Abstract
Background
Variant Creutzfeldt–Jakob disease (vCJD) is a prion disease thought to be acquired by the consumption of prion-contaminated beef products. To date, over 200 cases have been identified around the world, but mainly in the United Kingdom. Three cases have been identified in the United States; however, these subjects were likely exposed to prion infection elsewhere. Here we report on the first of these subjects.
Methodology/Principal Findings
Neuropathological and genetic examinations were carried out using standard procedures. We assessed the presence and characteristics of protease-resistant prion protein (PrPres) in brain and 23 other organs and tissues using immunoblots performed directly on total homogenate or following sodium phosphotungstate precipitation to increase PrPres detectability. The brain showed a lack of typical spongiform degeneration and had large plaques, likely stemming from the extensive neuronal loss caused by the long duration (32 months) of the disease. The PrPres found in the brain had the typical characteristics of the PrPres present in vCJD. In addition to the brain and other organs known to be prion positive in vCJD, such as the lymphoreticular system, pituitary and adrenal glands, and gastrointestinal tract, PrPres was also detected for the first time in the dura mater, liver, pancreas, kidney, ovary, uterus, and skin.
Conclusions/Significance
Our results indicate that the number of organs affected in vCJD is greater than previously realized and further underscore the risk of iatrogenic transmission in vCJD.
Introduction
Variant Creutzfeldt-Jakob disease (vCJD) was first reported in 1996 as a novel non-inherited form of prion disease [1]. Although the disease bears some of the classical features of the sporadic form of the human transmissible spongiform encephalopathies (TSE) or prion diseases, it has distinctive features [2]–[4]. Most remarkably, vCJD presents at an average age of 26 years [4]; histopathologically is characterized by the presence of plaques containing prion protein surrounded by vacuoles referred to as “florid” or “daisy” plaques [1]. Furthermore, the abnormal and pathogenic prion protein isoform (hereafter identified as PrPres) associated with vCJD has features that are unique among non-inherited human prion diseases [5]. PrPres is thought to be the major or lone component of the infectious agent of prion disease, the so called “prion”. However, occasionally not direct correlation has been reported between PrPres and infectivity [6].
The distinctive features of vCJD, along with its detection in the UK following the peak of the British epidemic of the prion disease bovine spongiform encephalopathy (BSE), pointed to the consumption of prion-contaminated beef products as the possible source of infection [1], [7]. Successful transmission to non-human primates and transgenic mice expressing the human prion protein (human PrP) with replication of major features of the vCJD phenotype, provided overwhelming evidence supporting the notion of cattle-to-human transmission [8]–[10]. These findings established vCJD as the first Western world prion disease to be acquired by oral infection. Kuru, discovered in the 1950s, was endemic among New Guinea tribes practicing ritualistic cannibalism [11]–[13].
The oral route of prion infection in vCJD raised the possibility that tissues and organs, beside the central nervous system (CNS), might also be affected. To date, PrPres has been reported in several tissues and organs outside the CNS of vCJD patients (Table 1) [14]–[19, P. Brown, unpublished data].
Table 1. Peripheral tissues shown to contain PrPres in vCJD1.
| Adrenal gland | Pituitary gland |
| Appendix | Rectum |
| Autonomic ganglia | Retina |
| Blood vessels | Skeletal muscle |
| Colon | Spinal ganglia |
| Ileum | Spleen |
| Jejunum | Thymus |
| Lymph nodes | Tonsil |
| Optic nerve | Trigeminal ganglia |
| Peripheral nerves |
Data obtained from immunoblotting and immunohistochemistry studies. Brown P., unpublished data; WHO Expert Committee Meeting, Baden, Austria, 18 May 2007 (updated through Jan 2009).
Although the amount of PrPres in non-neural tissues is small compared to that in the brain, the risk posed by the spread of even small amounts of PrPres has been underscored by the iatrogenic transmission of vCJD from blood donors in the preclinical phase of the disease [20].
We examined the main characteristics and tissue distribution of PrPres in a case of vCJD, in which the disease was most likely acquired in the UK but which is officially referred to as an American case because illness onset occurred in the US [21]. In an extensive autopsy examination, sodium phosphotungstate (NaPTA) precipitation, a highly sensitive method of PrPres detection [14], [22], was used to establish the presence and estimate the relative amounts of PrPres in several organs and tissues made available to the National Prion Disease Pathology Surveillance Center (NPDPSC).
Materials and Methods
Collection and Processing of Tissues
A whole body autopsy was performed within 20 hours from death. The National Prion Disease Pathology Surveillance Center (NPDPSC) received frozen and fixed tissue samples. Frozen tissue included slices from one cerebral and cerebellar hemisphere, portions of pituitary gland and dura mater as well as samples from the trachea, breast, heart, lung, esophagus, stomach, duodenum, jejunum, ileum, colon, liver, spleen, pancreas, adrenal gland, kidney, urinary bladder, uterus, ovary, mesenteric lymph nodes, diaphragm and skin. The skin was taken from the chest wall. In addition, paraffin blocks or sections from the same tissues were also received. Frozen tissues were stored at −80°C.
Histopathology and Prion Protein Immunohistochemistry
Histology and immunohistochemistry were carried out as previously described [23] on brain sections from frontal, temporal and parietal neocortices (the occipital cortex was unavailable), neo-striatum, thalamus, cerebellar hemisphere and on sections from all received tissues. Immunohistochemistry was carried out with the monoclonal antibody 3F4 to the PrP residues 109–112 [24].
Genetic Analysis
Genotyping was performed on genomic DNA extracted from blood as previously described [25].
Preparation of Tissue Homogenates
Tissue homogenates (TH) (10%, wt/vol) were prepared at 4°C in phosphate buffered saline (PBS) lacking Ca2+ and Mg2+, 1% Sarkosyl (pH 7.4), followed by centrifugation at 1000×g for 5 minutes to remove cellular debris. Excess of collagen was eliminated by homogenizing tissue and removing the white and dense fraction containing mainly collagen from the fraction rich in parenchymal tissue. Contamination of non-nervous tissue with brain tissue that might have occurred at autopsy was controlled by sampling the depth of the organs and discarding the tissue at the surface. Dura mater where this procedure was unsuitable was rinsed extensively with PBS before homogenization.
Sodium Phosphotungstate Precipitation (NaPTA)
Precipitation with NaPTA was carried out according to Wadsworth et al [14] with minor modifications. Briefly, 100 mg of wet tissue were homogenized (10% wt/vol) with PBS lacking Ca2+ and Mg2+, 2% Sarkosyl (pH 7.4), followed by centrifugation at 1000×g for 5 minutes to remove cellular debris. A fraction of the supernatant was collected and frozen for immunoblot analysis, whereas a second fraction of 500 µl was mixed with an equal volume of PBS prepared as above. Samples were adjusted to a final concentration of 50 units/ml of Benzonase and 1 µM of MgCl2 and incubated at 37°C for 30 minutes, followed by the addition of 81.3 µl of a pre-warmed solution containing 4% NaPTA and 170 mM MgCl2. After incubation for another 30 minutes at 37°C and constant agitation, samples were centrifuged at 16,000×g for 30 minutes. The supernatant was discarded whereas the pellet was re-suspended in 200 µl of PBS containing 0.1% Sarkosyl (pH 7.4) with the addition of 50 µl EDTA 250 mM (pH 8), in order to remove the white precipitate present in solution. After an additional centrifugation at 16,000×g for 30 minute, supernatants were discarded and the pellets re-suspended in 30 µl of PBS containing 0.1% Sarkosyl.
Immunoblot
Aliquots of TH or NaPTA-precipitated samples were either examined untreated or after treatment for 1 hour at 37°C with proteinase K (PK) (specific activity 44 units(U)/mg, Sigma Aldrich) at the concentration of 2 U/ml (1 U/ml corresponds to 23 µg/ml when PK specific activity is 44 U/mg) for 60 minutes at 37°C while constantly agitated. The reaction was terminated by addition of 3 mM of phenylmethylsulfonyl fluoride. Samples were diluted in sample buffer (final concentration: 3% sodium dodecyl sulfate [SDS], 4% ß-mercaptoethanol, 10% glycerol, 2 mM EDTA, 62.5 mM Tris, pH 6.8) and boiled for 10 minutes before loading. For deglycoyslation of the protein, samples were denatured and incubated in the presence of recombinant peptide N glycosidase F (PNGase F) according to the manufacturer's protocol (New England Biolabs). Protein samples were separated in 15% Tris-Glycine SDS-PAGE gels using gel electrophoresis apparatus holding running gels of different lengths (Criterion 7 cm and home made 15 cm high-resolution system, Bio-Rad). Proteins were transferred to Immobilon P (Millipore) for 2 h at 65 V, blocked in 5% (w/v) non-fat milk powder in TBS containing 0.1% (v/v) Tween-20 (TBST) (blocking solution), and incubated overnight at 4°C with selected antibodies. After several washes in TBST, membranes where incubated with a 1∶4,000 dilution of a peroxidase-conjugated secondary antibody in TBST for 60 minutes at room temperature, washed in TBST and visualized by enhanced chemiluminescence (Amersham ECL Plus, GE Healthcare) on Kodak BioMax XAR films (Eastman Kodak). Two antibodies to human PrP were used: the monoclonal antibody 3F4 (to residues 109–112) and the rabbit antisera 2301 (to residues 220–231).
Ethics Statement
This study was conducted according to the principles expressed in the Declaration of Helsinki. No Institutional Review Board review was required because federal regulations do not require approval of research on deceased patients by the Board. Written informed consent for use of patient information/tissue specimens for research purposes has been obtained.
Results
Clinical History
Clinical data on the present patient have been reported in detail [21]. Briefly, the patient lived in Britain until the age of 13 and immigrated to the US in 1992. In early November 2001, at the age of 22 years, the patient was evaluated for depression, emotional instability and memory loss, followed one month later by involuntary movements, gait disturbances and incontinence. During the ensuing three months, the patient's motor and cognitive deficits worsened, and confusion, hallucination, dysarthria, bradykinesia, and spasticity also occurred. The diagnosis of vCJD was made following brain magnetic resonance imaging and confirmed by immunoblot and immunohistochemistry of tonsil tissue. She received an experimental treatment with quinacrine for 3 months, but showed only minimal and transitory improvement. The patient died in June 2004, 32 months after the clinical onset.
Histopathological Examination
Both gray and white matter structures were severely atrophic with nearly total loss of neurons and replacement of the neuropil with prominent gemistocytic astrogliosis (Fig. 1A and B). Thus, the typical spongiform degeneration was not observed. Instead, there were irregular extracellular spaces consistent with the astroglial scarring present in the cerebral cortex (Fig. 1B). Macrophages were also present occasionally, especially in the white matter, and probably reflected Wallerian degeneration. The cerebral and cerebellar cortices and the basal ganglia were more affected than the thalamus. Many mono-centric plaques, often large and occasionally surrounded by “pseudo vacuoles”, were present preferentially in the deep cerebral cortex and superficial white matter as well as, to a lesser extent, in the cerebellar cortex and white matter (Fig. 1B). All the organs that had been examined (see Material and Methods for details) were unremarkable except for the kidney and the descending colon which evidenced lymphocytic inflammatory infiltrates (data not shown). In the kidney the infiltrates displayed a focal follicular pattern consistent with interstitial nephritis whereas in the descending colon the lymphocytic infiltrates were linear and located in the sub-mucosa.
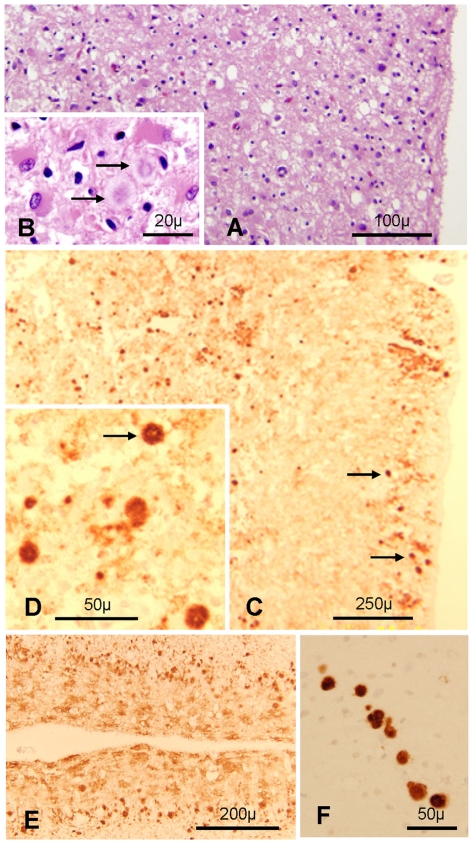
Figure 1. Histological and immunohistochemical features.
A and B. The cerebral cortex is markedly disrupted with prominent astrogliosis and presence of amyloid plaques (arrows in B); frontal cortex; H.E. C and D: PrP immunostaining shows the presence of many plaques (arrows) and plaque-like aggregates in superficial and deep cortical regions with a punctate background staining; frontal cortex. E: Distinctive spot-like PrP immunostaining in the molecular layer and many plaques especially evident in the Purkinje cell layer of the cerebellum. F: Plaques, often in a row are present in the superficial white matter; cerebellum. C–F: Monoclonal antibody (mAb) 3F4.
Immunohistochemical staining for PrP of brain sections revealed numerous well circumscribed as well as more diffuse PrP deposits consistent with unicentric plaques or early plaques (also called plaque-like) formations, which were especially prominent in the very superficial and deep cortical layers (Fig. 1C and D). Granular and “synaptic” immunostaining patterns were easily detectable in basal ganglia and thalamus. The cerebellum showed a leopard skin-like immunostaining and plaque-like patterns in the molecular and granule cell layer, respectively (Fig. 1E and F). Polarized light examination confirmed that the plaques contained amyloid (data not shown). No PrP immunostaining was detected in any of the tissues examined outside the brain.
Genetic analysis demonstrated methionine homozygosity at codon 129 and no mutations or other variations in the open reading frame of the PrP gene.
Characterization of Brain PrP
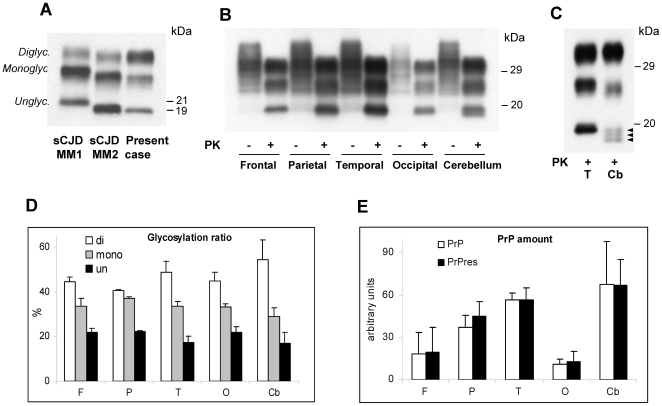
Immunoblot analyses of the PK-digested total homogenate (TH) from all cerebral cortices examined displayed the characteristic electrophoretic mobility and glycoform ratios of the PrPres described in vCJD (Fig. 2A) [5], [26]. In the cerebellum PrPres showed a slightly faster migration (Fig. 2B). When a high resolution gel (15%, 15 cm long) was used, the PrPres unglycosylated form in the cerebellum appeared to resolve into three bands, which included the band corresponding to the PrPres type 2 of 19 kDa and two additional bands that migrated about 0.5 kDa and 1 kDa faster (Fig. 2C). The upper band containing the diglycosylated PrP isoform was over-represented in all brain regions examined including the cerebellum (Fig. 2B and D). Total PrP and PrPres were best represented in the temporal cortex and cerebellum while they were present in the least amount least amount in the occipital cortex (Fig. 2B and E). In addition, we confirmed the presence of a 17 kDa PrPres fragment matching the anchorless PrPres type 2 fragment previously described in sporadic CJD (sCJD) and vCJD [27], whereas the 12/13 C-terminal fragment commonly present in sCJD was not detected (data not shown) [28]. These two findings are in agreement with the previously reported molecular characteristics of PrPres from vCJD [27]. To assess whether PrPres types 1 and 2 co-occurred in brain as previously reported [29], we digested the TH with a high concentration (32 U/ml) of PK and used high resolution gels (15%, 15 cm long), a technique that allows for the detection of even small amounts of PrPres type 1 and 2 (up to 3–5% of total PrPres) when they co-exist [30]. This procedure failed to demonstrate PrPres type 1 in the brain regions examined in this case (data not shown).
Figure 2. Detection and characterization of PrPres and PK-sensitive PrP in brain.
A: Immunoblot of total homogenates (TH), treated with proteinase K (PK), obtained from the frontal cortex of sCJDMM1, sCJDMM2 (representing PrPres types 1 and 2, respectively) and the present case showing the over-representation of the upper band (Diglyc.) containing the diglycosylated form, and the co-migration of the lowest band (Unglyc.), containing the unglycosylated form, with the corresponding band of sCJDMM2. B: Immunoblot of TH from the four regions of the cerebral cortex and the cerebellum, treated with PK as indicated. The cerebellar unglycosylated PrPres isoform generates a thicker and overall slightly faster migrating band than the corresponding PrPres from the cerebral cortex. C: A high-resolution immunoblot (15%, 15 cm long gel) confirms that the monoglycosylated and unglycosylated PrPres isoforms from the cerebellum have a faster electrophoretic mobility than the corresponding forms from the cerebral cortex, and shows that the cerebellar unglycosylated isoform resolves into three fragments including a 19 kDa band, corresponding to PrPres type 2, and two additional bands of slightly lower relative molecular weight (arrowheads); T: Temporal; Cb: Cerebellum. In A–C membranes were probed with the mAb 3F4. D and E: Ratios of the PrPres glycoforms (D) and of the total PrP and PrPres (E) obtained from the same brain regions examined in panel B. Each bar represents the mean ± SD of three densitometric determinations on each of two tissue samples.
Detection of PrPres in Non-Nervous Tissues
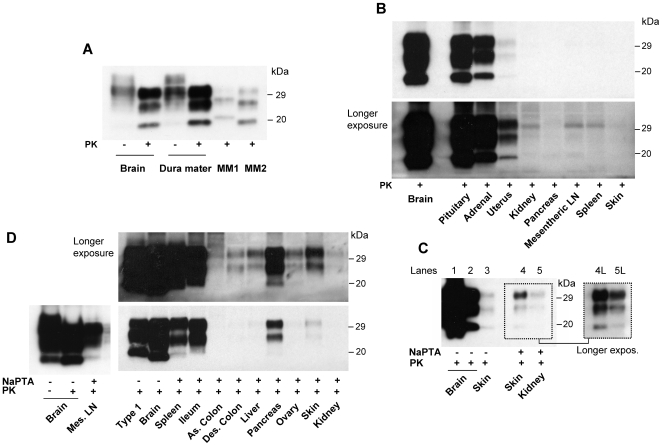
PrPres could be easily detected in the dura mater, the pituitary and adrenal glands, and the uterus using direct blotting of the TH (Table 2 and Fig. 3A and B). Detection in the skin required doubling the TH concentration (equivalent to 4 mg of wet tissue) but this procedure failed to reveal PrPres in other organs (Fig. 3B, 3C, and data not shown).
Table 2. Synopsis of PrPres analyses in the brain and other tissues.
| POSITIVE TISSUES | TH2 | NaPTA3 | PrPres4 | Glyc. ratio | NEGATIVE TISSUES |
| Brain | + | + | 100 | 47∶33∶20 | |
| Other Tissues | |||||
| Dura mater1 | + | + | 8.6 | 53∶34∶13 | Asc. Colon |
| Pituitary gland | + | + | 5.3 | 40∶41∶19 | Breast |
| Adrenal gland | + | + | 2.5 | 45∶39∶17 | Diaphragm |
| Uterus | + | + | 6·10−1 | 50∶38∶13 | Duodenum |
| Skin | + | + | 7·10−2 | 39∶43∶19 | Esophagus |
| Spleen | - | + | <7·10−2 | 60∶35∶6 | Heart |
| Ileum | - | + | <7·10−2 | 57∶40∶3 | Jejunum |
| Mesenteric LN | - | + | <7·10−2 | 48∶46∶11 | Left lung |
| Pancreas | - | + | <7·10−2 | 53∶36∶11 | Right lung |
| Liver | - | + | <7·10−2 | 57∶34∶9 | Stomach |
| Ovary | - | + | <7·10−2 | 58∶37∶5 | Trachea |
| Descending colon 5 | - | + | <7·10−2 | 62∶34∶5 | Urinary bladder |
| Kidney | - | + | <7·10−2 | 52∶35∶13 | |
In bold italic organs where PrPres had not been detected previously.
TH: PrPres searched in tissue homogenate.
NaPTA: PrPres searched following enrichment with sodium phosphotungstate precipitation.
Amount of PrPres expressed as percentage of PrPres present in the frontal cortex. Glyc. ratio: glycoform ratio expressed as percentage of the sum of the three isoforms and representing diglycosylated:monoglycosylated:unglycosylated forms. Data listed for tissue positive in both TH and NaPTA preparations were obtained from TH.
PrPres was previously reported in colon with no specification of the segment examined.
Figure 3. Detection of PrPres in non-nervous tissues.
A: PrPres from dura mater and frontal cortex (1∶24 dilution) from the present case is compared to PrPres of the frontal cortex from sCJDMM1 (type 1) and sCJDMM2 (type 2). B: Two film exposures of immunoblots from non-nervous tissues compared with that of the frontal cortex (1∶10 dilution). Pituitary gland, adrenal gland and uterus are clearly positive while the bands in the remaining preparations are considered to be non specific. C: PrPres from skin (double TH loading, equivalent to 4 mg of wet tissue) is barely detectable in TH (Lane 3) compared with frontal cortex TH, which is diluted 1∶4 (lane 1) or 1∶130 (lane 2). Skin PrPres is better detectable along with the kidney PrPres after sodium phosphotungstate (NaPTA) precipitation of PrPres (Lanes 4 and 5), especially after long exposure (Lanes 4L and 5L) (kidney TH loaded in double amount; probed with mAb 3F4). D: PrPres from mesenteric lymph nodes and other visceral organs recovered following NaPTA precipitation and compared with TH from the frontal cortex (1∶120 dilution) and sCJDMM1 following two film exposures. All organs but ascending colon are positive. Of note, unglycosylated isoform is underrepresented in all NaPTA precipitated samples as compared with that of the TH preparations (see panel A, lanes 3 and 4 and panel B). A–D: Membranes were probed with the mAb 3F4.
With NaPTA precipitation, we easily detected PrPres in the mesenteric lymph nodes, spleen, ileum, pancreas, skin, and to a lesser extent, in the descending colon, liver, ovary and kidney (Table 2 and Fig. 3D). The unequivocal identification of PrPres in the kidney required multiple sampling and an additional two-fold loading of the gel but these procedures failed to reveal PrPres in the ascending colon (Fig. 3C and D). Compared to direct TH blotting, NaPTA preparations often revealed a slower electrophoretic migration of up to 0.5 kDa (Fig. 3C, 3D and data not shown), as previously reported [14].
A significant over-representation of the diglycosylated form with a ratio comparable to that of the brain was apparently maintained in all the organs except the pituitary gland, the skin and some of the TH preparations from the uterus where diglycosylated and monoglycosylated isoforms had nearly the same concentration (Table 2, Fig. 3). Generally, in the NaPTA preparations the unglycosylated form was less well represented than in the TH preparations (Table 2, Fig. 3 and data not shown).
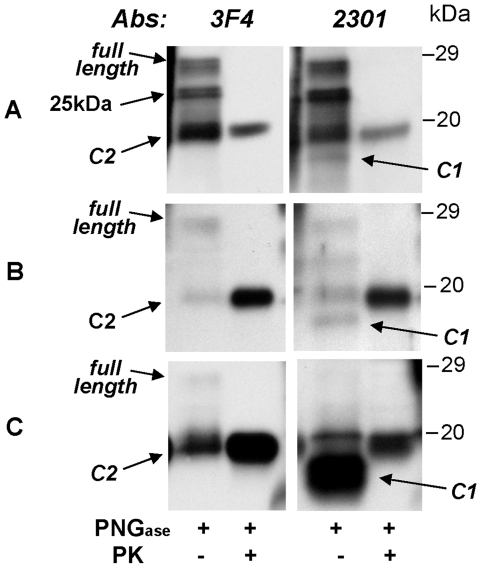
Notably, the antibody 2301 to the PrP C-terminal region revealed that in contrast to findings in the brain, most of the PK-sensitive PrP had the electrophoretic mobility of approximately 18 kDa (after deglycosylation) in all non-neural tissues, whereas the full length isoform appeared to be underrepresented (Fig. 4, data not shown). This finding was particularly prominent in the uterus but least evident in the pituitary gland (Fig. 4). Epitope mapping indicated that the 18 kDa fragment was truncated at the N-terminus matching the characteristics of the fragment identified as C1 (Fig. 4) [31]. In addition to being PK-sensitive, C1 could be easily distinguished from the unglycosylated form of PrPres detectable in PK untreated samples, named C2, which electrophoretically migrated to 19 kDa as in other organs (Fig. 4).
Figure 4. Characteristics of PK-sensitive PrP.
Immunoblot analysis of total homogenate from brain, pituitary gland and uterus are shown. The samples, with or without previous PK treatment, were deglycosylated with PNGase F. Membranes were probed with the mAbs 3F4 and 2301 as indicated. A: The brain has relatively large amounts of full-length isoform and PrPres C2 fragment but the N-terminus truncated PrPC fragment (C1) is poorly represented. In addition, brain preparation shows a previously unreported PK-sensitive fragment with molecular weight of 25 kDa (arrow), detectable only in deglycosylated samples, of undetermined origin. B: The C1 fragment is relatively better represented in the pituitary gland C: while it is overly abundant in the uterus.
Discussion
Our study confirms the diagnosis of vCJD in the present case, based on the characteristics of the PrPres and the methionine homozygosity at codon 129 of the PrP gene, the last feature being invariably present in vCJD [32]. However, we also observed two unusual features in this case. The first is the long disease duration of 32 months, which is more than twice the 14 month mean duration of the British cases of vCJD [3]. However, cases of up to 40 months duration after the diseases onset have been reported [3], [33]. The second unusual feature is the absence of typical spongiform degeneration which likely stemmed from the long duration of the disease. The long disease duration likely led to extensive loss of neurons, in which most of the vacuoles are formed, with ensuing astroglial scar [34].
As previously reported [21], the BSE exposure most likely occurred between the early eighties, when the BSE epidemic emerged in the UK, and 1992, when the patient immigrated to the US. This assumption is consistent with an incubation period of 9 to 21 years, which correlates well with the medium incubation period of 17 years estimated for the UK cases of vCJD [35].
The brain PrPres of the present case displayed the glycoform ratio and electrophoretic mobility characteristic of the PrPres associated with vCJD [5]. One exception is the cerebellum where the monoglycosylated and unglycosylated PrPres isoform migrated slightly faster than the PrPres from other brain regions and resolved in three bands. The variation in PrPres electrophoretic characteristics between the cerebellum and the cerebral cortex is not surprising for it has also been observed in sCJD [36]. Yet to our knowledge it has never been reported in vCJD. Finally, contrary to previous reports [29], PrPres type 1 did not co-occur with type 2. This discrepancy might stem from our rigorous PrP digestion with PK and from the use of different antibodies, an approach that rules out the possibility that partially cleaved fragments derived by the incomplete digestion of PrPSc be misinterpreted as the type 1 fragment [30], [37].
The major finding of the present study is the demonstration that PrPres is present in a number of non-CNS tissues and organs which previous studies had reported as free of PrPres (Table 1 and 2) [14]–[19, P. Brown, unpublished data]. These tissues include the dura mater, skin, liver, kidney, pancreas, descending colon, uterus and ovary (Table 2 and Fig. 3). The use of NaPTA, along with the long disease duration, may both have contributed to the undisputed detection of PrPres in these organs in this case. The glycoform ratio of the brain PrPres was not retained in every peripheral organ examined (Fig. 4). In the pituitary gland and the skin the diglycosylated and monoglycosylated PrPres isoforms were about equally represented thus the diglycosylated isoform was not dominant. On the other hand, electrophoretic mobility appeared to match that of the brain. Variations in the glycoform ratio could be assessed only on the TH because the glycoform ratio, as well the electrophoretic mobility, is affected by NaPTA enrichment [14].
The presence of prion in the human dura mater is not surprising because sCJD has been transmitted following transplantation of dura obtained from sCJD-affected cases [38]. However, to our knowledge this is the first immunoblot demonstration of PrPres in the dura mater in any prion disease. The detection of relatively large amounts of PrPres in the dura mater raises the possibility of contamination with brain tissue at autopsy. Although this possibility cannot be completely ruled out, extensive rinses in PBS were performed before homogenization in some experiments without observing a reduction in the amount of the PrPres detected.
Prion infectivity of kidney and liver has been demonstrated by bioassay in other human prion diseases [39], and PrPres has been observed in the kidney of scrapie infected sheep [40]. The presence of PrPres has also been reported in kidney, liver and pancreas of scrapie infected mice in association with lymphofollicular proliferation [41]. This last finding is relevant to the present case in which multiple lymphocytic infiltrates with follicular pattern were present in the kidney. However, contrary to this report, we observed no significant inflammatory reaction in any of the other tissues which contained PrPres. A puzzling finding of our study is the presence of PrPres albeit in small amounts in the kidney but not in the urinary bladder. This apparent discrepancy is relevant to the recent demonstrations of prion infectivity in urine of animals carrying experimental or naturally occurring prion diseases [42]–[46]. It would indicate that prion infectivity in urine is acquired from the kidney while the urinary bladder acts as a bystander. However the amount of PrPres we observed in the kidney was minimal, and might have not been sufficient to infect the urine and to propagate to the bladder in detectable amounts. Indeed we failed to demonstrate PrPres in the urine in the present case even after hundred-fold urine concentration (data not shown). Obviously more studies are needed to clarify this issue.
The present study also demonstrates for the first time the presence of PrPres in the skin in a human prion disease. Previously, PrPres has been detected in the skin from animals with experimental or naturally occurring scrapie [47] as well as in the antler velvet of elk affected by CWD [48].
Furthermore, it is remarkable that we observed PrPres in the uterus and the ovary, a finding which implicates the reproductive system, thereby raising the possibility of maternal transmission of vCJD. Vertical transmissibility of prion infection has been demonstrated in transgenic mice infected with BSE [49]. Related literature on human prion diseases is very scanty. Pregnancy completed to delivery has been reported in sCJD, iatrogenic CJD and vCJD [50], [51]; however, transmission to the progeny has not been examined in detail or confirmed in any of these cases. The first detailed determination of PrPC and PrPres in the reproductive and gestational tissues from a sCJD patient has been carried out only recently [51]. Although this study failed to detect PrPres, remarkably it showed that, in uterine tissue obtained at biopsy, most of the PK-sensitive PrP is truncated at the N-terminus and matches the C-terminal PrPC fragment C1 which is generated during normal PrPC metabolism [51]. Similarly, in the present case we observed that the C1-like fragment was largely predominant over the full-length PrPC in the uterus, and it was easily digested by PK but it was present along with a significant amount of characteristic vCJD PrPres (Fig. 4). Since the N-terminus of the PrPres type 2 associated with vCJD is at residues 92–99, the uterine PrPres must have formed from the full length PrPC rather than from C1, the N-terminus of which is at residues 111–112 [31], [52]. These findings raise the question of the origin of the PrPres found in the uterus, a question that is currently unanswered. A similar question may be raised for the urine, in which although the prion infectivity has been demonstrated in animals by bioassay [42]–[46], the only detected form of PrP under normal condition in animals and humans, is a fragment matching the C1 [53], [54, Notari et al., unpublished data].
All these considerations notwithstanding, the widespread presence of PrPres in visceral organs that we observed in the present case further reinforces the concerns over iatrogenic transmission of vCJD. These concerns are already compelling given the multiple reports of vCJD transmission by blood transfusion.
Acknowledgments
We are grateful to Dr Stephen Emancipator for the valuable help in the study of the kidney histology, Dr. José Luis Velajos for his valuable support, Dr. Kim Hummel for editorial assistance, and Mmes Diane Kofskey and Phyllis Scalzo, who provided the histological and immunochemical preparations.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: The study was supported by National Institutes of Health grant AG14359, Centers for Disease Control and Prevention (CCU515004), and the Charles S. Britton Fund to PG; grant NIH R01 NS062787 and the Creutzfeldt-Jakob Disease Foundation to WQZ, as for cost of supplies and salaries. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Will RG, Ironside JW, Zeidler M, Cousens S, Estebeiro K, et al. A new variant of Creutzfeldt-Jakob disease in the UK. Lancet. 1996;347:921–925. doi: 10.1016/s0140-6736(96)91412-9. [DOI] [PubMed] [Google Scholar]
- 2.Will RG, Zeidler M, Stewart GE, Macleod MA, Ironside JW, et al. Diagnosis of new variant Creutzfeldt-Jakob disease. Ann Neurol. 2000;47:575–582. [PubMed] [Google Scholar]
- 3.Will RG, Ward HJ. Clinical features of variant Creutzfeldt-Jakob disease. Curr Top Microbiol Immunol. 2004;284:121–132. doi: 10.1007/978-3-662-08441-0_5. [DOI] [PubMed] [Google Scholar]
- 4.Spencer MD, Knight RS, Will RG. First hundred cases of variant Creutzfeldt-Jakob disease: retrospective case note review of early psychiatric and neurological features. BMJ. 2002;324:1479–1482. doi: 10.1136/bmj.324.7352.1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Collinge J, Sidle KCL, Meads J, Ironside J, Hill AF. Molecular analysis of prion strain variation and the aetiology of ‘new variant’ CJD. Nature. 1996;383:685–690. doi: 10.1038/383685a0. [DOI] [PubMed] [Google Scholar]
- 6.Piccardo P, Manson JC, King D, Ghetti B, Barron RM. Accumulation of prion protein in the brain that is not associated with transmissible disease. Proc Natl Acad Sci U S A. 2007;(104):4712–4717. doi: 10.1073/pnas.0609241104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hill AF, Desbruslais M, Joiner S, Sidle KC, Gowland I, et al. The same prion strain causes vCJD and BSE. Nature. 1997;389:448–450. doi: 10.1038/38925. [DOI] [PubMed] [Google Scholar]
- 8.Lasmézas CI, Deslys JP, Demaimay R, Adjou KT, Lamoury F, et al. BSE transmission to macaques. Nature. 1996;381:743–744. doi: 10.1038/381743a0. [DOI] [PubMed] [Google Scholar]
- 9.Bruce ME, Will RG, Ironside JW, McConnell I, Drummond D, et al. Transmissions to mice indicate that ‘new variant’ CJD is caused by the BSE agent. Nature. 1997;389:498–501. doi: 10.1038/39057. [DOI] [PubMed] [Google Scholar]
- 10.Scott MR, Will R, Ironside J, Nguyen HO, Tremblay P, et al. Compelling transgenetic evidence for transmission of bovine spongiform encephalopathy prions to humans. Proc Natl Acad Sci U S A. 1999;96:15137–15142. doi: 10.1073/pnas.96.26.15137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gajdusek DC, Gibbs CJ, Alpers M. Experimental transmission of a Kuru-like syndrome to chimpanzees. Nature. 1966;209:794–796. doi: 10.1038/209794a0. [DOI] [PubMed] [Google Scholar]
- 12.Will RG. Acquired prion disease: iatrogenic CJD, variant CJD, kuru. Br Med Bull. 2003;66:255–265. doi: 10.1093/bmb/66.1.255. [DOI] [PubMed] [Google Scholar]
- 13.Liberski PP, Brown P. Kuru: a half-opened window onto the landscape of neurodegenerative diseases. Folia Neuropathol. 2004;42(Suppl A):3–14. [PubMed] [Google Scholar]
- 14.Wadsworth JD, Joiner S, Hill AF, Campbell TA, Desbruslais M, et al. Tissue distribution of protease resistant prion protein in variant Creutzfeldt-Jakob disease using a highly sensitive immunoblotting assay. Lancet. 2001;358:171–180. doi: 10.1016/s0140-6736(01)05403-4. [DOI] [PubMed] [Google Scholar]
- 15.Head MW, Northcott V, Rennison K, Ritchie D, McCardle L, et al. Prion protein accumulation in eyes of patients with sporadic and variant Creutzfeldt-Jakob disease. Invest Ophthalmol Vis Sci. 2003;44:342–346. doi: 10.1167/iovs.01-1273. [DOI] [PubMed] [Google Scholar]
- 16.Head MW, Ritchie D, Smith N, McLoughlin V, Nailon W, et al. Peripheral tissue involvement in sporadic, iatrogenic, and variant Creutzfeldt-Jakob disease: an immunohistochemical, quantitative, and biochemical study. Am J Pathol. 2004;(164):143–153. doi: 10.1016/S0002-9440(10)63105-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hilton DA, Sutak J, Smith ME, Penney M, Conyers L, et al. Specificity of lymphoreticular accumulation of prion protein for variant Creutzfeldt-Jakob disease. J Clin Pathol. 2004;(57):300–302. doi: 10.1136/jcp.2003.012278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peden AH, Ritchie DL, Head MW, Ironside JW. Detection and localization of PrPSc in the skeletal muscle of patients with variant, iatrogenic, and sporadic forms of Creutzfeldt-Jakob disease. Am J Pathol. 2006;168:927–935. doi: 10.2353/ajpath.2006.050788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peden AH, Ritchie DL, Uddin HP, Dean AF, Schiller KA, et al. Abnormal prion protein in the pituitary in sporadic and variant Creutzfeldt-Jakob disease. J Gen Virol. 2007;88:1068–1072. doi: 10.1099/vir.0.81913-0. [DOI] [PubMed] [Google Scholar]
- 20.Zou S, Fang CT, Schonberger LB. Transfusion transmission of human prion diseases. Transfus Med Rev. 2008;22:58–69. doi: 10.1016/j.tmrv.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 21.Belay ED, Sejvar JJ, Shieh WJ, Wiersma ST, Zou WQ, et al. Variant Creutzfeldt-Jakob disease death, United States. Emerg Infect Dis. 2005;11:1351–1354. doi: 10.3201/eid1109.050371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Safar J, Wille H, Itri V, Groth D, Serban H, et al. Eight prion strains have PrPSc molecules with different conformations. Nat Med. 1998;4:1157–1165. doi: 10.1038/2654. [DOI] [PubMed] [Google Scholar]
- 23.Pastore M, Chin SS, Bell KL, Dong Z, Yang Q, et al. Creutzfeldt-Jakob disease (CJD) with a mutation at codon 148 of prion protein gene: relationship with sporadic CJD. Am J Pathol. 2005;167:1729–1738. doi: 10.1016/S0002-9440(10)61254-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kascsak RJ, Rubenstein R, Merz PA, Tonna-DeMasi M, Fersko R, et al. Mouse polyclonal and monoclonal antibody to scrapie-associated fibril proteins. J Virol. 1987;61:3688–3693. doi: 10.1128/jvi.61.12.3688-3693.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gambetti P, Dong Z, Yuan J, Xiao X, Zheng M, et al. A novel human disease with abnormal prion protein sensitive to protease. Ann Neurol. 2008;63:697–708. doi: 10.1002/ana.21420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parchi P, Capellari S, Chen SG, Petersen RB, Gambetti P, et al. Typing prion isoforms. Nature. 1997;386:232–234. doi: 10.1038/386232a0. [DOI] [PubMed] [Google Scholar]
- 27.Notari S, Strammiello R, Capellari S, Giese A, Cescatti M, et al. Characterization of truncated forms of abnormal prion protein in Creutzfeldt-Jakob disease. J Biol Chem. 2008;283:30557–30565. doi: 10.1074/jbc.M801877200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zou WQ, Capellari S, Parchi P, Sy MS, Gambetti P, et al. Identification of novel proteinase K-resistant C-terminal fragments of PrP in Creutzfeldt-Jakob disease. J Biol Chem. 2003;278:40429–40436. doi: 10.1074/jbc.M308550200. [DOI] [PubMed] [Google Scholar]
- 29.Yull HM, Ritchie DL, Langeveld JP, van Zijderveld FG, Bruce ME, et al. Detection of type 1 prion protein in variant Creutzfeldt-Jakob disease. Am J Pathol. 2006;168:151–157. doi: 10.2353/ajpath.2006.050766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Notari S, Capellari S, Langeveld J, Giese A, Strammiello R, et al. A refined method for molecular typing reveals that co-occurrence of PrP Sc types in Creutzfeldt-Jakob disease is not the rule. Lab Invest. 2007;87:1103–1112. doi: 10.1038/labinvest.3700676. [DOI] [PubMed] [Google Scholar]
- 31.Chen SG, Teplow DB, Parchi P, Gambetti P, Autilio-Gambetti L. Truncated forms of the human prion protein in normal brain and prion diseases. J Biol Chem. 1995;270:19173–19180. doi: 10.1074/jbc.270.32.19173. [DOI] [PubMed] [Google Scholar]
- 32.Ironside JW, Head MW. Neuropathology and molecular biology of variant Creutzfeldt-Jakob disease. Curr Top Microbiol Immunol. 2004;284:133–159. doi: 10.1007/978-3-662-08441-0_6. [DOI] [PubMed] [Google Scholar]
- 33.The National CJD Surveillance Unit. Creutzfeldt-Jakob disease surveillance in the UK. Sixteenth annual report. 2007. Available: http://www.cjd.ed.ac.uk.
- 34.Bignami A, Forno LS. Status spongiosus in Jakob-Creutzfeldt disease. Electron microscopic study of a cortical biopsy. Brain. 1970;93:89–94. doi: 10.1093/brain/93.1.89. [DOI] [PubMed] [Google Scholar]
- 35.Valleron AJ, Boelle PY, Will R, Cesbron JY. Estimation of epidemic size and incubation time based on age characteristics of vCJD in the United Kingdom. Science. 2001;294:1726–1728. doi: 10.1126/science.1066838. [DOI] [PubMed] [Google Scholar]
- 36.Parchi P, Strammiello R, Notari S, Giese A, Langeveld J, et al. Incidence and spectrum of sporadic Creutzfeldt-Jakob disease variants with mixed phenotype and co-occurrence of PrPSc types: an updated classification. Acta Neruopathol: in press. 2009 doi: 10.1007/s00401-009-0585-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cali I, Castellani R, Alshekhlee A, Cohen Y, Blevins J, et al. Co-existence of scrapie prion protein types 1 and 2 in sporadic Creutzfeldt–Jakob disease: its effect on the phenotype and prion-type characteristics. Brain: in press. 2009 doi: 10.1093/brain/awp196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Noguchi-Shinohara M, Hamaguchi T, Kitamoto T, Sato T, Nakamura Y, et al. Clinical features and diagnosis of dura mater graft associated Creutzfeldt Jakob disease. Neurology. 2007;69:360–367. doi: 10.1212/01.wnl.0000266624.63387.4a. [DOI] [PubMed] [Google Scholar]
- 39.Brown P, Gibbs CJ, Jr, Rodgers-Johnson P, Asher DM, Sulima MP, et al. Human spongiform encephalopathy: the National Institutes of Health series of 300 cases of experimentally transmitted disease. Ann Neurol. 1994;35:513–529. doi: 10.1002/ana.410350504. [DOI] [PubMed] [Google Scholar]
- 40.Sisó S, Jeffrey M, Steele P, McGovern G, Martin S, Finlayson J, et al. Occurrence and cellular localization of PrPd in kidneys of scrapie-affected sheep in the absence of inflammation. J Pathol. 2008;215:126–134. doi: 10.1002/path.2336. [DOI] [PubMed] [Google Scholar]
- 41.Heikenwalder M, Zeller N, Seeger H, Prinz M, Klohn PC, et al. Chronic lymphocytic inflammation specifies the organ tropism of prions. Science. 2005;307:1107–1110. doi: 10.1126/science.1106460. [DOI] [PubMed] [Google Scholar]
- 42.Seeger H, Heikenwalder M, Zeller N, Kranich J, Schwarz P, et al. Coincident scrapie infection and nephritis lead to urinary prion excretion. Science. 2005;310:324–326. doi: 10.1126/science.1118829. [DOI] [PubMed] [Google Scholar]
- 43.Kariv-Inbal Z, Ben-Hur T, Grigoriadis NC, Engelstein R, Gabizon R. Urine from scrapie-infected hamsters comprises low levels of prion infectivity. Neurodegener Dis. 2006;3:123–128. doi: 10.1159/000094770. [DOI] [PubMed] [Google Scholar]
- 44.Gonzalez-Romero D, Barria MA, Leon P, Morales R, Soto C. Detection of infectious prions in urine. FEBS Lett. 2008;582:3161–3166. doi: 10.1016/j.febslet.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gregori L, Kovacs GG, Alexeeva I, Budka H, Rohwer RG. Excretion of transmissible spongiform encephalopathy infectivity in urine. Emerg Infect Dis. 2008;14:1406–1412. doi: 10.3201/eid1409.080259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Haley NJ, Seelig DM, Zabel MD, Telling GC, Hoover EA. Detection of CWD prions in urine and saliva of deer by transgenic mouse bioassay. PLoS One. 2009;4(3):e4848. doi: 10.1371/journal.pone.0004848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thomzig A, Schulz-Schaeffer W, Wrede A, Wemheuer W, Brenig B, et al. Accumulation of pathological prion protein PrPSc in the skin of animals with experimental and natural scrapie. PLoS Patho. 2007;3(5):e66. doi: 10.1371/journal.ppat.0030066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Angers RC, Seward TS, Napier D, Green M, Hoover E, et al. Chronic wasting disease prions in elk antler velvet. Emerg Infect Dis. 2009;15:696–703. doi: 10.3201/eid1505.081458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Castilla J, Brun A, Díaz-San Segundo F, Salguero FJ, Gutiérrez-Adán A, et al. Vertical transmission of bovine spongiform encephalopathy prions evaluated in a transgenic mouse model. J Virol. 2005;79:8665–8668. doi: 10.1128/JVI.79.13.8665-8668.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Murray K, Peters J, Stellitano L, Winstone A, Verity C, Will R. Is there evidence of vertical transmission of variant CJD? J Neurol Neurosurg Psych: in press. 2009 doi: 10.1136/jnnp.2009.172148. [DOI] [PubMed] [Google Scholar]
- 51.Xiao X, Miravalle L, Yuan J, McGeehan J, Dong Z, et al. Failure to detect the presence of prions in the uterine and gestational tissues from a Gravida with Creutzfeldt-Jakob disease. Am J Pathol. 2009;174:1602–1608. doi: 10.2353/ajpath.2009.081045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Parchi P, Zou W, Wang W, Brown P, Capellari S, et al. Genetic influence on the structural variations of the abnormal prion protein. Proc Natl Acad Sci USA. 2000;(97):10168–10172. doi: 10.1073/pnas.97.18.10168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Narang HK, Dagdanova A, Xie Z, Yang Q, Chen SG. Sensitive detection of prion protein in human urine. Exp Biol Med (Maywood) 2005;230:343–349. doi: 10.1177/153537020523000508. [DOI] [PubMed] [Google Scholar]
- 54.Andrievskaia O, Algire J, Balachandran A, Nielsen K. Prion protein in sheep urine. J Vet Diagn Invest. 2008;(20):141–146. doi: 10.1177/104063870802000201. [DOI] [PubMed] [Google Scholar]