Abstract
The reasons for hepatitis C treatment failure remain unknown but may be related to different host responses to therapy. In this study, we compared hepatic gene expression in patients prior to and during peginterferon and ribavirin therapy. In the on-treatment group, patients received either ribavirin for 72 hours prior to peginterferon alpha-2a injection or peginterferon alpha-2a for 24 hours, prior to biopsy. The patients were grouped into rapid responders (RRs) with a greater than 2-log drop and slow responders (SRs) with a less than 2-log drop in hepatitis C virus RNA by week 4. Pretreatment biopsy specimens were obtained from a matched control group. The pretreatment patients were grouped as RRs or SRs on the basis of the subsequent treatment response. Gene expression profiling was performed with Affymetrix microarray technology. Known interferon-stimulated genes (ISGs) were induced in treated patients. In the pretreatment group, future SRs had higher pretreatment ISG expression than RRs. On treatment, RRs and SRs had similar absolute ISG expression, but when it was corrected for the baseline expression with the pretreatment group, RRs showed a greater fold change in ISGs, whereas SRs showed a greater change in interferon (IFN)-inhibitory pathways. The patients pretreated with ribavirin had heightened induction of IFN-related genes and down-regulation of genes involved in IFN inhibition and hepatic stellate cell activation.
Conclusion
These data suggest that ISG inducibility is important for the treatment response and that ribavirin may improve outcomes by enhancing hepatic gene responses to peginterferon. Collectively, these mechanisms may provide a molecular basis for the improved efficacy of combination therapy.
Despite great advances in the treatment of chronic hepatitis C infection, the current therapy with peginterferon and ribavirin is effective in only about 50% of patients.1,2 The reasons for treatment failure are not well understood but likely are related to both viral and host factors.3 The viral genotype has the greatest impact on the treatment outcome. A sustained virological response (SVR) is achieved in 42%-46% of genotype 1 infections after a year of therapy, in contrast to rates of 80% in genotype 2 and 3 infections after just 6 months of treatment.1,2 In addition to the genotype, the baseline viral load is also an important factor, particularly in genotype 1 infection.2 Although numerous viral strategies for interfering with host viral defense mechanisms have been identified, none has been clearly shown to be responsible for the genotypic differences in the treatment response.
From large treatment trials, a number of host factors have been found to be associated with the treatment response. Gender and race are the most important factors. Men consistently respond less well to therapy than women, and African Americans have poorer outcomes than Caucasian populations.4 Age, obesity, and the degree of liver fibrosis also affect the treatment outcome.5 Although these factors have been consistently identified in multiple studies, the mechanism by which they affect the treatment outcome remains unknown.
To gain a further understanding of the host factors, microarray technology has been used to evaluate hepatic gene expression prior to antiviral therapy. Chen et al.6 found that pretreatment gene expression profiles from liver biopsies were predictive of the ultimate treatment outcome. Patients who did not respond to therapy showed up-regulation of numerous interferon-stimulated genes (ISGs) prior to treatment in comparison with both sustained responders and normal controls. Hepatic gene expression has not been reported in humans undergoing therapy. Several studies have reported gene expression in peripheral blood mononuclear cells (PBMCs) during the course of therapy; however, gene induction in PBMCs may not be entirely reflective of events in the liver.7–9
The addition of ribavirin to interferon (IFN) therapy significantly improves the treatment response rates; however, the mechanism by which this occurs is poorly understood. Numerous mechanisms of action for ribavirin have been proposed, including inosine-5-monophosphate dehydrogenase inhibition (IMPDH), direct viral inhibition, increased mutagenesis leading to error catastrophe, and promotion of a Th1 immune response.10 Although there is some experimental evidence to support all of these mechanisms, none accounts for the magnitude of the benefit seen with the addition of ribavirin.
In order to gain further understanding of the genetic factors that may contribute to the treatment response, we evaluated gene expression from liver biopsy samples from patients currently undergoing treatment with peginterferon. Half the patients also received ribavirin prior to liver biopsy, and this allowed us to assess the contribution of this agent to gene expression. Expression profiles from on-treatment patients were compared with those from pretreatment liver biopsy samples of a matched control population.
Materials and Methods
Study Subjects
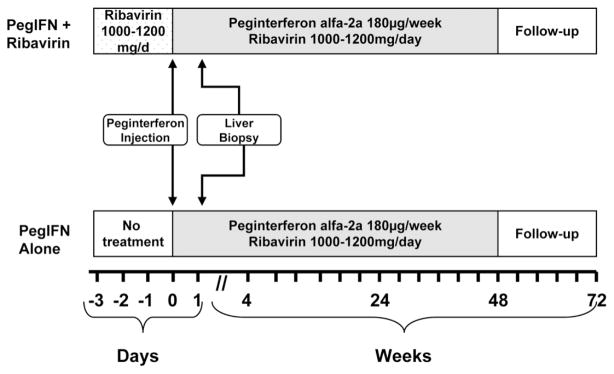
Adult patients evaluated at the University of North Carolina Liver Clinic and infected with hepatitis C virus (HCV) genotype 1 were eligible for enrollment. Patients with a hepatitis B or human immunodeficiency virus coinfection or a major systemic illness were excluded. All patients received 180 μg of peginterferon alpha-2a subcutaneously 24 hours prior to liver biopsy. The patients were randomized to receive peginterferon alone or to receive ribavirin prior to biopsy as well. Those randomized to receive ribavirin were given weight-based ribavirin (1000 mg/day for patients weighing less than 75 kg and 1200 mg/day for patients weighing more than 75 kg) for 72 hours prior to liver biopsy. A sample of biopsy tissue was snap-frozen in liquid nitrogen and stored at −80°C. The remainder of the biopsy was placed in formalin for a histological evaluation. After the liver biopsy, the patients continued on 180 μg of peginterferon per week plus weight-based ribavirin for 48 weeks (Fig. 1). All patients agreed to undergo a liver biopsy after starting antiviral therapy and signed an informed consent form. This investigator-initiated study was approved by the Institutional Review Board at the University of North Carolina Medical Center.
Fig. 1.
Study design. The patients in the on-treatment group were given either 180 μg of peginterferon-alpha 2a subcutaneously 24 hours prior to liver biopsy or 1000/1200 mg of ribavirin daily for 72 hours plus 180 μg of peginterferon-alpha 2a subcutaneously 24 hours prior to liver biopsy. After the biopsy, the patients were continued on the therapy for 48 weeks.
The HCV viral load was measured with Roche Taq-Man (Roche Molecular Systems, Alamaeda, CA) prior to therapy and serially during therapy. Those patients achieving at least a 2-log drop in HCV RNA by 4 weeks of therapy were deemed rapid responders (RRs). Patients with less than a 2-log drop in HCV RNA at 4 weeks were deemed slow responders (SRs). Standard definitions for relapse (HCV RNA–negative at 48 weeks with subsequent recurrent viremia), nonresponse [persistently HCV RNA–positive by polymerase chain reaction (PCR) throughout therapy], and SVR (HCV RNA–negative 6 months after the completion of therapy) were used to define the ultimate treatment outcome.
Stored liver tissue from pretreatment liver biopsies of patients with genotype 1 HCV infection performed at the Clinical Center of the National Institutes of Health were used as pretreatment controls. The same exclusion criteria were used for the pretreatment population, and patients were selected to match on-treatment patients for gender, race, age, ultimate treatment outcome, HCV genotype, baseline HCV viral load, and liver biopsy histological scores. Biopsy samples were snap-frozen in liquid nitrogen at the time of biopsy and stored at −80°C. All but 4 of the pretreatment patients were subsequently treated, and the on-treatment viral kinetics and treatment response were known. The patients were categorized as RRs or SRs with the same definitions used for the on-treatment group (rapid response ≥ 2-log drop in HCV RNA within the first 4 weeks of therapy, slow response < 2-log drop by 4 weeks).
RNA Extraction, Amplification, and Microarray Analysis
Hepatic tissue was placed in Trizol and mechanically ground with a piston until it was dissolved. RNA was then extracted with the RNeasy kit from Qiagen (Valencia, CA) according to the manufacturer’s instructions. RNA was quantified with a spectrophotometer, and the RNA quality was analyzed with an Agilent (Foster City, CA) bioanalyzer according to the manufacturer’s instructions. RNA was then amplified with an Agilent Enzo kit. Amplified complementary RNA was hybridized to an Affymetrix Human 133 Plus 2.0 microarray chip containing 54,675 gene transcripts. The chips were scanned, and the signal intensity was evaluated as previously described.11
Quantitative Real-Time PCR
A total of 14 genes were selected for real-time PCR confirmation and consisted of genes from major pathways identified by the microarray analysis. The selected genes were divided into 4 categories: (1) IFN-related genes [IFN-alpha receptor 2, interferon regulatory factor 7 (IRF7), ISG-15, signal transducer and activator of transcription 1 (STAT1), oligoadenylate synthetase 3 (OAS3), and myxovirus resistance 1 (Mx1)], (2) IFN-inhibitory genes [protein inhibitor of activated signal transducer and activator of transcription 4 (PIAS4) and protein phosphatase 2a catalytic subunit (PP2Ac)], (3) apoptosis-related genes (Fas and cytochrome C), and (4) genes related to hepatic stellate cell (HSC) activation [collagen type 1 alpha 2, CD36 (collagen type 1 receptor), and tissue inhibitor of metallopeptidase 2 (TIMP2)]. Primers and probes were obtained from Applied Biosystems (Foster City, CA). Real-time PCR was performed with TaqMan technology as described.12 All values were normalized for the glyceraldehyde 3-phosphate dehydrogenase expression level. All samples were repeated in duplicate, and mean expression values were used.
TaqMan confirmation was performed for all patient samples with adequate remaining RNA after the microarray analysis. In addition, a group of patient samples with inadequate RNA for the microarray analysis were included for real-time quantitative PCR. Samples analyzed only by PCR were divided by the treatment group and response similarly to those used for the microarray analysis. The gene expression patterns identified for confirmation by TaqMan were based on the initial cohort for which there was sufficient RNA to perform the microarray analysis. Because the latter group, for which only TaqMan was performed, was not included in the original microarray analysis, it served as a validation cohort.
Statistical Analysis
The signal intensity from gene transcripts was compared between groups with Partek (St. Louis, MO) software and Affymetrix (United States) MAS5 normalized log-signal comparisons. Before the groups were compared, the signal-to-noise ratio was evaluated by a source of variance analysis. This evaluates whether intergroup variation is greater than intragroup variation across all comparisons. Further analysis was considered only for comparisons in which between-group variation was significantly greater than within-group variation. For each comparison, models were developed to evaluate the effects of race and sex as confounding variables. The final selected model maximized the intergroup-to-intragroup differences or signal-to-noise ratio by minimizing the error of the model. For example, when the treatment response in the pretreatment group was compared, gender, race, and the interaction terms of gender, race, and response were evaluated in the model. The inclusion of gender and the interaction of gender and treatment response improved the model, giving an increased signal-to-noise ratio. The addition of race had no effect on the model and thus was not included in the final analysis. Similar model development was performed for all comparisons. After the best model was selected, groups were compared with an analysis of variance. Gene expression was compared between the treatment groups and by the treatment outcome. Only genes for which a signal was detected in at least 50% of the samples were included. Expression differences of at least 1.5-fold with P < 0.01 were considered significant. Because of the significant risk of false positives with this type of analysis for individual gene expression, known pathways were also compared with software from GeneGo, Inc. (Michigan). This evaluates the gene expression of all genes in an established signal transduction or other molecular pathway. The correction of an analysis of variance with Bonferroni or other corrections for multiple comparisons generally assumes the complete independence of each comparison. However, for a microarray analysis, this assumption is unlikely to be correct. If, for example, 7 genes in a particular pathway are up-regulated or down-regulated, this is much more likely to be a valid finding than if 7 random genes with unrelated functions are found to be differentially regulated. Although all genes found by fold-change and P-value cutoffs are listed, inferences were restricted to genes identified by a pathway analysis to be differentially regulated. Once gene lists for each comparison were established, supervised hierarchical clustering and heat maps were created with Partek software.
Results
Sufficient RNA with adequate quality for microarray analysis was available from 11 patients pretreated with peginterferon with or without ribavirin (on-treatment group) and from 19 patients with biopsies prior to therapy (pretreatment group; Table 1). The groups were matched by gender, race, and age, and all patients had a genotype 1 infection. The groups were also well matched for the initial HCV viral load and histological grade and stage (Table 1). Six patients in the on-treatment group achieved a rapid response (>2-log drop of HCV RNA by 4 weeks), whereas 5 had a slow response. Six treated patients ultimately achieved an SVR (5 with a rapid response and 1 with a slow response), 3 relapsed (all with a slow response), and 2 were lost to follow-up (1 with a rapid response and 1 with a slow response). In the pretreatment group, 5 patients had a rapid response, 10 had a slow response, and 4 were treatment-naive; 5 patients had an SVR (all with a rapid response), and 10 were nonresponders (all with a slow response). Six patients in the on-treatment group were treated with ribavirin in addition to peginterferon prior to liver biopsy, 4 of whom went on to achieve SVR. The additional patients used for real-time PCR for whom inadequate RNA was available for microarray analysis are described in Table 1.
Table 1.
Baseline Characteristics of the On-Treatment and Pretreatment Groups Used for Microarrays and Quantitative Real-Time PCR
| Microarray Only |
Microarray and Real-Time PCR |
|||
|---|---|---|---|---|
| On-Treatment Group (n = 11) | Pretreatment Group (n = 19) | On-Treatment Group (n = 21) | Pretreatment Group (n = 31) | |
| Gender (male/female) | 5/6 | 10/9 | 13/8 | 19/12 |
| Age | 45.7 ± 6.9 | 51.7 ± 4.5 | 44.9 ± 5.3 | 51.6 ± 6.0 |
| Ethnicity | ||||
| African American | 6 | 8 | 9 | 14 |
| Caucasian | 5 | 11 | 12 | 17 |
| Treatment response | ||||
| RR | 6 | 5 | 10 | 10 |
| SR | 5 | 10 | 11 | 14 |
| Naive | NA | 4 | NA | 7 |
| Baseline HCV RNA | ||||
| >6 log copies/mL | 6 | 6 | 13 | 16 |
| <6 log copies/mL | 5 | 13 | 8 | 15 |
| Biopsy stage* | ||||
| 0 | 3 | 3 | 7 | 4 |
| 1 | 5 | 10 | 10 | 18 |
| 2 | 3 | 1 | 3 | 4 |
| 3 | 0 | 5 | 1 | 5 |
| 4 | 0 | 0 | 0 | 0 |
| Biopsy grade* | ||||
| 0 | 0 | 0 | 0 | 0 |
| 1 | 3 | 5 | 9 | 10 |
| 2 | 8 | 9 | 12 | 15 |
| 3 | 0 | 5 | 0 | 6 |
| 4 | 0 | 0 | 0 | 0 |
The biopsy stages and grades follow the Batts and Ludwig scoring system. For RRs, there was a ≥2 log copies/mL decline in HCV RNA by 4 weeks of treatment. For SRs, there was a <2 log copies/mL decline in HCV RNA by 4 weeks of treatment. Abbreviations: HCV, hepatitis C virus; NA, not applicable; PCR, polymerase chain reaction; RR, rapid responder; SR, slow responder.
On-Treatment versus Pretreatment
Race and gender were found to contribute to the model for this comparison. After we controlled for the effects of gender and race, 6017 genes were differentially regulated between the on-treatment and pretreatment groups. Of genes that had detectable microarray signals in greater than 50% of the samples, a total of 364 genes differed by greater than 1.5-fold expression with a P value of less than 0.01. A summary of differing gene expression is shown in Table 2 and highlighted in a heat map in Fig. 2. A full list of genes is available in the supplementary material (Supplement to Table 2). Known ISGs were induced in the on-treatment group in comparison with the pretreatment group. Classical ISGs such as OAS3, Mx1, and ISG15 and IRFs were up-regulated. Other genes with known antiviral activities, including viperin, adenosine deaminase RNA-specific, phospholipid scramblase, and apolipoprotein B messenger RNA editing enzyme catalytic polypeptide-3A, were also induced in treated patients.13 ISGs provide the effector antiviral functions of the IFN response. Genes involved in IFN production, including IRF7 and retinoic acid–inducible gene I (RIG-I), were induced in the treated patients.
Table 2.
Selected Genes Induced by Peginterferon Treatment with a Fold Change Greater Than or Equal to 1.5 (P ≤ 0.01)
| Gene Category | Gene Name | Gene Symbol | Fold Change (On-Treatment/Pretreatment) | P |
|---|---|---|---|---|
| ISG | 2′,5′-Oligoadenylate synthetase 3 | OAS3 | 2.2 | 0.0001 |
| Adenosine deaminase, RNA-specific | ADAR | 1.5 | 2.6 × 10−7 | |
| Guanylate binding protein 1 | GBP1 | 2.3 | 9.2 × 10−6 | |
| IFN transmembrane protein 1 (9–27) | IFITM1 | 1.7 | 0.0009 | |
| IFN-alpha–inducible (ISG 15) | ISG15 | 1.8 | 0.001 | |
| IFN-induced protein 35 | IFI35 | 1.7 | 0.0001 | |
| IFN-induced protein 44 | IFI44 | 2.4 | 3.8 × 10−5 | |
| IFN-induced protein with TTPR 3 | IFIT3 | 1.7 | 0.004 | |
| IFN-induced protein with TTPR 5 | IFIT5 | 1.5 | 3.1 × 10−5 | |
| Myxovirus resistance 1 | Mx1 | 2.3 | 9.2 × 10−6 | |
| Myxovirus resistance 2 | Mx2 | 2.5 | 5.9 × 10−7 | |
| Phospholipid scramblase 1 | PLSCR1 | 3.0 | 4.6 × 10−8 | |
| Ubiquitin specific protease 18 | USP18 | 1.8 | 0.0009 | |
| Viperin | RSAD2 | 2.3 | 5.3 × 10−5 | |
| Apolipoprotein B messenger RNA editing enzyme catalytic polypeptide-3A | APOBEC3A | 3.8 | 6.9 × 10−8 | |
| IFN-related | IFN-alpha/beta receptor 2 | IFNAR2 | 1.7 | 0.0001 |
| IFN regulatory factor 7 | IRF7 | 2.5 | 6.0 × 10−5 | |
| RIG-I DEAD box polypeptide 58 | DDX58 | 1.6 | 0.0018 | |
| Signal transducer/activator transcription | STAT1 | 2.3 | 1.2 × 10−6 | |
| Immune | Beta-2-microglobulin | B2M | 2.3 | 3.2 × 10−5 |
| Chemokine ligand 8 | CCL8 | 4.6 | 1.16 × 10−8 | |
| Chemokine ligand 19 | CCL19 | 2.6 | 0.005 | |
| C-reactive protein | CRP | 4.0 | 0.0032 | |
| Interleukin 18 binding protein | IL18BP | 1.8 | 2.6 × 10−5 | |
| Interleukin 6 signal transducer | IL6ST | 2.3 | 7.5 × 10−6 | |
| Major histocompatibility complex F | HLA-F | 1.5 | 0.0002 | |
| N-myc and STAT interactor | NMI | 1.7 | 2.8 × 10−6 | |
| Nuclear factor of activated T cells | NFAT5 | 1.6 | 0.0002 | |
| Serum amyloid A1 | SAA1 | 10.5 | 7.0 × 10−5 |
Abbreviations: IFN, interferon; ISG, interferon-stimulated gene.
Fig. 2.
Heat map showing distinct gene expression patterns in pretreatment and on-treatment groups. The map is based on the 364 genes with a greater than 1.5-fold difference in the expression with P <0.01. Red indicates increased gene expression, and blue indicates decreased gene expression. The white line across and down the heat map provides a statistically significant separation of the 2 groups and up-regulated or down-regulated genes. Within the 2 groups, the treatment response is indicated as follows: N, naive treatment; RR, rapid responder; and SR, slow responder.
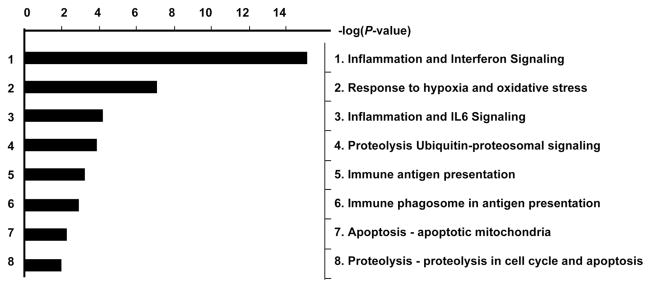
In addition to known ISGs, genes involved in the immune response, including interleukins, chemokines, major histocompatibility complex class I, and nonspecific factors such as beta-2-microglobulin and C-reactive protein, were also induced by peginterferon treatment. A pathway analysis revealed that genes involved in antigen presentation, oxidative stress, and apoptosis were also significantly up-regulated in on-treatment patients (Fig. 3). Although a number of genes were also down-regulated by the treatment, aside from genes involved in cellular proliferation such as the ras-oncogene family and tetraspanins, no clear pattern was apparent.
Fig. 3.
Gene pathways with significant differential gene expression between pretreatment and on-treatment patients. For each known pathway, the number of genes with differing expression between the groups was calculated, and a P value was determined on the basis of the likelihood of finding the given number of genes by chance alone. The inverse logarithm of the P value is shown on the x axis. Pathways with a cutoff of 2 (equivalent to a P value of 0.01) are shown. In addition to interferon signaling, pathways involved in immune responses and apoptosis were most affected by the interferon treatment.
Treatment Response
To evaluate whether pretreatment biopsies are of use for predicting treatment response, the future SRs (same as nonresponders) and RRs (same as sustained responders) in the pretreatment group were compared. The best model for this comparison included gender but not race. After we controlled for the effect of gender, 2765 genes were differentially regulated, and 220 were detectable in greater than 50% of the samples and differed by 1.5-fold or more with a P value of 0.01 or less. As previously reported,6 nonresponders had significantly higher expression of numerous ISGs than those who achieved an SVR when treated (Table 3 and Supplement to Table 3).
Table 3.
Selected Genes Differing Between Future RRs and SRs with a Fold Change Greater Than or Equal to 1.5 (P ≤ 0.01) in the Pretreatment Group
| Gene Category | Gene Name | Gene Symbol | Fold Change (RR/SR) | P |
|---|---|---|---|---|
| ISG | 2′,5′-Oligoadenylate synthetase 2*† | OAS2 | −2.4 | 0.02 |
| 2′,5′-Oligoadenylate synthetase 3*† | OAS3 | −2.4 | 0.01 | |
| IFN-induced transmembrane protein 1 | IFITM1 | −2.3 | 0.001 | |
| IFN-induced transmembrane protein 3 | IFITM3 | −1.5 | 0.004 | |
| IFN-alpha–inducible protein (ISG-15)*† | G1P2/ISG15 | −3.2 | 0.028 | |
| IFN-alpha–inducible protein (IFI-6–16)† | G1P3/IFI6 | −3.0 | 0.0016 | |
| IFN-alpha–inducible protein 27 | IFI27 | −3.7 | 0.004 | |
| IFN-induced protein 35 | IFI35 | −2.3 | 0.0048 | |
| IFN-induced protein 44 | IFI44 | −2.7 | 0.001 | |
| IFN-induced protein with TTPR 1† | IFIT1 | −2.8 | 0.01 | |
| IFN-induced protein with TTPR 3 | IFIT3 | −2.6 | 0.0075 | |
| Myxovirus resistance 1† | MX1 | −3.9 | 0.0004 | |
| Phospholipid scramblase 1 | PLSCR1 | −2.0 | 0.003 | |
| Ubiquitin-specific peptidase 18*† | USP18 | −1.7 | 0.042 | |
| Viperin† | RSAD2 | −3.3 | 0.0008 | |
| IFN-Related | RIG-I DEAD box polypeptide 58 | DDX58 | −2.0 | 0.01 |
| Signal transducer activator of transcription 1 | STAT1 | −2.0 | 0.0048 | |
| Other | Activating transcription factor 7 interacting protein 2† | ATF7IP2 | 1.7 | 0.0083 |
| Chemokine ligand 9 (MIG) | CXCL9 | 2.9 | 0.0062 | |
| Leucine aminopeptidase 3*† | LAP3 | −1.5 | 0.030 | |
| Ribosomal protein S28† | RPS28 | 1.98 | 0.0077 | |
| Syntaxin binding protein 5-like† | STXBP5L | 6.4 | 0.0003 |
P between 0.05 and 0.01.
Gene identified by Chen et al.6 as differing between future sustained responders and future nonresponders. Abbreviations: IFN, interferon; ISG, interferon-stimulated gene.
Gene expression patterns in patients who received treatment prior to liver biopsy were compared on the basis of their treatment response. Patients who achieved a 2-log or greater drop in HCV RNA by 4 weeks of therapy were deemed RRs and were compared to SRs. Early virological responses were compared rather than ultimate treatment outcomes to avoid issues of compliance and treatment tolerance. In addition, we reasoned that the early responses might be more reflective of altered gene expression by IFN than the ultimate treatment outcome. There was a good correlation between the early response and late response, with 5 of 6 RRs achieving an SVR. The other RR was lost to follow-up.
Similarly to the pretreatment samples, the best model for this comparison included gender but not race. After we controlled for the effect of gender, 2884 genes were differentially regulated between the RRs and SRs, and 179 genes were detectable in greater than 50% of the samples and differed by 1.5-fold or more with a P value of less than 0.01. A summary of the differentially regulated genes is shown in Table 4, and a full list is available in the supplementary material (Supplement to Table 4).
Table 4.
Direct Comparison of Selected Genes Differing Between RRs and SRs with a Fold Change Greater Than or Equal to 1.5 (P ≤ 0.01) in the On-Treatment Group
| Gene Category | Gene Name | Gene Symbol | Fold Change (RR/SR) | P |
|---|---|---|---|---|
| IFN-inhibitory | Protein phosphatase regulatory subunit (formerly 2A) | PPP2R3A | −8.4 | 0.0004 |
| Protein phosphatase 3 catalytic subunit (formerly 2B) | PPP3CB | −2.4 | 0.0005 | |
| Ubiquitin-specific peptidase 13 | USP13 | −2.7 | 3.0 × 10−5 | |
| Other | Insulin-like growth factor 1 | IGF1 | 2.7 | 0.0025 |
Abbreviations: IFN, interferon; RR, rapid responder; SR, slow responder.
No significant differences in the expression of known ISGs were noted between the groups. Genes involved in IFN-inhibitory pathways were up-regulated in SRs. PIAS1 interacts with STAT1 and prevents binding to the interferon-sensitive response element (ISRE), resulting in reduced ISG production.14 The ability of PIAS1 to interact with STAT1 is regulated by the methylation status of STAT1. PP2A has been reported to interfere with the methylation of STAT1, thereby increasing PIAS-STAT1 interactions and reducing STAT1-ISRE binding. PP2A was elevated slowly in comparison with RRs. In addition, ubiquitin-specific peptidase 13 (USP13) was 2.7-fold more highly expressed in SRs. USPs cleave conjugated ubiquitin or ubiquitin-like molecules from proteins, thus preventing them from being targeted for degradation in the proteosome. USP18 has specifically been shown to cleave conjugated ISG15 from target proteins. The silencing of USP18 improves IFN responsiveness in vitro, and USP18 was found to be up-regulated in pretreatment liver biopsies from future nonresponders by Chen et al.6 and in our cohort.15 Whether other USPs have similar IFN inhibitory activity is currently unknown.
Although the absolute gene expression level is important, the magnitude of gene induction from the baseline may also be relevant. Because patients could not be biopsied before and during the treatment, true treatment-related gene induction could not be evaluated. However, through the use of the pretreatment patients as the baseline for the on-treatment patients, a surrogate for gene induction was assessed. Thus, RRs in the on-treatment group were compared to RRs in the pretreatment group, and similarly, SRs were compared in each group. The fold change was calculated by the division of the mean expression value of the on-treatment group by the mean expression value of the pretreatment group because even after matching, a direct comparison would be inappropriate because of interindividual variation in gene expression. Because the pretreatment group and on-treatment group comprised different patients, this comparison is not a true measure of gene induction but rather is a surrogate measure of hepatic gene expressions in response to treatment. Using this comparison, after controlling for gender, we found that RRs had 873 genes differentially regulated by 1.5-fold or more with a P value of less than 0.01 between on-treatment and pretreatment groups, whereas SRs had 438 genes that differed by the treatment group (Table 5 and Supplement to Table 5).
Table 5.
Comparison of a Surrogate for the Fold Induction of Selected Genes Differing Between RRs and SRs with a Fold Change Greater Than or Equal to 1.5 (P ≤ 0.01) in the On-Treatment Group Versus the Pretreatment Group (On-Treatment RRs/Pretreatment RRs versus On-Treatment SRs/Pretreatment SRs)
| RRs (On-Treatment/Pretreatment) |
SRs (On-Treatment/Pretreatment) |
|||||
|---|---|---|---|---|---|---|
| Gene Category | Gene Name | Gene Symbol | Fold Change | P | Fold Change | P |
| ISG | 2′,5′-Oligoadenylate synthetase 1 | OAS1 | 6.7 | 0.0004 | 1.0 | NS |
| 2′,5′-Oligoadenylate synthetase 2 | OAS2 | 16.5 | 2.9 × 10−5 | 1.2 | NS | |
| 2′,5′-Oligoadenylate synthetase 3 | OAS3 | 8.8 | 0.0005 | 1.2 | NS | |
| 28-kD IFN-responsive protein | IFRG28 | 3.5 | 0.0003 | NC | NS | |
| Adenosine deaminase, RNA-specific | ADAR | 1.8 | 1.5 × 10−6 | NC | NS | |
| Guanylate binding protein 1 | GBP1 | 2.5 | 0.0003 | NC | NS | |
| IFN-induced transmembrane protein 1 (9–27) | IFITM1 | 5.4 | 0.0063 | 1.1 | NS | |
| IFN-induced transmembrane protein 2 | IFITM2 | 1.7 | 0.0004 | NC | NS | |
| IFN-induced with helicase C domain | IFIH1 | 3.4 | 1.3 × 10−5 | NC | NS | |
| IFN-stimulated exonuclease gene 20Kda | ISG20 | 4.6 | 0.0014 | NC | NS | |
| IFN-induced protein 35 | IFI35 | 4.6 | 0.0002 | 1.1 | NS | |
| IFN-induced 44-like | IFI44L | 57.9 | 0.0066 | 1.3 | NS | |
| IFN-induced protein with TTPR 1 | IFIT1 | 6.0 | 0.0003 | NC | NS | |
| IFN-induced protein with TTPR 2 | IFIT2 | 5.9 | 0.0022 | NC | NS | |
| IFN-induced protein with TTPR 3 | IFIT3 | 5.8 | 0.0005 | 1.1 | NS | |
| IFN-induced protein with TTPR 5 | IFIT5 | 3.0 | 0.0001 | 1.1 | NS | |
| IFN-stimulated gene 15 | ISG15 | 13.6 | 0.005 | 1.6 | 0.014* | |
| Myxovirus resistance 1 | Mx1 | 17.7 | 0.006 | 1.1 | NS | |
| Myxovirus resistance 2 | Mx2 | 5.7 | 2.7 × 10−5 | 1.6 | NS | |
| Viperin | RSAD2 | 12.1 | 0.0007 | 1.9 | 1.1 × 10−5 | |
| IFN-related | IFN-alpha 4 | IFNA4 | 3.4 | 0.0070 | NC | NS |
| IFN receptor | IFNAR2 | NC | NS | 2.4 | 0.0011 | |
| IFN regulatory factor 7 | IRF7 | 3.3 | 0.0040 | 1.2 | NS | |
| IFN-stimulated transcription factor | ISGF3G | 2.0 | 1.5 × 10−6 | NC | NS | |
| RIG-I DEAD box polypeptide 58 | DDX58 | 2.7 | 5.8 × 10−7 | NC | NS | |
| Signal transducer activator of transcription 1 | STAT1 | 5.2 | 0.0001 | 1.8 | 0.003 | |
| IFN-inhibitory | Protein inhibitor of activated STAT 2 | PIAS2 | NC | NS | 1.1* | 0.00001 |
| Protein inhibitor of activated STAT 4 | PIAS4 | NC | NS | 1.2* | 5.4 × 10−5 | |
| Protein phosphatase 2 regulatory subunit B | PPP2R2C | NC | NS | 3.5 | 0.0002 | |
| gamma (formerly 2A) | ||||||
| Protein phosphatase 2 regulatory subunit B | PPP2R3A | NC | NS | 6.6 | 0.0001 | |
| alpha (formerly 2A) | ||||||
| Small ubiquitin-like molecule 1 | SUMO1 | −1.6 | 0.006 | NC | NS | |
| Ubiquitin-specific peptidase 13 | USP13 | NC | NS | 2.3 | 0.0002 | |
| Ubiquitin-specific peptidase 18 | USP18 | 5.33 | 2.5 × 10−6 | NC | NS | |
| Immune | Chemokine (C-X-C motif) receptor 1 | CCR1 | 2.0 | 0.0023 | NC | NS |
| Chemokine (C-C motif) ligand 14 | CCL14 | −1.6 | 0.0002 | −2.8 | 0.0001 | |
| Chemokine (C-X-C motif) ligand 11 | CXCL11 | 5.4 | 0.0008 | NC | NS | |
| Complement component 1 subunit s1 | C1S | −7.1 | 3.9 × 10−6 | NC | NS | |
| Complement component 2 | C2 | −2.0 | 0.0041 | NC | NS | |
| Complement component 9 | C9 | 1.5 | 0.0013 | NC | NS | |
| Interleukin 6 receptor | IL6R | −6.4 | 9.0 × 10−7 | NC | NS | |
| Interleukin 13 receptor alpha 1 | IL13RA1 | −2.0 | 3.9 × 10−5 | −1.1 | NS | |
| Interleukin 17 receptor B | IL17RB | −2.1 | 0.0007 | −2.1 | 2.7 × 10−6 | |
| Interleukin 18 binding protein | IL18BP | 2.7 | 2.8 × 10−5 | NC | NS | |
| Other | Insulin receptor | INSR | −2.0 | 2.4 × 10−5 | NC | NS |
| Insulin-like growth factor 2 | IGF2 | −1.7 | 1.3 × 10−5 | NC | NS | |
| Integrin beta 1 | ITGB1 | −2.3 | 2.7 × 10−7 | NC | NS | |
| Leptin receptor | LEPR | −3.8 | 4.1 × 10−6 | −1.3 | NS | |
P between 0.05 and 0.01 or a fold change between 1.0 and 1.5. Abbreviations: IFN, interferon; ISG, interferon-stimulated gene; NC, no change in the gene expression detected between groups; NS, not statistically significant; RR, rapid responder; SR, slow responder.
With this surrogate measure, the most striking difference between RRs and SRs was in the IFN-related genes. Classical ISGs such as Mx1, OAS1-OAS3, ISG15, and viperin had a greater fold change between on-treatment and pretreatment RRs than SRs, as did proteins involved in the early IFN cascade such as STAT1. In addition, less well characterized ISGs (ISG20 and IFN-induced protein 35) and other IFN-related genes (IFN-induced proteins with TTPR 1–5, IFN transmembrane proteins 1 and 2, guanylate binding protein 1, and 28-kD IFN-responsive protein) also showed a larger fold change between the on-treatment and pretreatment groups among RRs. RIG-I and IRF7, which are involved in IFN production through the IRF3/7 pathway, also showed greater fold differences between treatment groups in RRs than SRs.16 This suggests that although the absolute level of expression of ISGs did not differ between the RRs and SRs, there was a greater fold difference, a surrogate for greater induction of ISGs in the RRs, and this may potentially account for their improved antiviral response.
In addition to IFN-related genes, several other pathways showed differences in the pretreatment expression versus the on-treatment expression in a comparison of RRs and SRs. IFN inhibitory pathways showed a greater fold-change difference in SRs than in RRs. PP2A expression was 6.6-fold higher (P = 0.0001) in on-treatment SRs versus pretreatment SRs but showed no change among the pretreatment and on-treatment RRs. The small ubiquitin-like modifier (SUMO) pathway was also affected. Like ubiquitin, SUMO binds to many proteins, targeting them for degradation, and it has been shown to bind STAT1, resulting in reduced IFN responsiveness.17 SUMO-1 was down-regulated in RRs. USP18 showed a greater fold change in RRs between pretreatment and on-treatment groups, and this likely reflected the fact that this ISG was significantly up-regulated in the pretreatment SRs.
Some immune-related genes also differed by the fold change between the groups. IP10 [chemokine (C-X-C motif) ligand 10] expression was higher in pretreatment RRs versus on-treatment RRs, whereas it was lower in on-treatment SRs. In studies of PBMCs, IP10 has been found to be elevated prior to treatment, with down-regulation during treatment correlating with viral clearance.18,19 Interleukin-6 was recently reported to be increased in HCV-infected chimpanzees and was postulated to interfere with IFN signaling possibly through a suppressor of cytokine signaling 3 (SOCS3)–mediated mechanism.20 Possibly in keeping with this observation, the expression of the interleukin-6 receptor was markedly lower in on-treatment RRs versus pretreatment RRs with no change in SRs. Other genes that differed between RRs and SRs but were of less clear significance include the insulin receptor, the leptin receptor, and insulin-like growth factor 1.
Ribavirin Versus No Ribavirin
Of the on-treatment patients, 5 received peginterferon alone, whereas 6 were treated with ribavirin for 72 hours followed by peginterferon, prior to liver biopsy. These 2 groups were compared to evaluate the independent effects of ribavirin on hepatic gene expression. Neither race nor gender contributed to the model. A total of 3645 genes were differentially regulated between the groups, and 563 genes differed by 1.5-fold or greater expression with a P value of 0.01. A summary of the differentially regulated genes is shown in Table 6, and a full list is available in the supplementary material (Supplement to Table 6). Gene expression patterns differed in patients that received ribavirin in 4 main categories with potential relevance to the treatment response. These included genes with effects on IFN signaling, IFN inhibition, HSC activation, and apoptosis.
Table 6.
Selected Genes Induced by Ribavirin with a Fold Change Greater Than or Equal to 1.5 (P ≤ 0.01) in the On-Treatment Group
| Gene Category | Gene Name | Gene Symbol | Fold Change (Ribavirin/Peginterferon Alone) | P |
|---|---|---|---|---|
| IFN-related | IFN-alpha/beta receptor 2* | IFNAR2 | 1.4 | 0.0012 |
| IFN regulatory factor 7* | IRF-7 | 1.7 | 0.035 | |
| IFN-stimulated transcription factor 3* | ISGF3 | 1.3 | 0.019 | |
| Janus kinase 1 | JAK1 | −2.8 | 0.0009 | |
| IFN-inhibitory | Protein phosphatase 2A* | PP2CA | −6.8 | 0.042 |
| Protein phosphatase 3 catalytic subunit (formerly 2B) | PPP3CA | −1.8 | 0.003 | |
| Small ubiquitin-like molecule 1* | SUMO1 | −1.7 | 0.031 | |
| SUMO-1 activating enzyme* | SAE1 | −1.7 | 0.048 | |
| SUMO-1 peptidase 3 | SENP3 | −2.8 | 0.0065 | |
| Suppressor of cytokine signaling 1* | SOCS1 | −1.7 | 0.035 | |
| HSC | CD 36 (collagen I receptor)* | CD36 | −2.7 | 0.047 |
| Collagen type I alpha 2* | COL1A2 | −1.5 | 0.021 | |
| CREBBP/EP 300 inhibitor 1* | CRI1 | −2.0 | 0.027 | |
| Latent TGF-β binding protein 2* | LTBP2 | −1.4 | 0.0066 | |
| Kruppel-like factor 9 | KLF9 | 1.9 | 0.0033 | |
| Matrix metallopeptidase 24 | MMP24 | 4.2 | 0.0026 | |
| Peroxisome proliferator-activated receptor gamma | PPARGC1B | 1.6 | 0.01 | |
| SMAD mothers against DPP homolog 4 | SMAD4 | −1.7 | 0.01 | |
| Tissue inhibitor of metallopeptidase 2* | TIMP2 | −1.7 | 0.031 | |
| Transforming growth factor beta 3 | TGFB3 | −3.2 | 0.0050 | |
| TGF-β receptor–associated protein | TGFBRAP1 | −3.9 | 0.0027 | |
| Apoptosis | Apoptosis caspase activation inhibitor* | AVEN | 5.7 | 0.013 |
| Apoptosis inhibitor 15* | AP15 | −1.7 | 0.032 | |
| Caspase 8* | CASP8 | 1.8 | 0.047 | |
| BCL-2 associate athanogene | BAG2 | −2.8 | 0.0047 | |
| Caspase recruitment domain family 12 | CARD12 | 4.1 | 0.010 | |
| P53-regulated apoptosis-inducing protein | P53AIP1 | −4.9 | 0.01 | |
| Programmed cell death 5* | PDCD5 | −1.3 | 0.005 | |
| Serine/threonine kinase 17b | STK17B | −3.9 | 0.01 | |
| TRAF2 and NCK interacting kinase | TNIK | −6.5 | 0.0078 |
P between 0.05 and 0.01 or a fold change between 1.0 and 1.5. Abbreviations: HSC indicates hepatic stellate cell; and IFN, interferon.
Patients treated with ribavirin and peginterferon showed greater induction of genes involved in the IFN signaling cascade than those treated with peginterferon alone. The IFN-alpha receptor was induced 1.4-fold in the ribavirin group, and although this did not meet the predefined threshold for significance, the P value was highly significant (P = 0.001). This level of induction early in the cascade may lead to important downstream effects. Similarly, IRF9, which binds to STAT1 dimers to form IFN-stimulated transcription factor 3, the transcriptional complex that leads to ISG production by binding to the ISRE,21 was up-regulated in ribavirin-treated patients 1.3-fold with a P value of 0.0002. IRF7, critical for endogenous IFN production, was also up-regulated in the ribavirin-treated patients.
In addition, ribavirin had effects on IFN-inhibitory pathways. PP2A was down-regulated in the ribavirin-treated group; however, although there was a marked reduction in expression by the fold change (−6.8), the P value (0.041) did not reach the 0.01 level of significance. When evaluated by real-time PCR, PP2A was found to be statistically significantly down-regulated in the ribavirin-treated patients (Fig. 4D). PP1C was also significantly down-regulated, and although it has not been shown to directly interfere with STAT1 methylation, given its homology with PP2A, it is possible that it has similar activity. SOCS1 also interferes with IFN signaling.22 SOCS1 was down-regulated in the ribavirin-treated patients. Finally, the SUMO pathway was affected by ribavirin. SUMO1, SUMO3, and the enzyme involved in SUMO activation, SUMO-activating enzyme 1, were down-regulated by ribavirin; however, SUMO-specific peptidase 3, which is responsible for SUMO degradation, was also down-regulated in the ribavirin-treated patients. Overall, ribavirin down-regulated IFN-inhibitory pathways, thus potentially enhancing the antiviral activity of IFN.
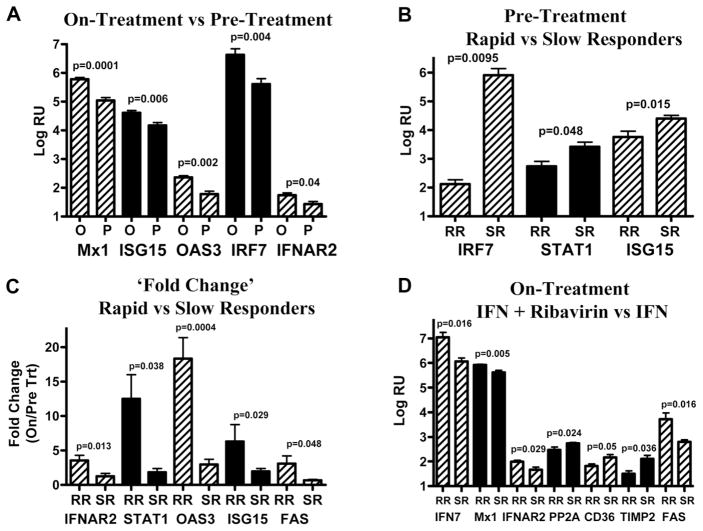
Fig. 4.
Analyses of the gene expression by a quantitative polymerase chain reaction. Selected genes from the microarray analysis were quantified by a TaqMan real-time polymerase chain reaction. (A) On-treatment versus pretreatment. (B) Future RRs versus SRs (pretreatment group). (C) RRs versus SRs (on-treatment group by the fold change). Gene expression in the on-treatment group was normalized for the baseline expression with the mean expression level in the pretreatment group. The fold change was calculated as on-treatment RRs/pretreatment RRs or on-treatment SRs/pretreatment SRs. (D) Peginterferon and ribavirin versus peginterferon. RR indicates rapid responder; and SR, slow responder.
The HSC is well established as the main cellular orchestrator of hepatic fibrosis.23 In response to liver injury, the HSC undergoes a phenotypic change from a quiescent cell to a proliferative myofibroblast-like activated cell that deposits extracellular matrix (ECM) leading to hepatic fibrosis. Studies have identified numerous triggers for HSC activation. Treatment with ribavirin led to down-regulation of a number of genes known to promote HSC activation. Transforming growth factor beta (TGF-β) is one of the most potent stimuli for HSC activation and is also produced by activated HSC.24 TGF-β1 and TGF-β3, the TGF-β receptor, and TGF-β receptor–associated protein 1 were all down-regulated in ribavirin-treated patients. Activated HSCs produce type I collagen.24 Both type I collagen and its receptor CD36 were down-regulated by ribavirin treatment. Peroxisome proliferator-activated receptor gamma (PPAR-γ) is expressed in HSC, and upon activation, PPAR-γ expression is reduced.23 In ribavirin-treated patients, PPAR-γ expression was increased, supporting decreased HSC activation. Matrix metalloproteinases degrade ECM deposited by HSC but are inhibited by the TIMP family. In ribavirin-treated patients, matrix metalloproteinase 24 was induced, and TIMP2 was down-regulated; this favored decreased ECM deposition. Ribavirin treatment resulted in the down-regulation of genes involved in HSC activation (TGF-β family) and markers of HSC activation (collagen type 1 and PPAR-γ), thus favoring less HSC activation and potentially less hepatic fibrosis.
Ribavirin treatment also affected apoptosis pathways. Caspase 8, the main regulator in the cytoplasmic Fas-associated death domain apoptosis cascade, was induced in ribavirin-treated patients. Caspase recruitment domain family member 12, an important promoter of apoptosis, was also up-regulated in the ribavirin group. There was a mix of up-regulation and down-regulation among other genes affecting apoptosis. On balance, ribavirin treatment appeared to promote apoptosis; however, given the complexity of the pathways, it is difficult to draw firm conclusions.
Real-Time PCR Confirmation
In addition to the use of real-time quantitative PCR to confirm the microarray findings in the original study group, tissue from patients for whom there was inadequate RNA to perform the microarray was also evaluated. Because the patterns for PCR confirmation were established only in those for whom the microarray was performed, the additional patients provided a validation cohort. To increase the numbers, groups were compared by the combination of patients from both cohorts, and the comparisons were performed within each group individually as well (Table 1). Not all genes were compared in all patient samples because of insufficient remaining RNA.
On-treatment patients in both the original and validation cohorts experienced the induction of STAT1, Mx1, OAS3, ISG15, IRF7, and the IFN-alpha receptor in comparison with untreated patients. Ribavirin-treated patients showed the induction of ISGs and the IFN-alpha receptor in comparison with patients that received peginterferon alone. An examination of IFN-inhibitory pathways showed that PP2A was down-regulated in ribavirin-treated patients. Markers of HSC activation, including CD36, collagen 1A, and TIMP2, were all down-regulated in ribavirin-treated patients. Fas was up-regulated in ribavirin-treated patients, and this suggested increased apoptosis. There was inadequate RNA to evaluate other apoptosis markers. Representative real-time PCR results are shown in Fig. 4.
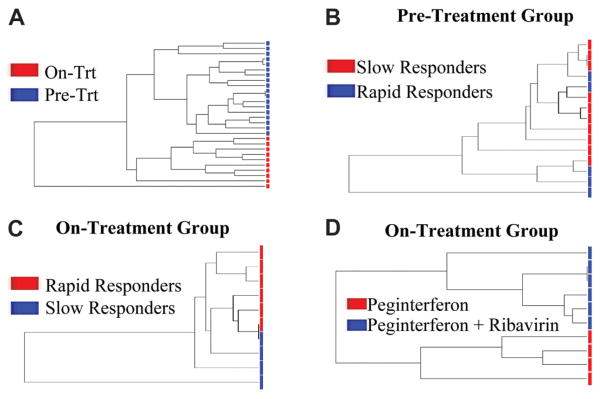
Cluster Analysis
With the gene lists generated for each subgroup, cluster analysis was performed and demonstrated that the groups separated into distinct gene expression groups, as shown in Fig. 5. Separation based on expression profiles was most distinct between pretreatment and on-treatment patients and between those receiving ribavirin and those treated with peginterferon alone. Although patients did separate on the basis of the treatment response in both pretreatment and on-treatment biopsy samples, the distinctness of the groups was not as great, as evidenced by closer common branch points.
Fig. 5.
Supervised hierarchical clustering by a gene list. The patients are categorized on the basis of gene expression profiles. The number of branch points between patients reflects the degree of similarity in the expression pattern. (A) On-treatment group versus the pre-treatment group. (B) Future slow responders versus rapid responders in the pretreatment group. (C) Rapid responders versus slow responders in the on-treatment group divided by the pretreatment group. (D) Peginterferon plus ribavirin versus peginterferon alone.
Discussion
In this study, peginterferon led to the hepatic induction of known ISGs and a large number of other genes. The list of induced genes was compared to that from published data on IFN-treated uninfected chimpanzees, primary human hepatocytes, and human PBMCs.7,9 A very similar pattern of ISG induction was seen. As in PBMCs, transcription factors such as activating transcription factor 7 and the proapoptotic interleukin-18 pathway were induced in the liver with peginterferon treatment.9 Genes involved in IFN production, including IRF7 and RIG-I, were also induced by treatment, and this suggested that therapeutic IFN may also promote endogenous IFN production. Other pathways induced by treatment included those involved in the oxidative stress response, apoptosis, and antigen presentation.
Although the lists of gene induced by peginterferon were similar between humans and chimpanzees, the level of fold induction between treated and untreated patients was lower in magnitude than the fold induction reported by Lanford et al.7 in chimpanzees. Most classical ISGs were up-regulated 1.5–3–fold in on-treatment biopsies in comparison with pretreatment samples, whereas Lanford et al. found induction levels of up to 47-fold. However, differences in the study design may explain these apparent discrepancies. The chimpanzees were uninfected at the baseline and served as their own controls. In contrast, in this study, the pretreatment group comprised HCV-infected individuals. The induction of ISGs from endogenous IFN is known to occur in chronically infected patients, and the elevation of the baseline value will greatly reduce the fold induction even if similar absolute levels of gene expression occur after treatment with IFN.7 With separation by the treatment response, this difference became more apparent, with greater induction in RRs than SRs, likely because of lower ISG expression at baseline in the future RRs. The timing of the evaluation may also be important. Lanford et al. were able to biopsy chimpanzees sequentially and found that after an initial surge in ISG expression at 4 hours, gene expression quickly dropped off within 24 hours. Unfortunately, the time course of hepatic gene induction during therapy in naive or infected humans is unknown, but it may be that there is a similar reduction in gene expression by 24 hours. A comparison of 24-hour gene expression after IFN between humans and chimpanzees was fairly similar, with the difference in the baseline ISG expression from chronic HCV infection in humans likely accounting for the differences.
Chen et al.6 previously reported that in pretreatment liver biopsies, nonresponders had higher hepatic ISG expression than sustained responders or uninfected controls. An examination of our pretreatment population revealed an identical pattern. All of the ISGs found by Chen et al. to be differentially regulated were also noted in our analysis. In addition, a number of other ISGs and IFN regulators, including STAT1 and RIG-I, were found to follow the same pattern of increased expression in future nonresponders. This result confirms the original findings, particularly given that not only were different cohorts examined but different microarray platforms (complementary DNA versus RNA) were also employed. To ensure that our choice of early virological response was reasonable, we analyzed the data on the basis of both early and ultimate responses and found similar results (data not shown).
The comparisons of RRs and SRs offer some clues to understanding IFN nonresponse. The first finding is that absolute ISG expression did not differ significantly between RRs and SRs on therapy. Coupled with higher pretreatment ISG expression in future SRs, this raises the question of whether SRs already have maximally induced ISGs and cannot respond further to therapeutic IFN. To evaluate this issue, we compared ISG expression between SRs before and during treatment. For most ISGs, the mean expression level was higher in the on-treatment SRs; however, this was not true for all ISGs. In addition, the magnitude of the difference was small, and some individual patients had pretreatment gene expression levels comparable to those found in on-treatment SRs. In contrast, among RRs, on-treatment ISG expression was universally higher than pretreatment levels, and the fold change was much greater than that in SRs. The effect on global gene expression also differed, with almost twice as many genes with greater than 1.5-fold induction in RRs than SRs. Together, these data suggest that all patients likely achieve maximal ISG induction on peginterferon, but SRs already have high and possibly even maximal ISG expression prior to treatment. Although SRs may be able to induce ISGs further with treatment, they gain little additional benefit from therapy, and this results in a slow response and ultimately nonresponse.
Although ISG expression was similar between RRs and SRs on therapy, differences were seen in IFN inhibitory pathways. PP2A levels are higher in patients infected with HCV than in healthy controls, and both in vitro and in vivo expression of this protein results in hypomethylation of STAT1, which results in greater interaction with PIAS1, thus reducing STAT1-ISRE binding and subsequent ISG expression.14 PP2A expression was 8-fold greater in SRs. Furthermore, in contrast to ISGs, IFN-inhibitory pathways showed a greater change in the expression level between on-treatment and pretreatment SRs than RRs. The inhibition of IFN activity may be critical for circumventing the effects of endogenous and therapeutic IFN. A proposed schematic is shown in Fig. 6.
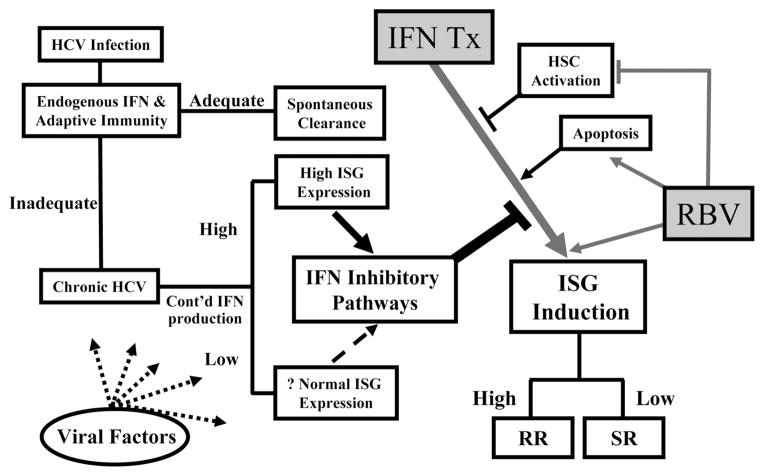
Fig. 6.
Postulated scheme of the importance of gene expression before and during peginterferon and ribavirin treatment and its effect on treatment outcome. The strength of the adaptive immune response and early endogenous IFN production likely determine whether the initial HCV infection is cleared or becomes chronic. High ISG expression, presumably from increased endogenous IFN production, is accompanied by up-regulation of IFN-inhibitory pathways. This leads to reduced ISG induction with IFN treatment. Ribavirin enhances the efficacy of IFN directly through up-regulation of the IFN receptor and indirectly through effects on apoptosis and possibly HSCs. Ultimately, the degree of ISG induction with therapy may determine treatment outcome. HCV indicates hepatitis C virus; HSC, hepatic stellate cell; IFN, interferon; ISG, interferon-stimulated gene; RR, rapid responder; and SR, slow responder.
In contrast to the relative lack of ISG induction in the livers of SRs, ISGs were recently reported to be strongly induced in PBMC from SRs, albeit somewhat less so than in RRs, after treatment with IFN.25 The pattern of response to treatment among SRs is very similar to the pattern recently described in HCV-infected chimpanzees. Huang et al.20 found that like human nonresponders, chimpanzees had high baseline hepatic but normal PBMC ISG expression levels. When they were treated with IFN, ISG induction in PBMCs from infected chimpanzees was found to be at levels only slightly lower than those in naive animals. However, in the liver, ISG induction was almost completely abrogated in the infected chimpanzees, and little or no reduction in HCV RNA was seen. As in human SRs, increased expression of IFN-inhibitory pathways was also seen in the infected animals. The similarities between these patterns of response suggest that the chimpanzee may serve as a relevant model for understanding IFN nonresponse.
Despite its clear effectiveness, the mechanisms by which ribavirin improves the response to IFN are poorly understood. Taylor et al.9 found few differences in gene expression in PBMCs in patients treated with ribavirin. In contrast, a comparison of hepatic gene expression in patients receiving ribavirin and peginterferon with those receiving peginterferon alone revealed differing gene expression patterns, potentially offering some important insights into the mechanism by which ribavirin affects the treatment response. The most direct effect of ribavirin was the induction of genes involved in the IFN cascade. In addition to increased expression of the IFN-alpha receptor, IFN-stimulated transcription factor 3 and IRF7, which promotes endogenous IFN production, were also induced in ribavirin-treated patients. The effect on IFN signaling is in keeping with that reported by Zhang et al.,26 who found that ribavirin led to up-regulation of ISGs and a reporter gene driven by the ISRE promoter in a respiratory syncytial virus infection. Notably, in this experimental system, ribavirin increased ISRE activity only in the setting of endogenous IFN production, with no effect seen with ribavirin treatment alone. This is analogous to the situation seen in the treatment of HCV, in which ribavirin has little effect as a monotherapy but leads to important synergistic improvements in treatment when combined with peginterferon. The confirmation of the induction of IFN-alpha receptor and IRF7 expression by real-time PCR in the cohort not examined by a microarray adds further weight to the importance of this mechanism. This suggests that ribavirin may contribute to the antiviral response by making cells more responsive to IFN through the receptor and furthermore by increasing the production of endogenous IFN. The down-regulation of IFN-inhibitory pathways by ribavirin may further enhance the IFN response.
Aside from direct effects on the IFN response, ribavirin treatment altered gene expression in pathways affecting HSC activation, which may reduce hepatic fibrogenesis. In addition to known HSC activators, numerous other genes identified by microarray studies of HSC activation were also down-regulated by ribavirin.23,24 This may account for the finding of reduced hepatic fibrosis in a subset of patients treated with long-term ribavirin monotherapy.27 Although the mechanism is unclear, increased hepatic fibrosis is associated with a poorer treatment response.28,29 Perhaps by reducing fibrogenesis, at least in some patients, ribavirin further improves IFN efficacy.
Ribavirin also appears to have an effect on apoptotic pathways. Although there were mixed effects, caspase 8, the main activator of the Fas-mediated apoptosis pathway, was up-regulated in ribavirin-treated patients. This is in keeping with a previous report from Schlosser et al.,30 who showed that IFN and ribavirin treatment of Hep G2 cells resulted in increased caspase 8 expression and increased Fas-mediated apoptosis. The finding that apoptotic pathways are also up-regulated in responders to therapy suggests that apoptosis may be a critical component of the response to HCV treatment.31
This study has some limitations. The sample size in all groups, particularly for ribavirin, is relatively small; however, it is comparable to the size of many microarray studies. To provide some validation, the cohort of patients with inadequate RNA for a microarray was evaluated by real-time PCR with generally confirmatory results. The liver biopsies were performed 24 hours after the first dose of peginterferon. Up-regulation and down-regulation of IFN pathways occur very rapidly, and even by 24 hours, some important effects may have been missed.7 In addition, the lack of multiple biopsies also prevents the collection of longitudinal data on gene expression, which may show important dynamic changes during the course of therapy. Use of the pre-treatment group as a baseline for the on-treatment group is not ideal, but given the infeasibility of biopsying patients before and during treatment, it is the best available alternative. Despite the use of a P value of 0.01 for significance, the possibility of false positive findings due to multiple comparisons remains. To minimize this issue, a fold-change threshold was included, and signaling pathways and individual gene expression were also compared. Finally, important findings were confirmed with real-time PCR.
In summary, we have shown that peginterferon treatment leads to the induction of known ISGs and many other genes. We have confirmed previous findings showing increased ISG expression in pretreatment liver biopsies of nonresponders. On treatment, we found that RRs and SRs have similar ISG expression but SRs have up-regulation of IFN inhibitory pathways. Evaluating a surrogate for treatment-induced gene induction by controlling for baseline expression in the pretreatment group, we found that RRs have higher levels of induction of ISGs, whereas IFN-inhibitory pathways are induced to a greater degree in SRs. Finally, ribavirin appears to augment the IFN response and down-regulate genes involved in HSC activation.
Supplementary Material
Acknowledgments
Supported in part by a grant from Hoffmann-La Roche, by a grant from the General Clinical Research Center of the University of North Carolina (RR 000046), by a Midcareer Investigator Award in Patient-Oriented Research (DK06614; to M.W.F.), Doris Duke Fellowship (LMS) and by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases (National Institutes of Health).
We thank Karen Dougherty, A.N.P., Roshan Shrestha, M.D., and George Poy for their contributions to this study.
Abbreviations
- ECM
extracellular matrix
- HCV
hepatitis C virus
- HSC
hepatic stellate cell
- IFN
interferon
- IRF
interferon regulatory factor
- ISG
interferon-stimulated gene
- ISRE
interferon-sensitive response element
- Mx
myxovirus resistance
- OAS
oligoadenylate synthetase
- PBMC
peripheral blood mononuclear cell
- PCR
polymerase chain reaction
- PIAS
protein inhibitor of activated signal transducer and activator of transcription
- PP2A
protein phosphatase 2A
- PPAR-γ
peroxisome proliferator-activated receptor gamma
- RIG-I
retinoic acid–inducible gene I
- RR
rapid responder
- SOCS
suppressor of cytokine signaling
- SR
slow responder
- STAT
signal transducer and activator of transcription
- SUMO
small ubiquitin-like modifier
- SVR
sustained virological response
- TGF-β
transforming growth factor beta
- TIMP
tissue inhibitor of metallopeptidase
- USP
ubiquitin-specific peptidase
Footnotes
Potential conflict of interest: Dr. Fried is a consultant for and received grants from Roche. Dr. Zacks is on the speakers’ bureau of Roche.
Supplementary material for this article can be found on the Hepatology Web site (http://interscience.wiley.com/jpages/0270-9139/suppmat/index.html).
References
- 1.Manns MP, McHutchison JG, Gordon SC, Rustgi VK, Shiffman M, Reindollar R, et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet. 2001;358:958–965. doi: 10.1016/s0140-6736(01)06102-5. [DOI] [PubMed] [Google Scholar]
- 2.Fried MW, Shiffman ML, Reddy KR, Smith C, Marinos G, Goncales FL, Jr, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347:975–982. doi: 10.1056/NEJMoa020047. [DOI] [PubMed] [Google Scholar]
- 3.Hofmann WP, Zeuzem S, Sarrazin C. Hepatitis C virus-related resistance mechanisms to interferon alpha-based antiviral therapy. J Clin Virol. 2005;32:86–91. doi: 10.1016/j.jcv.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 4.Conjeevaram HS, Fried MW, Jeffers LJ, Terrault NA, Wiley-Lucas TE, Afdhal N, et al. Peginterferon and ribavirin treatment in African American and Caucasian American patients with hepatitis C genotype 1. Gastroenterology. 2006;131:470–477. doi: 10.1053/j.gastro.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 5.Ferenci P. Predictors of response to therapy for chronic hepatitis C. Semin Liver Dis. 2004;24(suppl 2):25–31. doi: 10.1055/s-2004-832925. [DOI] [PubMed] [Google Scholar]
- 6.Chen L, Borozan I, Feld J, Sun J, Tannis LL, Coltescu C, et al. Hepatic gene expression discriminates responders and nonresponders in treatment of chronic hepatitis C viral infection. Gastroenterology. 2005;128:1437–1444. doi: 10.1053/j.gastro.2005.01.059. [DOI] [PubMed] [Google Scholar]
- 7.Lanford RE, Guerra B, Lee H, Chavez D, Brasky KM, Bigger CB. Genomic response to interferon-alpha in chimpanzees: implications of rapid downregulation for hepatitis C kinetics. HEPATOLOGY. 2006;43:961–972. doi: 10.1002/hep.21167. [DOI] [PubMed] [Google Scholar]
- 8.Asahina Y, Izumi N, Uchihara M, Noguchi O, Nishimura Y, Inoue K, et al. Interferon-stimulated gene expression and hepatitis C viral dynamics during different interferon regimens. J Hepatol. 2003;39:421–427. doi: 10.1016/s0168-8278(03)00287-3. [DOI] [PubMed] [Google Scholar]
- 9.Taylor MW, Grosse WM, Schaley JE, Sanda C, Wu X, Chien SC, et al. Global effect of PEG-IFN-alpha and ribavirin on gene expression in PBMC in vitro. J Interferon Cytokine Res. 2004;24:107–118. doi: 10.1089/107999004322813354. [DOI] [PubMed] [Google Scholar]
- 10.Lau JY, Tam RC, Liang TJ, Hong Z. Mechanism of action of ribavirin in the combination treatment of chronic HCV infection. HEPATOLOGY. 2002;35:1002–1009. doi: 10.1053/jhep.2002.32672. [DOI] [PubMed] [Google Scholar]
- 11.Cope L, Hartman SM, Gohlmann HW, Tiesman JP, Irizarry RA. Analysis of Affymetrix GeneChip data using amplified RNA. Biotechniques. 2006;40:165–166. 168, 170. doi: 10.2144/000112057. [DOI] [PubMed] [Google Scholar]
- 12.Bleicher KB, Pippert TR, Glaab WE, Skopek TR, Sina JF, Umbenhauer DR. Use of real-time gene-specific polymerase chain reaction to measure RNA expression of three family members of rat cytochrome P450 4A. J Biochem Mol Toxicol. 2001;15:133–142. doi: 10.1002/jbt.10. [DOI] [PubMed] [Google Scholar]
- 13.Helbig KJ, Lau DT, Semendric L, Harley HA, Beard MR. Analysis of ISG expression in chronic hepatitis C identifies viperin as a potential antiviral effector. HEPATOLOGY. 2005;42:702–710. doi: 10.1002/hep.20844. [DOI] [PubMed] [Google Scholar]
- 14.Duong FH, Filipowicz M, Tripodi M, La Monica N, Heim MH. Hepatitis C virus inhibits interferon signaling through up-regulation of protein phosphatase 2A. Gastroenterology. 2004;126:263–277. doi: 10.1053/j.gastro.2003.10.076. [DOI] [PubMed] [Google Scholar]
- 15.Randall G, Chen L, Panis M, Fischer AK, Lindenbach BD, Sun J, et al. Silencing of USP18 potentiates the antiviral activity of interferon against hepatitis C virus infection. Gastroenterology. 2006;131:1584–1591. doi: 10.1053/j.gastro.2006.08.043. [DOI] [PubMed] [Google Scholar]
- 16.Foy E, Li K, Wang C, Sumpter R, Jr, Ikeda M, Lemon SM, et al. Regulation of interferon regulatory factor-3 by the hepatitis C virus serine protease. Science. 2003;300:1145–1148. doi: 10.1126/science.1082604. [DOI] [PubMed] [Google Scholar]
- 17.Ungureanu D, Vanhatupa S, Gronholm J, Palvimo JJ, Silvennoinen O. SUMO-1 conjugation selectively modulates STAT1-mediated gene responses. Blood. 2005;106:224–226. doi: 10.1182/blood-2004-11-4514. [DOI] [PubMed] [Google Scholar]
- 18.Diago M, Castellano G, Garcia-Samaniego J, Perez C, Fernandez I, Romero M, et al. Association of pretreatment serum interferon gamma inducible protein 10 levels with sustained virological response to peginterferon plus ribavirin therapy in genotype 1 infected patients with chronic hepatitis C. Gut. 2006;55:374–379. doi: 10.1136/gut.2005.074062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lagging M, Romero AI, Westin J, Norkrans G, Dhillon AP, Pawlotsky JM, et al. IP-10 predicts viral response and therapeutic outcome in difficult-to-treat patients with HCV genotype 1 infection. HEPATOLOGY. 2006;44:1617–1625. doi: 10.1002/hep.21407. [DOI] [PubMed] [Google Scholar]
- 20.Huang Y, Feld JJ, Sapp RK, Nanda S, Lin JH, Blatt LM, et al. Defective hepatic response to interferon and activation of suppressor of cytokine signaling 3 in chronic hepatitis C. Gastroenterology. 2007;132:733–744. doi: 10.1053/j.gastro.2006.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Feld JJ, Hoofnagle JH. Mechanism of action of interferon and ribavirin in treatment of hepatitis C. Nature. 2005;436:967–972. doi: 10.1038/nature04082. [DOI] [PubMed] [Google Scholar]
- 22.Brand S, Zitzmann K, Dambacher J, Beigel F, Olszak T, Vlotides G, et al. SOCS-1 inhibits expression of the antiviral proteins 23,53-OAS and MxA induced by the novel interferon-lambdas IL-28A and IL-29. Biochem Biophys Res Commun. 2005;331:543–548. doi: 10.1016/j.bbrc.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 23.Eng FJ, Friedman SL. Transcriptional regulation in hepatic stellate cells. Semin Liver Dis. 2001;21:385–395. doi: 10.1055/s-2001-17553. [DOI] [PubMed] [Google Scholar]
- 24.Mann DA, Smart DE. Transcriptional regulation of hepatic stellate cell activation. Gut. 2002;50:891–896. doi: 10.1136/gut.50.6.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taylor MW, Tsukahara T, Brodsky L, Schaley J, Sanda C, Stephens MJ, et al. Changes in gene expression during peginterferon and ribavirin therapy of chronic hepatitis C distinguish responders from non responders to antiviral therapy. J Virol. 2007;81:3391–3401. doi: 10.1128/JVI.02640-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang Y, Jamaluddin M, Wang S, Tian B, Garofalo RP, Casola A, et al. Ribavirin treatment up-regulates antiviral gene expression via the interferon-stimulated response element in respiratory syncytial virus-infected epithelial cells. J Virol. 2003;77:5933–5947. doi: 10.1128/JVI.77.10.5933-5947.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hoofnagle JH, Ghany MG, Kleiner DE, Doo E, Heller T, Promrat K, et al. Maintenance therapy with ribavirin in patients with chronic hepatitis C who fail to respond to combination therapy with interferon alfa and ribavirin. HEPATOLOGY. 2003;38:66–74. doi: 10.1053/jhep.2003.50258. [DOI] [PubMed] [Google Scholar]
- 28.Everson GT, Jensen DM, Craig JR, van Leeuwen DJ, Bain VG, Ehrinpreis MN, et al. Efficacy of interferon treatment for patients with chronic hepatitis C: comparison of response in cirrhotics, fibrotics, or nonfibrotics. HEPATOLOGY. 1999;30:271–276. doi: 10.1002/hep.510300116. [DOI] [PubMed] [Google Scholar]
- 29.Heathcote EJ, Shiffman ML, Cooksley WG, Dusheiko GM, Lee SS, Balart L, et al. Peginterferon alfa-2a in patients with chronic hepatitis C and cirrhosis. N Engl J Med. 2000;343:1673–1680. doi: 10.1056/NEJM200012073432302. [DOI] [PubMed] [Google Scholar]
- 30.Schlosser SF, Schuler M, Berg CP, Lauber K, Schulze-Osthoff K, Schmahl FW, et al. Ribavirin and alpha interferon enhance death receptor-mediated apoptosis and caspase activation in human hepatoma cells. Antimicrob Agents Chemother. 2003;47:1912–1921. doi: 10.1128/AAC.47.6.1912-1921.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Volkmann X, Cornberg M, Wedemeyer H, Lehner F, Manns MP, Schulze-Osthoff K, et al. Caspase activation is required for antiviral treatment response in chronic hepatitis C virus infection. HEPATOLOGY. 2006;43:1311–1316. doi: 10.1002/hep.21186. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.