Abstract
Exposure to fine particulate matter (PM, mean aerodynamic diameter ≤ 2.5 μm) has been shown to be a risk factor for cardiovascular disease mortality and may contribute to acute coronary events such as myocardial infarction (MI). There is sufficient reason to believe that smaller particles, such as nanoparticles, might be even more detrimental than larger-sized particles due to their increased surface area and higher pulmonary deposition. Our lab showed that nanoparticle inhalation impairs endothelium-dependent arteriolar vasodilation in skeletal muscle. However, it is not known if coronary microvascular endothelial function is affected in a similar manner. Rats were exposed to filtered air (control) or TiO2 nanoparticles (primary particle diameter, ~21 nm) via inhalation at concentrations that produced measured depositions (10 μg) relevant to ambient air pollution. Subepicardial arterioles (~150 μm in diameter) were isolated and responses to transmural pressure, flow-induced dilation (FID), acetylcholine, the Ca2+ ionophore A23187, and sodium nitroprusside (SNP) assessed. Myogenic responsiveness was preserved between groups. In addition, there was no difference in the vasodilation to SNP, signifying that smooth muscle sensitivity to nitric oxide (NO) is unaffected by nano-TiO2 exposure. However, inhalation of nano-TiO2 produced an increase in spontaneous tone in coronary arterioles and also impaired endothelium-dependent FID. In addition, ACh- and A23187-induced vasodilation was also blunted in arterioles after inhalation of nano-TiO2. Data showed that nanoparticle exposure significantly impairs endothelium-dependent vasodilation in subepicardial arterioles. Such disturbances in coronary microvascular function are consistent with the cardiac events associated with particle pollution exposure.
Keywords: coronary, microcirculation, inhalation toxicology, nanoparticle, endothelium-dependent
INTRODUCTION
The association between exposure to ambient particulate matter (PM) and cardiovascular disease is well known ( Dominici et al, 2005; Kodavanti et al, 2002). PM exposure affects tissues and organs outside the respiratory tract, as evidenced by the elevated occurrence of cardiovascular dysfunction on high pollution days (Pope et al. 2004; Samet et al. 2000). Exposure to airborne pollutants, such as PM, significantly increased all-cause morbidity and mortality (Dockery 2001; Goldberg et al. 2001; Dominici et al. 2005), and led to myocardial ischemia, malignant ventricular arrythmias, or coronary thrombosis (Brook et al. 2004). . Pulmonary inflammation, bradycardia, and cardiac arrythmias were proposed as mechanisms for PM-dependent cardiovascular mortality (Godleski et al. 2000; Watkinson et al. 2001; Kodavanti et al, 2002), but detrimental changes in coronary microvascular reactivity may contribute to compromised cardiac function and may also be a vital indicator of impending risk in this regard.
While the pulmonary effects of PM exposure are well-studied, the effect(s) of exposure to specific components of such particles on the systemic circulation are poorly understood. Exposure to fine PM (mean aerodynamic diameter ≤ 2.5 μm) was found to be a risk factor for cardiovascular disease mortality, (Pope et al. 2004) and contributed to acute coronary events, such as unstable angina and MI (Pope et al. 2006). However, there is sufficient reason to believe that the smallest particles, such as ultrafine (UF) PM and nanoparticles, are important in morbidity and mortality associations (Araujo et al. 2008) otherwise attributed to larger-sized particles due to their increased surface area and higher pulmonary deposition (Dreher 2004). Nano-sized titanium dioxide (TiO2) particles, for example, fall within the definition of a nanoparticle (a particle with a mean aerodynamic diameter ≤ 100 nm ) and are most commonly used as photocatalysts to clean air and water (Sun et al. 2004) and as antibacterial agents (Shieh et al. 2006). Once in the lung, nanoparticles and their chemical constituents may initiate a variety of inflammatory responses similar to those reported after exposure to environmental particles (Chen et al. 2006; de Haar et al. 2006; Grassian et al. 2007; Sager et al. 2008) and may translocate to systemic sites within 24 hr of deposition (Oberdorster et al. 2004; Wallenborn et al. 2007).
Currently, there are limited studies investigating the potential adverse health and systemic vascular effects of exposure to nano-sized particles. Our lab previously showed that pulmonary exposure to PM impaired vasodilator capacity in the microcirculation of the spinotrapezius muscle (Nurkiewicz et al. 2006), and this microvascular dysfunction was augmented after nanoparticle inhalation (Nurkiewicz et al. 2008). However, far less is known regarding the effects of nanoparticle inhalation on the microvessels which constitute the majority of vascular resistance in the coronary circulation [i.e., arterioles < 200 μm in luminal diameter (Chilian et al. 1986)].
Coronary microvessels differ from skeletal muscle arterioles in that local metabolic feedback, specifically the production of relaxing and constricting factors, is the primary regulator of coronary blood flow. Due to the high oxygen extraction at rest (approximately 75%), increases in cardiac metabolism must be met by an immediate increase in coronary blood flow. Under normal physiological conditions, the majority of coronary resistance (up to 75%) resides in arterioles (Chilian et al. 1986). Subsequently, it is the role of coronary resistance arteries and arterioles to accurately respond to local metabolic stimuli and continuously deliver oxygen-rich blood. When the responsiveness of these vessels becomes dysfunctional, increased oxygen demands can not be met and ischemia results. Potential alterations in coronary microvessel reactivity after nanoparticle exposure have not been investigated. Furthermore, given the importance of the coronary resistance arterioles in the distribution and regulation of blood flow in the heart, it is critical to investigate possible alterations in their reactivity after nanoparticle exposure. In the current study, it was postulated that nanoparticle inhalation impairs endothelium-dependent responses in the coronary microcirculation. Therefore, the purpose of this study was to determine if nanoparticle exposure alters the vasoreactivity of coronary arterioles to myogenic, flow-dependent and pharmacological stimuli.
MATERIALS AND METHODS
Experimental animals
Specific pathogen free male Sprague Dawley [Hla:(SD)CVF] rats (10-12 wk old) were purchased from Hilltop Laboratories (Scottsdale, PA) and housed in an AAALAC approved animal facility at the National Institute for Occupational Safety and Health. Rats were housed in laminar flow cages under controlled temperature and humidity conditions and a 12 hr light/dark cycle. Food and water were provided ad libitum. Rats were acclimated for 5 days before use and certified free of endogenous viral pathogens, parasites, mycoplasms, Helicobacter and CAR bacillus. To ensure that all methods were performed humanely and with regard to alleviation of suffering, all experimental procedures were approved by the Animal Care and Use Committees of the National Institute for Occupational Safety and Health, and West Virginia University.
Inhalation exposure
The inhalation exposure system used for particle exposures in the current experiments has been previously described (Nurkiewicz et al. 2008). Briefly, the system contains a fluidized-bed powder generator, an animal exposure chamber, and assorted aerosol monitoring and control devices that are collectively capable of generating and characterizing nanoparticle aerosols. Nano-TiO2 powders were obtained from DeGussa (Aeroxide TiO2, P25, Parsippany, NJ). This powder is 80% anatase, and 20% rutile, with a primary particle size of 21 nm. These proportions of anatase and rutile TiO2 have been independently verified (Hurum et al. 2005; Vasiliev et al. 2008). Prior to aerosol generation, the dry, nano-TiO2 particles had BET surface areas (Brunauer 1938) of 48.08 m2/g (Sager et al. 2008). The count mode diameter (CMD) of the aerosolized nano-TiO2 was 100 nm (Nurkiewicz et al. 2008). Rats were housed in the exposure chamber for 240 min at aerosol concentrations of 6 mg/m3. These conditions produced actual lung burdens of 10 μg which were previously measured in ashed lung tissue. The complete aerosol/exposure profile and particle depositions/burdens for the nano-TiO2 group were reported by Nurkiewicz et al ( 2008). The 10 μg dose was the EC50 for nanoparticles and was defined as the lung burden that produced ~50% impairment of microvascular reactivity in previous experiments with the rat spinotrapezius muscle. For the purpose of consistency among ongoing studies, this lung burden has been used throughout multiple experimental series (Nurkiewicz et al. 2008; 2009).
Subepicardial arteriole isolation
Twenty-four hr after exposure, rats were anesthetized (thiopental, 100 mg/kg, i.p.), and heart removed from the chest. The heart was flushed of excess blood and placed in a dissecting dish with physiological salt solution [PSS (in mmol/l): 129.8 NaCl, 5.4 KCl, 0.5 NaH2PO4, 0.83 MgSO4, 19 NaHCO3, 1.8 CaCl2, and 5.5 glucose] and chilled to 4° C. Coronary resistance arterioles from the left anterior descending (LAD) artery distribution were isolated and transferred to a vessel chamber (Living Systems Instrumentation, Burlington, VT) containing fresh PSS oxygenated with normoxic gas (21% O2-5% CO2-74% N2), cannulated with glass micropipettes, and secured with nylon suture (10-0 ophthalmic, Alcon, U.K.). Coronary arterioles were not chosen based on a specific branch order of the LAD, but rather that they met the requirements of passive diameters ≤ 150 μm (which corresponded to 3rd - 4th order arterioles). Arterioles were pressurized to 45 mm Hg (Chilian et al. 1986) with PSS using a servo controlled peristaltic pump (Living Systems Instrumentation, Burlington, VT) and superfused with oxygenated 37° C PSS at a rate of 10 mL/min. Vessel diameter was measured with a video caliper (Colorado Video, Boulder, CO). Vessels without leaks were allowed to develop spontaneous tone (≥ 20% less initial diameter).
The orders of the following experimental periods were randomized in each vessel to ensure that responses were neither interactive nor time-dependent.
Active pressure response
After steady-state spontaneous tone was achieved, pressure was decreased to 0 mm Hg. Myogenic responsiveness was measured by increasing luminal pressure, and arteriolar diameter was subsequently measured. Pressure was increased from 0 to 90 mm Hg in increments of 15 mm Hg, and then decreased in 15 mm Hg increments back down to 0 mm Hg.
Response to flow
Arterioles were exposed to graded increases in intraluminal flow at constant intraluminal pressure (45 mm Hg). Diameter measurements were determined by increasing perfusate flow by increments of 5 up to 25 μL/min (Huang et al. 2000) using a dual pump servo null pressure indicator (Flow Indicator and FC pump, Living Systems Instrumentation, Burlington, VT), which maintains the intraluminal pressure constant by simultaneously increasing pressure on one side of the vessel while decreasing pressure on the other side.
Pharmacological evaluation of the endothelium
Endothelium-dependent arteriolar dilation was evaluated by exposing vessels to increasing concentrations of ACh (1×10−9 – 1×10−4 M) or A23187, a calcium ionophore (1×10−9 – 1×10−6 M) in the superfusate. Response curves to A23187 were not elevated above 1×10−6 M because previous in vivo reports from our lab indicated that greater concentrations of A23187 render the vessel unable to achieve steady-state diameter following vasodilation in a timely manner.
Response to SNP
To evaluate vascular smooth muscle responsiveness to NO, the concentration-response to SNP (1×10−9 – 1×10−3 M), a NO donor, was determined. At the conclusion of each experiment, the vessels were washed with Ca2+-free PSS every 15 min for one hr to obtain maximal passive diameter and wall thickness at 45 mm Hg.
Formulas and statistical analysis
Data are expressed as means ± standard error. Spontaneous tone was calculated by the following equation:
where DM is the maximal diameter recorded at 45 mm Hg under Ca2+-free PSS as described above, and DI is the initial steady-state diameter achieved prior to experimental period. Vessels were used for experiments only if spontaneous tone ≥ 20% was achieved.
Active responses to pressure changes were normalized to the maximal diameter according to the following formula (Murphy 1980; Shipley and Muller-Delp 2005)
where DSS is the steady-state arteriolar diameter during each pressure step. Normalized diameter is a unit-less variable.
The experimental responses to flow, ACh, A23187, and SNP are expressed using the following equation:
where DSS is the steady-state arteriolar diameter during the experimental period, DCon is the control diameter recorded immediately prior to experimental period. All experimental periods were at least 2 min in duration, and all steady-state diameters were collected for at least a one-min period. Vasodilation is represented as “% relaxation” because this equation normalizes for potential differences in baseline diameter at the start of a response curve.
Shear stress was calculated from volumetric flow (Q) according to the following equation:
where η is viscosity (0.8 cp), Q is volumetric flow rate (measured with a calibrated flow indicator, Living Systems Instrumentation, Burlington, VT), and r is vessel radius.
Wall thickness (WT) was calculated from measurements of both inner (ID) and outer (OD) steady-state vessel diameters during Ca2+-free wash by the equation
Wall-to-lumen ratio (WLR) was calculated by dividing the wall thickness (WT) by the inner vessel diameter (ID) as
Flow-diameter and concentration-diameter curves were evaluated by two-way repeated measures ANOVA in order to detect differences within and between factors. Pairwise comparisons were made by post-hoc analysis (Bonferroni) when a significant main effect was found. T-tests were used for comparisons of animal and vessel characteristics, and sensitivity (EC50) to ACh, A23187, and SNP. Significance was set at p<0.05.
RESULTS
Animal and Vessel Characteristics
Heart weight and left ventricle weight were increased after nano-TiO2 exposure (Table 1). Body weight or heart weight-to-body weight ratio was not altered after exposure (Table 1). Nano-TiO2 exposure increased spontaneous arteriolar tone achieved prior to interventions, but did not alter other vessel characteristics when compared to sham-control rats, such as maximal diameter, wall thickness, and WLR ratio (Table 1).
Table 1.
Animal and arteriolar characteristics of sham-control and nano-TiO2 exposed rats.
|
Sham-control |
Nano-TiO2 |
|
|---|---|---|
| Animal Characteristics | ||
| N | 22 | 26 |
| Age (wks) | 10.9 ± 0.3 | 11.2 ± 0.3 |
| Body weight (g) | 300 ± 11 | 304 ± 9 |
| Heart muscle weight (mg) | 1016 ± 22 | 1106 ± 25* |
| Left ventricle weight (mg) | 799 ± 19 | 858 ± 21* |
| Heart wt / Body wt (mg/g) | 3.06 ± 0.05 | 3.18 ± 0.06 |
| Vessel Characteristics | ||
| n | 27 | 31 |
| Maximal diameter (DM, μm) | 152 ± 4 | 151 ± 5 |
| Steady-state diameter (DI, μm) | 124 ± 4 | 112 ± 6* |
| Wall thickness (WT, μm) | 20.0 ± 1.2 | 18.6 ± 1.9 |
| WLR | 0.13 ± 0.00 | 0.13 ± 0.00 |
| Spontaneous tone (%) | 23 ± 1 | 30 ± 3* |
N, number of rats; n, number of vessels. Values are means ± SE.
P≤0.05 sham-control versus nano-TiO2.
Active Pressure Response
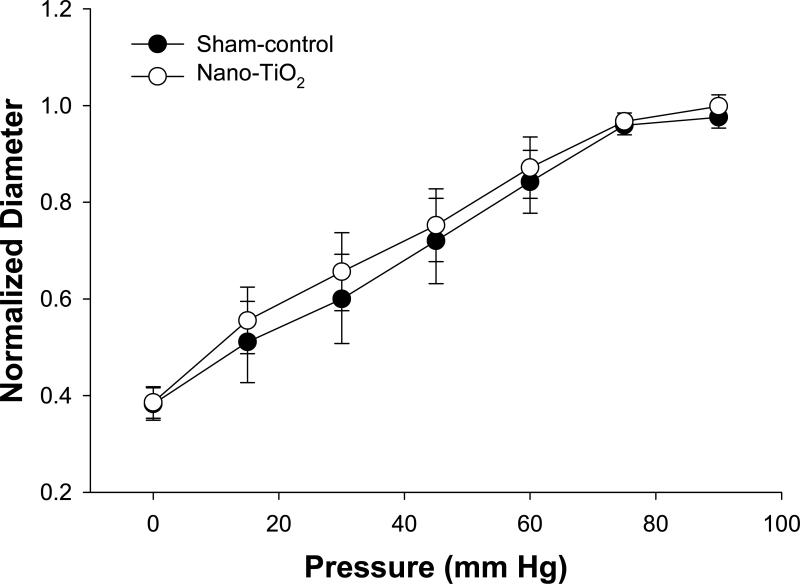
Figure 1 displays the pressure response from 0 – 90 mm Hg, which represents the physiological pressure range that normal coronary microvessels typically experience (Chilian et al. 1986). Myogenic responsiveness of coronary arterioles was not different between sham-control and nano-TiO2 rats (Figure 1). Further, the individual pressure-response at each stage was not different between groups (Figure 1). This suggests that the ability of these arterioles to sense and transduce changes in transmural pressure is unaffected by pulmonary nanoparticle exposure. Similarly, the ability to respond to changes in arteriolar wall tension is unaffected by nanoparticle exposure (data not shown).
Figure 1.
Active pressure responses of coronary arterioles of sham-control rats (n = 10) and nano-TiO2 exposed rats (n = 11). Values are means ± S.E.
Vasodilation to shear stress
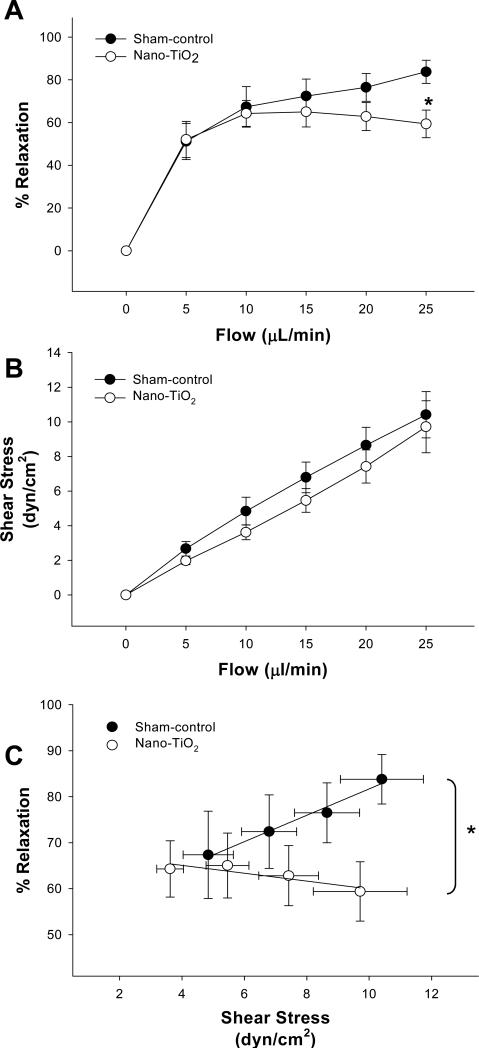
Coronary arterioles from sham-control rats displayed greater vasodilation at the highest flow rate (25 μl/min) when compared to those from nano-TiO2-exposed rats (Figure 2A). The shear stress that the vascular endothelium is exposed to is largely a function of vessel radius; therefore, shear stress was calculated in coronary arterioles from sham-control and nano-TiO2 rats at each level of volumetric flow. Figure 2B shows identical positive linear relationships between volumetric flow and shear stress in coronary arterioles from both groups (first-order regression line slope: sham-control = 0.38, nano-TiO2 = 0.38). Shear stress in arterioles from sham-control and nano-TiO2 rats was comparable throughout step-wise increases in intraluminal flow (Figure 2B). Figure 2C shows the relationship between % relaxation and increases in shear stress. The slope of the first-order regression line fit to the nano-TiO2 data was significantly less than the slope from sham-control animals (Fig 2C). This indicates that arterioles from rats exposed to nano-TiO2 are either less responsive to changes in shear stress, or their inherent ability to sense changes in shear stress is impaired (or both).
Figure 2.
A) Vasodilatory responses to increases in intraluminal flow in sham-control rats (n = 10) and rats exposed to nano-TiO2 (n = 10). Values are means ± S.E. *p<0.05 sham-control versus nano-TiO2 at 25 μL/min
B) Linear relationship between calculated shear stress and increases in intraluminal flow in coronary arterioles. C) Vasodilation as a function of shear stress. Equations of 1st-order regression lines are sham-control: y = 2.86× + 53.01, r2 = 0.98 and nano-TiO2, y = −0.85× + 68.49, r2 = 0.80. Vasodilation is characterized as % Relaxation. Values are means ± S.E.
*p<0.05 sham-control regression line versus nano-TiO2 regression line.
Vasodilator responses to ACh, A23187
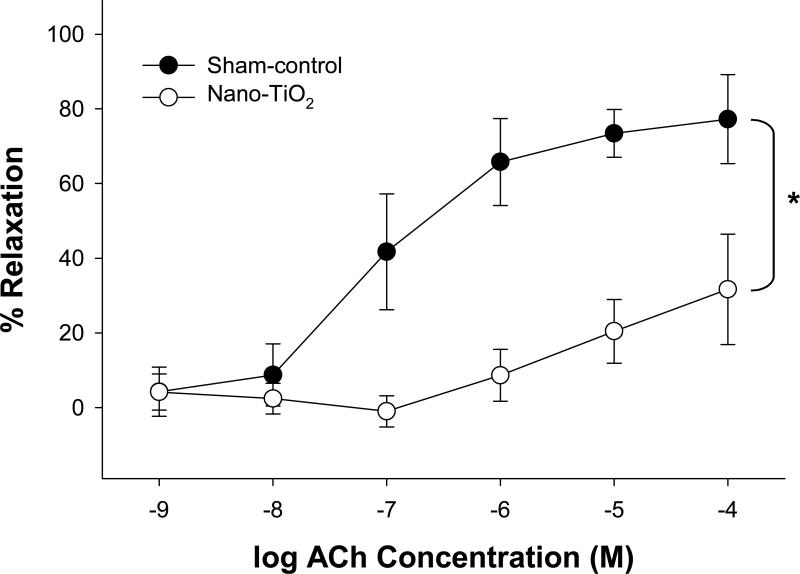
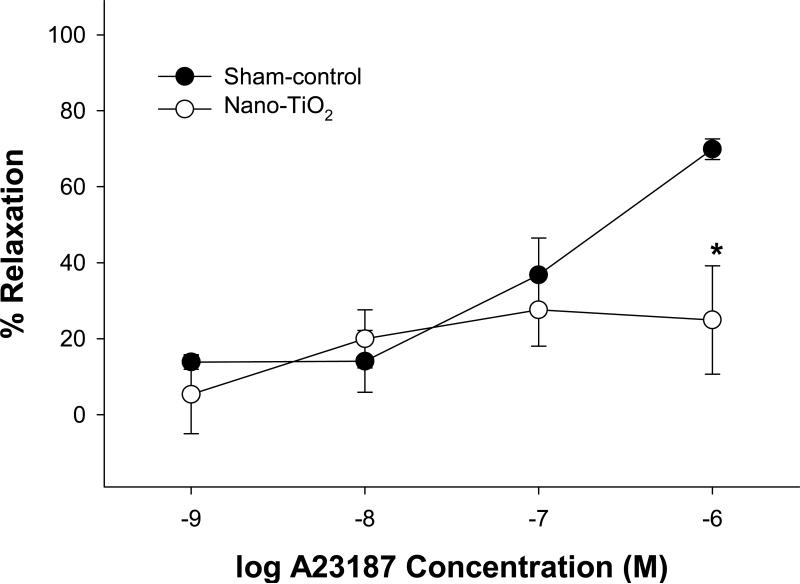
ACh-induced vasodilation was impaired in coronary arterioles from rats exposed to nano-TiO2 (Figure 3). Similarly, nanoparticle exposure produced a decrease in maximal vasodilation to A23187 (1×10−6 M), a Ca2+ ionophore (Figure 4), but this was only at the highest concentration of A23187. Data indicate that arterioles from nano-TiO2 rats are less responsive to endothelium-dependent agonists such as ACh and A23187. Higher concentrations of A23187 were not used in the current study because arterioles exposed to such doses tend not to fully redevelop vascular tone after such a stimulus (Personal communication).
Figure 3.
Endothelium-dependent vasodilation to ACh was decreased in rats exposed to nano-TiO2 (n = 8) compared to arterioles from sham-control rats (n = 18). Vasodilation is characterized as % Relaxation. Values are means ± S.E. *p<0.05 sham-control versus nano-TiO2.
Figure 4.
Coronary arterioles from rats exposed to nano-TiO2 (n = 6) display a blunted vasodilation to the highest concentration of A23187 (1×10−6 M) as compared to sham-control (n = 10). Vasodilation is characterized as % Relaxation. Values are means ± S.E. *p<0.05 sham-control versus nano-TiO2.
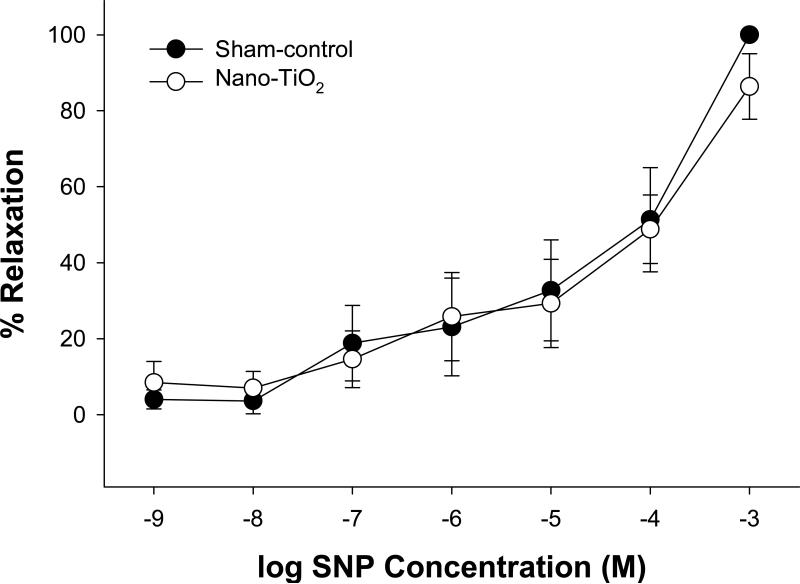
Smooth muscle responsiveness
To determine whether the nanoparticle-dependent impairment of vasodilation in coronary arterioles was due to a decrease in smooth muscle responsiveness to NO, vasoreactivity to SNP was measured. SNP elicited similar vasodilatory response-curves in coronary arterioles from sham-control and nano-TiO2 (Figure 5). This suggests that nanoparticle exposure does not alter vascular smooth muscle NO sensitivity in coronary arterioles.
Figure 5.
Vasodilation to SNP, an NO donor, was similar between coronary arterioles from rats exposed to nano-TiO2 (n = 8) and those from sham-control rats (n = 8). Vasodilation is characterized as % Relaxation. Values are means ± S.E.
DISCUSSION
Alterations in coronary microvessel reactivity after pulmonary nanoparticle exposure have not been previously identified. When the reactivity of these resistance vessels become dysfunctional, increased oxygen demands can not be met and overall cardiac metabolic activity is limited and/or decreased. Such alterations in cardiac physiology are consistent with ischemic events (ACSM 2005). Events such as these were found to be increased after PM exposure (Goldberg et al. 2000; Schwartz and Morris 1995). Because coronary dysfunction in healthy people is unlikely to lead to observation or realization of clinical symptoms, it is more likely that PM-induced dysfunction contributes to ongoing disease or exacerbates events by diminishing collateral reserve. The present study showed that coronary arterioles from rats exposed to nano-TiO2 demonstrated reduced vasoreactivity to shear stress, ACh, and A23187, while smooth muscle responsiveness to NO remained unaltered. All of these changes are consistent with endothelial dysfunction. However, it is important to indicate that each of these stimuli operates through distinctly different cellular mechanisms. Because this is the first study to examine the relationship between nanoparticle exposure and coronary microvessel reactivity; a thorough investigation of these mechanisms need to be performed in subsequent studies.
While certain inhaled particles may be capable of exiting the lung in a limited context, there is currently not a large body of evidence that supports the translocation of particles en masse into the systemic circulation. Further, the likelihood of direct interaction of particles and endothelial cells is not high due to a relatively low pulmonary deposition (10 μg) in relation to the overall size of the rats used in the study (approximately 300 g). Despite this disproportionate relationship, the current study did not identify if nanoparticles are on or in the endothelial cells of the preparation. As such, it is not possible to distinguish between direct effects of nanoparticles and more global systemic response. However, if this possibility is entertained in the hypothetical context of an immediate, 100% particle migration; dilution in the systemic circulation would be marked, and subsequent tissue-particle interaction would be further opposed by the vast endothelial surface area of the collective vascular endothelium, thus rendering a direct effect between migrated particles and the systemic endothelium unlikely. Because this is an in vitro preparation, the influence of nerves on microvascular function has most likely been removed. While it is not possible to discount the possibility that the nerves have primed cells in the microvascular wall and altered arteriolar function that persists in vitro, there is currently no evidence to support this possibility after particle exposure. Therefore, it appears an inflammatory effect is likely mediating the resultant dysfunction in coronary arterioles that follows pulmonary nanoparticle exposure.
Increases in intraluminal flow produce profound changes in the caliber of coronary resistance arterioles via endothelial transduction of shear stress (Kuo et al. 1990); thus, flow-induced dilation is critically important in regulating coronary vascular resistance. This is the first study to show that under similar levels of longitudinal shear stress (Figure 2B), coronary arterioles from nanoparticle-exposed animals exhibited impaired vasodilation as compared to those from sham-control rats (Figure 2A). Coronary microvessels of similar size to those used in the present study were found to actively respond to a range of shear stress (0-10 dyn/cm2) (Kuo et al. 1995), indicating that the flow rates correspond to physiologically significant levels of shear stress. In studies with humans, it was shown that air pollution exposure diminished brachial blood flow in response to endothelium-dependent agonists (O'Neill et al. 2005; Tornqvist et al. 2007). Further, endothelial cell dysfunction was suspected to be the primary cause of reduced dilation to flow (as defined by ischemic responses) after exposure to urban air pollution in humans (Briet et al. 2007). Similarly, occupational exposure to PM carries the same risk as environmental exposure, but perhaps at higher deposition rates. Since occlusion-based studies in conduit vessels correlate poorly with myocardial microvascular perfusion (Bottcher et al. 2001), it is difficult to extrapolate findings with conduits in the arm to direct coronary microvascular effects. Flow-mediated arteriolar dilation is heavily dependent upon endothelial cell integrity in the coronary microcirculation (Kuo et al. 1990); therefore, the current results support the link between nanoparticle exposure and endothelial cell dysfunction via direct experiments with the target tissue in question.
In the present study, endothelium-dependent dilations to ACh and A23187 were decreased in coronary arterioles from nano-TiO2 exposed rats. This is in accordance with previous reports from our lab which showed an impaired dilation to A23187 in the spinotrapezius microcirculation after exposure to fine combustion particles (Nurkiewicz et al. 2006) or nano-TiO2 (Nurkiewicz et al. 2008; 2009). This is also consistent with other reports that indicate endothelium-dependent vasoreactivity is impaired after exposure to PM. Cozzi et al. (2006) showed a reduction in ACh-induced vasodilation in aortas after intratracheal (IT) instillation with UF PM. Similarly, IT instillation of larger particles (PM10) produced an impairment in ACh-induced dilation in rabbit carotid arteries (Tamagawa et al. 2008). Other pollutants such as diesel exhaust or its related gaseous components exert comparable deleterious effects as evidenced by decreased ACh-induced vasodilation (Hansen et al. 2007) or enhanced vasoconstriction (Campen et al. 2005). However, this is the first investigation to show that exposure to relatively non-inflammatory levels of a nanoparticle (TiO2) negatively impacted endothelium-dependent vasodilation in the coronary microcirculation. Because overt pulmonary inflammation was not present at the 10 μg deposition dose used herein or with higher doses used in other studies (Nurkiewicz et al. 2006, 2008; Sager et al. 2008), yet considerable coronary dysfunction was, it was postulated that inflammatory mechanisms reside in the microvascular wall and continue to exert their effects in vitro. Therefore, future studies need to characterize this inflammation in the microvascular wall and assess its intensity relative to the pulmonary exposure. Given the association between the function of this critical level of circulation and cardiac function, the potential link between pulmonary exposure and ischemic events is apparent.
Under conditions of reduced perfusion pressure in the heart, autoregulatory adjustments through vasodilation contribute to maintenance of blood flow to the myocardium. Autoregulatory adjustments are crucial in helping to prevent edema and microvascular damage by preserving capillary hydrostatic pressure when coronary perfusion pressure swells by shielding capillaries from excessive systemic pressures during diastole. Besides vasodilation to metabolic stimuli, another autoregulatory mechanism that participates in controlling coronary blood flow is the myogenic response, or the rapid and maintained constriction of a blood vessel in response to pressure elevation. Consistent with a previous study in mesenteric arteries (Knuckles et al. 2008), the present results showed no differences in myogenic responsiveness after nanoparticle exposure. Since a functional endothelium was reported to influence arteriolar myogenic responsiveness (Kuo et al. 1988; Nurkiewicz and Boegehold 1999), and resting spontaneous tone was increased after exposure (Table 1), it was anticipated that arterioles from rats exposed to nanoparticles exhibit heightened myogenic responsiveness. However, myogenic responsiveness was not altered after nanoparticle exposure (Figure 1). Because the isolated vessel technique removes the influence of nerves and circulating vasoactive agents, it can not be concluded that myogenic responsiveness is not altered by nanoparticle exposure. Therefore, continued investigation at a more mechanistic level is indicated.
Even though smooth muscle contractile function (assessed via myogenic responsiveness) was unaffected by nano-TiO2 exposure, coronary arterioles from nano-TiO2 rats displayed an enhanced spontaneous tone prior to the start of experiments. This is in contrast to previous reports from our lab evaluating microvessels of the spinotrapezius muscle, which showed similar spontaneous tone after exposure to either fine or nano-TiO2 (Nurkiewicz et al. 2006; 2008; 2009). Obvious points of disparity between our previous findings and the present study include a different microvascular bed (spinotrapezius muscle), younger rats (by 3-4 weeks), and use of an in vivo experimental setup.
An unexpected result from the present study was the observed increase in left ventricular and heart weight in rats exposed to nano-TiO2 (Table 1).. The acute rise in heart and left ventricle weight after particle exposure may be due to altered balance of fluids in the heart (i.e. an increase in inter- or intracellular fluid volume). Clearly, hydration status effects the weight of any tissue in the body (Wallace et al. 1970). However, alterations in microvascular permeability are consistent with the functional changes in reactivity reported herein (He et al. 2006). Nurkiewicz et al (2008) observed potential breaches in endothelial integrity after nanoparticle exposure. In these experiments, luminal infusion of A23187 in animals exposed to nano-TiO2 produced arteriolar constriction (rather than dilation); which is highly consistent with the Ca2+ ionophore interacting with vascular smooth muscle (rather than the endothelium alone). It is important to note that the current findings in the heart are preliminary. This specific hypothesis requires continued, more rigorous experiments in order to confirm the present results.
In regards to the question of relevant human exposures, it is important to relate the current exposure paradigm to those encountered in an occupational setting. NIOSH has recently proposed recommended exposure limits (REL) of 0.1 mg/m3 for nano-TiO2 (NIOSH 2009). Unreported levels of nanoparticles have been measured to be as high as 1.4 mg/m3 in the occupational workplace (NIOSH Field Team, personal communication). Therefore, a worker exposed at this level could achieve a pulmonary burden equivalent to 10 μg in the rat, as used in the present study, within 5 years [normalized for alveolar epithelial surface area (Stone et al. 1992) and taking human pulmonary deposition of a 100 nm particle to be 45% (Kreyling 2003)].
CONCLUSIONS
Although the results of the present study are compelling, experimental limitations exist. The isolated arteriole technique offers the benefit of studying the reactivity of microvessels in the absence of extravascular influences, such as nerves and circulating factors. This allows us to manipulate and record responses to local autoregulatory and metabolic influences, such as the myogenic response and shear stress. The inability of the coronary vasculature to provide and distribute adequate blood flow in response to increased myocardial demand likely contributes to compromised cardiac function after PM exposure. The current results show that nanoparticle inhalation significantly increases spontaneous tone while impairing endothelium-dependent vasodilation in subepicardial arterioles. Altered microvascular permeability may also result from such exposures. Collectively, these findings are consistent with the notion that nanoparticle exposure induces microvascular dysfunction that may contribute to ischemic cardiac events.
Acknowledgements
The authors thank Carroll McBride and Kimberly Wix for their expert technical assistance in this study, and Travis Knuckles, Ph.D, for his help in reviewing this manuscript.
This work was supported by National Institutes of Health/National Institute for Environmental Health Sciences [grant number R01-ES015022 (to TRN)]; and Health Effects Institute Award #4730 (TRN).
Abbreviations
- ACh
acetylcholine
- A23187
calcium ionophore
- FID
flow-induced dilation
- LAD
left anterior descending artery
- MAP
mean arterial pressure
- MI
myocardial infarction
- N
number of rats
- n
number of vessels
- NIOSH
National Institute for Occupational Safety and Health
- NO
nitric oxide
- OSHA
Occupational Safety and Health Administration
- PEL
permissible exposure limit
- PM
particulate matter
- REL
recommended exposure limits
- SNP
sodium nitroprusside
- TiO2
titanium dioxide
- UF
ultrafine
- WLR
wall-to-lumen ratio
- WT
wall thickness
Footnotes
Disclaimers
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the National Institute for Occupational Safety and Health.
Research described in this article was conducted under contract to the Health Effects Institute (HEI), an organization jointly funded by the United States Environmental Protection Agency (EPA) (Assistance Award No. R-82811201) and certain motor vehicle and engine manufacturers. The contents of this article do not necessarily reflect the views of HEI, or its sponsors, nor do they necessarily reflect the views and policies of the EPA or motor vehicle and engine manufacturers. The authors declare they have no competing interests.
REFERENCES
- ACSM . Guidelines for Exercise Testing and Prescription. American College of Sports Medicine; Indianapolis, IN: 2005. American College of Sports Medicine. [Google Scholar]
- Araujo JA, Barajas B, Kleinman M, Wang X, Bennett BJ, Gong KW, Navab M, Harkema J, Sioutas C, Lusis AJ, Nel AE. Ambient particulate pollutants in the ultrafine range promote early atherosclerosis and systemic oxidative stress. Circ Res. 2008;102:589–596. doi: 10.1161/CIRCRESAHA.107.164970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottcher M, Madsen MM, Refsgaard J, Buus NH, Dorup I, Nielsen TT, Sorensen K. Peripheral flow response to transient arterial forearm occlusion does not reflect myocardial perfusion reserve. Circulation. 2001;103:1109–1114. doi: 10.1161/01.cir.103.8.1109. [DOI] [PubMed] [Google Scholar]
- Briet M, Collin C, Laurent S, Tan A, Azizi M, Agharazii M, Jeunemaitre X, Alhenc-Gelas F, Boutouyrie P. Endothelial function and chronic exposure to air pollution in normal male subjects. Hypertension. 2007;50:970–976. doi: 10.1161/HYPERTENSIONAHA.107.095844. [DOI] [PubMed] [Google Scholar]
- Brook RD, Franklin B, Cascio W, Hong Y, Howard G, Lipsett M, Luepker R, Mittleman M, Samet J, Smith SC, Jr., Tager I. Air pollution and cardiovascular disease: a statement for healthcare professionals from the Expert Panel on Population and Prevention Science of the American Heart Association. Circulation. 2004;109:2655–2671. doi: 10.1161/01.CIR.0000128587.30041.C8. [DOI] [PubMed] [Google Scholar]
- Brunauer S, Emmett PH, Teller E. Adsorption of gases in multimolecular layers. J. Am. Chem. Soc. 1938;60:309–319. [Google Scholar]
- Campen MJ, Babu NS, Helms GA, Pett S, Wernly J, Mehran R, McDonald JD. Nonparticulate components of diesel exhaust promote constriction in coronary arteries from ApoE-/- mice. Toxicol Sci. 2005;88:95–102. doi: 10.1093/toxsci/kfi283. [DOI] [PubMed] [Google Scholar]
- Chen HW, Su SF, Chien CT, Lin WH, Yu SL, Chou CC, Chen JJ, Yang PC. Titanium dioxide nanoparticles induce emphysema-like lung injury in mice. FASEB J. 2006;20:2393–2395. doi: 10.1096/fj.06-6485fje. [DOI] [PubMed] [Google Scholar]
- Chilian WM, Eastham CL, Marcus ML. Microvascular distribution of coronary vascular resistance in beating left ventricle. Am J Physiol. 1986;251:H779–H788. doi: 10.1152/ajpheart.1986.251.4.H779. [DOI] [PubMed] [Google Scholar]
- de Haar C, Hassing I, Bol M, Bleumink R, Pieters R. Ultrafine but not fine particulate matter causes airway inflammation and allergic airway sensitization to co-administered antigen in mice. Clin Exp Allergy. 2006;36:1469–1479. doi: 10.1111/j.1365-2222.2006.02586.x. [DOI] [PubMed] [Google Scholar]
- Dockery DW. Epidemiologic evidence of cardiovascular effects of particulate air pollution. Environ Health Persp. 2001;109(Suppl 4):483–486. doi: 10.1289/ehp.01109s4483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominici F, McDermott A, Daniels M, Zeger SL, Samet JM. Revised analysis of the national morbidity, mortality and air pollution study: Mortality among residents in 90 cities. J. Toxicol. Environ. Health A. 2005;68:1071–1092. doi: 10.1080/15287390590935932. [DOI] [PubMed] [Google Scholar]
- Dreher KL. Health and environmental impact of nanotechnology: toxicological assessment of manufactured nanoparticles. Toxicol Sci. 2004;77:3–5. doi: 10.1093/toxsci/kfh041. [DOI] [PubMed] [Google Scholar]
- Godleski JJ, Verrier RL, Koutrakis P, Catalano P, Coull B, Reinisch U, Lovett EG, Lawrence J, Murthy GG, Wolfson JM, Clarke RW, Nearing BD, Killingsworth C. Mechanisms of morbidity and mortality from exposure to ambient air particles. Res Rep Health Eff Inst. 2000:5–88. discussion 89-103. [PubMed] [Google Scholar]
- Goldberg MS, Bailar JC, 3rd, Burnett RT, Brook JR, Tamblyn R, Bonvalot Y, Ernst P, Flegel KM, Singh RK, Valois MF. Identifying subgroups of the general population that may be susceptible to short-term increases in particulate air pollution: a time-series study in Montreal, Quebec. Res Rep Health Eff Inst. 2000:7–113. discussion 115-120. [PubMed] [Google Scholar]
- Goldberg MS, Burnett RT, Bailar JC, 3rd, Tamblyn R, Ernst P, Flegel K, Brook J, Bonvalot Y, Singh R, Valois MF, Vincent R. Identification of persons with cardiorespiratory conditions who are at risk of dying from the acute effects of ambient air particles. Environ Health Persp. 2001;109(Suppl 4):487–494. doi: 10.1289/ehp.01109s4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grassian VH, O'Shaughnessy P T, Adamcakova-Dodd A, Pettibone JM, Thorne PS. Inhalation exposure study of titanium dioxide nanoparticles with a primary particle size of 2 to 5 nm. Environ Health Persp. 2007;115:397–402. doi: 10.1289/ehp.9469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen CS, Sheykhzade M, Moller P, Folkmann JK, Amtorp O, Jonassen T, Loft S. Diesel exhaust particles induce endothelial dysfunction in apoE−/− mice. Toxicol Appl Pharmacol. 2007;219:24–32. doi: 10.1016/j.taap.2006.10.032. [DOI] [PubMed] [Google Scholar]
- He P, Zhang H, Zhu L, Jiang Y, Zhou X. Leukocyte-platelet aggregate adhesion and vascular permeability in intact microvessels: role of activated endothelial cells. Am J Physiol Heart Circ Physiol. 2006;291:H591–H599. doi: 10.1152/ajpheart.01228.2005. [DOI] [PubMed] [Google Scholar]
- Huang A, Sun D, Koller A, Kaley G. 17beta-estradiol restores endothelial nitric oxide release to shear stress in arterioles of male hypertensive rats. Circulation. 2000;101:94–100. doi: 10.1161/01.cir.101.1.94. [DOI] [PubMed] [Google Scholar]
- Hurum DC, Gray KA, Rajh T, Thurnauer MC. Recombination pathways in the Degussa P25 formulation of TiO2: surface versus lattice mechanisms. J Phys Chem B. 2005;109:977–980. doi: 10.1021/jp045395d. [DOI] [PubMed] [Google Scholar]
- Knuckles TL, Lund AK, Lucas SN, Campen MJ. Diesel exhaust exposure enhances venoconstriction via uncoupling of eNOS. Toxicol Appl Pharmacol. 2008;230:346–351. doi: 10.1016/j.taap.2008.03.010. [DOI] [PubMed] [Google Scholar]
- Kodavanti UP, Schlaweiler MC, Ledbetter a.D., Hauser R, Christiani DC, McGee J, Richards JR, Costa DL. Temporal association between pulmonary and systemic effects of particulate matter in healthy and cardiovascular compromised rats. J. Toxicol. Environ. Health A. 2002;65:1545–1569. doi: 10.1080/00984100290071667. [DOI] [PubMed] [Google Scholar]
- Kreyling W. Deposition, retention, and clearance of ultrafine particles. BIA-Workshop, Ultrafine Aerosols at Workplaces. 2003 http://www.hvbg.de/e/bia/pub/rep/rep04/pdf_datei/biar0703/topic_a.pdf.
- Kuo L, Davis M, Chilian WM. Endothelium-dependent, flow-induced dilation of isolated coronary arterioles. Am J Physiol. 1990;259:H1063–H1070. doi: 10.1152/ajpheart.1990.259.4.H1063. [DOI] [PubMed] [Google Scholar]
- Kuo L, Davis MJ, Chilian WM. Myogenic activity in isolated subepicardial and subendocardial coronary arterioles. Am J Physiol. 1988;255:H1558–H1562. doi: 10.1152/ajpheart.1988.255.6.H1558. [DOI] [PubMed] [Google Scholar]
- Kuo L, Davis MJ, Chilian WM. Longitudinal gradients for endothelium-dependent and -independent vascular responses in the coronary microcirculation. Circulation. 1995;92:518–525. doi: 10.1161/01.cir.92.3.518. [DOI] [PubMed] [Google Scholar]
- Murphy RA. Handbook of Physiology. The Cardiovascular System. The Vascular System. II. Am. Physiol. Soc.; Bethesda, MD: 1980. Mechanics of vascular smooth muscle. . pp. 325–352. section 2. [Google Scholar]
- NIOSH NIOSH Current Intelligence Bulletin: Evaluation of Health Hazard and Recommendation for Occupational Exposure to Titanium Dioxide. 2009 http://www.cdc.gov/niosh/review/public/TIO2/pdfs/TIO2Draft.pdf.
- Nurkiewicz TR, Boegehold MA. Limitation of arteriolar myogenic activity by local nitric oxide: segment-specific effect of dietary salt. Am J Physiol. 1999;277:H1946–H1955. doi: 10.1152/ajpheart.1999.277.5.H1946. [DOI] [PubMed] [Google Scholar]
- Nurkiewicz TR, Porter DW, Barger M, Millecchia L, Rao KM, Marvar PJ, Hubbs AF, Castranova V, Boegehold MA. Systemic microvascular dysfunction and inflammation after pulmonary particulate matter exposure. Environ Health Persp. 2006;114:412–419. doi: 10.1289/ehp.8413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurkiewicz TR, Porter DW, Hubbs AF, Cumpston JL, Chen BT, Frazer DG, Castranova V. Nanoparticle inhalation augments particle-dependent systemic microvascular dysfunction. Part Fibre Toxicol. 2008;5:1. doi: 10.1186/1743-8977-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurkiewicz TR, Porter DW, Hubbs AF, Stone S, Chen BT, Frazer DG, Boegehold MA, Castranova V. Pulmonary nanoparticle exposure disrupts systemic microvascular nitric oxide signaling. Toxicol Sci. 2009 doi: 10.1093/toxsci/kfp051. IN PRESS, http://toxsci.oxfordjournals.org/cgi/reprint/kfp051v051. [DOI] [PMC free article] [PubMed]
- O'Neill MS, Veves A, Zanobetti A, Sarnat JA, Gold DR, Economides PA, Horton ES, Schwartz J. Diabetes enhances vulnerability to particulate air pollution-associated impairment in vascular reactivity and endothelial function. Circulation. 2005;111:2913–2920. doi: 10.1161/CIRCULATIONAHA.104.517110. [DOI] [PubMed] [Google Scholar]
- Oberdorster G, Sharp Z, Atudorei V, Elder A, Gelein R, Kreyling W, Cox C. Translocation of inhaled ultrafine particles to the brain. Inhal Toxicol. 2004;16:437–445. doi: 10.1080/08958370490439597. [DOI] [PubMed] [Google Scholar]
- Pope CA, 3rd, Burnett RT, Thurston GD, Thun MJ, Calle EE, Krewski D, Godleski JJ. Cardiovascular mortality and long-term exposure to particulate air pollution: epidemiological evidence of general pathophysiological pathways of disease. Circulation. 2004;109:71–77. doi: 10.1161/01.CIR.0000108927.80044.7F. [DOI] [PubMed] [Google Scholar]
- Pope CA, 3rd, Muhlestein JB, May HT, Renlund DG, Anderson JL, Horne BD. Ischemic heart disease events triggered by short-term exposure to fine particulate air pollution. Circulation. 2006;114:2443–2448. doi: 10.1161/CIRCULATIONAHA.106.636977. [DOI] [PubMed] [Google Scholar]
- Sager TM, Kommineni C, Castranova V. Pulmonary response to intratracheal instillation of ultrafine versus fine titanium dioxide: role of particle surface area. Part Fibre Toxicol. 2008;5:17. doi: 10.1186/1743-8977-5-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samet JM, Dominici F, Curriero FC, Coursac I, Zeger SL. Fine particulate air pollution and mortality in 20 U.S. cities, 1987-1994. N Engl J Med. 2000;343:1742–1749. doi: 10.1056/NEJM200012143432401. [DOI] [PubMed] [Google Scholar]
- Schwartz J, Morris R. Air pollution and hospital admissions for cardiovascular disease in Detroit, Michigan. Am J Epidemiol. 1995;142:23–35. doi: 10.1093/oxfordjournals.aje.a117541. [DOI] [PubMed] [Google Scholar]
- Shieh KJ, Li M, Lee YH, Sheu SD, Liu YT, Wang YC. Antibacterial performance of photocatalyst thin film fabricated by defection effect in visible light. Nanomedicine. 2006;2:121–126. doi: 10.1016/j.nano.2006.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipley RD, Muller-Delp JM. Aging decreases vasoconstrictor responses of coronary resistance arterioles through endothelium-dependent mechanisms. Cardiovasc Res. 2005;66:374–383. doi: 10.1016/j.cardiores.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Stone KC, Mercer RR, Freeman BA, Chang LY, Crapo JD. Distribution of lung cell numbers and volumes between alveolar and nonalveolar tissue. Am Rev Respir Dis. 1992;146:454–456. doi: 10.1164/ajrccm/146.2.454. [DOI] [PubMed] [Google Scholar]
- Sun D, Meng TT, Loong TH, Hwa TJ. Removal of natural organic matter from water using a nano-structured photocatalyst coupled with filtration membrane. Water Sci Technol. 2004;49:103–110. [PubMed] [Google Scholar]
- Tamagawa E, Bai N, Morimoto K, Gray C, Mui T, Yatera K, Zhang X, Xing L, Li Y, Laher I, Sin DD, Man SF, van Eeden SF. Particulate matter exposure induces persistent lung inflammation and endothelial dysfunction. Am J Physiol Lung Cell Mol Physiol. 2008;295:L79–L85. doi: 10.1152/ajplung.00048.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tornqvist H, Mills NL, Gonzalez M, Miller MR, Robinson SD, Megson IL, Macnee W, Donaldson K, Soderberg S, Newby DE, Sandstrom T, Blomberg A. Persistent endothelial dysfunction in humans after diesel exhaust inhalation. Am J Respir Crit Care Med. 2007;176:395–400. doi: 10.1164/rccm.200606-872OC. [DOI] [PubMed] [Google Scholar]
- Vasiliev PO, Faure B, Ng JB, Bergstrom L. Colloidal aspects relating to direct incorporation of TiO2 nanoparticles into mesoporous spheres by an aerosol-assisted process. J Colloid Interface Sci. 2008;319:144–151. doi: 10.1016/j.jcis.2007.11.013. [DOI] [PubMed] [Google Scholar]
- Wallace WM, Goldstein K, Taylor A, Teree TM. Thermal dehydration of the rat: distribution of losses among tissues. Am J Physiol. 1970;219:1544–1548. doi: 10.1152/ajplegacy.1970.219.6.1544. [DOI] [PubMed] [Google Scholar]
- Wallenborn JG, McGee JK, Schladweiler MC, Ledbetter AD, Kodavanti UP. Systemic translocation of particulate matter-associated metals following a single intratracheal instillation in rats. Toxicol Sci. 2007;98:231–239. doi: 10.1093/toxsci/kfm088. [DOI] [PubMed] [Google Scholar]
- Watkinson WP, Campen MJ, Nolan JP, Costa DL. Cardiovascular and systemic responses to inhaled pollutants in rodents: effects of ozone and particulate matter. Environ Health Persp. 2001;109(Suppl 4):539–546. doi: 10.1289/ehp.01109s4539. [DOI] [PMC free article] [PubMed] [Google Scholar]