Abstract
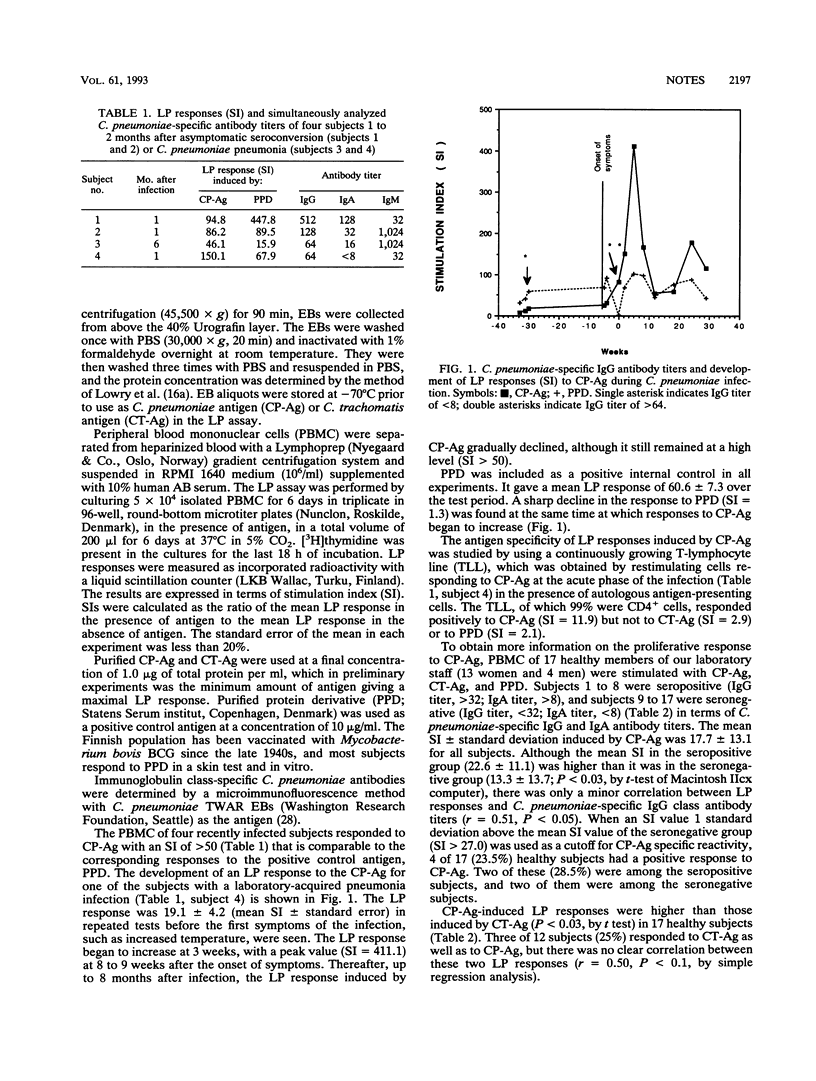
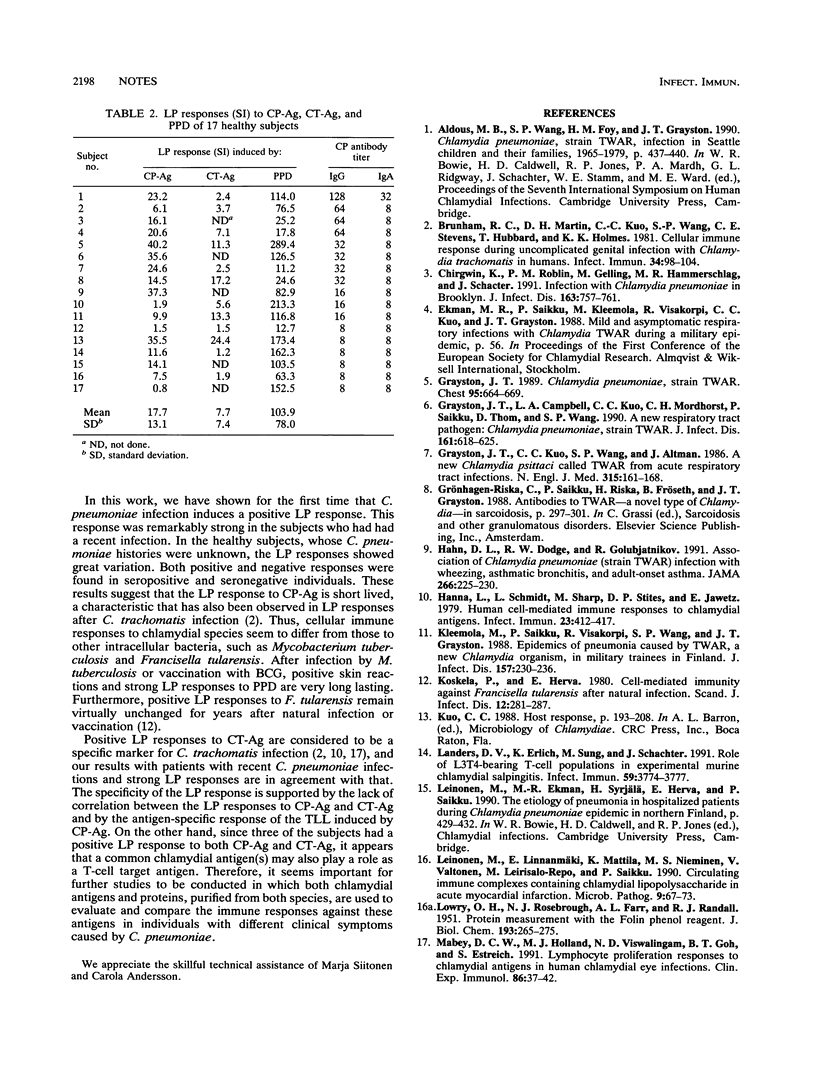
The purpose of the present study was to analyze Chlamydia pneumoniae-induced, antigen-specific, cell-mediated immunity. Peripheral blood mononuclear cells of four persons infected with C. pneumoniae Kajaani 6 and 17 healthy volunteers were stimulated with antigen composed of whole elementary bodies of C. pneumoniae Kajaani 6 (CP-Ag). Definitive antigen-specific lymphoproliferation (LP) responses were developed after recent infection. The LP responses of healthy people to CP-Ag varied considerably. There was no clear correlation between LP responses to CP-Ag and those to an antigen prepared from Chlamydia trachomatis serotype L2 (r > or = 0.50, P < 0.1). A larger study is required to demonstrate whether the LP responses to CP-Ag can be used for the diagnosis of C. pneumoniae infection.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brunham R. C., Martin D. H., Kuo C. C., Wang S. P., Stevens C. E., Hubbard T., Holmes K. K. Cellular immune response during uncomplicated genital infection with Chlamydia trachomatis in humans. Infect Immun. 1981 Oct;34(1):98–104. doi: 10.1128/iai.34.1.98-104.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin K., Roblin P. M., Gelling M., Hammerschlag M. R., Schachter J. Infection with Chlamydia pneumoniae in Brooklyn. J Infect Dis. 1991 Apr;163(4):757–761. doi: 10.1093/infdis/163.4.757. [DOI] [PubMed] [Google Scholar]
- Grayston J. T., Campbell L. A., Kuo C. C., Mordhorst C. H., Saikku P., Thom D. H., Wang S. P. A new respiratory tract pathogen: Chlamydia pneumoniae strain TWAR. J Infect Dis. 1990 Apr;161(4):618–625. doi: 10.1093/infdis/161.4.618. [DOI] [PubMed] [Google Scholar]
- Grayston J. T. Chlamydia pneumoniae, strain TWAR. Chest. 1989 Mar;95(3):664–669. doi: 10.1378/chest.95.3.664. [DOI] [PubMed] [Google Scholar]
- Grayston J. T., Kuo C. C., Wang S. P., Altman J. A new Chlamydia psittaci strain, TWAR, isolated in acute respiratory tract infections. N Engl J Med. 1986 Jul 17;315(3):161–168. doi: 10.1056/NEJM198607173150305. [DOI] [PubMed] [Google Scholar]
- Hahn D. L., Dodge R. W., Golubjatnikov R. Association of Chlamydia pneumoniae (strain TWAR) infection with wheezing, asthmatic bronchitis, and adult-onset asthma. JAMA. 1991 Jul 10;266(2):225–230. [PubMed] [Google Scholar]
- Hanna L., Schmidt L., Sharp M., Stites D. P., Jawetz E. Human cell-mediated immune responses to chlamydial antigens. Infect Immun. 1979 Feb;23(2):412–417. doi: 10.1128/iai.23.2.412-417.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleemola M., Saikku P., Visakorpi R., Wang S. P., Grayston J. T. Epidemics of pneumonia caused by TWAR, a new Chlamydia organism, in military trainees in Finland. J Infect Dis. 1988 Feb;157(2):230–236. doi: 10.1093/infdis/157.2.230. [DOI] [PubMed] [Google Scholar]
- Koskela P., Herva E. Cell-mediated immunity against Francisella tularensis after natural infection. Scand J Infect Dis. 1980;12(4):281–287. doi: 10.3109/inf.1980.12.issue-4.08. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Landers D. V., Erlich K., Sung M., Schachter J. Role of L3T4-bearing T-cell populations in experimental murine chlamydial salpingitis. Infect Immun. 1991 Oct;59(10):3774–3777. doi: 10.1128/iai.59.10.3774-3777.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leinonen M., Linnanmäki E., Mattila K., Nieminen M. S., Valtonen V., Leirisalo-Repo M., Saikku P. Circulating immune complexes containing chlamydial lipopolysaccharide in acute myocardial infarction. Microb Pathog. 1990 Jul;9(1):67–73. doi: 10.1016/0882-4010(90)90042-o. [DOI] [PubMed] [Google Scholar]
- Mabey D. C., Holland M. J., Viswalingam N. D., Goh B. T., Estreich S., Macfarlane A., Dockrell H. M., Treharne J. D. Lymphocyte proliferative responses to chlamydial antigens in human chlamydial eye infections. Clin Exp Immunol. 1991 Oct;86(1):37–42. doi: 10.1111/j.1365-2249.1991.tb05770.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qvigstad E., Digranes S., Thorsby E. Antigen-specific proliferative human T-lymphocyte clones with specificity for Chlamydia trachomatis. Scand J Immunol. 1983 Oct;18(4):291–297. doi: 10.1111/j.1365-3083.1983.tb01800.x. [DOI] [PubMed] [Google Scholar]
- Qvigstad E., Skaug K., Hirschberg H. Characterization of Chlamydia trachomatis serotypes by human T-lymphocyte clones. Scand J Immunol. 1985 Mar;21(3):215–220. doi: 10.1111/j.1365-3083.1985.tb01423.x. [DOI] [PubMed] [Google Scholar]
- Qvigstad E., Skaug K., Thorsby E. Proliferative human T cell responses to Chlamydia trachomatis in vitro. Acta Pathol Microbiol Immunol Scand C. 1983 Jun;91(3):203–209. [PubMed] [Google Scholar]
- Ramsey K. H., Rank R. G. Resolution of chlamydial genital infection with antigen-specific T-lymphocyte lines. Infect Immun. 1991 Mar;59(3):925–931. doi: 10.1128/iai.59.3.925-931.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rank R. G., Soderberg L. S., Sanders M. M., Batteiger B. E. Role of cell-mediated immunity in the resolution of secondary chlamydial genital infection in guinea pigs infected with the agent of guinea pig inclusion conjunctivitis. Infect Immun. 1989 Mar;57(3):706–710. doi: 10.1128/iai.57.3.706-710.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saikku P., Leinonen M., Mattila K., Ekman M. R., Nieminen M. S., Mäkelä P. H., Huttunen J. K., Valtonen V. Serological evidence of an association of a novel Chlamydia, TWAR, with chronic coronary heart disease and acute myocardial infarction. Lancet. 1988 Oct 29;2(8618):983–986. doi: 10.1016/s0140-6736(88)90741-6. [DOI] [PubMed] [Google Scholar]
- Saikku P., Leinonen M., Tenkanen L., Linnanmäki E., Ekman M. R., Manninen V., Mänttäri M., Frick M. H., Huttunen J. K. Chronic Chlamydia pneumoniae infection as a risk factor for coronary heart disease in the Helsinki Heart Study. Ann Intern Med. 1992 Feb 15;116(4):273–278. doi: 10.7326/0003-4819-116-4-273. [DOI] [PubMed] [Google Scholar]
- Saikku P., Wang S. P., Kleemola M., Brander E., Rusanen E., Grayston J. T. An epidemic of mild pneumonia due to an unusual strain of Chlamydia psittaci. J Infect Dis. 1985 May;151(5):832–839. doi: 10.1093/infdis/151.5.832. [DOI] [PubMed] [Google Scholar]
- Thom D. H., Grayston J. T. Infections with Chlamydia pneumoniae strain TWAR. Clin Chest Med. 1991 Jun;12(2):245–256. [PubMed] [Google Scholar]
- Wesslén L., Påhlson C., Friman G., Fohlman J., Lindquist O., Johansson C. Myocarditis caused by Chlamydia pneumoniae (TWAR) and sudden unexpected death in a Swedish elite orienteer. Lancet. 1992 Aug 15;340(8816):427–428. doi: 10.1016/0140-6736(92)91509-7. [DOI] [PubMed] [Google Scholar]


