Abstract
A disintegrin and metalloprotease with a thrombospondin type 1 motifs 13 (ADAMTS-13) regulates hemostasis by cleaving the folded A2 domain of von Willebrand factor (VWF). The cleavage is regulated by forces as it occurs in flowing blood. We tested the hypothesis that force-induced A2 domain unfolding facilitates cleavage using atomic force microscopy to pull single VWF A1A2A3 tridomain polypeptides by platelet glycoprotein Ibα or antibodies to measure time, distance, and force. Structural destabilization of A1A2A3 was induced by 5- to 80-pN forces, manifesting as an abrupt molecular length increase distributed around 20 and 50 nm, probably because of uncoupling A1A2A3 (or partially unfolding A2) and fully unfolding A2, respectively. Time required to destabilize A1A2A3 first increased (catch), reaching a maximum of 0.2 seconds at 20pN, then decreased (slip) with increasing force, independent of ADAMTS-13. The time required to rupture A1A2A3 exhibited a similar catch-slip behavior when pulled by glycoprotein Ibα but only slip behavior when pulled by antibody, which was progressively shortened by increasing concentration of ADAMTS-13 after (but not before) structural destabilization, indicating that cleavage of A2 requires the force-induced A2 unfolding. Analysis with a model for single-substrate trimolecular enzymatic kinetics estimated a cleavage rate kcat of 2.9 (± 59) seconds and a Kd of 5.6 (± 3.4) nM for ADAMTS-13/A1A2A3 binding. These findings quantify the mechanical regulation of VWF cleavage by ADAMTS-13 at the level of single A1A2A3 tridomain.
Introduction
von Willebrand factor (VWF) is critical to the tethering of circulating platelets to the sites of vascular injury.1,2 On stimulation, endothelial cells secrete ultra-large (UL) and highly adhesive forms of VWF multimers3 that form long string-like structures to tether and aggregate platelets to endothelial cells in vitro4 and in vivo,5 potentially causing systemic or localized thromboembolism as found in thrombotic thrombocytopenia purpura.6 The hyperactive and prothrombotic properties are significantly reduced when ULVWF is cleaved by ADAMTS-13 (A Disintegrin and Metalloprotease with ThromboSpondin motif 13)7 at the Tyr1605-Met1606 peptide bond in the A2 domain. Cleavage reduces ULVWF multimers to smaller forms4,8 that require immobilization to surfaces, modulators, or high fluid shear stresses to bind platelets.4,7,9,10
Fluid shear stress has long been considered critical to the activation of circulating VWF, presumably by inducing conformational changes to expose A1 domain for binding of platelet glycoprotein (GP) Ibα.10,11 However, whether and how mechanical forces affect VWF proteolysis by ADAMTS-13 is less clear. Because VWF proteolysis takes place in rapidly flowing blood, it is subjected to physical forces that could regulate the cleavage kinetics.4,8,12 This hypothesis has been increasingly supported by experimental evidence. For example, under static conditions, denaturing agents such as urea are required for ADAMTS-13 to cleave VWF multimers9,13 but not isolated A2 domain or its derivative peptides.14–17 Shear stress, which induces VWF conformational changes,18 has also been shown to accelerate VWF cleavage in plasma.8 Recently, we have shown that increasing fluid shear enhances cleavage of a VWF A1A2A3 tridomain that is coated on microspheres and mediates platelet agglutination in a buffer system.19 A recent study used optical tweezers to measure the mechanoenzymatic cleavage of isolated A2 domain by ADAMTS-13.20
We hypothesize that force regulates VWF cleavage by ADAMTS-13 via disrupting noncovalent interactions between and/or within the A domains to destabilize the protein structure and expose the cryptic cleavage site in the A2 domain. To test this hypothesis, we used atomic force microscopy (AFM) to apply controlled forces to induce structural destabilization in an A1A2A3 tridomain polypeptide and to examine whether this impacts its cleavage by ADAMTS-13.
Methods
The extracellular fragment of GPIbα (glycocalicin)10 was purified from platelets as described.21 Recombinant A1A2A3 with a 6-histidine tag at the C-terminus22 and ADAMTS-1323 were respectively produced as previously described. In addition, a truncation ADAMTS-13 mutant lacking the catalytic domain (DisC) was generated by polymerase chain reaction, expressed Chinese Hamster Ovary cells (ATCC), and purified through an Ni column.23 Anti-His monoclonal antibody (mAb) was from Sigma-Aldrich. Anti-A1 mAb CR1 was a generous gift from M. C. Berndt (University College Cork, Cork, Ireland).
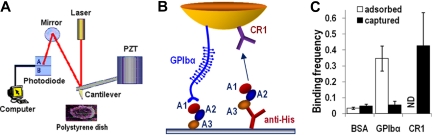
The AFM (Figure 1A) was built in our laboratory as previously described.19 It was functionalized in 2 ways (Figure 1B): (1) A1A2A3 was adsorbed on a polystyrene surface and tested by an AFM tip coated with glycocalicin; and (2) A1A2A3 was captured by an anti-His mAb preadsorbed on a polystyrene dish and tested by an AFM tip coated with the mAb CR1.
Figure 1.
Atomic force microscopy (AFM) setup. (A) AFM schematic. (B) Functionalization of AFM. Molecules depicted represent a composite of 2 sets adsorbed or captured on the AFM tip or the polystyrene dish. A1A2A3 tridomain was directly adsorbed or captured by anti-His mAb preadsorbed on the surface. GPIbα or CR1 was adsorbed on the cantilever tip. (C) Binding specificity. Immobilized A1A2A3 bound GPIbα- or CR1-coated cantilever tips but not BSA-coated tips. ND indicates not done. GPIbα did not bind captured A1A2A3 probably because of its different conformation from adsorbed A1A2A3.
AFM tips were incubated with 10 μL per tip of GPIbα (10 μg/mL), CR1 (15 μg/mL), or bovine serum albumin (BSA; 1%) at 4°C overnight, rinsed, and soaked in phosphate-buffered saline (PBS) containing 1% BSA to block nonspecific binding. Polystyrene dishes were thoroughly cleaned with absolute ethanol and dried with argon gas before protein adsorption. Surfaces were incubated with 10 μL per spot of A1A2A3 or anti-His mAb (15 μg/mL) at 4°C overnight and washed 2 times with PBS. The anti-His mAb–coated surfaces were further incubated with 10 μL per spot of A1A2A3 (5 μg/mL) at room temperature for 1 hour. Dishes were then filled with PBS containing 1% BSA in the absence or presence of ADAMTS-13 (1.25-10 μg/mL) without or with 5mM ethylenediaminetetraacetic acid (EDTA).
AFM experiments were performed using a feedback control routine to clamp the force at a preset level. The AFM tip was first driven by a piezoelectric translator (PZT) to touch the surface (Figure 1A). After sensing a compressive force (< 30pN) from the photodiode, the cantilever was retracted 20 nm and held there for 1 second to allow for bond formation. The cantilever was further retracted to detect binding from the tensile force measured by the photodiode. Lifting the tip from the surface in the bond formation phase usually reduced nonspecific binding to less than 5% of the test cycles when the AFM tip was coated with BSA. Specific binding was confirmed by more than 5-fold increase in adhesion frequency (Figure 1C). If retraction did not generate a tensile force (ie, no adhesion) or if the force so generated could not reach a preset level (ie, premature rupture), the cantilever was fully retracted to the starting position and the cycle repeated. If the preset level of force was reached during retraction, the cantilever would stop to clamp the force at that level. The cantilever would retract again after a sudden loss of structural stability (manifested as an abrupt force drop) and to accommodate any drift. This process was repeated until the preset force could no longer be resumed after final rupture. The cantilever was retracted to the starting position and the cycle repeated.
To measure the cleavage kinetics under static conditions, purified ADAMTS-13 (58.8nM) was incubated with different concentrations of A1A2A3 (10, 20, 30, and 40nM) for 3 hours in the buffer containing 1.6M urea, 5mM Tris-HCl, and 5mM NaCl, pH 8.15. The reaction solution was separated on 10% sodium dodecyl sulfate electrophoresis under reducing conditions. The cleavage of A1A2A3 was detected by a polyclonal VWF antibody (Dako North America) and quantified by densitometry. The data were analyzed by the Michaelis-Menten equation24 using the Lineweaver-Burk plot.
Results
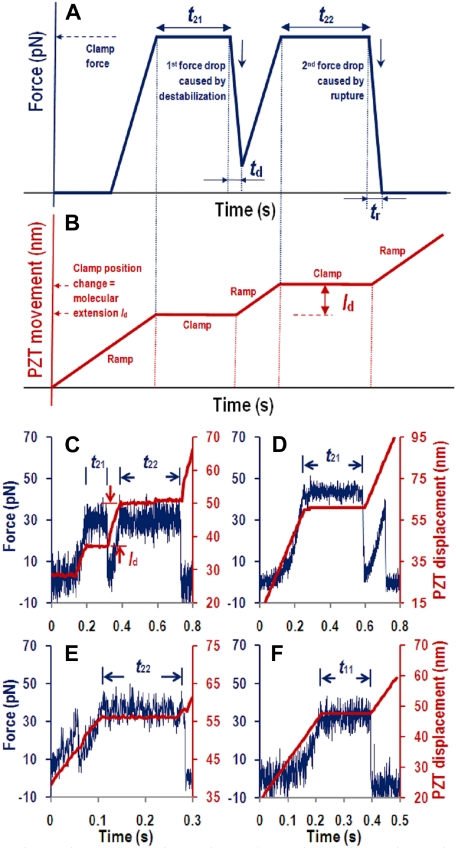
We used AFM to quantify how force impacted the A1A2A3 structural stability, dissociation from GPIbα or CR1 (or anti-His mAb), and/or cleavage by ADAMTS-13. The experiment is schematically illustrated in Figure 2A and B. Four representative raw datasets are presented in Figure 2C through F.
Figure 2.
Force curves. (A-B) Schematic illustrations. (C-F) Example data. The PZT retracted the cantilever base linearly to a preset position (B,C-F, red lines and right ordinate) and then held it there to stretch the molecules with a constant force (A,C-F, blue curves and left ordinate) to measure various times characteristic of molecular structural destabilization or rupture induced by this force. (C) A curve of 2 force drops (indicated by 2 in the first subscript of tij) and 2 periods at constant force (indicated by 1 and 2 in the second subscript of tij) with respective durations of time-to-destabilization t21 and time-to-rupture t22. Force drop triggered via feedback control further retraction of the PZT attempting to bring the force back to the preset level, which was successful after the first force drop by retracting a distance ld but was unsuccessful after the second force drop even by long-distance retraction, indicating rupture. (D) A curve of 2 force drops but only 1 period at constant force with duration of t21 but no t22 resulting from premature rupture occurred before force was resumed to the preset level. (E) A curve of 2 force drops but only 1 period at constant force with duration of t22 but no t21 resulting from premature destabilization occurred before arriving at the preset force. (F) A curve of 1 force drop (indicated by 1 in the first subscript of tij, i, j = 1 or 2) and 1 period at constant force (indicated by 1 in the second subscript of tij) with duration of time-to-rupture t11. Data shown were acquired by GPIbα-pulling, but those obtained by CR1-pulling were similar.
Measurements from force-clamp experiment
Two AFM readouts, cantilever force and PZT movement, are drawn in Figure 2A and Figure 2B, respectively, to illustrate the observations and their interpretations. The experimental traces (curves) begin when the PZT retracts the cantilever with a linear ramp (Figure 2B). After moving through a dead space with zero force, the immobilized A1A2A3 picked up by the GPIbα- or CR1-bearing AFM tip begins to pull and bend the cantilever with an increasing force (Figure 2A). Once arriving at the clamped force, the PZT stops to hold the cantilever still. The force remains constant for a period (t21) and then drops abruptly during a brief period (td). This triggers the feedback control loop to retract the PZT with a second ramp to resume the force to the clamped level. After another constant-force period (t22), the force drops again during another brief period (tr), which triggers the PZT with a third ramp (Figure 2B), but the force does not respond (Figure 2A). A force drop indicates a sudden increase in the distance between the cantilever tip and the surface, which may result from either destabilization or rupture of the molecular structure. Other possibilities will be excluded based on other experimental evidence (see “Force-extension curves reveal A1A2A3 structural destabilization” and “Discussion”). Destabilization is identified when force increases again during the second PZT ramp, whereas a lack of force response indicates a molecular rupture because there is no more molecular connection to pull on the tip to bend the cantilever toward the surface. Rupture may result from either A1A2A3 dissociation from GPIbα (or anti-A1 or anti-His mAbs) or cleavage by ADAMTS-13 when it is present.
Our measurements include the clamped force f and the various times mentioned in Figure 2A as well as molecular length increment ld in Figure 2B. ld is measured from the increase in the clamp distance. It is used to characterize the molecular structural changes. The duration t21 of the first force plateau (indicated by 1 in the second subscript of tij; the first subscript indicates the total number of force drops) is time to destabilization because it measures how long the structure can support the force before losing stability. The duration t22 of the second force plateau (indicated by 2 in the second subscript of tij) is time to rupture because it measures how long the structure can support the force before rupture. Other 2 temporal parameters of interest are the durations of the destabilization and rupture processes from the beginning to the end, td and tr, respectively.
Representative experimental data corresponding to the schematic illustrations of Figure 2A and B are shown in Figure 2C, where force (blue curve, left ordinate) and PZT movement (red curve, right ordinate) are plotted together. Two additional types of 2 force drop events were also observed. In Figure 2D, rupture occurred before arriving at the clamped force during the second ramp, which allowed measurement for t21, ld, td, and tr, but not t22. In Figure 2E, destabilization occurred before arriving at the clamp force, which allowed measurement for ld, td, t22, and tr, but not t21. These 2 force drop events are of primary interest because they include force-induced structural destabilization (first force drop) and molecular rupture (second force drop), the focus of this study. Events with a single force drop are also of interest because they provide a control for molecular rupture without structural destabilization. These include rupture occurring after (Figure 2F) or before (not shown) a single force-clamp period where the time to rupture is denoted as t11. Events with more than 2 abrupt force drops were very rare. They were recorded for frequency analysis only (supplemental Figure 1, available on the Blood website; see the Supplemental Materials link at the top of the online article).
Measurements of force, distance, and time from the 2 AFM readouts illustrated in Figure 2 represent mechanical and kinetic characteristics of a single A1A2A3 tridomain interacting with the pulling molecule. Analysis of ensembles of these data will allow us to extract information regarding the force regulation of the kinetics of GPIbα/A1 (or A1/CR1 or His/anti-His) bond dissociation, A1A2A3 uncoupling and A2 unfolding, and A2 cleavage by ADAMTS-13 when ADAMTS-13 was added to the system.
Force-extension curves reveal A1A2A3 structural destabilization
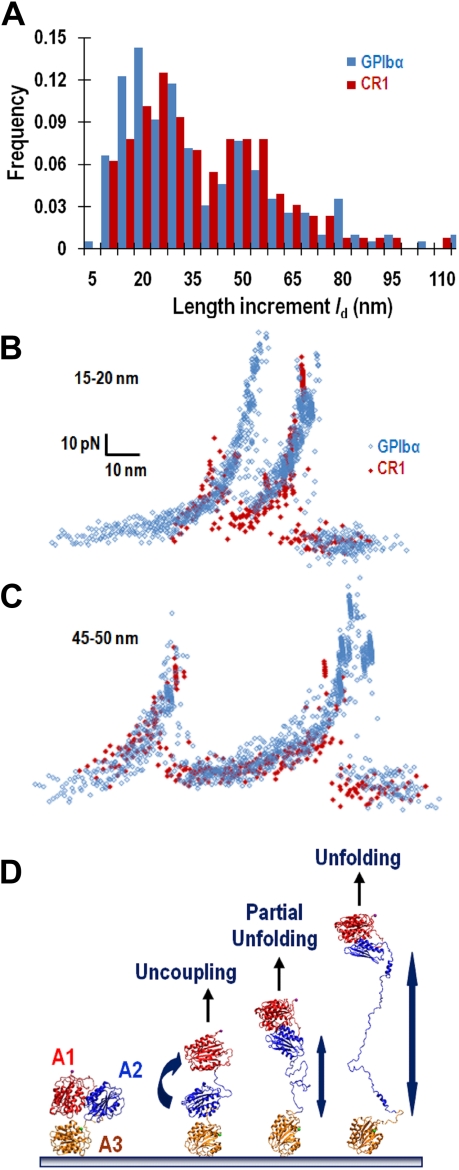
We analyzed the length increment ld by histogram to properly interpret the first force drop in a 2–force drop event. The ld distribution exhibited 2 major peaks regardless of whether the A1A2A3 were adsorbed or captured by anti-His mAb and pulled by GPIbα or CR1 (Figure 3A). When the force-time and displacement-time traces (Figure 2C and 2E, blue and red curves, respectively) were converted into force-molecular extension curves by eliminating the time, we observed a sawtooth pattern that was similar to the widely reported signature of protein domain unfolding.25 Overlaying a large number of force-molecular extension curves with similar ld values reveals a good alignment in the ascending segments for both the 20- and 50-nm-length increment groups, regardless of the methods used to pull A1A2A3 (Figure 3B-C). This indicates that identical molecular segments were stretched by similar loading processes. One probable segment is the A2 domain as it is the common segment pulled by the 2 methods. Other segments are doubtful because mAb-captured A1A2A3 assumes the same conformation as full-length VWF in solution and did not bind GPIbα, whereas adsorption results in a conformational change in A1A2A3 that exposes the GPIbα binding site (Figure 1C), similar to the full-length VWF.26 These results also indicate that the first force drop was caused by structural destabilization of a single molecular linkage, not by rupture of 1 of 2 parallel bonds that mediated a double-bond adhesion event. The latter possibility is not supported by the finding that pulling adsorbed (therefore random oriented) A1A2A3 by GPIbα resulted in force-molecular extension curves that are well aligned with those generated using CR1 to pull A1A2A3 captured at the C-terminal His-tag of the A3 domain by an anti-His mAb (Figure 3B-C).
Figure 3.
Analysis of the length increase resulted from loss of stability. (A) Histograms of molecular length increment, ld, obtained using adsorbed A1A2A3 pulled by GPIbα (blue bars) or anti-His mAb captured A1A2A3 pulled by CR1 (red bars). (B-C) Force-time and displacement-time curves with 2 force drops were converted to force–molecular extension curves: 16 and 10 curves, respectively, obtained by GPIbα (blue) and CR1 (red) pulling with short (ld ∼ 10-20 nm) length increase (B) as well as 10 and 8 curves (obtained by the 2 pulling methods with the same color codes) with long (ld ∼ 45-50 nm) length increase (C) are overlaid by aligning the first ascending segment. (D) Possible modes of structural destabilization. A model for the native A1A2A3 structure is shown in the far left panel. Force may disrupt the interdomain interactions to uncouple the quaternary structure of the A1A2A3 tridomain (second panel from left) or disrupt the intradomain interactions to partially (third panel from left) or fully (far right panel) unfold the tertiary structure of the A2 domain.
The spread in ld values (Figure 3A) may result from nonuniform orientations of the immobilized A1A2A3 and GPIbα (and CR1) and/or nonuniform alignment of the molecular linkage relative to the pulling direction. The 2 peaks in the ld distribution may reflect different modes of structural destabilization, as depicted in Figure 3D. One mode might be unfolding of the tertiary structure of the A2 domain (far right panel), as ld of approximately 50 nm is consistent with the contour length increase predicted for A2 unfolding.27 The short length increase (ld ∼ 20 nm) might represent either partial unfolding of the A2 domain or uncoupling of the quaternary structure of the A1A2A3 tridomain by disrupting the domain-domain interactions (2 middle panels). Unfolding of A1 or A3 is improbable because each domain contains a disulfide bond between cystines at the N- and C-termini that makes it strongly resistant to unfolding.27
ADAMTS-13 effects on time measurements
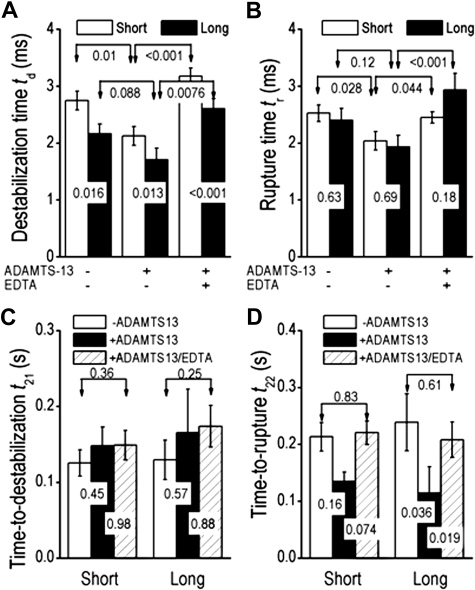
We quantified the ADAMTS-13 effects on GPIbα-pulling–induced A1A2A3 structural change by comparing the characteristic measurements (Figure 2) of the force-clamp experiment in the absence and presence of 5 μg/mL ADAMTS-13 without and with 5mM EDTA. The hypothesis regarding different modes of structural destabilization of A1A2A3 prompted us to segregate the data into 2 groups by ld less than 35 nm (short) and ld more than 35 nm (long).
The destabilization time td was longer for the short group than for the long group (Figure 4A), indicating 2 speeds, ld/td, for the short and long groups and 2 modes of destabilization. By comparison, the rupture time tr was indifferent to the ld values (Figure 4B). ADAMTS-13 shortened the destabilization time td (Figure 4A) regardless of the short or long groups. The effect was blocked by the divalent calcium chelater EDTA, which inhibits the VWF-cleavage activity of ADAMTS-13 as indicated by the significantly prolonged td value. However, the time-to-destabilization t21 was not affected by ADAMTS-13 and EDTA (Figure 4C). These data suggest that ADAMTS-13 exerts its effect only after, but not before, the onset of structural destabilization. By comparison, both the rupture time tr (Figure 4B) and the time-to-rupture t22 (Figure 4D) were significantly reduced by the addition of ADAMTS-13, and the effect was divalent cation-dependent because EDTA abolished the ADAMTS-13 effect.
Figure 4.
Impact of mode of destabilization. (A-B) Comparison of destabilization times (A) or rupture times (B) for molecules with structural destabilization that yielded short (ld < 35 nm, open bars) and long (ld > 35 nm, closed bars) length increases in the absence or presence of 5 μg/mL ADAMTS-13 without or with 5mM EDTA. (C-D) Comparison of time-to-destabilization t21 (C) or time-to-rupture t22 (D) measured in the absence (open bars) or presence of ADAMTS-13 without (closed bars) or with (hatched bars) EDTA for molecules with structural destabilization that yielded short or long length increases. Data were acquired by GPIbα-pulling and presented as mean ± SEM of several tens of measurements. P values of Student t test are shown to indicate the statistical significance (or lack thereof) of the differences.
Two hypotheses could explain the observed shortening of t22: (1) ADAMTS-13 accelerated the dissociation of GPIbα/A1 bond, and/or (2) ADAMTS-13 cleaved the A2 domain. The second hypothesis was supported by the analysis of running adhesion frequencies from repeated contacts between the same GPIbα-bearing AFM tip and the same location on an A1A2A3-coated surface. The repeated contacts most probably tested the same pair of interacting molecules repeatedly. The running frequency remained stable in the absence of ADAMTS-13, indicating no loss of functionality over time (supplemental Figure 2). In contrast, adhesion frequencies starting at 2 different initial values declined rapidly in the presence of 5 μg/mL ADAMTS-13, suggesting that ADAMTS-13 cleaved A2 to progressively reduce the number of intact A1A2A3 polypeptides capable of mediating adhesion in repeated tests.28 With the addition of EDTA, the running adhesion frequency became stable again and was indistinguishable from that in the absence of ADAMTS-13, providing an important control for the cleavage effects.
Interestingly, ADAMTS-13 resulted in a much greater reduction in t22 in the long compared with the short groups. Indeed, the t22 reduction of the long group was significant, whereas that of the short group was not (Figure 4D). As illustrated in Figure 3D, the results suggest that cleaving the Tyr1605-Met1606 peptide bond may be more efficient in a fully unfolded A2 domain (as indicated by the long ld value) than a partially unfolded A2 or uncoupled A1A2A3 (as indicated by the short ld value).
Effects of ADAMTS-13 concentration
We varied the ADAMTS-13 concentration to further test the hypothesis that structural destabilization of A1A2A3 facilitates its cleavage by ADAMTS-13. These experiments used A1A2A3 adsorbed on polystyrene surfaces pulled by GPIbα-bearing AFM tips. As a control, t11, time-to-rupture of single force drop events, was found indifferent to ADAMTS-13 at the 4 concentrations tested and to the catalytic domain deletion mutant DisC that had no VWF-cleaving activity (Figure 5A). The data suggest that ADAMTS-13 could not cleave A2 before structural destabilization under our experimental conditions. Time-to-destabilization t21 was also indifferent to the changes in ADAMTS-13 concentration and to the inactive DisC (Figure 5B). However, time-to-rupture t22 for the long group (Figure 5D), but not the short group (Figure 5C), decreased with increasing ADAMTS-13 concentration that was saturated at 2.5 μg/mL (17nM). This effect requires the VWF-cleaving activity of ADAMTS-13 as it was not observed in the presence of DisC at a concentration (5 μg/mL or 37nM) that was more than doubled the saturation concentration of WT ADAMTS-13.
Figure 5.
Effects of ADAMTS-13 concentration. Time-to-rupture t11 (A), time-to-destabilization t21 (B), and time-to-rupture t22 of the short (C) and long (D) groups were plotted versus ADAMTS-13 concentration. With the exception of t22, which was shortened by ADAMTS-13 in a dose-dependent manner, other time parameters were indifferent to the changing ADAMTS-13 concentration. DisC is a control construct without VWF-cleaving activity. Data were acquired by GPIbα-pulling and presented as mean ± SEM of several tens of measurements.
ADAMTS-13 catalytic rates for mechanically and chemically denatured A1A2A3
We used a recently developed model for single-substrate trimolecular enzymatic kinetics (W. Chen and C.Z., manuscript submitted November 2009) to analyze the ADAMTS-13 dose-dependent data of A1A2A3 cleavage in Figure 5D. This model extends the Michaelis-Menten ensemble-averaged kinetics of a bimolecular enzyme-substrate system24 to the single-substrate kinetics of a trimolecular system in which a receptor (GPIbα) binds and stretches a substrate (VWF) to expose its cleavage site before dissociation, allowing an enzyme (ADAMTS-13) to bind and cleave it (rupture). The counterpart to the Michaelis-Menten equation is:
where [ADAMTS-13] is ADAMTS-13 concentration, kcat is catalytic rate constant, Kd is the equilibrium dissociation constant of ADAMTS-13/A1A2A3 binding, and t220 denotes t22 value at 0 ADAMTS-13 concentration. Equation 1 was fit to the data in Figure 5D, resulting in a very good agreement (Figure 6A). The estimated values are kcat = 2.9 ± 0.59 seconds and Kd = 5.6 ± 3.4nM.
Figure 6.
Enzymatic kinetics. (A) Data from Figure 5D (points) were replotted using molar concentration and fitted by Equation 1 (curve). (B) Lineweaver-Burk plot of reciprocal initial rate of substrate reduction versus reciprocal substrate concentration. The data (points) were fitted by a straight line. The best-fit parameters are indicated. The goodness of fit is indicated by R2 (B) or adjusted R2 (A).
To ensure the VWF-cleaving activity of the recombinant ADAMTS-13 used in the AFM experiment and to obtain an independent measurement of the catalytic rate, we performed standard stress-free enzymatic assay using A1A2A3 chemically denatured by urea. The result was analyzed by the Lineweaver-Burk plot (Figure 6B), which linearizes the Michaelis-Menten equation. The estimated catalytic rate is kcat = 0.015 (± 0.018) seconds and the Michaelis constant is Km = 1.3 (± 1.6) μM.
Force-dependent kinetics of A1A2A3 dissociation and cleavage by GPIbα-pulling
In vivo, VWF is subject to different forces depending on whether it attaches to the vascular surface or flowing platelets and on where this occurs in the circulation. We thus used a range of clamped forces to induce A1A2A3 destabilization, bond dissociation, and cleavage. The 3 time parameters quantifying these processes (t11, t21, and t22) were sorted according to force regardless of the EDTA ld values. This is in contrast to Figures 4 and 5, which lump data measured at all forces.
In the absence of ADAMTS-13, A2 would not be cleaved so the time-to-rupture (t11 and t22) represents time-to-dissociation, or lifetime, of GPIbα/A1A2A3 bonds. It is evident from Figure 7A that the t11-versus-force plot showed catch-slip bonds, where t11 first increased (catch) to reach a maximum and then decreased (slip) as force increased. This is expected based on our recent observation of catch-slip bonds for GPIbα interacting with an isolated A1 domain and VWF multimers.19 Interestingly, the time-to-destabilization t21-versus-force data (Figure 7B) demonstrated the first observation of catch-slip bonds in the kinetic process of protein structural destabilization. The time-to-rupture t22-versus-force curve exhibited a similar shape as the t11-versus-force curve, that is, catch-slip bonds (Figure 7C), indicating that the force-dependent dissociation kinetics of the GPIbα/A1A2A3 interaction was not affected by the structural destabilization of A1A2A3.
Figure 7.
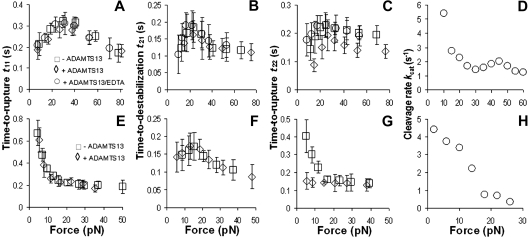
Force-dependent kinetics of destabilization and rupture. Time-to-rupture t11 (A,E) or t22 (C,G) or time-to-destabilization t21 (B,F) is plotted versus force measured in the absence (□) or presence of 5 μg/mL ADAMTS-13 without (◊) or with (○) 5mM EDTA for A1A2A3 adsorbed directly on polystyrene dish and pulled by GPIbα (A-C) or captured by preadsorbed anti-His mAb and pulled by CR1 (E-G). Data are mean ± SEM of several tens of measurements for each point. The rate of cleavage as a function of force was calculated by kcat = 1/t22 − 1/t220 denotes using the respective data in panel C (D) or panel G (H).
However, addition of 5 μg/mL ADAMTS-13 significantly shortened the time-to-rupture t22 (Figure 7C) at all forces tested, consistent with Figures 4D and 5D. The effect was again blocked by EDTA. By contrast, ADAMTS-13 did not affect the t11-versus-force (Figure 7A) and t21-versus-force (Figure 7B) curves, consistent with Figures 4C and 5A and B.
Force-dependent kinetics of A1A2A3 dissociation and cleavage by mAb-pulling
We also performed experiments using CR1 to pull A1A2A3 captured by anti-His mAb in a range of forces to obtain data parallel to those in Figure 7A through C and are plotted in Figure 7E through G. The rationale is as follows: in the absence of ADAMTS-13, the force dependence of the t11 (Figure 7A open squares) and t22 (Figure 7C open squares) curves are characteristic of the GPIbα/A1A2A3 interaction, whereas that of the t21 curve (Figure 7B open squares) is interpreted as characteristic of the intramolecular interactions that stabilize the A1A2A3 structure. However, the similar catch-slip bond pattern of all 3 curves in Figure 7A through C raised an alternative interpretation that the t21 curve might also have represented the GPIbα/A1A2A3 bond characteristic should the first force drop reflect the rupture of 1 of 2 parallel members of a double-bond adhesion event. The CR1 experiment would allow us to discriminate these 2 possible interpretations because antigen/antibody dissociations have been observed to exhibit slip bonds only,29–31 which predicts slip bond t11- and t22-versus-force curves in Figure 7E and G that are different from the catch-slip bond curves in Figure 7A and C. Should t21 be characteristic of A1A2A3 destabilization, pulling by CR1 would result in a t21 curve different from the t11 and t22 curves, but similar to the t21 curve in Figure 7B. Had the first force drop reflected the rupture of 1 of 2 parallel members of a double-bond adhesion event, pulling by CR1 would have resulted in similar curves for t11, t21, and t22, but different from the t21 curve in Figure 7B, because, like t11 and t22, t21 would have been characteristic of A1A2A3/antibody dissociation. Furthermore, the effect (or lack thereof) of ADAMTS-13 on the t11, t21, and t22 curves can be confirmed because this effect should not depend on the method of pulling. Thus, these experiments allow us to confirm or contrast, depending on the time parameter, the observations in Figure 7A and C, thereby greatly strengthening our conclusions.
Figure 7E shows that the bond lifetime t11-versus-force curve in the absence of ADAMTS-13 exhibited ordinary slip bonds as expected for an antigen/antibody interaction.29–31 The differential data depicted in Figure 7A and E demonstrate that the force-dependent dissociation kinetics are specific to the 2 distinct interactions (GPIbα/A1 vs antigen/antibody). However, similar to Figure 7B, the t21-versus-force curve generated with the antibody capturing method also showed catch-slip bonds (Figure 7F), strongly suggesting that the force-dependent kinetics of structural destabilization is governed by intramolecular, not intermolecular interactions between A1A2A3 and the pulling molecule. The force-dependent time-to-rupture after structural destabilization (t22-vs-force) curve showed slip bonds (Figure 7G), which is similar to the force-dependent time-to-rupture before structural destabilization (t11-vs-force, Figure 7E), but different from the t22 curve in Figure 7C. The agreement between Figure 7E and G indicates that the force-dependent dissociation kinetics of the antigen/antibody interaction is not affected by structural destabilization of A1A2A3, consistent with the agreement between Figure 7A and C.
ADAMTS-13 had no effect on the curves of t11 versus force (Figure 7E) and t21 versus force (Figure 7F) obtained by CR1-pulling, consistent with the lack of ADAMTS-13 effect on the curves of t11 versus force (Figure 7A) and t21 versus force (Figure 7B) obtained by GPIbα-pulling. Thus, the dissociation kinetics of GPIbα/A1A2A3 and the antigen/antibody bonds as well as the kinetics of A1A2A3 destabilization do not depend on ADAMTS-13 and how A1A2A3 is captured and pulled. However, addition of 5 μg/mL ADAMTS-13 again shifted the t22-versus-force curve downward toward shorter lifetimes (Figure 7G), similar to the ADAMTS-13 effect on the t22-versus-force curve observed in Figure 7C. Taken together, these data consistently demonstrate that ADAMTS-13 cleaves A2 after, but not before, the A1A2A3 tridomain is structurally destabilized by a mechanical force regardless of how A1A2A3 was immobilized and pulled.
Force-dependent cleavage rate estimated by 2 methods
The 5 μg/mL of ADAMTS-13 used in these experiments corresponds to a saturation concentration (Figures 5D, 6A). Taking the limit of [ADAMTS-13] → ∞ reduces Equation 1 to kcat = 1/t22 − 1/t220. The force-dependent kcat can thus be calculated from the data in Figure 7C by subtracting 1/t22 in the presence of ADAMTS-13 from that in the absence of ADAMTS-13 at each force bin (Figure 7D). The kcat can be similarly calculated from the data in Figure 7G, which is plotted versus force in Figure 7H. Plots in Figure 7D and H both show a decreasing ADAMTS-13 cleavage rate with increasing force. This agreement attests to the reliability of our data and conclusion.
Discussion
The goal of this work was to study the structural stability of the A1A2A3 tridomain under mechanical forces and how the structural destabilization facilitates the tridomain cleavage by ADAMTS-13 at the level of single molecular triad. AFM was used to apply controlled force to a single A1A2A3 polypeptide and to characterize the resulting molecular stretch, structural destabilization, and rupture. We found that cleavage of A2 occurred after, but not before, a loss of structural stability in the A1A2A3 tridomain. Structural destabilization was manifested as an abrupt increase of molecular length by usually approximately 20 or 50 nm (Figure 3A-C) in milliseconds (Figure 4A). It could be induced by a pulling force as low as 5pN for as short as one-tenth of a second (Figure 7B,F). The structural destabilization with long (ld > 35 nm, indicating a full A2 unfolding) but not short (ld < 35 nm, indicating a partial A2 unfolding or A1A2A3 tridomain uncoupling) length increments facilitated A1A2A3 cleavage by ADAMTS-13 in a dose-dependent manner (Figure 5C-D), which was well described by a model of single-substrate trimolecular enzymatic kinetics (Figure 6A). At a saturation concentration of 5 μg/mL, ADAMTS-13 cleaved A1A2A3 several times per second, but the catalytic rate decreased with increasing force (Figure 7D,H). These data provide detailed quantitative characterization the force-regulated VWF cleavage by ADAMTS-13.
We conclude that the first abrupt force drop in the 2 force drop events (eg, those exemplified in Figure 2C-E) is caused by structural destabilization of a single A1A2A3 tridomain, not rupture of one of 2 parallel bonds as in a double-bond adhesion, based on 3 sets of analyses. First, the characteristics of force versus molecular extension curves are consistent with the structural destabilization, but not bond rupture (Figure 3B-C). Second, we compared the experimental frequencies of occurrence of 0 (no adhesion), 1 (eg, Figure 2F), 2 (eg, Figure 2C-E), and 3 (not shown) force drops with the Poisson distribution of matched frequency of no adhesion events (supplemental Figure 1). Frequencies of sequential ruptures of multiple independent bonds in parallel are predicted by the Poisson distribution.32,33 It is evident from supplemental Figure 1 that, regardless of the pulling methods, the experimental distributions have consistently lower frequencies of 1 force drop event per adhesion, but higher frequencies of 2 and 3 force drop events than the Poisson distribution. This result does not support the alternative hypothesis that the abrupt force drop represents rupture of a bond in an adhesion mediated by multiple bonds in parallel. Third, the 2 methods used to pull A1A2A3 generated qualitatively different time-to-rupture t11 curves (Figure 7A,E), reflecting distinct dissociation kinetics for the 2 types of molecular interactions (receptor/ligand vs antigen/antibody). Pulling by GPIbα resulted in catch-slip bonds (Figure 7A), whereas pulling by mAb resulted in slip bonds (Figure 7E). If the first force drops in 2 force drop events were caused by rupturing 1 of 2 parallel members in a double bond, the 2 t21 curves in Figure 7B and F should have had the respective shapes match their corresponding t11 curves (Figure 7A and Figure 7E, respectively).29 However, whereas the catch-slip bond behavior seen in Figure 7B is coincidentally similar to Figure 7A, the same behavior observed in Figure 7F is distinctly different from Figure 7E. These results are sufficient to rule out the alternative hypothesis.
The time-to-destabilization versus force data (Figure 7B,F) represent the first experimental demonstration of catch-slip bonds for an intramolecular interaction that stabilizes the quaternary structure of the A1A2A3 tridomain and/or the tertiary structure of the A2 domain. Although this may seem surprising at first glance, catch bonds for intramolecular interactions should be as natural as for intermolecular interactions that stabilize a receptor/ligand complexes as recently demonstrated in several systems.19,29–31,34,35 The reason is that both are governed by the same types of noncovalent atomic-level interactions. Unfolding kinetics was also recently studied using optical tweezers to pull isolated A2 domains with different ramp speeds.20 The unfolding forces were analyzed with the Bell model,36 which assumes exponential decay of the time to unfold with increasing force, which precludes catch bonds. Using their results, it can be calculated that t21 = 0.16 and 1.8 × 10−5 seconds at 10 and 20pN. The first value is comparable to and the second value is 4 orders of magnitude smaller than our measurements (Figure 7B,F), underscoring the lack of reliability of predicting bond lifetime at constant force using off-rate calculated from rupture forces.
The hypothesis that the reduction in time to rupture t22 (Figures 4D, 5D, and 7C,G) was caused by ADAMTS-13 cleavage of A1A2A3 was supported by 5 lines of experimental evidence: (1) t22, but not t11 or t21, was reduced by ADAMTS-13 in a dose-dependent manner; (2) the running frequency plots showed gradual loss of function (supplemental Figure 2); (3) the similar effects were detected regardless of whether GPIbα or an antibody was used to pull A1A2A3; (4) the effects were blocked by chelating divalent cations; and (5) the effects were not observed when the proteolytically inactive DisC was tested.
We evaluated the A1A2A3 cleavage rate by ADAMTS-13 by fitting Equation 1 to the data of dose-dependent reduction of t22 (Figure 6A), yielding a kcat = 2.9 (± 0.59) seconds and Kd = 5.6 (± 3.4) nM. The Kd value is comparable with the value of 14 (± 1.2) nM previously determined by a binding assay.37 The kcat value is comparable with the value of 4.5 seconds estimated by applying the same model (W. Chen and C.Z., manuscript submitted November 2009) to our recent experiment in which beads bearing A1A2A3 were mixed with platelets in a shear flow to allow heterotypic agglutination in the absence and presence of ADAMTS-1319 but is 20-fold faster than a recently reported value of 0.14 (± 0.01) seconds obtained by pulling an isolated A2 domain with optical tweezers.20 In contrast, the static enzyme-linked immunosorbent assay of partially denatured A1A2A3 polypeptide by 1.6M of urea yielded a kcat = 0.015 (± 0.018) seconds and Km = 1.3 (± 1.6) μM.
The exact causes for the discrepant kcat values require further investigation, but a couple potential causes could be considered. First, although urea is required to cleave the A1A2A3 tridomain under static condition,22 it may also partially inhibit the ADAMTS-13 activity. This hypothesis is supported by a previous measurement of kcat for cleaving a 73-amino acid A2 peptide measured in the absence of urea.38 The estimated 1.3 (± 0.1) seconds value is significantly higher than our 0.015 (± 0.018) seconds value measured using urea to unfold A1A2A3, but much closer to our 2.9 (± 0.56) seconds value measured using force to destabilize A1A2A3. Second, the ADAMTS-13 cleavage site may be more exposed in an isolated A2 domain than in the A1A2A3 tridomain. This hypothesis is consistent with our previous data showing that urea is required to facilitate ADAMTS-13 cleavage of A1A2A3, but not of A2,14,22 suggesting that the cleavage site in the A2 domain may be protected by the adjacent A1 and A3 domains. Together, these results suggest that the A1 and A3 domains play a critical role in regulating the VWF binding and cleaving activities of ADAMTS-13.
In conclusion, we also observed that the cleavage rate decreased with increasing force (Figure 7D,H). This is consistent with the recently reported disulfide bond reduction rates by thioredoxin on the I27 domain of human cardiac titin, which also decrease with increasing force.39 Furthermore, the 5- to 80-pN forces used to destabilize A1A2A3 (Figure 7) correspond to the forces that are required to anchor a platelet to the vascular surface subjected to 100/s to 1600/s wall shear rates,19 which occur in veins and arteries. These also correspond to the forces pull on a VWF multimer that crosslinks a platelet to a 3-μm-diameter thrombus in a flow field of 700/s to 11 000/s shear rates.19 Thus, the forces, the force-induced A1A2A3 structural destabilization, and the force-regulated VWF cleavage by ADAMTS-13 are physiologically important to regulating the adhesion activity of VWF during hemostasis and thrombosis.
Acknowledgments
The authors thank Michael Berndt for providing anti-A1 mAb (CR1), Carol Sun for the purification of glycocalicin, Cecilia Martin for the expression and purification of recombinant A1A2A3 tridomain, Chalmette Ball for the expression and purification of ADAMTS-13, Fang Kong for programming of the AFM force-clamp routine, Wei Chen for modeling of A1A2A3 structure and calculating the enzymatic kinetics, Hui-Chun Yeh for performing the static kinetic assay of VWF cleavage by ADAMTS-13, and Jizhong Lou for helpful discussions.
This work was supported by the National Institutes of Health (grants HL093723 and HL091020, C.Z.; grant HL072886, M.A.C.; and grant HL71895, J.-f.D.).
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: C.Z. designed research; T.W. and J.L. performed research and analyzed data; M.A.C. and J.-f.D. contributed new reagents; and T.W., J.-f.D., and C.Z. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Cheng Zhu, Georgia Institute of Technology, Coulter Department of Biomedical Engineering, 315 Ferst Dr, Atlanta, GA 30332; e-mail: cheng.zhu@bme.gatech.edu; or Jing-fei Dong, Baylor College of Medicine, Thrombosis Research Section, Department of Medicine, Houston, TX 77030; e-mail jfdong@bcm.tmc.edu.
References
- 1.Sadler JE. Biochemistry and genetics of von Willebrand factor. Annu Rev Biochem. 1998;67:395–424. doi: 10.1146/annurev.biochem.67.1.395. [DOI] [PubMed] [Google Scholar]
- 2.Savage B, Almus-Jacobs F, Ruggeri ZM. Specific synergy of multiple substrate-receptor interactions in platelet thrombus formation under flow. Cell. 1998;94(5):657–666. doi: 10.1016/s0092-8674(00)81607-4. [DOI] [PubMed] [Google Scholar]
- 3.Sporn LA, Marder VJ, Wagner DD. Inducible secretion of large, biologically potent von Willebrand factor multimers. Cell. 1986;46(2):185–190. doi: 10.1016/0092-8674(86)90735-x. [DOI] [PubMed] [Google Scholar]
- 4.Dong JF, Moake JL, Nolasco L, et al. ADAMTS-13 rapidly cleaves newly secreted ultralarge von Willebrand factor multimers on the endothelial surface under flowing conditions. Blood. 2002;100(12):4033–4039. doi: 10.1182/blood-2002-05-1401. [DOI] [PubMed] [Google Scholar]
- 5.Chauhan AK, Motto DG, Lamb CB, et al. Systemic antithrombotic effects of ADAMTS13. J Exp Med. 2006;203(3):767–776. doi: 10.1084/jem.20051732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moake JL, Rudy CK, Troll JH, et al. Unusually large plasma factor VIII:von Willebrand factor multimers in chronic relapsing thrombotic thrombocytopenic purpura. N Engl J Med. 1982;307(23):1432–1435. doi: 10.1056/NEJM198212023072306. [DOI] [PubMed] [Google Scholar]
- 7.Zheng X, Chung D, Takayama TK, Majerus EM, Sadler JE, Fujikawa K. Structure of von Willebrand factor-cleaving protease (ADAMTS13), a metalloprotease involved in thrombotic thrombocytopenic purpura. J Biol Chem. 2001;276(44):41059–41063. doi: 10.1074/jbc.C100515200. [DOI] [PubMed] [Google Scholar]
- 8.Tsai HM, Sussman, Nagel RL. Shear stress enhances the proteolysis of von Willebrand factor in normal plasma. Blood. 1994;83(8):2171–2179. [PubMed] [Google Scholar]
- 9.Tsai HM. Physiologic cleavage of von Willebrand factor by a plasma protease is dependent on its conformation and requires calcium ion. Blood. 1996;87(10):4235–4244. [PubMed] [Google Scholar]
- 10.Dong JF, Berndt MC, Schade A, McIntire LV, Andrews RK, Lopez JA. Ristocetin-dependent, but not botrocetin-dependent, binding of von Willebrand factor to the platelet glycoprotein Ib-IX-V complex correlates with shear-dependent interactions. Blood. 2001;97(1):162–168. doi: 10.1182/blood.v97.1.162. [DOI] [PubMed] [Google Scholar]
- 11.Kroll MH, Hellums JD, McIntire LV, Schafer AI, Moake JL. Platelets and shear stress. Blood. 1996;88(5):1525–1541. [PubMed] [Google Scholar]
- 12.Dong JF. Cleavage of ultra-large von Willebrand factor by ADAMTS-13 under flow conditions. J Thromb Haemost. 2005;3(8):1710–1716. doi: 10.1111/j.1538-7836.2005.01360.x. [DOI] [PubMed] [Google Scholar]
- 13.Furlan M, Robles R, Lamie B. Partial purification and characterization of a protease from human plasma cleaving von Willebrand factor to fragments produced by in vivo proteolysis. Blood. 1996;87(10):4223–4234. [PubMed] [Google Scholar]
- 14.Whitelock JL, Nolasco L, Bernardo A, Moake J, Dong JF, Cruz MA. ADAMTS-13 activity in plasma is rapidly measured by a new ELISA method that uses recombinant VWF-A2 domain as substrate. J Thromb Haemost. 2004;2(3):485–491. doi: 10.1111/j.1538-7836.2004.00601.x. [DOI] [PubMed] [Google Scholar]
- 15.Cruz MA, Whitelock J, Dong JF. Evaluation of ADAMTS-13 activity in plasma using recombinant von Willebrand factor A2 domain polypeptide as substrate. Thromb Haemost. 2003;90(6):1204–1209. doi: 10.1160/TH03-06-0398. [DOI] [PubMed] [Google Scholar]
- 16.Kokame K, Nobe Y, Kokubo Y, Okayama A, Miyata T. FRETS-VWF73, a first fluorogenic substrate for ADAMTS13 assay. Br J Haematol. 2005;129(1):93–100. doi: 10.1111/j.1365-2141.2005.05420.x. [DOI] [PubMed] [Google Scholar]
- 17.Wu JJ, Fujikawa K, McMullen BA, Chung DW. Characterization of a core binding site for ADAMTS-13 in the A2 domain of von Willebrand factor. Proc Natl Acad Sci U S A. 2006;103(49):18470–18474. doi: 10.1073/pnas.0609190103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Siedlecki CA, Lestini BJ, Kottke-Marchant KK, Eppell SJ, Wilson DL, Marchant RE. Shear-dependent changes in the three-dimensional structure of human von Willebrand factor. Blood. 1996;88(8):2939–2950. [PubMed] [Google Scholar]
- 19.Yago T, Lou J, Wu T, et al. Platelet glycoprotein Ibα forms catch bonds with human WT vWF but not with type 2B von Willebrand disease vWF. J Clin Invest. 2008;118:3195–3207. doi: 10.1172/JCI35754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang X, Halvorsen K, Zhang CZ, Wong WP, Springer TA. Mechanoenzymatic cleavage of the ultralarge vascular protein von Willebrand factor. Science. 2009;324(5932):1330–1334. doi: 10.1126/science.1170905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Romo GM, Dong JF, Schade AJ, et al. The glycoprotein Ib-IX-V complex is a platelet counterreceptor for P-selectin. J Exp Med. 1999;190(6):803–814. doi: 10.1084/jem.190.6.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Auton M, Cruz AA, Moake JL. Conformational stability and domain unfolding of the von Willebrand factor A domains. J Mol Biol. 2006;366:986–1000. doi: 10.1016/j.jmb.2006.10.067. [DOI] [PubMed] [Google Scholar]
- 23.Tao Z, Peng Y, Nolasco L, et al. Recombinant CUB-1 domain polypeptide inhibits the cleavage of ULVWF strings by ADAMTS13 under flow conditions. Blood. 2005;106(13):4139–4145. doi: 10.1182/blood-2005-05-2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Michaelis L, Menten ML. The kinetics of the inversion effect. Biochem Z. 1913;49:333–369. [Google Scholar]
- 25.Rief M, Gautel M, Oesterhelt F, Fernandez JM, Gaub HE. Reversible unfolding of individual titin immunoglobulin domains by AFM. Science. 1997;276(5315):1109–1112. doi: 10.1126/science.276.5315.1109. [DOI] [PubMed] [Google Scholar]
- 26.Martin C, Morales LD, Cruz MA. Purified A2 domain of von Willebrand factor binds to the active conformation of von Willebrand factor and blocks the interaction with platelet glycoprotein Ibα. J Thromb Haemost. 2007;5(7):1363–1370. doi: 10.1111/j.1538-7836.2007.02536.x. [DOI] [PubMed] [Google Scholar]
- 27.Chen W, Lou J, Zhu C. Molecular dynamics simulated unfolding of von Willebrand factor A domains by force. Cell Mol Bioeng. 2009;2(1):75–86. [Google Scholar]
- 28.Chesla SE, Selvaraj P, Zhu C. Measuring two-dimensional receptor-ligand binding kinetics by micropipette. Biophys J. 1998;75(3):1553–1572. doi: 10.1016/S0006-3495(98)74074-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marshall BT, Long M, Piper JW, Yago T, McEver RP, Zhu C. Direct observation of catch bonds involving cell-adhesion molecules. Nature. 2003;423(6936):190–193. doi: 10.1038/nature01605. [DOI] [PubMed] [Google Scholar]
- 30.Sarangapani KK, Yago T, Klopocki AG, et al. Low force decelerates L-selectin dissociation from P-selectin glycoprotein ligand-1 and endoglycan. J Biol Chem. 2004;279(3):2291–2298. doi: 10.1074/jbc.M310396200. [DOI] [PubMed] [Google Scholar]
- 31.Kong F, Garcia AJ, Mould AP, Humphries MJ, Zhu C. Demonstration of catch bonds between an integrin and its ligand. J Cell Biol. 2009;185(7):1275–1284. doi: 10.1083/jcb.200810002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Long M, Zhao H, Huang KS, Zhu C. Kinetic measurements of cell surface E-selectin/carbohydrate ligand interactions. Ann Biomed Eng. 2001;29(11):935–946. doi: 10.1114/1.1415529. [DOI] [PubMed] [Google Scholar]
- 33.Zhu C, Long M, Bongrand P. Measuring receptor/ligand interaction at the single bond level: experimental and interpretive issues. Ann Biomed Eng. 2002;30:305–314. doi: 10.1114/1.1467923. [DOI] [PubMed] [Google Scholar]
- 34.Guo B, Guilford WH. Mechanics of actomyosin bonds in different nucleotide states are tuned to muscle contraction. Proc Natl Acad Sci U S A. 2006;103(26):9844–9849. doi: 10.1073/pnas.0601255103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yakovenko O, Sharma S, Forero M, et al. FimH forms catch bonds that are enhanced by mechanical force due to allosteric regulation. J Biol Chem. 2008;283(17):11596–11605. doi: 10.1074/jbc.M707815200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bell GI. Models for the specific adhesion of cells to cells. Science. 1978;200(4342):618–627. doi: 10.1126/science.347575. [DOI] [PubMed] [Google Scholar]
- 37.Majerus EM, Anderson PJ, Sadler JE. Binding of ADAMTS13 to von Willebrand factor. J Biol Chem. 2005;280(23):21773–21778. doi: 10.1074/jbc.M502529200. [DOI] [PubMed] [Google Scholar]
- 38.Gao W, Anderson PJ, Majerus EM, Tuley EA, Sadler JE. Exosite interactions contribute to tension-induced cleavage of von Willebrand factor by the antithrombotic ADAMTS13 metalloprotease. Proc Natl Acad Sci U S A. 2006;103(50):19099–19104. doi: 10.1073/pnas.0607264104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wiita AP, Perez-Jimenez R, Walther KA, et al. Probing the chemistry of thioredoxin catalysis with force. Nature. 2007;450(7166):124–127. doi: 10.1038/nature06231. [DOI] [PMC free article] [PubMed] [Google Scholar]