Abstract
Objectives
The aim of this paper is to review current investigations on functional assessments of osseointegration and assess correlations to the peri-implant structure.
Material and methods
The literature was electronically searched for studies of promoting dental implant osseointegration, functional assessments of implant stability, and finite element (FE) analyses in the field of implant dentistry, and any references regarding biological events during osseointegration were also cited as background information.
Results
Osseointegration involves a cascade of protein and cell apposition, vascular invasion, de novo bone formation and maturation to achieve the primary and secondary dental implant stability. This process may be accelerated by alteration of the implant surface roughness, developing a biomimetric interface, or local delivery of growth-promoting factors. The current available preclinical and clinical biomechanical assessments demonstrated a variety of correlations to the peri-implant structural parameters, and functionally integrated peri-implant structure through FE optimization can offer strong correlation to the interfacial biomechanics.
Conclusions
The progression of osseointegration may be accelerated by alteration of the implant interface as well as growth factor applications, and functional integration of peri-implant structure may be feasible to predict the implant function during osseointegration. More research in this field is still needed.
Keywords: finite element analysis, growth factor, bone-implant interactions
Introduction
Osseointegration, which histologically is defined as “direct bone-to-implant contact”, is believed to provide rigid fixation of a dental implant within the alveolar bone and may promote the long-term success of dental implants (Franchi et al., 2005, Joos et al., 2006). The processes of osseointegration involve an initial interlocking between alveolar bone and the implant body (primary implant stability), and later, biological fixation through continuous bone apposition (contact osteogenesis) and remodeling toward the implant (secondary implant stability) (Berglundh et al., 2003).
Stiffness of the tissue-implant interface and implant-supporting tissues are considered as the main determinant factors in osseointegration (Ramp and Jeffcoat, 2001, Turkyilmaz et al., 2008). While the structure and heterogeneity of mineralization affects the stiffness of bone (Hoffler et al., 2000), Johansson et al. (Johansson et al., 1998) demonstrated that biomechanical testing may be a more suitable indicator to evaluate the dynamic changes of osseointegration than any single structural parameter. However, biomechanical testing, such as push-out and pull-out measurements, is destructive and only available for preclinical use (Berzins et al., 1997). Therefore, the clinical value of non-destructive measurements, such as resonance frequency analysis (RFA) or damping characteristics (Periotest® technique), are still limited due to the lower resolution and higher variability during examinations (Aparicio et al., 2006). Thus, it is still of interest to develop effective approaches to functionally assess osseointegration for the evaluation of peri-implant wound healing and prognosis of implant therapy.
By reviewing the sequences of osseointegration and current efforts on promoting osseointegration, this paper is concentrated on the scientific significance of preclinical biomechanical testing and has characterized the state-of-the-art clinical functional assessments as well as the model analysis. According to the development of modern medical imaging techniques and mechanical modeling, the relationship between structural and biomechanical parameters were also described.
Timing of Osseointegration
While it has been demonstrated that excessive mobility may cause fibrous tissue formation and lead to failure of osseointegration (Huiskes et al., 1997, Lioubavina-Hack et al., 2006), in order to limit the micromotion and achieve primary stability of the implant, a slightly undersized osteotomy is usually prepared for press-fitting of the implant. However, a ~60 micrometer gap between the implant and host bone has been noted under microscopic investigations (Colnot et al., 2007, Futami et al., 2000), and depending on the extent of injury to the host bone, this gap may later extend to 100-500 micrometers (Eriksson et al., 1984). Therefore, this gap is filled with blood and forms a water layer incorporated with hydrated ions on the implant surfaces immediately after implant placement (Berglundh et al., 2003, Park and Davies, 2000). The small proteins adsorbed on the surface are subsequently replaced by larger proteins based on the ‘Vroman effect’. Although different implant surface properties may affect the composition and conformational states of the binding proteins, the biological aggregates on the surface interact with the cell extensions, cell membrane, membrane-bound proteins or receptors, and initial cell attachment eventually establishes on the implant surface (Kasemo and Gold, 1999). The interface area is first occupied by red blood cells, inflammatory cells, and degenerating cellular elements, then is gradually replaced with spindle-shaped or flattened cells, concurrent with initiation of osteolysis on the host bone surface until day 3 (Futami et al., 2000). Osteoblasts begin to attach and deposit collagen matrix at this stage (Meyer et al., 2004).
Early bone formation is not evident until days 5-7 (Berglundh et al., 2003, Colnot et al., 2007) and is consistent with the sequence of appositional matrix deposition and calcification from the lamina limitans of host bone onto the implant surface (Marco et al., 2005). Most of the interfacial zone is occupied by provisional matrix rich in collagen fibrils and vasculature, and woven bone can be observed around the vascular areas by day seven (Berglundh et al., 2003). Through continuous deposition, trabecular bone fills the initial gap and arranges in a three-dimensional network at day 14 (Franchi et al., 2005). The do novo formation of primary bone spongiosa offers not only a biological fixation to ensure secondary implant stability (Ferguson et al., 2006) but also a biological scaffold for cell attachment and bone deposition (Franchi et al., 2005). After 28 days, delineated bone marrow space and thickened bone trabeculae with parallel-fibered and lamellar bone can be found within the interfacial area. After 8 to 12 weeks, the interfacial area appears histologically to be completely replaced by mature lamellar bone in direct contact with titanium (Berglundh et al., 2003).
Implant Surface Alteration to Accelerate Osseointegration
The chemical composition or charges of the implant interface on the implant surface were shown to affect initial cell attachment (Kasemo and Gold, 1999). This has aroused great interest on implant surface modification as a way to accelerate the rate of osseointegration (Junker et al., 2009, Wennerberg and Albrektsson, 2009).
Surface Roughness
Depending on the scale of the features and based on the proposal of Wennerberg and Albrektsson (Wennerberg and Albrektsson, 2009), surface roughness can be divided into four categories (Lang and Jepsen, 2009):
Smooth surfaces: Sa value < 0.5 μm (e.g. polished abutment surface)
Minimally rough surfaces: Sa value 0.5 to < 1.0 μm (e.g. turned implants)
Moderately rough surfaces: Sa value 1.0 to < 2.0 μm (e.g. most commonly used types)
Rough surfaces: Sa value ≥ 2.0 μm (e.g. plasma sprayed surfaces).
Moderate roughness and roughness is associated with implant geometry, such as screw structure, and macroporous surface treatments. Previous studies demonstrated that this type of roughness allowed for bone ongrowth and provided mechanical interlocking shortly after implant placement (Berglundh et al., 2003, Franchi et al., 2005). Higher BIC and removal torque force suggested enhanced secondary stability compared to smooth and minimally rough implants (Buser et al., 1991, Wennerberg et al., 1996).
There are two main theories regarding the influence of implant surface microtopography on peri-implant tissue formation – 1) the surface energy and 2) the distortional strain. The smaller grain size on the surface results in higher surface energy, which is more favorable for cell adherence (Kilpadi and Lemons, 1994, Kim et al., 2008). Bowers and colleagues (Bowers et al., 1992) first demonstrated that the moderate roughness with sandblasted and acid-etching treatments significantly promoted cell attachment. Anselme and Bigerelle (Anselme and Bigerelle, 2005) later investigated long-term osteoblast adherence and behavior in vitro and demonstrated that a low amplitude of the surface roughness induced cell spreading more intimately than the rougher one. Therefore, the microtopography of the implant surface also influences differentiation events by providing the distortional signals. While osteoblastic cells show a cuboidal shape with polarized nuclei, the inactive bone-lining cells tended to have a flattened morphology without polarization (Kieswetter et al., 1996). Later studies further demonstrated that minor distortional strain and low compressive hydrostatic stress on mesenchymal stem cells were most likely for promoting osteogenic differentiation, whereas excessive distortional strain resulted in fibrogenesis as well as chondrogenesis, due to significant hydrostatic pressure (Andreykiv et al., 2008). Based on the mesenchymal cell size of about 5 to 12 μm in length, surface microtopographic pits with a 4 μm diameter and 1.5 μm depth are thought to be optimal for cells to attach and subsequently differentiate on the implant surface (Hansson and Norton, 1999, Schwartz et al., 1999).
Based on the large proportion of grain boundaries increasing surface energy, significant enhancement of cell attachment, proliferation, viability, spreading, and early osteogenic differentiation on these nano-/ultrafine-grained structures has been demonstrated in several investigations (Misra et al., 2009, Puckett et al., 2008, Brett et al., 2004). However, reproducible surface roughness on a nanoscale level is difficult to achieve, thus optimal surface nanotopography for rapid osseointegration is still not achievable (Le Guehennec et al., 2007).
Surface Coating and Biomimetic Approaches
Another category of implant surface modification is to coat the implant with layers of bioactive materials. One approach is to coat the titanium surface of implants with calcium phosphates, mainly composed of hydroxyapatite (HA), by plasma-spraying. The calcium phosphates are released to the peri-implant area after implantation and precipitated biological apatites, which serve as matrices for subsequent osteogenic cell attachment and growth (Junker et al., 2009, Le Guehennec et al., 2007). Compared to a titanium surface without coating, osteogenic cells attach, proliferate, and differentiate on the HA-coated surface (Knabe et al., 2004), and result in superior initial rates of osseointegration in vivo (Geurs et al., 2002). However, the delamination of the coating and particle release from the implant surface causes long-term failure in some studies (Chang et al., 1999, Lee et al., 2000a). To prevent this, recent investigations have focused on depositing HA onto the implant surface through biomimetic approaches, such as electrodeposition or immersion in SBF (Le Guehennec et al., 2007).
Implant surfaces may be also coated with biomolecules, such as bio-adhesive motifs or growth factors, to enhance osseointegration. The RGD sequence from fibronectin is the most commonly used bio-adhesive motif, which binds adhesion receptors and promotes cell adhesion (Shakesheff et al., 1998). RGD-functionalized, tissue-engineered constructs have shown improvement during early bone ingrowth and matrix mineralization in vivo (Alsberg et al., 2001, Lütolf et al., 2003). However, RGD immobilization on titanium implant surfaces has not improved bone-implant contact nor osteoblast differentiation (Schliephake et al., 2002, Tosatti et al., 2004), presumably due to neglecting the conformation-dependent effects and absence of crucial modulatory domains from the native fibronectin, thus diminishing the RGD signals through non-specific adsorption of plasma protein and interactions with inflammatory components (Garcia and Reyes, 2005).
Growth Factor Delivery to Accelerate Osseointegration
The rate of osseointegration is dependent on the commitment, replication, and differentiation of osteoprogenitor cells, and on interfacial tissue maturation (Brunski et al., 2000, Marie, 2003). Since growth factors, such as BMP and platelet-derived growth factor (PDGF), enhance osteogenesis and were suggested to regenerate the periodontal and dentoalveolar tissues (Ramseier et al., 2006, Taba et al., 2005), several of those biomolecules were also introduced to accelerate peri-implant wound healing and osseointegration (Table 1).
Table 1.
The Modes of Growth Factor Delivery for Promoting Dental Implant Osseointegration
| Growth Factor | Mechanisms | Delivery Mode | Reference |
|---|---|---|---|
| BMPs (−2 & −7) | Osteogenic lineage differentiation | Recombinant protein | (Barboza et al., 2004, Bianchi et al., 2004, Brandao et al., 2002, Cochran et al., 1999, Nevins et al., 1996) |
| Gene delivery | (Dunn et al., 2005) | ||
| PDGF-BB | Mitogenesis and chemotaxis of mesenchymal and osteogenic cells populations |
Recombinant protein | (Lee et al., 2000b, Nevins et al., 2005) |
| Gene delivery | (Jin et al., 2004, Chang et al., 2009a) | ||
| TGF-β | Mitogenesis of osteoblasts | Recombinant protein | (Ng et al., 2008, Xu et al., 2008) |
| IGFs (−1 & −2) | Collagen matrix production and stabilization, mitogenesis |
Recombinant protein | (Giustina et al., 2008) |
| FGF-2 | Mitogenesis and anti-apoptosis of osteoprogenitor cells | Recombinant protein | (Kitamura et al., 2008, Marie, 2003) |
| PDGF-BB/IGF-1 | Combinational effects of dual growth factors | Recombinant protein | (Becker et al., 1992, Stefani et al., 2000) |
| BMP-2/VEGF | Combinational effects of dual growth factors | Recombinant protein | (Huang et al., 2005, Patel et al., 2008) |
| BMP-2/FGF-2 | Combinational effects of dual growth factors | Recombinant protein | (Lan et al., 2006) |
| BMP-2/TGF-β | Combinational effects of dual growth factors | Recombinant protein | (Sumner et al., 2006) |
Abbreviations: BMP: bone morphogenetic protein; PDGF: platelet-derived growth factor; TGF: transforming growth factor; IGF: insulin-like growth factor, FGF: fibroblast growth factor; VEGF: vascular-endothelial growth factor
Bone Morphogenetic Proteins (BMPs)
Belonging to the transforming growth factor-beta (TGF-β) superfamily, BMPs have been proven to drive the multipotent cells into an osteogenic lineage and promote extracellular matrix formation through the Smad signaling pathway (Chen et al., 2004). Among all of the BMPs isoforms, BMP-2 and BMP-7 are the most commonly investigated. BMP can induce ectopic and periosteal bone formation in vivo (Chang et al., 2007, Hak et al., 2006). Within the dental field, BMP has been shown to promote tooth extraction socket healing, peri-implant wound healing, and sinus floor and alveolar ridge augmentation in preclinical studies (Barboza et al., 2004, Brandao et al., 2002, Cochran et al., 1999, Nevins et al., 1996, Dunn et al., 2005, Nakashima and Reddi, 2003). Some investigations have also reported that BMP exhibits superior short- but not long-term effects over controls (Jones et al., 2006, Jovanovic et al., 2007, Matin et al., 2001). In clinical trials, BMP tended to accelerate extraction socket and alveolar ridge augmentation compared to collagen vehicle alone within the period of 4-6 months (Bianchi et al., 2004, Howell et al., 1997). However, no significant difference could be found between BMP application and bone grafting in the treatment of sinus floor and alveolar ridge augmentation (Boyne et al., 2005, Jung et al., 2003).
Platelet-Derived Growth Factors (PDGFs)
PDGF is a potent mitogen and chemotactic factor for cells of mesenchymal origin, including periodontal ligament (PDL) cells and osteoblasts (Graves et al., 1994). PDGF can also regulate the expression of vascular endothelial growth factor (VEGF) to promote angiogenesis and is reported as an essential hormone in the healing process of soft tissue and bone (Hollinger et al., 2008). PDGF exists as a dimer form (-AA, -AB, -BB, -CC, and -DD) and signals through binding to tyrosine kinase receptors, termed PDGF receptors alpha and beta (Seifert et al., 1989), with PDGF-BB the most widely used isoform of PDGF based on its capability to bind to all known PDGF receptor isotypes (Hollinger et al., 2008).
PDGF plays an indirect role in osteogenesis by recruiting and expanding the osteogenic cell populations, and subsequent differentiation of those cells is achieved by BMPs (Chaudhary and Hruska, 2001, Cho et al., 2002). In vivo investigations also indicate that applying PDGF to denuded tooth root surfaces increase proliferation of PDL cells, osteoblasts, and perivascular cells, and accelerate alveolar bone regeneration (Giannobile et al., 1996, Park et al., 1995, Wang et al., 1994). A multicenter clinical trial validated PDGF-BB is capable of promoting periodontal defect regeneration (Nevins et al., 2005). Furthermore, a significant amount of in vivo bone regeneration was also noted in a ‘pure’ orthopaedic environment such as the calvarial or femoral critical-sized osteomtomy using a combination of calcium phosphate graft and PDGF (Lee et al., 2000b, Nash et al., 1994). Combination of PDGF and insulin-like growth factor-1 (IGF-1) had shown to stimulate bone regeneration around the press-fit titanium implants (Becker et al., 1992, Lynch et al., 1991). Recently Chang et al. demonstrated the PDGF protein or gene delivery was capable of accelerating oral implant osseointegration in vivo as well as improving biomechanical properties (Chang et al., 2009a).
On the other hand, the possible inhibitory effects to osteogenesis have also been documented. Kono and colleague reported that PDGF treatment negatively regulates osteogenic differentiation (Kono et al., 2007), and Tokunaga et al demonstrated that specifically the PDGF receptor beta had a determinable effect on mesenchymal cell differentiation (Tokunaga et al., 2008). Therefore, the bidirectional effect on osteogenesis is associated with the expression profile of PDGF, with pulse PDGF application stimulating osteogenesis while continuous PDGF exposure elicits an inhibitory effect (Hsieh and Graves, 1998).
Other Growth Factors and Combinations
Besides BMP and PDGF, there are several growth factors being investigated for accelerating osteogenesis, such as transforming growth factor-beta (TGF-β), insulin-like growth factor (IGF), and fibroblast growth factor (FGF) (Andrades et al., 1999, Mukherjee and Rotwein, 2009). TGF-β has been proposed as an osteoinductive factor based on its ability to promote proliferation of osteoblasts (Macdonald et al., 2007). However, studies also demonstrate that TGF-β enhances chondrogenesis rather than osteogenesis in MSCs (Ng et al., 2008, Xu et al., 2008). IGF-1 and IGF-2 regulate the bone formation process through increasing type I collagen synthesis, decreasing collagen degradation, modestly enhancing mitogenesis, and stabilizing α-catenin, a key regulator in Wnt pathway of osteogenic differentiation (Giustina et al., 2008). FGF-2 promotes mitogenesis and reduces apoptosis of osteoprogenitor cells, which increases the population of functional osteoblasts, but induces apoptosis in more differentiated osteoblasts, thus limiting the early increase of mature cells in the osteoblast pool (Marie, 2003). A recent clinical investigation demonstrated that FGF-2 significantly increased the alveolar bone height after 36 weeks in patients with periodontitis suggesting that FGF-2 could be a potential stimulator for bone regeneration (Kitamura et al., 2008).
The process of osteogenesis is regulated through several growth factors, and cross-talk most likely exists among them (Marie, 2003, Singhatanadgit et al., 2006). Thus, combination of growth factors is a viable approach to amplify osteogenesis. The first approach was proposed based on the synergistic effects on wound healing using a combination of PDGF-BB and IGF-1 (Lynch et al., 1989a). This combination exhibited greater alveolar bone and cementum regeneration than single growth factor application (Giannobile et al., 1996, Lynch et al., 1989b), and promoted initial dental implant osseointegration in later investigations (Becker et al., 1992, Lynch et al., 1991, Stefani et al., 2000). The combination of angiogenic (ie., VEGF) and osteogenic growth factors (ie., BMP) promoted bone regeneration (Huang et al., 2005, Patel et al., 2008), and dual delivery of BMP/TGF-β or BMP/FGF also enhanced osseointegration in vivo (Lan et al., 2006, Sumner et al., 2006). However, application should be controlled by sequential release profile of the growth factors in order to maximize the beneficial effects of combinatorial delivery (Kempen et al., 2009).
Preclinical Biomechanical Assessments for Osseointegration
Tensional Test
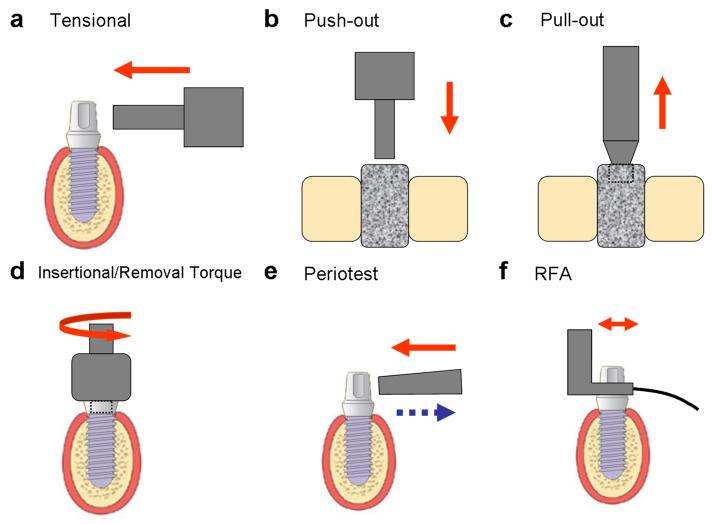
The interfacial tensile strength was originally measured by detaching the implant plate from the supporting bone (Kitsugi et al., 1996) (Table 2). Brånemark later modified this technique by applying the lateral load to the cylindrical fixture (Brånemark et al., 1998) (Fig 1a). However, they also addressed the difficulties of translating the test results to any area-independent mechanical properties.
Table 2.
Current Biomechanical Assessments for Dental Implant Osseointegration
| Methodology | Destructive | Clinical use | Property Investigated |
Parameters | Reference |
|---|---|---|---|---|---|
| Tensional test | Yes | No | Lateral resistance | Maximal lateral load | (Brånemark et al., 1998, Kitsugi et al., 1996) |
| Push-out/Pull-out | Yes | No | Interfacial shear | Maximal force Interfacial stiffness |
(Berzins et al., 1997, Brunski et al., 2000) |
| Removal Torque | Yes | No | Interfacial shear | Loosening torque Torque load |
(Johansson et al., 1998, Meredith et al., 1997) |
| Cutting Resistance/Insertional Torque |
No | Yes | Interfacial shear | Peak insertional torque Torque load |
(Friberg et al., 1995, O’Sullivan et al., 2000) |
| Periotest | No | Yes | Damping | Periostest value (PTV) | (Aparicio et al., 2006, Schulte and Lukas, 1993) |
| Resonance Frequency Analysis | No | Yes | Vibration/Damping | Implant stability quotient (ISQ) |
(Friberg et al., 1999, Meredith et al., 1997, Turkyilmaz et al., 2009) |
Figure 1. Biomechanical assessments for oral implant osseointegration.
(a) tensional test, (b) push-out test, (c) pull-out test, (d) insertional/removal torque test, (e) Periotest, and (e) resonance frequency analysis (RFA).
Push-out/Pull-out Test
The ‘push-out’ or ‘pull-out’ test is the most commonly used approach to investigate the healing capabilities within the bone-implant interface (Brunski et al., 2000, Kempen et al., 2009). In the typical push-out or pull-out test, a cylinder-type implant is placed transcortically or intramedullarly in bone structures and then removed by applying a force parallel to the interface (Fig 1b-c). The maximum load capability (or failure load) is defined as the maximum force on the force-displacement plot, and the interfacial stiffness is visualized as the slope of a tangent approximately at the linear region of the force-displacement curve prior to breakpoint (Brunski et al., 2000, Lütolf et al., 2003) (Table 2). Therefore, the general loading capacity of the interface (or interfacial shear strength) can be measured by dividing the maximum force by the area of implant in contact with the host bone (Berzins et al., 1997). However, the push-out and pull-out tests are only applicable for non-threaded cylinder type implants, whereas most of clinically available fixtures are of threaded design, and their interfacial failures are solely dependent on shear stress without any consideration for either tensile or compressive stresses (Brunski et al., 2000).
Removal Torque
The removal torque refers to the torsional force necessary for unscrewing the fixture (Fig 1d) and was first investigated by Johansson and coworkers (Johansson et al., 1998). The removal torque value was recorded using a torque manometer calibrated in Newton-centimeters (Ncm). This technique primarily focuses on interfacial shear properties (Table 2). However, the results may be affected by implant geometry and topography (Meredith et al., 1997, Yeo et al., 2008).
Combination of Push-out/pull-out and Removal Torque
This combinational trial was introduced by Brånemark and colleagues by applying torsional force until reaching the maximum torque and then pulling the implant out (Brånemark et al., 1998). In this investigation, the removal torque was related to the interfacial bonding capability, and the pull-out strength was related to the shear properties from the implant-supporting structure.
Clinical Biomechanical Assessments for Osseointegration
Cutting Resistance/Insertional Torque
The cutting resistance refers to the energy required in cutting of a unit volume of bone (Friberg et al., 1995) while the insertional torque occurs during the fixture tightening procedure (Ueda et al., 1991). Both of these measurements consider the lateral compression force and friction at the interface during implant insertion and are mainly influenced by the tolerance of the fixture thread design (O’Sullivan et al., 2000). Many researchers also used the peak insertional torque value, which is generated during the last fixture tightening step, as an indicator of primary implant stability (Table 2). A positive correlation between insertional and removal torque is evident however, any relationship between the cutting resistance and the peak insertional torque is still unclear (Molly, 2006).
Periotest®
Significant deformation of the bone-implant unit is not measurable for most clinical situations. To overcome this limitation, damping characteristics, or the dynamic tissue recovery processes after loading, have been recommended for noninvasive assessment of osseointegration (Aparicio et al., 2006). A Periotest® (Siemens, Bensheim, Germany) was originally designed to assess the damping characteristics of the periodontal ligament (PDL) by calculating the contact time between the test subject and the percussion rod (Figure 1e) and are reported as Periotest value (PTV) (Schulte and Lukas, 1993) (Table 2).
The main limitation of the Periotest® is a lack of sensitivity in evaluating osseointegration, whereby the range of PTV in osseointegrated implants falls to a narrow zone (−5 to +5) within a wide scale (−8 to +50) (Olive and Aparicio, 1990). This could be accounted for by physical differences between periodontium and the bone-implant interface, because bone is much stiffer and does not allow for significant deformation as compared to the soft tissue of the periodontium (Meredith et al., 1997). Moreover, results may also be influenced by the position and direction of the percussion rod (Schulte and Lukas, 1992).
Resonance Frequency Analysis (RFA)
RFA was first introduced by Meredith and co-workers (Meredith et al., 1997). An L-shaped transducer connected to the implant was utilized to provide a high frequency mechanical vibration and record the frequency and amplitude of the signal received (Fig 1f). The resonance frequency was thus defined as the peak of the frequency-amplitude plot and converted to a value representing stiffness of the bone-implant interface. Currently, Osstell® (Integration Diagnostic AB, Goteborg, Sweden), a commercialized product utilizing the concept of RFA, has translated the resonance frequency ranging from 3000 to 8500 Hz as the implant stability quotient (ISQ) of 0 to 100 (Atsumi et al., 2007) (Table 2).
While moderate to strong correlation is found between cutting resonance and ISQ value upon implant placement (Friberg et al., 1999, Turkyilmaz et al., 2008), and because of the noninvasive nature of the measurement, RFA has been widely used for clinically assessing osseointegration, as well as for prognostic evaluation (Aparicio et al., 2006, Meredith et al., 1997, Oates et al., 2009). However, the latter aspect still has to be questioned (Aparicio et al., 2006).
Relevance of the Peri-Implant Structure to Interfacial Biomechanics
Considering that intrinsic properties of the peri-implant bone may affect the stiffness of bone-implant interface (Bischof et al., 2004, Brunski, 1992), a number of studies have been initiated to provide insights of correlation between peri-implant structure and implant stability (Tables 3 and 4).
Table 3.
Correlation between Biomechanical Testing and Peri-Implant Structures (Primary Stability)
| Methodology | Model | Structure Assessment |
Structural Parameters |
Correlation | Reference (s) |
|---|---|---|---|---|---|
| PO | Canine | Histology | CBT | r=0.44 * | (Salmoria et al., 2008) |
| IT | Human cadaver | CT (3D) | BMD | r=0.690 * | (Turkyilmaz et al., 2009) |
| RFA | Human cadaver | CT (3D) | BMD | r=0.557 * | (Turkyilmaz et al., 2009) |
| IT | Human cadaver | Micro-CT (3D) | Tb.Th Tb.N Tb.Sp |
r=0.825 * r=0.718 * r=−0.795 * |
(Akça et al., 2006) |
| RFA | Human cadaver | Micro-CT (3D) | Tb.Th Tb.N Tb.Sp |
N.S. for any of parameter | (Akça et al., 2006) |
| IT | Human cadaver | CT (3D) | BMD | r2=0.81 * | (Homolka et al., 2002) |
| IT | Human | CT (3D) | BMD | r=0.10-0.83 * | (Turkyilmaz et al., 2007) |
| RFA | Human | CT (3D) | BMD | r=0.34-0.91 * | (Turkyilmaz et al., 2007) |
| IT | Human cadaver | CT (3D) | BMD | r=0.86 * | (Beer et al., 2003) |
| IT | Human | CT (3D) | BMD (ID<4 mm) BMD (ID>4 mm) |
r=0.33-0.59 * r=0.05-0.29 |
(Turkyilmaz et al., 2006) |
| RT | Human cadaver | Calipers | CBT TBT |
p<0.05 * N.S. |
(Niimi et al., 1997) |
p<0.05
Abbreviations: IT: insertional torque; PO: pull-out; PS: push-out; RFA: resonance frequency analysis; CT: computed tomography; CBT: cortical bone thickness; BIC: bone-implant contact; BVD: bone-volume density; BMD: bone mineral density; BV/TV: bone volume/total volume; ID: implant diameter; TBT: total bone thickness; Tb.Th: trabecular thickness; Tb.N: trabecular number; Tb.Sp: trabecular separation; Conn.D: connectivity density; N.S.: no significant difference (p>0.05)
Table 4.
Correlation between Biomechanical Testing and Peri-Implant Structures (Secondary Stability)
| Methodology | Model | Structure Assessment | Structural Parameters | Correlation | Reference (s) |
|---|---|---|---|---|---|
| IT | Human | CT (2D) | CBT | r=0.320* | (Motoyoshi et al., 2007) |
| PO | Canine | Histology | CBT | N.S. | (Salmoria et al., 2008) |
| RFA | Human | Histology | BIC | p=0.016 * | (Scarano et al., 2006) |
| RFA | Canine | Histology | BIC BVD |
r=0.128, p=0.264 r=0.206, p=0.072 |
(Schliephake et al., 2002) |
| Periotest | Canine | Radiography | BIC | r=0.38 * | (Sykaras et al., 2004) |
| Periotest | Canine | Histology & radiography | BIC (His) BIC (Rad) BVD (His) |
r2=0.72 * r2=0.88 * r2=0.80 * |
(Ramp and Jeffcoat, 2001) |
| RFA | Porcine | Histology | BIC | r=0.221 * | (Ito et al., 2008) |
| RT | Rodent | Histology | BIC TBT |
r=0.78-0.84 * r=0.68-0.76 * |
(Brånemark et al., 1997) |
| PO | Rodent | Histology | TBT | r=0.87 * | (Brånemark et al., 1997) |
| PO | Rodent | Micro-CT (3D) | BIC BV/TV Tb.Th Tb.N Conn.D |
r2=0.52 (FL)* 0.24 (IS)* r2=0.72 (FL)* 0.43 (IS)* r2=0.60 (FL)* 0.31 (IS)* r2=0.47 (FL)* 0.32 (IS)* r2=0.37 (FL)* 0.28 (IS)* |
(Gabet et al., 2006) |
| PS | Rodent | Micro-CT (3D) & FE optimization | BV BMC BMD FBAM FCAM |
r= 0.67 (OA)* 0.34 (OS)* r= 0.70 (OA)* 0.71 (OS)* r= 0.61 (OA)* 0.62 (OS)* r= 0.96 (OA)* 0.84 (OS)* r= 0.74 (OA)* 0.95 (OS)* |
(Chang et al., 2009b) |
p<0.05
highest correlation coefficient for each parameter
Abbreviations: IT: insertional torque; PO: pull-out; PS: push-out; RFA: resonance frequency analysis; CT: computed tomography; CBT: cortical bone thickness; BIC: bone-implant contact; BV: bone volume; BVD: bone-volume density; BMC: bone mineral content; BMD: bone mineral density; BV/TV: bone volume/total volume; FBAM: functional bone apparent modulus; FCAM: functional composite tissue apparent modulus; ID: implant diameter; FL: failure load; IS: interfacial stiffness; OS: implant placing in osteotomy hole with osseous defect situation (0.6×1 mm circumferential); OA: implant placing in osteotomy-alone without any surrounding defect situation; TBT: total bone thickness; Tb.Th: trabecular thickness; Tb.N: trabecular number; Tb.Sp: trabecular separation; Conn.D: connectivity density; N.S.: no significant difference (p>0.05)
Correlations between Primary Implant Stability and Peri-Implant Structures
Considering that intrinsic properties of the peri-implant bone may affect the stiffness of bone-implant interface (Bischof et al., 2004, Brunski et al., 2000), a number of studies have undertaken to provide insight into the correlation between peri-implant structure and implant stability (Tables 3 and 4).
Correlations between Primary Implant Stability and Peri-Implant Structures
The relationship between the primary implant stability and peri-implant structures was first reported by Niimi and colleagues (Niimi et al., 1997). These authors applied torque to implants within the fibulae, iliac crest, and scapula of human cadavers and found that the removal torque value was significantly correlated to cortical bone thickness but was not associated with the trabecular bone area based on histological sections. This same correlation was also observed in a later investigation using implant pull out methods from dog mandibulae (Salmoria et al., 2008).
Primary implant stability may also be correlated to the bone mineral density (BMD) by analyzing and interpreting three-dimensional (3D) computed tomography (CT) images (Homolka et al., 2002, Turkyilmaz et al., 2008), and is strongly correlated with increasing implant diameter (Turkyilmaz et al., 2008). Akça and coworkers also found significant correlation between the trabecular bone structure and the insertional torque value (Akça et al., 2006). However, most of these investigations also revealed that the insertional torque value tended to be more sensitive to the peri-implant structure than the ISQ value (Table 3).
Correlations between Secondary Implant Stability and Peri-Implant Structures
An early preclinical study demonstrated a similar tendency of change in removal torque value and bone-implant contact over a period of time (Johansson et al., 1998), demonstrating that results could be influenced by implant topography or metal biocompatibility (Johansson et al., 1998, Wennerberg and Albrektsson, 2009). However, a relationship between the amount of bone within the threaded area and the removal torque value was not made clear from these approaches (Wennerberg et al., 1996). Because of the inability to perform biomechanical testing and structural analysis on the same specimens due to, at that time, a lack of reliable clinical biomechanical assessments or more definitive imaging techniques, careful review of those results appears to be necessary.
Measuring specimens during and after implant removal, Brånemark and co-workers demonstrated that the total bone thickness (TBT) 50 μm from the interface and bone implant contact area (BIC) were significantly correlated to the maximal and breakpoint torque, and the TBT also strongly correlated to the subsequent pull-out force (Brånemark et al., 1998). The correlation between insertional torque value and cortical bone thickness was recently reported (Motoyoshi et al., 2007). However, the opposite result was found from a study on dog mandibles, where the pull-out force was correlated to primary implant stability, but this correlation became non-significant in the latter healing stages (Salmoria et al., 2008).
Using non-destructive biomechanical assessments (ie., Periotest®, RFA) on dog mandibles, a high correlation was found between the mechanical impedance from the Periotest® and BIC as well as bone density from histology and radiography at 3 months post-implantation (Ramp and Jeffcoat, 2001). Significant correlations between PTV and BIC based on histology were also found (Sykaras et al., 2004). However, using a different treatment modality such as the pull-out test, the PTV was not sensitive to the osseous wound repair (Sykaras et al., 2004). Significant, but weak correlations between ISQ values and BIC were shown in some reports (Itoh et al., 2003, Scarano et al., 2006), whereas others failed to demonstrate such correlations (Schliephake et al., 2002). Moreover, recent investigations utilizing micro-CT technology also demonstrated a variety of moderate to strong correlations between the structural parameters (ie, BIC, bone volume, trabecular bone thickness, trabecular number, and connectivity density) and pull-out results, and different treatment strategies resulted in similar a correlation between the biomechanical and structural properties (Gabet et al., 2006).
Model Analysis for Osseointegration
Finite Element (FE) Analysis
FE analysis had been extensively used as a tool of functional assessments in the field of implant density over the past 2 decades (Geng et al., 2001). The FE model was built based on the pre-determined geometry of tissue and implant, material properties, and boundary conditions. Through applying the loading situation and numerical iteration, the functional performance of dental implant system could be expressed as specific values or gradient distribution of stress and strain in the model (Van Staden et al., 2006). Thus, FE analysis had been utilized to investigate the functional influence the implant geometry (Himmlova et al., 2004), material properties of implant (Yang and Xiang, 2007), quality of implant-supporting tissue (Petrie and Williams, 2007, Sevimay et al., 2005), fixture-prosthesis connection (Akça et al., 2003), and the loading condition (Mellal et al., 2004, Natali et al., 2006).
The bone-implant interface was considered as the boundary condition, and usually assigned as the pre-determined situation in FE model. Thus, the interfacial biomechanics have not been directly assessed from FE analysis (Van Staden et al., 2006). Therefore, in most of the FE model, the assignment of material properties was based on the theoretical value or references, and a simplified model following reasonable assumptions was usually suggested to reduce the complexity of iteration and assure the numerical convergence. The numerical artifacts may somewhat influence the accuracy of evaluations (Ladd and Kinney, 1998). Thus, the results from FE analyses should be carefully interpreted, and the experimental validation should be necessary if possible.
Functional Apparent Moduli
Homogenization of the mechanical properties to calculate the effective stiffness of bone was first introduced by Hollister and colleagues (Hollister et al., 1994). They acquired three-dimensional trabecular bone architecture from micro-CT imaging and investigated the stress and strain distribution of the elements under simulated loading conditions to calculate the effective Young’s modulus of the bulk specimen. The effective modulus revealed significant agreement with experimental results. Utilizing the concept of homogenization, later investigations by heterogeneous micro-elastic properties assignments demonstrated that the non-uniform mineral density and trabecular architecture could influence the effective tissue modulus (Morgan et al., 2004, Renders et al., 2008, van der Linden et al., 2001).
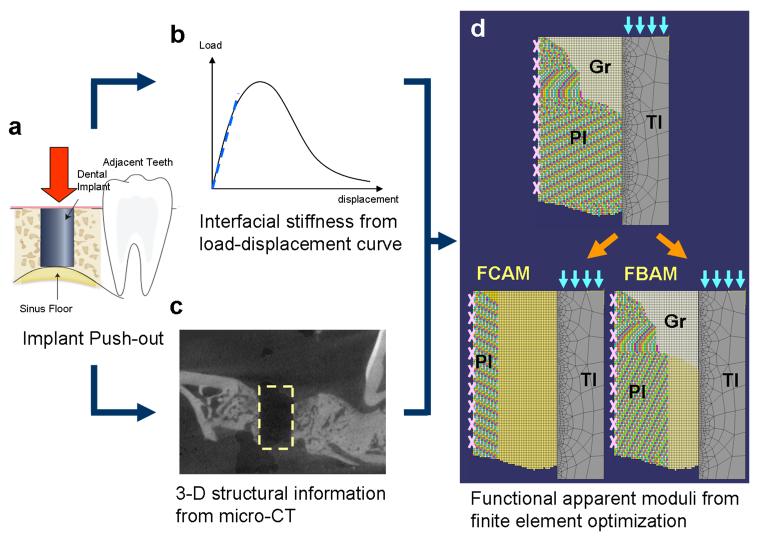
According to the unavailability of functionally evaluating peri-implant tissue, our group utilized the homogenization theory to calculate the effective stiffness of peri-implant tissue under loading from dental implant (Fig 2), whereas the functional bone apparent modulus (FBAM) represented the effective modulus of bone architecture, and functional composite tissue modulus (FCAM) for effective modulus of whole tissue within the wound (Chang et al., 2009b). Compared with individual structural parameters, the results indicated that the bone repair in early stage was to provide significant resistance to support the dental implant rather than fill the wound space or maturation. A much stronger correlation to interfacial biomechanics than all the other structural parameters was also noted (Table 4).
Figure 2. The in vivo finite element homogenization procedures for functional apparent moduli.
(a) The cylinder implant was pushed out (following the direction of the red arrow) from the jaw bone, and (b) the load-displacement relationship was recorded for calculating the interfacial stiffness (dash line, referred to the slope of the curve before the yielding point). (c) The three-dimensional (3-D) peri-implant structure was identified after removing the implant. (d) The finite element model was developed from projecting the peri-implant structure and interfacial information (microscopic model, upper panel) with the suspension boundary condition (pink marks on the peri-implant tissue border). In the optimizing model (lower panels), the peri-implant layer of interest was homogenized (yellow peri-implant regions), and the effective stiffness was calculated from the numerical approximation (to the microscopic model) under the implant loading condition (light blue arrows). Abbreviations: TI: titanium implant; PI: peri-implant tissue; Gr: granulation tissue in peri-implant area; FBAM: functional bone apparent modulus; FCAM: functional composite tissue apparent modulus
Conclusions
Although several approaches are available to assess implant stability (at the implant or surrounding host bone regions), limitations still exist to date, and no definite link between the function and peri-implant structure can be established. Functional apparent modulus through FE optimization is feasible to evaluate peri-implant osseous wound repair as well as interfacial biomechanics. Hence, integration of peri-implant structure may be necessary to predict the interfacial properties. However, further confirmation through preclinical and clinical models is still needed for investigating the mechanism involved in osseointegration and bone regeneration associated with oral implants.
Acknowledgments
Funding: This study was supported by the AO Foundation Switzerland and NIH/NIDCR DE 13397.
Reference
- Akça K, Cehreli MC, Iplikcioglu H. Evaluation of the mechanical characteristics of the implant-abutment complex of a reduced-diameter morse-taper implant. A nonlinear finite element stress analysis. Clinical Oral Implants Research. 2003;14:444–454. doi: 10.1034/j.1600-0501.2003.00828.x. [DOI] [PubMed] [Google Scholar]
- Akça K, Chang TL, Tekdemir I, Fanuscu MI. Biomechanical aspects of initial intraosseous stability and implant design: a quantitative micro-morphometric analysis. Clinical Oral Implants Research. 2006;17:465–472. doi: 10.1111/j.1600-0501.2006.01265.x. [DOI] [PubMed] [Google Scholar]
- Alsberg E, Anderson KW, Albeiruti A, Franceschi RT, Mooney DJ. Cell-interactive alginate hydrogels for bone tissue engineering. Journal of Dental Research. 2001;80:2025–2029. doi: 10.1177/00220345010800111501. [DOI] [PubMed] [Google Scholar]
- Andrades JA, Han B, Becerra J, Sorgente N, Hall FL, Nimni ME. A recombinant human TGF-beta1 fusion protein with collagen-binding domain promotes migration, growth, and differentiation of bone marrow mesenchymal cells. Experimental Cell Research. 1999;250:485–498. doi: 10.1006/excr.1999.4528. [DOI] [PubMed] [Google Scholar]
- Andreykiv A, van Keulen F, Prendergast PJ. Computational mechanobiology to study the effect of surface geometry on peri-implant tissue differentiation. J Biomechanical Engineering. 2008;130:051015. doi: 10.1115/1.2970057. [DOI] [PubMed] [Google Scholar]
- Anselme K, Bigerelle M. Topography effects of pure titanium substrates on human osteoblast long-term adhesion. Acta Biomaterialia. 2005;1:211–222. doi: 10.1016/j.actbio.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Aparicio C, Lang NP, Rangert B. Validity and clinical significance of biomechanical testing of implant/bone interface. Clinical Oral Implants Research. 2006;17(Suppl 2):2–7. doi: 10.1111/j.1600-0501.2006.01365.x. [DOI] [PubMed] [Google Scholar]
- Atsumi M, Park SH, Wang HL. Methods used to assess implant stability: current status. International Journal of Oral and Maxillofacial Implants. 2007;22:743–754. [PubMed] [Google Scholar]
- Barboza EP, Caula AL, Fde O. Caula, de Souza RO, Neto L. Geolas, Sorensen RG, Li XJ, Wikesjo UM. Effect of recombinant human bone morphogenetic protein-2 in an absorbable collagen sponge with space-providing biomaterials on the augmentation of chronic alveolar ridge defects. Journal of Periodontology. 2004;75:702–708. doi: 10.1902/jop.2004.75.5.702. [DOI] [PubMed] [Google Scholar]
- Becker W, Lynch SE, Lekholm U, Becker BE, Caffesse R, Donath K, Sanchez R. A comparison of ePTFE membranes alone or in combination with platelet-derived growth factors and insulin-like growth factor-I or demineralized freeze-dried bone in promoting bone formation around immediate extraction socket implants. Journal of Periodontology. 1992;63:929–940. doi: 10.1902/jop.1992.63.11.929. [DOI] [PubMed] [Google Scholar]
- Beer A, Gahleitner A, Holm A, Tschabitscher M, Homolka P. Correlation of insertion torques with bone mineral density from dental quantitative CT in the mandible. Clinical Oral Implants Research. 2003;14:616–620. doi: 10.1034/j.1600-0501.2003.00932.x. [DOI] [PubMed] [Google Scholar]
- Berglundh T, Abrahamsson I, Lang NP, Lindhe J. De novo alveolar bone formation adjacent to endosseous implants. Clinical Oral Implants Research. 2003;14:251–262. doi: 10.1034/j.1600-0501.2003.00972.x. [DOI] [PubMed] [Google Scholar]
- Berzins A, Shah B, Weinans H, Sumner DR. Nondestructive measurements of implant-bone interface shear modulus and effects of implant geometry in pull-out tests. J Biomedical Materials Research. 1997;34:337–340. doi: 10.1002/(sici)1097-4636(19970305)34:3<337::aid-jbm8>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Bianchi J, Fiorellini JP, Howell TH, Sekler J, Curtin H, Nevins ML, Friedland B. Measuring the efficacy of rhBMP-2 to regenerate bone: a radiographic study using a commercially available software program. International Journal of Periodontics and Restorative Dentistry. 2004;24:579–587. [PubMed] [Google Scholar]
- Bischof M, Nedir R, Szmukler-Moncler S, Bernard JP, Samson J. Implant stability measurement of delayed and immediately loaded implants during healing. Clinical Oral Implants Research. 2004;15:529–539. doi: 10.1111/j.1600-0501.2004.01042.x. [DOI] [PubMed] [Google Scholar]
- Bowers KT, Keller JC, Randolph BA, Wick DG, Michaels CM. Optimization of surface micromorphology for enhanced osteoblast responses in vitro. International Journal of Oral and Maxillofacial Implants. 1992;7:302–310. [PubMed] [Google Scholar]
- Boyne PJ, Lilly LC, Marx RE, Moy PK, Nevins M, Spagnoli DB, Triplett RG. De novo bone induction by recombinant human bone morphogenetic protein-2 (rhBMP-2) in maxillary sinus floor augmentation. Journal of Oral and Maxillofacial Surgery. 2005;63:1693–1707. doi: 10.1016/j.joms.2005.08.018. [DOI] [PubMed] [Google Scholar]
- Brandao AC, Brentegani LG, Novaes AB, Jr., Grisi MF, Souza SL, Junior M. Taba, Salata LA. Histomorphometric analysis of rat alveolar wound healing with hydroxyapatite alone or associated to BMPs. Brazilian Dental Journal. 2002;13:147–154. doi: 10.1590/s0103-64402002000300001. [DOI] [PubMed] [Google Scholar]
- Brånemark R, Ohrnell LO, Nilsson P, Thomsen P. Biomechanical characterization of osseointegration during healing: an experimental in vivo study in the rat. Biomaterials. 1997;18:969–978. doi: 10.1016/s0142-9612(97)00018-5. [DOI] [PubMed] [Google Scholar]
- Brånemark R, Ohrnell LO, Skalak R, Carlsson L, Branemark PI. Biomechanical characterization of osseointegration: an experimental in vivo investigation in the beagle dog. Journal of Orthopaedic Research. 1998;16:61–69. doi: 10.1002/jor.1100160111. [DOI] [PubMed] [Google Scholar]
- Brett PM, Harle J, Salih V, Mihoc R, Olsen I, Jones FH, Tonetti M. Roughness response genes in osteoblasts. Bone. 2004;35:124–133. doi: 10.1016/j.bone.2004.03.009. [DOI] [PubMed] [Google Scholar]
- Brunski JB. Biomechanical factors affecting the bone-dental implant interface. Clinical Materials. 1992;10:153–201. doi: 10.1016/0267-6605(92)90049-y. [DOI] [PubMed] [Google Scholar]
- Brunski JB, Puleo DA, Nanci A. Biomaterials and biomechanics of oral and maxillofacial implants: current status and future developments. International Journal of Oral and Maxillofacial Implants. 2000;15:15–46. [PubMed] [Google Scholar]
- Buser D, Schenk RK, Steinemann S, Fiorellini JP, Fox CH, Stich H. Influence of surface characteristics on bone integration of titanium implants. A histomorphometric study in miniature pigs. Journal of Biomedical Materials Research. 1991;25:889–902. doi: 10.1002/jbm.820250708. [DOI] [PubMed] [Google Scholar]
- Chang PC, Liu BY, Liu CM, Chou HH, Ho MH, Liu HC, Wang DM, Hou LT. Bone tissue engineering with novel rhBMP2-PLLA composite scaffolds. Journal of Biomedical Materials Research A. 2007;81:771–780. doi: 10.1002/jbm.a.31031. [DOI] [PubMed] [Google Scholar]
- Chang PC, Seol YJ, Cirelli JA, Pellegrini GR, Jin Q, Franco LM, Goldstein SA, Chandler LA, Sosnowski BK, Giannobile WV. PDGF-B Gene Therapy Accelerates Bone Engineering and Oral Implant Osseointegration. Gene Therapy. 2009a doi: 10.1038/gt.2009.117. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang PC, Seol YJ, Kikuchi N, Goldstein SA, Giannobile WV. In vivo FEA predicts functional oral implant osseointegration. Journal of Biomechanical Engineering. 2009b In press. [Google Scholar]
- Chang YL, Lew D, Park JB, Keller JC. Biomechanical and morphometric analysis of hydroxyapatite-coated implants with varying crystallinity. Journal of Oral and Maxillofacial Surgery. 1999;57:1096–1108. doi: 10.1016/s0278-2391(99)90333-6. discussion 1108-1099. [DOI] [PubMed] [Google Scholar]
- Chaudhary LR, Hruska KA. The cell survival signal Akt is differentially activated by PDGF-BB, EGF, and FGF-2 in osteoblastic cells. Journal of Cellular Biochemistry. 2001;81:304–311. [PubMed] [Google Scholar]
- Chen D, Zhao M, Mundy GR. Bone morphogenetic proteins. Growth Factors. 2004;22:233–241. doi: 10.1080/08977190412331279890. [DOI] [PubMed] [Google Scholar]
- Cho TJ, Gerstenfeld LC, Einhorn TA. Differential temporal expression of members of the transforming growth factor beta superfamily during murine fracture healing. J Bone Miner Res. 2002;17:513–520. doi: 10.1359/jbmr.2002.17.3.513. [DOI] [PubMed] [Google Scholar]
- Cochran DL, Schenk R, Buser D, Wozney JM, Jones AA. Recombinant human bone morphogenetic protein-2 stimulation of bone formation around endosseous dental implants. Journal of Periodontology. 1999;70:139–150. doi: 10.1902/jop.1999.70.2.139. [DOI] [PubMed] [Google Scholar]
- Colnot C, Romero DM, Huang S, Rahman J, Currey JA, Nanci A, Brunski JB, Helms JA. Molecular analysis of healing at a bone-implant interface. Journal of Dental Research. 2007;86:862–867. doi: 10.1177/154405910708600911. [DOI] [PubMed] [Google Scholar]
- Dunn CA, Jin Q, Taba M, Jr., Franceschi RT, Rutherford R. Bruce, Giannobile WV. BMP gene delivery for alveolar bone engineering at dental implant defects. Molecular Therapy. 2005;11:294–299. doi: 10.1016/j.ymthe.2004.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson RA, Albrektsson T, Magnusson B. Assessment of bone viability after heat trauma. A histological, histochemical and vital microscopic study in the rabbit. Scandinavian Journal of Plastic and Reconstructive Surgery. 1984;18:261–268. doi: 10.3109/02844318409052849. [DOI] [PubMed] [Google Scholar]
- Ferguson SJ, Broggini N, Wieland M, de Wild M, Rupp F, Geis-Gerstorfer J, Cochran DL, Buser D. Biomechanical evaluation of the interfacial strength of a chemically modified sandblasted and acid-etched titanium surface. Journal of Biomedical Materials Research A. 2006;78:291–297. doi: 10.1002/jbm.a.30678. [DOI] [PubMed] [Google Scholar]
- Franchi M, Fini M, Martini D, Orsini E, Leonardi L, Ruggeri A, Giavaresi G, Ottani V. Biological fixation of endosseous implants. Micron. 2005;36:665–671. doi: 10.1016/j.micron.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Friberg B, Sennerby L, Meredith N, Lekholm U. A comparison between cutting torque and resonance frequency measurements of maxillary implants. A 20-month clinical study. International Journal of Oral and Maxillofacial Surgery. 1999;28:297–303. [PubMed] [Google Scholar]
- Friberg B, Sennerby L, Roos J, Johansson P, Strid CG, Lekholm U. Evaluation of bone density using cutting resistance measurements and microradiography: an in vitro study in pig ribs. Clinical Oral Implants Research. 1995;6:164–171. doi: 10.1034/j.1600-0501.1995.060305.x. [DOI] [PubMed] [Google Scholar]
- Futami T, Fujii N, Ohnishi H, Taguchi N, Kusakari H, Ohshima H, Maeda T. Tissue response to titanium implants in the rat maxilla: ultrastructural and histochemical observations of the bone-titanium interface. Journal of Periodontology. 2000;71:287–298. doi: 10.1902/jop.2000.71.2.287. [DOI] [PubMed] [Google Scholar]
- Gabet Y, Muller R, Levy J, Dimarchi R, Chorev M, Bab I, Kohavi D. Parathyroid hormone 1-34 enhances titanium implant anchorage in low-density trabecular bone: a correlative micro-computed tomographic and biomechanical analysis. Bone. 2006;39:276–282. doi: 10.1016/j.bone.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Garcia AJ, Reyes CD. Bio-adhesive surfaces to promote osteoblast differentiation and bone formation. Journal of Dental Research. 2005;84:407–413. doi: 10.1177/154405910508400502. [DOI] [PubMed] [Google Scholar]
- Geng JP, Tan KB, Liu GR. Application of finite element analysis in implant dentistry: a review of the literature. Journal of Prosthetic Dentistry. 2001;85:585–598. doi: 10.1067/mpr.2001.115251. [DOI] [PubMed] [Google Scholar]
- Geurs NC, Jeffcoat RL, McGlumphy EA, Reddy MS, Jeffcoat MK. Influence of implant geometry and surface characteristics on progressive osseointegration. International Journal of Oral and Maxillofacial Implants. 2002;17:811–815. [PubMed] [Google Scholar]
- Giannobile WV, Hernandez RA, Finkelman RD, Ryan S, Kiritsy CP, D’Andrea M, Lynch SE. Comparative effects of platelet-derived growth factor-BB and insulin-like growth factor-I, individually and in combination, on periodontal regeneration in Macaca fascicularis. Journal of Periodontal Research. 1996;31:301–312. doi: 10.1111/j.1600-0765.1996.tb00497.x. [DOI] [PubMed] [Google Scholar]
- Giustina A, Mazziotti G, Canalis E. Growth hormone, insulin-like growth factors, and the skeleton. Endocrine Reviews. 2008;29:535–559. doi: 10.1210/er.2007-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves DT, Kang YM, Kose KN. Growth factors in periodontal regeneration. Compendium Supplement. 1994:S672–677. quiz S714-677. [PubMed] [Google Scholar]
- Hak DJ, Makino T, Niikura T, Hazelwood SJ, Curtiss S, Reddi AH. Recombinant human BMP-7 effectively prevents non-union in both young and old rats. Journal of Orthopaedic Research. 2006;24:11–20. doi: 10.1002/jor.20022. [DOI] [PubMed] [Google Scholar]
- Hansson S, Norton M. The relation between surface roughness and interfacial shear strength for bone-anchored implants. A mathematical model. Journal of Biomechanics. 1999;32:829–836. doi: 10.1016/s0021-9290(99)00058-5. [DOI] [PubMed] [Google Scholar]
- Himmlova L, Dostalova T, Kacovsky A, Konvickova S. Influence of implant length and diameter on stress distribution: a finite element analysis. Journal of Prosthetic Dentistry. 2004;91:20–25. doi: 10.1016/j.prosdent.2003.08.008. [DOI] [PubMed] [Google Scholar]
- Hoffler CE, Moore KE, Kozloff K, Zysset PK, Brown MB, Goldstein SA. Heterogeneity of bone lamellar-level elastic moduli. Bone. 2000;26:603–609. doi: 10.1016/s8756-3282(00)00268-4. [DOI] [PubMed] [Google Scholar]
- Hollinger JO, Hart CE, Hirsch SN, Lynch S, Friedlaender GE. Recombinant human platelet-derived growth factor: biology and clinical applications. Journal of Bone and Joint Surgery. American volume. 2008;90(Suppl 1):48–54. doi: 10.2106/JBJS.G.01231. [DOI] [PubMed] [Google Scholar]
- Hollister SJ, Brennan JM, Kikuchi N. A homogenization sampling procedure for calculating trabecular bone effective stiffness and tissue level stress. Journal of Biomechanics. 1994;27:433–444. doi: 10.1016/0021-9290(94)90019-1. [DOI] [PubMed] [Google Scholar]
- Homolka P, Beer A, Birkfellner W, Nowotny R, Gahleitner A, Tschabitscher M, Bergmann H. Bone mineral density measurement with dental quantitative CT prior to dental implant placement in cadaver mandibles: pilot study. Radiology. 2002;224:247–252. doi: 10.1148/radiol.2241010948. [DOI] [PubMed] [Google Scholar]
- Howell TH, Fiorellini J, Jones A, Alder M, Nummikoski P, Lazaro M, Lilly L, Cochran D. A feasibility study evaluating rhBMP-2/absorbable collagen sponge device for local alveolar ridge preservation or augmentation. International Journal of Periodontics and Restorative Dentistry. 1997;17:124–139. [PubMed] [Google Scholar]
- Hsieh SC, Graves DT. Pulse application of platelet-derived growth factor enhances formation of a mineralizing matrix while continuous application is inhibitory. Journal of Cellular Biochemistry. 1998;69:169–180. doi: 10.1002/(sici)1097-4644(19980501)69:2<169::aid-jcb7>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Huang YC, Kaigler D, Rice KG, Krebsbach PH, Mooney DJ. Combined angiogenic and osteogenic factor delivery enhances bone marrow stromal cell-driven bone regeneration. Journal of Bone and Mineral Research. 2005;20:848–857. doi: 10.1359/JBMR.041226. [DOI] [PubMed] [Google Scholar]
- Huiskes R, Van Driel WD, Prendergast PJ, Soballe K. A biomechanical regulatory model for periprosthetic fibrous-tissue differentiation. Journal of Material Science. Materials in Medicine. 1997;8:785–788. doi: 10.1023/a:1018520914512. [DOI] [PubMed] [Google Scholar]
- Ito Y, Sato D, Yoneda S, Ito D, Kondo H, Kasugai S. Relevance of resonance frequency analysis to evaluate dental implant stability: simulation and histomorphometrical animal experiments. Clinical Oral Implants Research. 2008;19:9–14. doi: 10.1111/j.1600-0501.2007.01419.x. [DOI] [PubMed] [Google Scholar]
- Itoh K, Suzuki S, Kuroda T. Effects of local administration of insulin-like growth factor-I on mandibular condylar growth in rats. Journal of Medical and Dental Sciences. 2003;50:79–85. [PubMed] [Google Scholar]
- Jin Q, Anusaksathien O, Webb SA, Printz MA, Giannobile WV. Engineering of tooth-supporting structures by delivery of PDGF gene therapy vectors. Molecular Therapy. 2004;9:519–526. doi: 10.1016/j.ymthe.2004.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson CB, Han CH, Wennerberg A, Albrektsson T. A quantitative comparison of machined commercially pure titanium and titanium-aluminum-vanadium implants in rabbit bone. International Journal of Oral and Maxillofacial Implants. 1998;13:315–321. [PubMed] [Google Scholar]
- Jones AA, Buser D, Schenk R, Wozney J, Cochran DL. The effect of rhBMP-2 around endosseous implants with and without membranes in the canine model. Journal of Periodontology. 2006;77:1184–1193. doi: 10.1902/jop.2006.050337. [DOI] [PubMed] [Google Scholar]
- Joos U, Wiesmann HP, Szuwart T, Meyer U. Mineralization at the interface of implants. International Journal of Oral and Maxillofacial Surgery. 2006;35:783–790. doi: 10.1016/j.ijom.2006.03.013. [DOI] [PubMed] [Google Scholar]
- Jovanovic SA, Hunt DR, Bernard GW, Spiekermann H, Wozney JM, Wikesjo UM. Bone reconstruction following implantation of rhBMP-2 and guided bone regeneration in canine alveolar ridge defects. Clinical Oral Implants Research. 2007;18:224–230. doi: 10.1111/j.1600-0501.2006.01324.x. [DOI] [PubMed] [Google Scholar]
- Jung RE, Glauser R, Scharer P, Hammerle CH, Sailer HF, Weber FE. Effect of rhBMP-2 on guided bone regeneration in humans. Clinical Oral Implants Research. 2003;14:556–568. doi: 10.1034/j.1600-0501.2003.00921.x. [DOI] [PubMed] [Google Scholar]
- Junker R, Dimakis A, Thoneck M, Jansen J. Effects of implant surface coatings and composition on bone integration. Clinical Oral Implants Research. 2009;20(Suppl 4):187–208. doi: 10.1111/j.1600-0501.2009.01777.x. [DOI] [PubMed] [Google Scholar]
- Kasemo B, Gold J. Implant surfaces and interface processes. Advances in Dental Research. 1999;13:8–20. doi: 10.1177/08959374990130011901. [DOI] [PubMed] [Google Scholar]
- Kempen DH, Lu L, Heijink A, Hefferan TE, Creemers LB, Maran A, Yaszemski MJ, Dhert WJ. Effect of local sequential VEGF and BMP-2 delivery on ectopic and orthotopic bone regeneration. Biomaterials. 2009;30:2816–2825. doi: 10.1016/j.biomaterials.2009.01.031. [DOI] [PubMed] [Google Scholar]
- Kieswetter K, Schwartz Z, Dean DD, Boyan BD. The role of implant surface characteristics in the healing of bone. Critical Reviews in Oral Biology and Medicine. 1996;7:329–345. doi: 10.1177/10454411960070040301. [DOI] [PubMed] [Google Scholar]
- Kilpadi DV, Lemons JE. Surface energy characterization of unalloyed titanium implants. Journal of Biomedical Materials Research. 1994;28:1419–1425. doi: 10.1002/jbm.820281206. [DOI] [PubMed] [Google Scholar]
- Kim TN, Balakrishnan A, Lee BC, Kim WS, Dvorankova B, Smetana K, Park JK, Panigrahi BB. In vitro fibroblast response to ultra fine grained titanium produced by a severe plastic deformation process. Journal of Material Science. Materials in Medicine. 2008;19:553–557. doi: 10.1007/s10856-007-3204-5. [DOI] [PubMed] [Google Scholar]
- Kitamura M, Nakashima K, Kowashi Y, Fujii T, Shimauchi H, Sasano T, Furuuchi T, Fukuda M, Noguchi T, Shibutani T, Iwayama Y, Takashiba S, Kurihara H, Ninomiya M, Kido J, Nagata T, Hamachi T, Maeda K, Hara Y, Izumi Y, Hirofuji T, Imai E, Omae M, Watanuki M, Murakami S. Periodontal tissue regeneration using fibroblast growth factor-2: randomized controlled phase II clinical trial. PLoS ONE. 2008;3:e2611. doi: 10.1371/journal.pone.0002611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitsugi T, Nakamura T, Oka M, Yan WQ, Goto T, Shibuya T, Kokubo T, Miyaji S. Bone bonding behavior of titanium and its alloys when coated with titanium oxide (TiO2) and titanium silicate (Ti5Si3) Journal of Biomedical Materials Research. 1996;32:149–156. doi: 10.1002/(SICI)1097-4636(199610)32:2<149::AID-JBM1>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Knabe C, Howlett CR, Klar F, Zreiqat H. The effect of different titanium and hydroxyapatite-coated dental implant surfaces on phenotypic expression of human bone-derived cells. Journal of Biomedical Materials Research A. 2004;71:98–107. doi: 10.1002/jbm.a.30130. [DOI] [PubMed] [Google Scholar]
- Kono SJ, Oshima Y, Hoshi K, Bonewald LF, Oda H, Nakamura K, Kawaguchi H, Tanaka S. Erk pathways negatively regulate matrix mineralization. Bone. 2007;40:68–74. doi: 10.1016/j.bone.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Ladd AJ, Kinney JH. Numerical errors and uncertainties in finite-element modeling of trabecular bone. Journal of Biomechanics. 1998;31:941–945. doi: 10.1016/s0021-9290(98)00108-0. [DOI] [PubMed] [Google Scholar]
- Lan J, Wang Z, Wang Y, Wang J, Cheng X. The effect of combination of recombinant human bone morphogenetic protein-2 and basic fibroblast growth factor or insulin-like growth factor-I on dental implant osseointegration by confocal laser scanning microscopy. Journal of Periodontology. 2006;77:357–363. doi: 10.1902/jop.2006.050016. [DOI] [PubMed] [Google Scholar]
- Lang NP, Jepsen S. Implant surfaces and design. Consensus report of working group 4. Clinical Oral Implants Research. 2009;20(Suppl 4):230–233. doi: 10.1111/j.1600-0501.2009.01771.x. [DOI] [PubMed] [Google Scholar]
- Le Guehennec L, Soueidan A, Layrolle P, Amouriq Y. Surface treatments of titanium dental implants for rapid osseointegration. Dental Materials. 2007;23:844–854. doi: 10.1016/j.dental.2006.06.025. [DOI] [PubMed] [Google Scholar]
- Lee JJ, Rouhfar L, Beirne OR. Survival of hydroxyapatite-coated implants: a meta-analytic review. Journal of Oral and Maxillofacial Surgery. 2000a;58:1372–1379. doi: 10.1053/joms.2000.18269. discussion 1379-1380. [DOI] [PubMed] [Google Scholar]
- Lee YM, Park YJ, Lee SJ, Ku Y, Han SB, Klokkevold PR, Chung CP. The bone regenerative effect of platelet-derived growth factor-BB delivered with a chitosan/tricalcium phosphate sponge carrier. Journal of Periodontology. 2000b;71:418–424. doi: 10.1902/jop.2000.71.3.418. [DOI] [PubMed] [Google Scholar]
- Lioubavina-Hack N, Lang NP, Karring T. Significance of primary stability for osseointegration of dental implants. Clinical Oral Implants Research. 2006;17:244–250. doi: 10.1111/j.1600-0501.2005.01201.x. [DOI] [PubMed] [Google Scholar]
- Lütolf MP, Weber FE, Schmoekel HG, Schense JC, Kohler T, Muller R, Hubbell JA. Repair of bone defects using synthetic mimetics of collagenous extracellular matrices. Nature Biotechnology. 2003;21:513–518. doi: 10.1038/nbt818. [DOI] [PubMed] [Google Scholar]
- Lynch SE, Buser D, Hernandez RA, Weber HP, Stich H, Fox CH, Williams RC. Effects of the platelet-derived growth factor/insulin-like growth factor-I combination on bone regeneration around titanium dental implants. Results of a pilot study in beagle dogs. Journal of Periodontology. 1991;62:710–716. doi: 10.1902/jop.1991.62.11.710. [DOI] [PubMed] [Google Scholar]
- Lynch SE, Colvin RB, Antoniades HN. Growth factors in wound healing. Single and synergistic effects on partial thickness porcine skin wounds. Journal of Clinical Investigations. 1989a;84:640–646. doi: 10.1172/JCI114210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch SE, Williams RC, Polson AM, Howell TH, Reddy MS, Zappa UE, Antoniades HN. A combination of platelet-derived and insulin-like growth factors enhances periodontal regeneration. Journal of Clinical Periodontology. 1989b;16:545–548. doi: 10.1111/j.1600-051x.1989.tb02334.x. [DOI] [PubMed] [Google Scholar]
- Macdonald KK, Cheung CY, Anseth KS. Cellular delivery of TGFbeta1 promotes osteoinductive signalling for bone regeneration. Journal of Tissue Engineering and Regenerative Medicine. 2007;1:314–317. doi: 10.1002/term.31. [DOI] [PubMed] [Google Scholar]
- Marco F, Milena F, Gianluca G, Vittoria O. Peri-implant osteogenesis in health and osteoporosis. Micron. 2005;36:630–644. doi: 10.1016/j.micron.2005.07.008. [DOI] [PubMed] [Google Scholar]
- Marie PJ. Fibroblast growth factor signaling controlling osteoblast differentiation. Gene. 2003;316:23–32. doi: 10.1016/s0378-1119(03)00748-0. [DOI] [PubMed] [Google Scholar]
- Matin K, Nakamura H, Irie K, Ozawa H, Ejiri S. Impact of recombinant human bone morphogenetic protein-2 on residual ridge resorption after tooth extraction: an experimental study in the rat. International Journal of Oral and Maxillofacial Implants. 2001;16:400–411. [PubMed] [Google Scholar]
- Mellal A, Wiskott HW, Botsis J, Scherrer SS, Belser UC. Stimulating effect of implant loading on surrounding bone. Comparison of three numerical models and validation by in vivo data. Clinical Oral Implants Research. 2004;15:239–248. doi: 10.1111/j.1600-0501.2004.01000.x. [DOI] [PubMed] [Google Scholar]
- Meredith N, Shagaldi F, Alleyne D, Sennerby L, Cawley P. The application of resonance frequency measurements to study the stability of titanium implants during healing in the rabbit tibia. Clinical Oral Implants Research. 1997;8:234–243. doi: 10.1034/j.1600-0501.1997.080310.x. [DOI] [PubMed] [Google Scholar]
- Meyer U, Joos U, Mythili J, Stamm T, Hohoff A, Fillies T, Stratmann U, Wiesmann HP. Ultrastructural characterization of the implant/bone interface of immediately loaded dental implants. Biomaterials. 2004;25:1959–1967. doi: 10.1016/j.biomaterials.2003.08.070. [DOI] [PubMed] [Google Scholar]
- Misra RD, Thein-Han WW, Pesacreta TC, Hasenstein KH, Somani MC, Karjalainen LP. Cellular response of preosteoblasts to nanograined/ultrafine-grained structures. Acta Biomaterialia. 2009;5:1455–1467. doi: 10.1016/j.actbio.2008.12.017. [DOI] [PubMed] [Google Scholar]
- Molly L. Bone density and primary stability in implant therapy. Clinical Oral Implants Research. 2006;17(Suppl 2):124–135. doi: 10.1111/j.1600-0501.2006.01356.x. [DOI] [PubMed] [Google Scholar]
- Morgan EF, Bayraktar HH, Yeh OC, Majumdar S, Burghardt A, Keaveny TM. Contribution of inter-site variations in architecture to trabecular bone apparent yield strains. Journal of Biomechanics. 2004;37:1413–1420. doi: 10.1016/j.jbiomech.2003.12.037. [DOI] [PubMed] [Google Scholar]
- Motoyoshi M, Yoshida T, Ono A, Shimizu N. Effect of cortical bone thickness and implant placement torque on stability of orthodontic mini-implants. International Journal of Oral and Maxillofacial Implants. 2007;22:779–784. [PubMed] [Google Scholar]
- Mukherjee A, Rotwein P. Akt promotes BMP2-mediated osteoblast differentiation and bone development. Joural of Cell Science. 2009;122:716–726. doi: 10.1242/jcs.042770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashima M, Reddi AH. The application of bone morphogenetic proteins to dental tissue engineering. Nature Biotechnology. 2003;21:1025–1032. doi: 10.1038/nbt864. [DOI] [PubMed] [Google Scholar]
- Nash TJ, Howlett CR, Martin C, Steele J, Johnson KA, Hicklin DJ. Effect of platelet-derived growth factor on tibial osteotomies in rabbits. Bone. 1994;15:203–208. doi: 10.1016/8756-3282(94)90709-9. [DOI] [PubMed] [Google Scholar]
- Natali AN, Pavan PG, Ruggero AL. Analysis of bone-implant interaction phenomena by using a numerical approach. Clinical Oral Implants Research. 2006;17:67–74. doi: 10.1111/j.1600-0501.2005.01162.x. [DOI] [PubMed] [Google Scholar]
- Nevins M, Giannobile WV, McGuire MK, Kao RT, Mellonig JT, Hinrichs JE, McAllister BS, Murphy KS, McClain PK, Nevins ML, Paquette DW, Han TJ, Reddy MS, Lavin PT, Genco RJ, Lynch SE. Platelet-derived growth factor stimulates bone fill and rate of attachment level gain: results of a large multicenter randomized controlled trial. Journal of Periodontology. 2005;76:2205–2215. doi: 10.1902/jop.2005.76.12.2205. [DOI] [PubMed] [Google Scholar]
- Nevins M, Kirker-Head C, Nevins M, Wozney JA, Palmer R, Graham D. Bone formation in the goat maxillary sinus induced by absorbable collagen sponge implants impregnated with recombinant human bone morphogenetic protein-2. International Journal of Periodontics and Restorative Dentistry. 1996;16:8–19. [PubMed] [Google Scholar]
- Ng F, Boucher S, Koh S, Sastry KS, Chase L, Lakshmipathy U, Choong C, Yang Z, Vemuri MC, Rao MS, Tanavde V. PDGF, TGF-beta, and FGF signaling is important for differentiation and growth of mesenchymal stem cells (MSCs): transcriptional profiling can identify markers and signaling pathways important in differentiation of MSCs into adipogenic, chondrogenic, and osteogenic lineages. Blood. 2008;112:295–307. doi: 10.1182/blood-2007-07-103697. [DOI] [PubMed] [Google Scholar]
- Niimi A, Ozeki K, Ueda M, Nakayama B. A comparative study of removal torque of endosseous implants in the fibula, iliac crest and scapula of cadavers: preliminary report. Clinical Oral Implants Research. 1997;8:286–289. doi: 10.1034/j.1600-0501.1997.080406.x. [DOI] [PubMed] [Google Scholar]
- O’Sullivan D, Sennerby L, Meredith N. Measurements comparing the initial stability of five designs of dental implants: a human cadaver study. Clinical Implant Dentistry and Related Research. 2000;2:85–92. doi: 10.1111/j.1708-8208.2000.tb00110.x. [DOI] [PubMed] [Google Scholar]
- Oates TW, Dowell S, Robinson M, McMahan CA. Glycemic control and implant stabilization in type 2 diabetes mellitus. J Dent Res. 2009;88:367–371. doi: 10.1177/0022034509334203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olive J, Aparicio C. Periotest method as a measure of osseointegrated oral implant stability. International Journal of Oral and Maxillofacial Implants. 1990;5:390–400. [PubMed] [Google Scholar]
- Park JB, Matsuura M, Han KY, Norderyd O, Lin WL, Genco RJ, Cho MI. Periodontal regeneration in class III furcation defects of beagle dogs using guided tissue regenerative therapy with platelet-derived growth factor. Journal of Periodontology. 1995;66:462–477. doi: 10.1902/jop.1995.66.6.462. [DOI] [PubMed] [Google Scholar]
- Park JY, Davies JE. Red blood cell and platelet interactions with titanium implant surfaces. Clinical Oral Implants Research. 2000;11:530–539. doi: 10.1034/j.1600-0501.2000.011006530.x. [DOI] [PubMed] [Google Scholar]
- Patel ZS, Young S, Tabata Y, Jansen JA, Wong ME, Mikos AG. Dual delivery of an angiogenic and an osteogenic growth factor for bone regeneration in a critical size defect model. Bone. 2008;43:931–940. doi: 10.1016/j.bone.2008.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrie CS, Williams JL. Probabilistic analysis of peri-implant strain predictions as influenced by uncertainties in bone properties and occlusal forces. Clinical Oral Implants Research. 2007;18:611–619. doi: 10.1111/j.1600-0501.2007.01384.x. [DOI] [PubMed] [Google Scholar]
- Puckett S, Pareta R, Webster TJ. Nano rough micron patterned titanium for directing osteoblast morphology and adhesion. International Journal of Nanomedicine. 2008;3:229–241. doi: 10.2147/ijn.s2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramp LC, Jeffcoat RL. Dynamic behavior of implants as a measure of osseointegration. International Journal of Oral and Maxillofacial Implants. 2001;16:637–645. [PubMed] [Google Scholar]
- Ramseier CA, Abramson ZR, Jin Q, Giannobile WV. Gene therapeutics for periodontal regenerative medicine. Dental Clinic of North America. 2006;50:245–263. ix. doi: 10.1016/j.cden.2005.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renders GA, Mulder L, Langenbach GE, van Ruijven LJ, van Eijden TM. Biomechanical effect of mineral heterogeneity in trabecular bone. Journal of Biomechanics. 2008;41:2793–2798. doi: 10.1016/j.jbiomech.2008.07.009. [DOI] [PubMed] [Google Scholar]
- Salmoria KK, Tanaka OM, Guariza-Filho O, Camargo ES, de Souza LT, Maruo H. Insertional torque and axial pull-out strength of mini-implants in mandibles of dogs. American Journal of Orthodontics and Dentofacial Orthopedics. 2008;133:790, e715–722. doi: 10.1016/j.ajodo.2007.12.020. [DOI] [PubMed] [Google Scholar]
- Scarano A, Degidi M, Iezzi G, Petrone G, Piattelli A. Correlation between implant stability quotient and bone-implant contact: a retrospective histological and histomorphometrical study of seven titanium implants retrieved from humans. Clinical Implant Dentistry and Related Research. 2006;8:218–222. doi: 10.1111/j.1708-8208.2006.00022.x. [DOI] [PubMed] [Google Scholar]
- Schliephake H, Scharnweber D, Dard M, Rossler S, Sewing A, Meyer J, Hoogestraat D. Effect of RGD peptide coating of titanium implants on periimplant bone formation in the alveolar crest. An experimental pilot study in dogs. Clin Oral Implants Res. 2002;13:312–319. doi: 10.1034/j.1600-0501.2002.130312.x. [DOI] [PubMed] [Google Scholar]
- Schulte W, Lukas D. The Periotest method. International Dental Journal. 1992;42:433–440. [PubMed] [Google Scholar]
- Schulte W, Lukas D. Periotest to monitor osseointegration and to check the occlusion in oral implantology. Journal of Oral Implantology. 1993;19:23–32. [PubMed] [Google Scholar]
- Schwartz Z, Lohmann CH, Oefinger J, Bonewald LF, Dean DD, Boyan BD. Implant surface characteristics modulate differentiation behavior of cells in the osteoblastic lineage. Advanced Dental Research. 1999;13:38–48. doi: 10.1177/08959374990130011301. [DOI] [PubMed] [Google Scholar]
- Seifert RA, Hart CE, Phillips PE, Forstrom JW, Ross R, Murray MJ, Bowen-Pope DF. Two different subunits associate to create isoform-specific platelet-derived growth factor receptors. Journal of Biological Chemistry. 1989;264:8771–8778. [PubMed] [Google Scholar]
- Sevimay M, Turhan F, Kilicarslan MA, Eskitascioglu G. Three-dimensional finite element analysis of the effect of different bone quality on stress distribution in an implant-supported crown. Journal of Prosthetic Dentistry. 2005;93:227–234. doi: 10.1016/j.prosdent.2004.12.019. [DOI] [PubMed] [Google Scholar]
- Shakesheff K, Cannizzaro S, Langer R. Creating biomimetic micro-environments with synthetic polymer-peptide hybrid molecules. Journal of Biomaterials Science. Polymer Edition. 1998;9:507–518. doi: 10.1163/156856298x00596. [DOI] [PubMed] [Google Scholar]
- Singhatanadgit W, Salih V, Olsen I. Up-regulation of bone morphogenetic protein receptor IB by growth factors enhances BMP-2-induced human bone cell functions. Journal of Cellular Physiology. 2006;209:912–922. doi: 10.1002/jcp.20799. [DOI] [PubMed] [Google Scholar]
- Stefani CM, Machado MA, Sallum EA, Sallum AW, Toledo S, Nociti FH., Jr. Platelet-derived growth factor/insulin-like growth factor-1 combination and bone regeneration around implants placed into extraction sockets: a histometric study in dogs. Implant Dentistry. 2000;9:126–131. doi: 10.1097/00008505-200009020-00004. [DOI] [PubMed] [Google Scholar]
- Sumner DR, Turner TM, Urban RM, Virdi AS, Inoue N. Additive enhancement of implant fixation following combined treatment with rhTGF-beta2 and rhBMP-2 in a canine model. Journal of Bone and Joint Surgery. American volume. 2006;88:806–817. doi: 10.2106/JBJS.E.00846. [DOI] [PubMed] [Google Scholar]
- Sykaras N, Iacopino AM, Triplett RG, Marker VA. Effect of recombinant human bone morphogenetic protein-2 on the osseointegration of dental implants: a biomechanics study. Clinical Oral Investigestions. 2004;8:196–205. doi: 10.1007/s00784-004-0270-7. [DOI] [PubMed] [Google Scholar]
- Taba M, Jr., Jin Q, Sugai JV, Giannobile WV. Current concepts in periodontal bioengineering. Orthodontics and Craniofacial Research. 2005;8:292–302. doi: 10.1111/j.1601-6343.2005.00352.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokunaga A, Oya T, Ishii Y, Motomura H, Nakamura C, Ishizawa S, Fujimori T, Nabeshima Y, Umezawa A, Kanamori M, Kimura T, Sasahara M. PDGF receptor beta is a potent regulator of mesenchymal stromal cell function. Journal of Bone and Mineral Research. 2008;23:1519–1528. doi: 10.1359/jbmr.080409. [DOI] [PubMed] [Google Scholar]
- Tosatti S, Schwartz Z, Campbell C, Cochran DL, VandeVondele S, Hubbell JA, Denzer A, Simpson J, Wieland M, Lohmann CH, Textor M, Boyan BD. RGD-containing peptide GCRGYGRGDSPG reduces enhancement of osteoblast differentiation by poly(L-lysine)-graft-poly(ethylene glycol)-coated titanium surfaces. Journal of Biomedical Materials Research A. 2004;68:458–472. doi: 10.1002/jbm.a.20082. [DOI] [PubMed] [Google Scholar]
- Turkyilmaz I, Sennerby L, McGlumphy EA, Tozum TF. Biomechanical Aspects of Primary Implant Stability: A Human Cadaver Study. Clinical Implant Dentistry and Related Research. 2008 doi: 10.1111/j.1708-8208.2008.00097.x. [DOI] [PubMed] [Google Scholar]
- Turkyilmaz I, Sennerby L, McGlumphy EA, Tozum TF. Biomechanical Aspects of Primary Implant Stability: A Human Cadaver Study. Clinical Implant Dentistry and Related Research. 2009;11:113–119. doi: 10.1111/j.1708-8208.2008.00097.x. [DOI] [PubMed] [Google Scholar]
- Turkyilmaz I, Tozum TF, Tumer C, Ozbek EN. Assessment of correlation between computerized tomography values of the bone, and maximum torque and resonance frequency values at dental implant placement. Journal of Oral Rehabilitation. 2006;33:881–888. doi: 10.1111/j.1365-2842.2006.01692.x. [DOI] [PubMed] [Google Scholar]
- Turkyilmaz I, Tumer C, Ozbek EN, Tozum TF. Relations between the bone density values from computerized tomography, and implant stability parameters: a clinical study of 230 regular platform implants. J Clinical Periodontology. 2007;34:716–722. doi: 10.1111/j.1600-051X.2007.01112.x. [DOI] [PubMed] [Google Scholar]
- Ueda M, Matsuki M, Jacobsson M, Tjellstrom A. Relationship between insertion torque and removal torque analyzed in fresh temporal bone. International Journal of Oral and Maxillofacial Implants. 1991;6:442–447. [PubMed] [Google Scholar]
- van der Linden JC, Birkenhager-Frenkel DH, Verhaar JA, Weinans H. Trabecular bone’s mechanical properties are affected by its non-uniform mineral distribution. Journal of Biomechanics. 2001;34:1573–1580. doi: 10.1016/s0021-9290(01)00146-4. [DOI] [PubMed] [Google Scholar]
- Van Staden RC, Guan H, Loo YC. Application of the finite element method in dental implant research. Computer Methods in Biomechanics Biomedical Engineering. 2006;9:257–270. doi: 10.1080/10255840600837074. [DOI] [PubMed] [Google Scholar]
- Wang HL, Pappert TD, Castelli WA, Chiego DJ, Jr., Shyr Y, Smith BA. The effect of platelet-derived growth factor on the cellular response of the periodontium: an autoradiographic study on dogs. Journal of Periodontology. 1994;65:429–436. doi: 10.1902/jop.1994.65.5.429. [DOI] [PubMed] [Google Scholar]
- Wennerberg A, Albrektsson T. Effects of titanium surface topography on bone integration. Clinical Oral Implants Research. 2009;20(Suppl 4):174–186. doi: 10.1111/j.1600-0501.2009.01775.x. [DOI] [PubMed] [Google Scholar]
- Wennerberg A, Albrektsson T, Lausmaa J. Torque and histomorphometric evaluation of c.p. titanium screws blasted with 25- and 75-microns-sized particles of Al2O3. Journal of Biomedical Materials Research. 1996;30:251–260. doi: 10.1002/(SICI)1097-4636(199602)30:2<251::AID-JBM16>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Xu Y, James AW, Longaker MT. Transforming growth factor-beta1 stimulates chondrogenic differentiation of posterofrontal suture-derived mesenchymal cells in vitro. Plastic and Reconstructive Surgery. 2008;122:1649–1659. doi: 10.1097/PRS.0b013e31818cbf44. [DOI] [PubMed] [Google Scholar]
- Yang J, Xiang HJ. A three-dimensional finite element study on the biomechanical behavior of an FGBM dental implant in surrounding bone. Journal of Biomechanics. 2007;40:2377–2385. doi: 10.1016/j.jbiomech.2006.11.019. [DOI] [PubMed] [Google Scholar]
- Yeo IS, Han JS, Yang JH. Biomechanical and histomorphometric study of dental implants with different surface characteristics. Journal of Biomedical Materials Research B. Applied Biomaterials. 2008;87:303–311. doi: 10.1002/jbm.b.31104. [DOI] [PubMed] [Google Scholar]