. Thus, we suspect that a variety of bacterial TLR ligands, such as peptidoglycans, LPS, and flagellins, are translocated through the damaged gut in chronic HIV infection and may drive immune activation and monocyte TF expression in this setting. Interestingly, although IL-6 expression can be induced by LPS and flagellin in vitro (data not shown), and the plasma level of IL-6 was an independent predictor of mortality in the SMART study,13 IL-6 does not seem to be on the causal pathway of TF induction by microbial products. We propose that HIV replication and systemic translocation of microbial products from the damaged gut, and the subsequent immune activation, contribute to a procoagulant state in HIV-infected patients that is due, at least in part, to increased surface expression of TF on circulating monocytes.
Acknowledgments
The authors thank the members of the Cleveland Immunopathogenesis Consortium for their comments and contributions to the generation of this work.
This work was supported by the Center for AIDS Research at Case Western Reserve University (AI-36219) and the National Institutes of Health (grants AI-076174 and AI-68636).
Footnotes
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: N.T.F., E.M., W.J., and M.K. performed experiments; N.T.F. and A.A.L. organized figures; B.R. performed statistical analysis; R.A. provided patient samples; M.M.L., E.M., N.T.F., and S.F.S. designed experiments; and all authors contributed to the writing of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Michael M. Lederman, 2109 Adelbert Rd, 1048B, BRB Bldg, Cleveland, OH 44106; e-mail: MXL6@case.edu.
References
- 1.Tabib A, Leroux C, Mornex JF, Loire R. Accelerated coronary atherosclerosis and arteriosclerosis in young human-immunodeficiency-virus-positive patients. Coron Artery Dis. 2000;11(1):41–46. doi: 10.1097/00019501-200002000-00008. [DOI] [PubMed] [Google Scholar]
- 2.Matta F, Yaekoub AY, Stein PD. Human immunodeficiency virus infection and risk of venous thromboembolism. Am J Med Sci. 2008;336(5):402–406. doi: 10.1097/MAJ.0b013e31816dd2fd. [DOI] [PubMed] [Google Scholar]
- 3.Hsue PY, Lo JC, Franklin A, et al. Progression of atherosclerosis as assessed by carotid intima-media thickness in patients with HIV infection. Circulation. 2004;109(13):1603–1608. doi: 10.1161/01.CIR.0000124480.32233.8A. [DOI] [PubMed] [Google Scholar]
- 4.Hsue PY, Hunt PW, Sinclair E, et al. Increased carotid intima-media thickness in HIV patients is associated with increased cytomegalovirus-specific T-cell responses. AIDS. 2006;20(18):2275–2283. doi: 10.1097/QAD.0b013e3280108704. [DOI] [PubMed] [Google Scholar]
- 5.Hsue PY, Giri K, Erickson S, et al. Clinical features of acute coronary syndromes in patients with human immunodeficiency virus infection. Circulation. 2004;109(3):316–319. doi: 10.1161/01.CIR.0000114520.38748.AA. [DOI] [PubMed] [Google Scholar]
- 6.Friis-Moller N, Weber R, Reiss P, et al. Cardiovascular disease risk factors in HIV patients: association with antiretroviral therapy. Results from the DAD study. AIDS. 2003;17(8):1179–1193. doi: 10.1097/01.aids.0000060358.78202.c1. [DOI] [PubMed] [Google Scholar]
- 7.d'Arminio A, Sabin CA, Phillips AN, et al. Cardio- and cerebrovascular events in HIV-infected persons. AIDS. 2004;18(13):1811–1817. doi: 10.1097/00002030-200409030-00010. [DOI] [PubMed] [Google Scholar]
- 8.Kaplan RC, Kingsley LA, Gange SJ, et al. Low CD4+ T-cell count as a major atherosclerosis risk factor in HIV-infected women and men. AIDS. 2008;22(13):1615–1624. doi: 10.1097/QAD.0b013e328300581d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mangili A, Gerrior J, Tang AM, et al. Risk of cardiovascular disease in a cohort of HIV-infected adults: a study using carotid intima-media thickness and coronary artery calcium score. Clin Infect Dis. 2006;43(11):1482–1489. doi: 10.1086/509575. [DOI] [PubMed] [Google Scholar]
- 10.Mooser V. Atherosclerosis and HIV in the highly active antiretroviral therapy era: towards an epidemic of cardiovascular disease? AIDS. 2003;17(suppl 1):S65–S69. doi: 10.1097/00002030-200304001-00009. [DOI] [PubMed] [Google Scholar]
- 11.Periard D, Cavassini M, Taffe P, et al. High prevalence of peripheral arterial disease in HIV-infected persons. Clin Infect Dis. 2008;46(5):761–767. doi: 10.1086/527564. [DOI] [PubMed] [Google Scholar]
- 12.Stein JH. Cardiovascular risks of antiretroviral therapy. N Engl J Med. 2007;356(17):1773–1775. doi: 10.1056/NEJMe078037. [DOI] [PubMed] [Google Scholar]
- 13.Kuller LH, Tracy R, Belloso W, et al. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med. 2008;5(10):e203. doi: 10.1371/journal.pmed.0050203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brenchley JM, Price DA, Schacker TW, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006;12(12):1365–1371. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- 15.Marchetti G, Bellistri GM, Borghi E, et al. Microbial translocation is associated with sustained failure in CD4+ T-cell reconstitution in HIV-infected patients on long-term highly active antiretroviral therapy. AIDS. 2008;22(15):2035–2038. doi: 10.1097/QAD.0b013e3283112d29. [DOI] [PubMed] [Google Scholar]
- 16.Jiang W, Lederman MM, Hunt P, et al. Plasma levels of bacterial DNA correlate with immune activation and the magnitude of immune restoration in persons with antiretroviral-treated HIV infection. J Infect Dis. 2009;199(8):1177–1185. doi: 10.1086/597476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Funderburg N, Luciano AA, Jiang W, Rodriguez B, Sieg SF, Lederman MM. Toll-like receptor ligands induce human T cell activation and death, a model for HIV pathogenesis. PLoS One. 2008;3(4):e1915. doi: 10.1371/journal.pone.0001915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meier A, Alter G, Frahm N, et al. MyD88-dependent immune activation mediated by human immunodeficiency virus type 1-encoded toll-like receptor ligands. J Virol. 2007;81(15):8180–8191. doi: 10.1128/JVI.00421-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Drake TA, Morrissey JH, Edgington TS. Selective cellular expression of tissue factor in human tissues: implications for disorders of hemostasis and thrombosis. Am J Pathol. 1989;134(5):1087–1097. [PMC free article] [PubMed] [Google Scholar]
- 20.Niemetz J. Tissue-factor-endowed leukocytes do cause thrombosis. Blood. 2005;106(4):1506. doi: 10.1182/blood-2005-03-1163. author reply 1507. [DOI] [PubMed] [Google Scholar]
- 21.Kitchens RL, Thompson PA. Modulatory effects of sCD14 and LBP on LPS-host cell interactions. J Endotoxin Res. 2005;11(4):225–229. doi: 10.1179/096805105X46565. [DOI] [PubMed] [Google Scholar]
- 22.Giorgi JV, Hultin LE, McKeating JA, et al. Shorter survival in advanced human immunodeficiency virus type 1 infection is more closely associated with T lymphocyte activation than with plasma virus burden or virus chemokine coreceptor usage. J Infect Dis. 1999;179(4):859–870. doi: 10.1086/314660. [DOI] [PubMed] [Google Scholar]
- 23.Deeks SG, Kitchen CM, Liu L, et al. Immune activation set point during early HIV infection predicts subsequent CD4+ T-cell changes independent of viral load. Blood. 2004;104(4):942–947. doi: 10.1182/blood-2003-09-3333. [DOI] [PubMed] [Google Scholar]
- 24.Semeraro N, Biondi A, Lorenzet R, Locati D, Mantovani A, Donati MB. Direct induction of tissue factor synthesis by endotoxin in human macrophages from diverse anatomical sites. Immunology. 1983;50(4):529–535. [PMC free article] [PubMed] [Google Scholar]
- 25.Oeth PA, Parry GC, Kunsch C, Nantermet P, Rosen CA, Mackman N. Lipopolysaccharide induction of tissue factor gene expression in monocytic cells is mediated by binding of c-Rel/p65 heterodimers to a kappa B-like site. Mol Cell Biol. 1994;14(6):3772–3781. doi: 10.1128/mcb.14.6.3772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martin DM, Boys CW, Ruf W. Tissue factor: molecular recognition and cofactor function. FASEB J. 1995;9(10):852–859. doi: 10.1096/fasebj.9.10.7615155. [DOI] [PubMed] [Google Scholar]
- 27.Furie B, Furie BC. Mechanisms of thrombus formation. N Engl J Med. 2008;359(9):938–949. doi: 10.1056/NEJMra0801082. [DOI] [PubMed] [Google Scholar]
- 28.Mackman N. Role of tissue factor in hemostasis and thrombosis. Blood Cells Mol Dis. 2006;36(2):104–107. doi: 10.1016/j.bcmd.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 29.Yan ZQ, Hansson GK. Innate immunity, macrophage activation, and atherosclerosis. Immunol Rev. 2007;219:187–203. doi: 10.1111/j.1600-065X.2007.00554.x. [DOI] [PubMed] [Google Scholar]
- 30.Weber C, Zernecke A, Libby P. The multifaceted contributions of leukocyte subsets to atherosclerosis: lessons from mouse models. Nat Rev Immunol. 2008;8(10):802–815. doi: 10.1038/nri2415. [DOI] [PubMed] [Google Scholar]
- 31.Taylor FB, Jr, Chang A, Ruf W, et al. Lethal E. coli septic shock is prevented by blocking tissue factor with monoclonal antibody. Circ Shock. 1991;33(3):127–134. [PubMed] [Google Scholar]
- 32.Creasey AA, Chang AC, Feigen L, Wun TC, Taylor FB, Jr, Hinshaw LB. Tissue factor pathway inhibitor reduces mortality from Escherichia coli septic shock. J Clin Invest. 1993;91(6):2850–2860. doi: 10.1172/JCI116529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leatham EW, Bath PM, Tooze JA, Camm AJ. Increased monocyte tissue factor expression in coronary disease. Br Heart J. 1995;73(1):10–13. doi: 10.1136/hrt.73.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jude B, Zawadzki C, Susen S, Corseaux D. Relevance of tissue factor in cardiovascular disease. Arch Mal Coeur Vaiss. 2005;98(6):667–671. [PubMed] [Google Scholar]
- 35.Al-Saady NM, Leatham EW, Gupta S, Kwan JT, Eastwood JB, Seymour CA. Monocyte expression of tissue factor and adhesion molecules: the link with accelerated coronary artery disease in patients with chronic renal failure. Heart. 1999;81(2):134–140. doi: 10.1136/hrt.81.2.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Annex BH, Denning SM, Channon KM, et al. Differential expression of tissue factor protein in directional atherectomy specimens from patients with stable and unstable coronary syndromes. Circulation. 1995;91(3):619–622. doi: 10.1161/01.cir.91.3.619. [DOI] [PubMed] [Google Scholar]