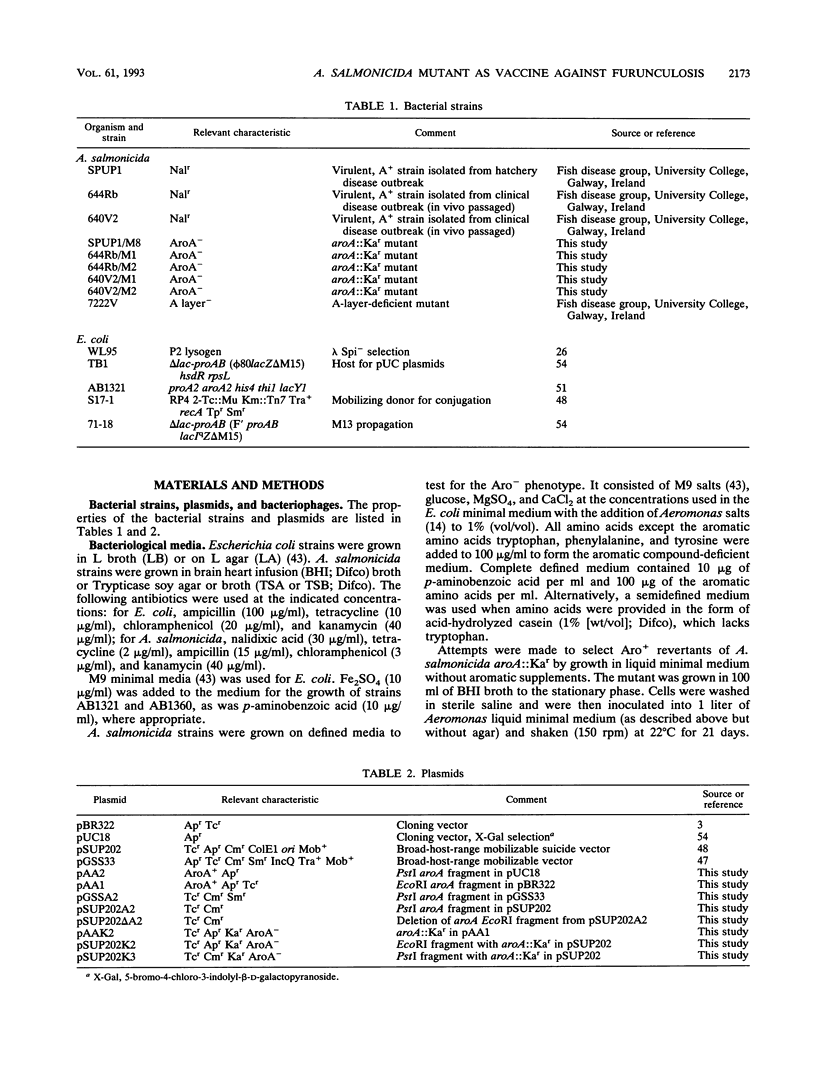
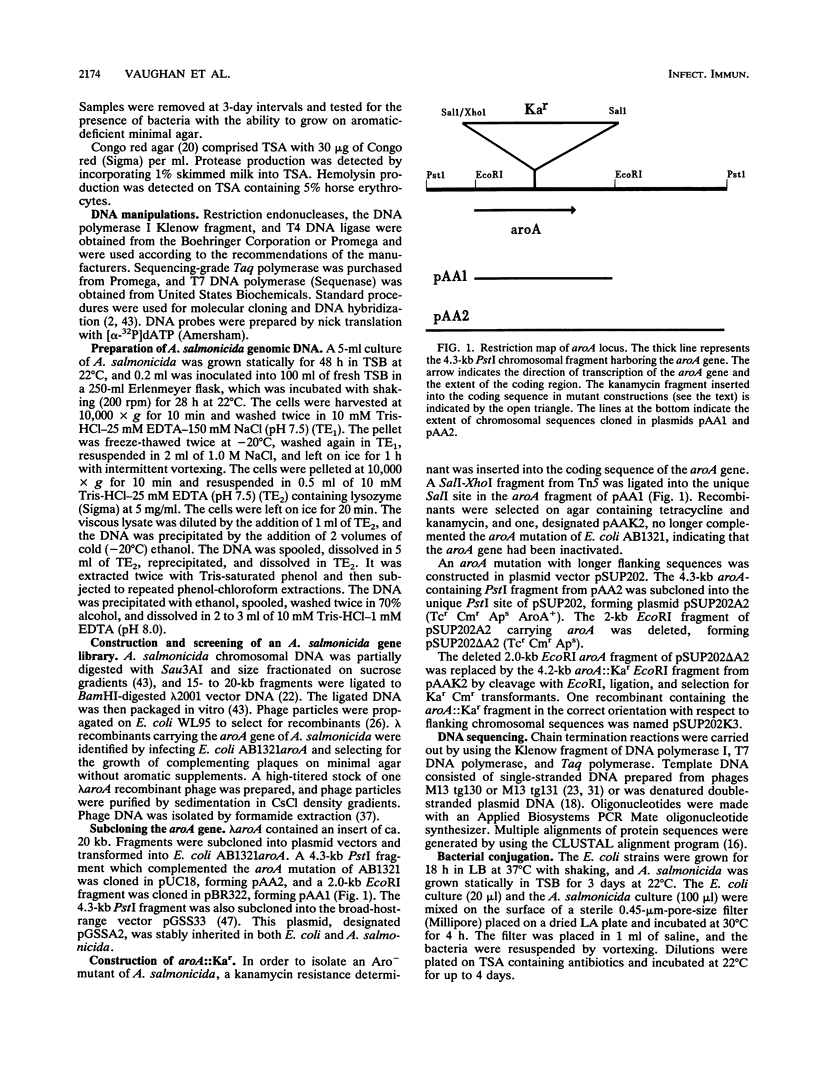
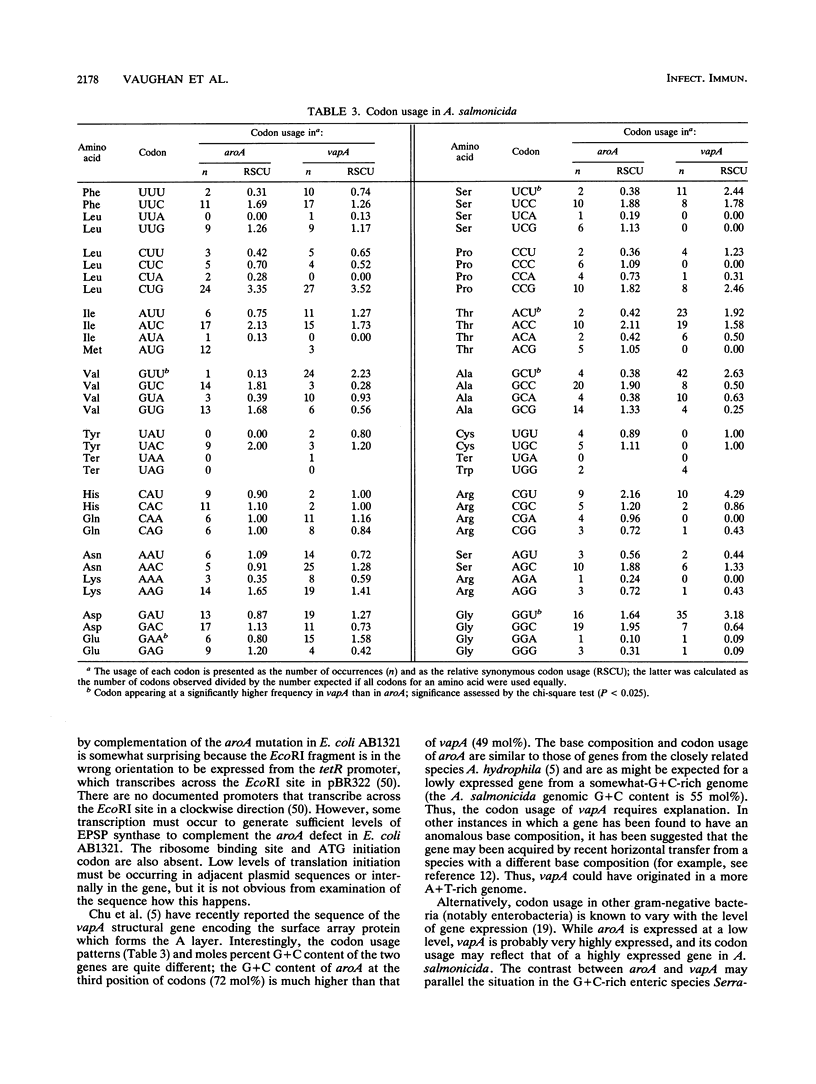
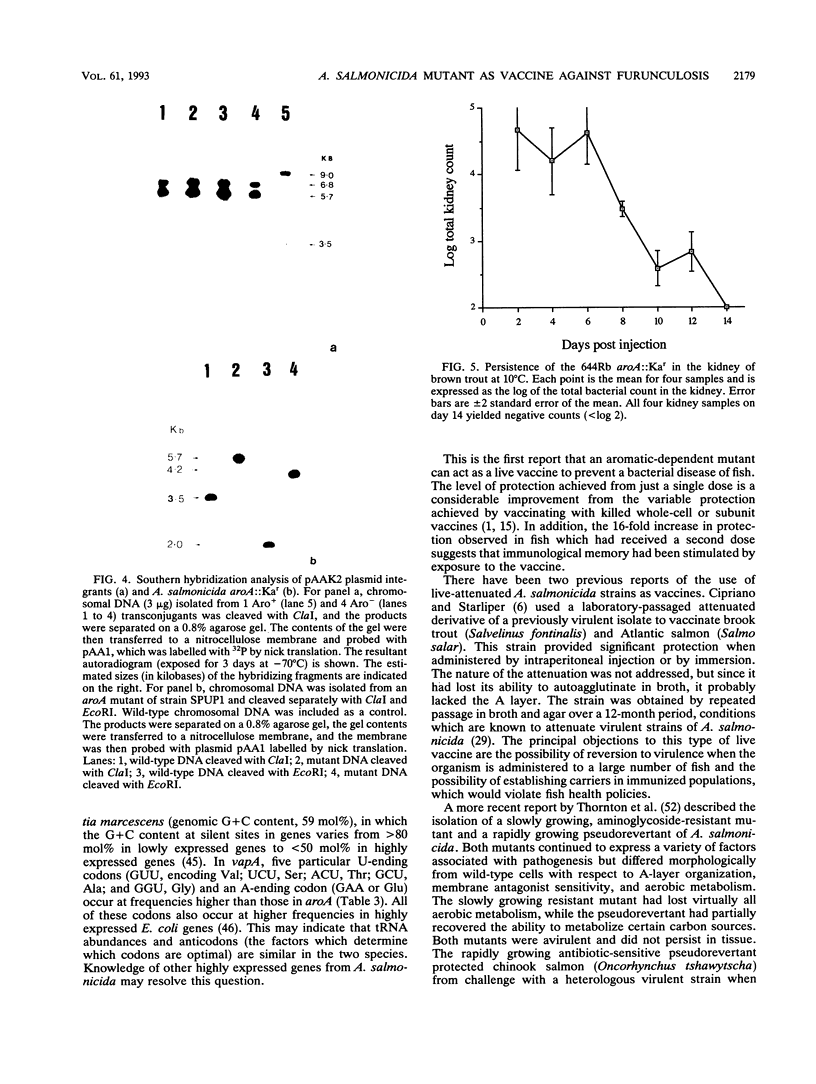
Abstract
Aeromonas salmonicida is the etiological agent of furunculosis in salmonid fish. The disease is responsible for severe economic losses in intensively cultured salmon and trout. Bacterin vaccines provide inadequate protection against infection. We have constructed an aromatic-dependent mutant of A. salmonicida in order to investigate the possibility of an effective live-attenuated vaccine. The aroA gene of A. salmonicida was cloned in Escherichia coli, and the nucleotide sequence was determined. The codon usage pattern of aroA was found to be quite distinct from that of the vapA gene coding for the surface array protein layer (A layer). The aroA gene was inactivated by inserting a fragment expressing kanamycin resistance within the coding sequence. The aroA::Kar mutation was introduced into the chromosome of virulent A. salmonicida 644Rb and 640V2 by allele replacement by using a suicide plasmid delivery system. The aroA mutation did not revert at a detectable frequency (< 10(-11). The mutation resulted in attenuation when bacteria were injected intramuscularly into Atlantic salmon (Salmo salar L.). Introduction of the wild-type aroA gene into the A. salmonicida mutants on a broad-host-range plasmid restored virulence. A. salmonicida mutant 644Rb aroA::Kar persisted in the kidney of brown trout (Salmo trutta L.) for 12 days at 10 degrees C. Vaccination of brown trout with 10(7) CFU of A. salmonicida 644Rb aroA by intraperitoneal injection resulted in a 253-fold increase in the 50% lethal dose (LD50) compared with unvaccinated controls challenged with a virulent clinical isolate 9 weeks later. A second vaccination after 6 weeks increased the LD50 by a further 16-fold.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Bowe F., O'Gaora P., Maskell D., Cafferkey M., Dougan G. Virulence, persistence, and immunogenicity of Yersinia enterocolitica O:8 aroA mutants. Infect Immun. 1989 Oct;57(10):3234–3236. doi: 10.1128/iai.57.10.3234-3236.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu S., Cavaignac S., Feutrier J., Phipps B. M., Kostrzynska M., Kay W. W., Trust T. J. Structure of the tetragonal surface virulence array protein and gene of Aeromonas salmonicida. J Biol Chem. 1991 Aug 15;266(23):15258–15265. [PubMed] [Google Scholar]
- Felmlee T., Pellett S., Welch R. A. Nucleotide sequence of an Escherichia coli chromosomal hemolysin. J Bacteriol. 1985 Jul;163(1):94–105. doi: 10.1128/jb.163.1.94-105.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRIFFIN P. J., SNIESZKO S. F., FRIDDLE S. B. Pigment formation by Bacterium salmonicida. J Bacteriol. 1953 Jun;65(6):652–659. doi: 10.1128/jb.65.6.652-659.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin H. G., Griffin A. M. Cloning and DNA sequence analysis of the serC-aroA operon from Salmonella gallinarum; evolutionary relationships between the prokaryotic and eukaryotic aroA-encoded enzymes. J Gen Microbiol. 1991 Jan;137(1):113–121. doi: 10.1099/00221287-137-1-113. [DOI] [PubMed] [Google Scholar]
- Higgins D. G., Sharp P. M. CLUSTAL: a package for performing multiple sequence alignment on a microcomputer. Gene. 1988 Dec 15;73(1):237–244. doi: 10.1016/0378-1119(88)90330-7. [DOI] [PubMed] [Google Scholar]
- Hoiseth S. K., Stocker B. A. Aromatic-dependent Salmonella typhimurium are non-virulent and effective as live vaccines. Nature. 1981 May 21;291(5812):238–239. doi: 10.1038/291238a0. [DOI] [PubMed] [Google Scholar]
- Ikemura T. Codon usage and tRNA content in unicellular and multicellular organisms. Mol Biol Evol. 1985 Jan;2(1):13–34. doi: 10.1093/oxfordjournals.molbev.a040335. [DOI] [PubMed] [Google Scholar]
- Ishiguro E. E., Ainsworth T., Trust T. J., Kay W. W. Congo red agar, a differential medium for Aeromonas salmonicida, detects the presence of the cell surface protein array involved in virulence. J Bacteriol. 1985 Dec;164(3):1233–1237. doi: 10.1128/jb.164.3.1233-1237.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivins B. E., Welkos S. L., Knudson G. B., Little S. F. Immunization against anthrax with aromatic compound-dependent (Aro-) mutants of Bacillus anthracis and with recombinant strains of Bacillus subtilis that produce anthrax protective antigen. Infect Immun. 1990 Feb;58(2):303–308. doi: 10.1128/iai.58.2.303-308.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karn J., Matthes H. W., Gait M. J., Brenner S. A new selective phage cloning vector, lambda 2001, with sites for XbaI, BamHI, HindIII, EcoRI, SstI and XhoI. Gene. 1984 Dec;32(1-2):217–224. doi: 10.1016/0378-1119(84)90049-0. [DOI] [PubMed] [Google Scholar]
- Kieny M. P., Lathe R., Lecocq J. P. New versatile cloning and sequencing vectors based on bacteriophage M13. Gene. 1983 Dec;26(1):91–99. doi: 10.1016/0378-1119(83)90039-2. [DOI] [PubMed] [Google Scholar]
- Lee K. K., Ellis A. E. Glycerophospholipid:cholesterol acyltransferase complexed with lipopolysaccharide (LPS) is a major lethal exotoxin and cytolysin of Aeromonas salmonicida: LPS stabilizes and enhances toxicity of the enzyme. J Bacteriol. 1990 Sep;172(9):5382–5393. doi: 10.1128/jb.172.9.5382-5393.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loenen W. A., Brammar W. J. A bacteriophage lambda vector for cloning large DNA fragments made with several restriction enzymes. Gene. 1980 Aug;10(3):249–259. doi: 10.1016/0378-1119(80)90054-2. [DOI] [PubMed] [Google Scholar]
- Maskell D. J., Morrissey P., Dougan G. Cloning and nucleotide sequence of the aroA gene of Bordetella pertussis. J Bacteriol. 1988 Jun;170(6):2467–2471. doi: 10.1128/jb.170.6.2467-2471.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra T. K. DNA sequencing: a new strategy to create ordered deletions, modified M13 vector, and improved reaction conditions for sequencing by dideoxy chain termination method. Methods Enzymol. 1987;155:119–139. doi: 10.1016/0076-6879(87)55012-1. [DOI] [PubMed] [Google Scholar]
- Munn C. B., Ishiguro E. E., Kay W. W., Trust T. J. Role of surface components in serum resistance of virulent Aeromonas salmonicida. Infect Immun. 1982 Jun;36(3):1069–1075. doi: 10.1128/iai.36.3.1069-1075.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nnalue N. A., Stocker B. A. Test of the virulence and live-vaccine efficacy of auxotrophic and galE derivatives of Salmonella choleraesuis. Infect Immun. 1987 Apr;55(4):955–962. doi: 10.1128/iai.55.4.955-962.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Callaghan D., Maskell D., Liew F. Y., Easmon C. S., Dougan G. Characterization of aromatic- and purine-dependent Salmonella typhimurium: attention, persistence, and ability to induce protective immunity in BALB/c mice. Infect Immun. 1988 Feb;56(2):419–423. doi: 10.1128/iai.56.2.419-423.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Gaora P., Maskel D., Coleman D., Cafferkey M., Dougan G. Cloning and characterisation of the serC and aroA genes of Yersinia enterocolitica, and construction of an aroA mutant. Gene. 1989 Dec 7;84(1):23–30. doi: 10.1016/0378-1119(89)90135-2. [DOI] [PubMed] [Google Scholar]
- Roberts M., Maskell D., Novotny P., Dougan G. Construction and characterization in vivo of Bordetella pertussis aroA mutants. Infect Immun. 1990 Mar;58(3):732–739. doi: 10.1128/iai.58.3.732-739.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp P. M., Cowe E., Higgins D. G., Shields D. C., Wolfe K. H., Wright F. Codon usage patterns in Escherichia coli, Bacillus subtilis, Saccharomyces cerevisiae, Schizosaccharomyces pombe, Drosophila melanogaster and Homo sapiens; a review of the considerable within-species diversity. Nucleic Acids Res. 1988 Sep 12;16(17):8207–8211. doi: 10.1093/nar/16.17.8207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp P. M. Processes of genome evolution reflected by base frequency differences among Serratia marcescens genes. Mol Microbiol. 1990 Jan;4(1):119–122. doi: 10.1111/j.1365-2958.1990.tb02020.x. [DOI] [PubMed] [Google Scholar]
- Sharpe G. S. Broad host range cloning vectors for gram-negative bacteria. Gene. 1984 Jul-Aug;29(1-2):93–102. doi: 10.1016/0378-1119(84)90170-7. [DOI] [PubMed] [Google Scholar]
- Stalker D. M., Hiatt W. R., Comai L. A single amino acid substitution in the enzyme 5-enolpyruvylshikimate-3-phosphate synthase confers resistance to the herbicide glyphosate. J Biol Chem. 1985 Apr 25;260(8):4724–4728. [PubMed] [Google Scholar]
- Stüber D., Bujard H. Organization of transcriptional signals in plasmids pBR322 and pACYC184. Proc Natl Acad Sci U S A. 1981 Jan;78(1):167–171. doi: 10.1073/pnas.78.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAYLOR A. L., THOMAN M. S. THE GENETIC MAP OF ESCHERICHIA COLI K-12. Genetics. 1964 Oct;50:659–677. doi: 10.1093/genetics/50.4.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton J. C., Garduño R. A., Newman S. G., Kay W. W. Surface-disorganized, attenuated mutants of Aeromonas salmonicida as furunculosis live vaccines. Microb Pathog. 1991 Aug;11(2):85–99. doi: 10.1016/0882-4010(91)90002-r. [DOI] [PubMed] [Google Scholar]
- Verma N. K., Lindberg A. A. Construction of aromatic dependent Shigella flexneri 2a live vaccine candidate strains: deletion mutations in the aroA and the aroD genes. Vaccine. 1991 Jan;9(1):6–9. doi: 10.1016/0264-410x(91)90308-s. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]




