Abstract
The cost of developing new drugs is a major obstacle for pharmaceutical companies and academia with many drugs identified in the drug discovery process failing approval for clinical use due to lack of intended effect or because of severe side effects. Since the early 1990s, high throughput screening of drug compounds has increased enormously in capacity but has not resulted in a higher success rate of the identified drugs. Thus, there is a need for methods that can identify biologically relevant compounds and more accurately predict in vivo effects early in the drug discovery process. To address this, we developed a proximity ligation-based assay for high content screening of drug effects on signaling pathways. As a proof of concept, we used the assay to screen through a library of previously identified kinase inhibitors, including six clinically used tyrosine kinase inhibitors, to identify compounds that inhibited the platelet-derived growth factor (PDGF) receptor β signaling pathway in stimulated primary human fibroblasts. Thirteen of the 80 compounds were identified as hits, and the dose responses of these compounds were measured. The assay exhibited a very high Z′ factor (0.71) and signal to noise ratio (11.7), demonstrating excellent ability to identify compounds interfering with the specific signaling event. A comparison with regular immunofluorescence detection of phosphorylated PDGF receptor demonstrated a far superior ability by the in situ proximity ligation assay to reveal inhibition of receptor phosphorylation. In addition, inhibitor-induced perturbation of protein-protein interactions of the PDGF signaling pathway could be quantified, further demonstrating the usefulness of the assay in drug discovery.
Screening for new drug compounds typically starts out with primary high throughput binding assays in a cell-free environment to identify possible drug candidates in a large library of compounds. Interesting compounds are then further characterized in secondary cell-based assays to validate the hits and remove false positives. These secondary assays include for example functional assays, reporter gene assays, and phenotypic assays for cellular processes (for a review, see An and Tolliday (1)), and methods such as high content microscopy (2), flow cytometry (3), and transcriptional profiling (4) are used.
Characterization of direct functional effects of drug compounds on cells often relies on using genetically modified cell lines with ectopically expressed fusion-tagged proteins. However, the use of primary cells in drug screening and drug target validation provides important advantages over immortalized cell lines because they more closely resemble in vivo conditions and thus provide more biologically relevant results (3, 5). It may also enable studies of how different cell types respond to treatment, e.g. cancer versus normal cells or cells from different lineages, to determine possible side effects. Furthermore, if cells from patients are used, drug effects can be evaluated on a per patient basis, paving the way for personalized medicine.
When studying proteins or post-translational modifications (PTMs)1 in genetically unmodified cells, immunofluorescence (IF)-based methods, which rely on the specific binding of a fluorophore-labeled antibody to the target protein or PTM, are typically used. Although this is a simple and useful approach, it has some drawbacks such as low sensitivity with scarce proteins, problems with cellular autofluorescence, and difficulty of robust quantification. However, the biggest problem with antibody-based detection is the poor target selectivity exhibited by many antibodies (6). In addition, IF cannot be used to study protein-protein interactions. Thus, more sensitive and selective methods for studying proteins are needed.
The in situ proximity ligation assay (PLA) is a highly selective and sensitive method for detecting proteins, protein-protein interactions, and post-translational modifications of proteins, and it has been applied to a range of different biological systems (7–11). The method utilizes dual target recognition of the protein or protein complex by a pair of antibodies to which oligonucleotides have been attached. If the two antibodies bind epitopes that are in close proximity, the oligonucleotides will also be brought into proximity and can be used as templates for the enzymatic joining of two additional linear oligonucleotides into a DNA circle (Fig. 1 a). This DNA circle can then be replicated using rolling circle amplification (RCA) using one of the antibody-attached oligonucleotides as a primer. The RCA product is a long, single-stranded chain of concatemeric copies of the circle that collapses into a bundle of DNA and can be detected by hybridizing fluorophore-labeled oligonucleotides to the repeated sequences (Fig. 1b). The result is a brightly fluorescent, submicrometer-sized spot that can easily be detected by fluorescence microscopy and quantified by image analysis (Fig. 1c). By using oligonucleotide-conjugated antibodies specific for immunoglobulins from two different species, target protein recognition can be performed utilizing primary antibodies from the corresponding species, simplifying the adaptation of the assay to different targets (9).
Fig. 1.
The principle of in situ proximity ligation assay. a, phosphorylated PDGFRβ was recognized by a rabbit monoclonal antibody binding the receptor and a pan-specific mouse monoclonal antibody binding phosphotyrosine residues. The primary antibodies were bound by species-specific antibodies conjugated to oligonucleotides. When in proximity, the oligonucleotides could be used as templates for the joining of two additional linear oligonucleotides into a DNA circle. One of the antibody-attached oligonucleotides was extended by rolling circle amplification using the DNA circle as a template to generate a submicrometer-sized bundle of DNA. b, by hybridizing fluorophore-labeled oligonucleotides to the repeated sequences of the amplification product, the antibody binding event could be visualized by microscopy as bright fluorescent spots. c, typical results from the detection of phosphorylated PDGFRβ in primary human fibroblasts. Red indicates in situ PLA signals, green indicates cytoplasmic staining, and blue indicates cell nuclei.
To show that in situ PLA can be used for screening and target validation of drug compounds in primary cells, we set up an assay to screen for compounds that inhibit platelet-derived growth factor receptor (PDGFR) β signaling pathways in primary human fibroblasts stimulated with PDGF-BB. We adapted in situ PLA to high content analysis techniques by performing the reactions in 96-well plates with image acquisition and quantification by a Cellomics ArrayScan II automated fluorescence microscope, greatly increasing assay throughput and reducing hands-on time.
EXPERIMENTAL PROCEDURES
Drug Compound Library
The Screen-Well™ Kinase Inhibitor Library (BIOMOL International/Enzo Life Sciences, Plymouth Meeting, PA), consisting of 80 kinase inhibitors supplied as 10 mm solutions in DMSO, was used for the screening. In addition, the tyrosine kinase inhibitors erlotinib, gefitinib, lapatinib, sorafenib, sunitinib (all from LC Laboratories, Woburn, MA), and imatinib (a kind gift from Novartis) were included. Before use, the compounds were diluted to 100 μm with sterile water.
Cell Culturing and Stimulation
Human primary fibroblast cells (GM008402, Coriell) were cultured in MEM supplemented with 10% FBS, 50 units/ml penicillin, 50 μg/ml streptomycin, and 1 mm l-glutamine (all from Sigma-Aldrich) at 37 °C and 5% CO2. The cells were loosened by trypsin treatment and seeded at a concentration of 10,000 cells/well in a flat bottom 96-well plate (PerkinElmer Life Sciences ViewPlate 96). After an overnight incubation, the cells were treated with 2 μm CellTracker Green 5-chloromethylfluorescein diacetate (Invitrogen) for 30 min in MEM at 37 °C before being serum-starved overnight with MEM with 0.5% FBS. The cells were treated for 60 min at 37 °C with drug compounds dissolved in DMSO and diluted to the indicated concentrations in MEM with 0.5% FBS at a volume of 50 μl. The DMSO concentration was kept at 0.1% for all wells. The cells were then put on ice to cool, and 50 μl of 100 ng/ml PDGF-BB (Peprotech, Rocky Hill, NJ) dissolved in MEM was added to all wells. The cells were stimulated for 60 min on ice, washed with cold PBS, and fixed on ice with cold 70% ethanol for 60 min.
Detection of Phosphorylated PDGF Receptor by in Situ PLA
To reduce the risk of unspecific binding by the antibodies, the cells were incubated with blocking solution (1× PBS with 5 mm EDTA, 20% goat serum, 2.5 μg/ml sonicated salmon sperm DNA, 25 mm l-cysteine, and 0.1% Tween 20) for 2 h at 37 °C. The blocking solution was then replaced with the primary antibodies (rabbit monoclonal PDGFRβ (28E1) and mouse monoclonal phosphotyrosine antibody (P-Tyr-100), both from Cell Signaling Technology) diluted 1:60 and 1:4,000 in blocking solution, respectively. The cells were incubated with the primary antibodies overnight at 4 °C and then washed three times with PBS with 0.1% Tween 20. To detect the primary antibodies with in situ PLA, secondary proximity probes binding rabbit and mouse immunoglobulin (PLA probe rabbit PLUS and PLA probe mouse MINUS, Olink Bioscience) were diluted 1:50 and 1:5 in blocking solution, respectively. The cells were incubated with the proximity probe solution for 60 min at 37 °C and then washed once with 10 mm Tris-HCl, pH 7.5 with 0.1% Tween 20 and then twice with TBST (1× TBS with 0.05% Tween 20). The detection of the bound proximity probes was performed with in situ PLA detection kit 613 (Olink Bioscience) according to the manufacturer's instructions. Briefly, the cells were incubated with 1× hybridization mixture for 15 min, washed once with TBST, incubated with 1× ligation mixture with 0.025 units/μl T4 DNA ligase for 15 min, washed twice with TBST, incubated with 1× RCA mixture with 0.0125 units/μl ϕ 29 polymerase for 90 min, washed twice with TBST, and then incubated with 1× detection mixture for 60 min. Finally, the cells were washed twice with TBST and then once with TBS. All reactions were performed at 37 °C with 50 μl of reaction mixture/well and with 240-rpm orbital shaking.
Detection of PDGF Receptor Interactions with PI 3-Kinase by in Situ PLA
Interaction between PDGFRβ and PI 3-kinase was performed as described for the detection of phosphorylated PDGFR described above, but instead of the phosphotyrosine antibody a mouse monoclonal PI 3-kinase antibody p85α (Ab6, Abcam) was used. The PI 3-kinase antibody was diluted 1:200. For the control, a mouse monoclonal actin antibody (Cedarlane Laboratories) diluted 1:100 was used.
Detection of Phosphorylated PDGF Receptor by Immunofluorescence
To compare the results of in situ PLA staining of phosphorylated PDGFRβ with normal immunofluorescence, cells were set up as was described for the in situ PLA staining. However, instead of staining with both primary antibodies, only one primary antibody was used. The pan-specific phosphotyrosine antibody was tested at the dilution used in the in situ PLA experiment (1:4,000) and at 1:200, and the mouse monoclonal phosphospecific PDGFRβ Tyr(P)-751 (Cell Signaling Technology) antibody was tested at a dilution of 1:500 and 1:5,000. Instead of using secondary proximity probes to detect the bound primary antibodies, a Texas Red-labeled donkey anti-mouse immunoglobulin antibody was used (Jackson ImmunoResearch Laboratories) at a dilution of 1:100 in blocking solution. The cells were incubated with the secondary antibody for 60 min at 37 °C and washed twice with TBST and then finally with TBS. Hoechst 33342 was included in the secondary antibody incubation step to stain the cell nuclei.
Image Acquisition and Analysis
Images of the cells stained with in situ PLA were acquired with a Cellomics ArrayScan II with the XF53 filter set using the neuronal profiling module v3.5 for image analysis. The nuclear staining and the CellTracker dye were used to delineate the cells, whereas spot counting was used to quantify the number of in situ PLA signals per cell. For the immunofluorescence-stained cells, the same image analysis settings were used as for the in situ PLA analyses. However, instead of counting spots, the total fluorescence intensity per cell in the Texas Red channel was measured. Approximately 1,000 cells were analyzed in each well for all reactions. Dose-response curves were fitted to a four-parameter logistic equation using GraphPad PRISM 5.0 to estimate the IC50 values of each tested compound. Z′ factors were calculated according to the published formula by Zhang et al. (12), i.e. Z′ factor = 1 − (3·(spos + sneg)/(|μpos − μneg|). Signal to noise ratio was calculated as SNR = (|μpos − μneg|)/ where μpos is the mean of the positive controls, μneg is the mean of the negative, and spos and sneg are the standard deviations of the positive and negative controls, respectively. To compare cells treated with imatinib with the control cells, Student's t test was used (two-tailed).
RESULTS
Comparison between in Situ PLA and Immunofluorescence-based Detection
We compared our assay with conventional IF-based detection of phosphorylated PDGFRβ in primary human fibroblasts that had been treated with the well known PDGFR inhibitor imatinib or with 0.1% DMSO as a negative control before being stimulated with PDGF-BB (Fig. 2). For the in situ PLA reactions, we used one primary antibody specific for phosphorylated tyrosine residues and one antibody directed against PDGFRβ to enable highly selective studies of the amount and distribution of phosphorylated PDGFRβ in the cells. The IF-based detection with fluorophore-labeled secondary antibodies was performed with either the pan-specific phosphotyrosine antibody used in the in situ PLA assay or with an antibody specific for phosphorylated PDGFRβ. The antibodies were used at the same concentrations used for the in situ PLA reactions, but other concentrations were also tested with similar results (data not shown). When characterizing screening assays, the Z′ factor is often estimated as a measure of the reliability and suitability of the assay for screening purposes, taking into account the dynamic range and variability of the assay (12). For it to be considered excellent, the Z′ factor of an assay must be above 0.5, whereas assays with a value between 0 and 0.5 are considered poor, and assays with Z′ factors below 0 are considered unusable. Our in situ PLA-based assay attained a very high Z′ factor of 0.71 and a signal to noise ratio of 11.6 despite it being a cell-based assay with all the inherent heterogeneity and variation of primary cells. Although all three assays recorded significant differences between the drug-treated cells and the control cells (in situ PLA, p < 0.0001; IF pan-Tyr(P), p < 0.0001; IF Tyr(P)-751, p = 0.0114), IF-based assays using either the phosphospecific PDGFRβ or the pan-specific phosphotyrosine antibody were found not to be useful for screening purposes, having Z′ factors of −3.1 and 0.25, respectively.
Fig. 2.
Comparison between in situ PLA and immunofluorescence-based detection of phosphorylated PDGFRβ. In situ PLA was compared with immunofluorescence-based detection as a means of detecting inhibition of phosphorylation of PDGFRβ by either treating cells with 10 μm imatinib or leaving them untreated as controls, stimulating them with PDGF-BB, and then analyzing the difference by in situ PLA and immunofluorescence with fluorophore-labeled secondary antibodies. The phosphospecific primary antibodies used were either a pan-specific phosphotyrosine antibody (pan pY) or an antibody specific for phosphorylated PDGFRβ (PDGFRβ pY751). In addition, for the in situ PLA reaction a PDGFRβ-specific antibody was used. All signals were normalized to the mean of the controls. The box plots indicate the median and range of the detected signals (n = 8).
Screening for Inhibitors of PDGFR Signaling
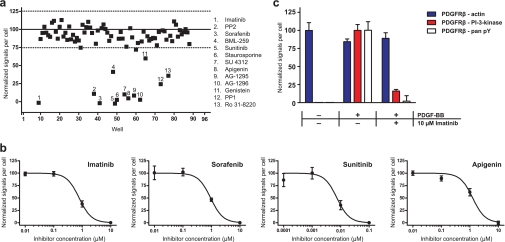
Having set up the assay, we used it to screen though a library of previously identified small molecule kinase inhibitors, including six clinically used tyrosine kinase inhibitors such as the aforementioned PDGFR inhibitor imatinib and the epidermal growth factor receptor inhibitor erlotinib. The cells were incubated in a 96-well plate for 1 h with the compounds at a concentration of 10 μm with 0.1% DMSO before being stimulated with PDGF-BB and analyzed by in situ PLA. As a result, 13 of the 86 compounds were identified as hits with our selection criteria, i.e. having a signal of more than three standard deviations below the mean of the negative controls (Fig. 3a). All hit compounds except BML-259 have previously been reported to have PDGFR inhibitory effects. However, the inhibitory effect of BML-259 on PDGFR phosphorylation could not be verified in subsequent experiments, and it was therefore regarded as a false positive. As expected, the clinically used PDGFR inhibitors (imatinib, sorafenib, and sunitinib) all showed strong inhibition of PDGFRβ phosphorylation, whereas the epidermal growth factor receptor inhibitors (erlotinib, lapatinib, and gefitinib) did not show any inhibition. The hit compounds were further studied by measuring the cellular dose responses of different compound concentrations by in situ PLA (Fig. 3b). The IC50 values of the effects of the compounds on the amount of phosphorylated PDGFRβ were estimated from these results and are presented in Table I
Fig. 3.
In situ PLA-based screening and target validation of a library of kinase inhibitors. a, in situ PLA was used to screen a library of kinase inhibitors for compounds that inhibited phosphorylation of the PDGFRβ in stimulated human primary fibroblasts. The cells were incubated for 1 h with inhibitors at a concentration of 10 μm, stimulated with 50 ng/ml PDGF-BB for 1 h at 4°C, and then fixed and analyzed by in situ PLA. The solid line indicates the mean of the negative controls, and the dashed lines indicate ±3 standard deviations from the mean. The signals have been normalized to the mean of the negative and positive controls. b, hit compounds from the library screen with a signal more than three standard deviations below the mean of the stimulated, uninhibited negative controls were diluted and investigated at different concentrations by in situ PLA to validate the hits. Non-linear curves were fitted to the dose-response data by a four-parameter logistic equation from which IC50 values were estimated (Table I). c, the effect of imatinib on PDGFRβ interactions with PI 3-kinase was monitored by in situ PLA in unstimulated and stimulated cells and in cells stimulated in the presence of imatinib by using one primary antibody specific for PDGFRβ and one specific for PI 3-kinase. As a control, interactions between PDGFRβ and actin were also monitored. Error bars indicate one standard deviation of triplicate reactions.
Table I. IC50 values calculated for the hit compounds.
To calculate the IC50 values, the cells were treated with the compounds at four different concentrations in triplicate reactions, and the amount of phosphorylated PDGFRβ was measured by in situ PLA. Non-linear curves were fitted to the dose-response data by a four-parameter logistic equation to estimate the IC50 values.
| Compound name or ID number | IC50 |
|---|---|
| μm | |
| Imatinib | 0.70 |
| Sorafenib | 0.93 |
| Sunitinib | 0.0065 |
| AG-1296 | 0.55 |
| SU 4312 | 2.29 |
| Apigenin | 1.28 |
| PP2 | 1.51 |
| Staurosporine | 0.031 |
| Ro 31-8220 | 1.05 |
| AG-1295 | 3.18 |
| PP1 | 1.31 |
| Genistein | >10 |
Inhibitor Effects on Protein-Protein Interactions
Upon ligand binding and phosphorylation, PDGFRβ activates a number of signaling pathways by associating with proteins such as PI 3-kinase, Ras GTPase-activating protein, and phospholipase C-γ to translate signals at the cell surface into cellular functions (13). To investigate whether perturbations of such interactions could be monitored by in situ PLA, we replaced the pan-specific phosphotyrosine antibody by an antibody specific for PI 3-kinase and investigated the level of interactions in unstimulated, stimulated, and imatinib-treated stimulated cells (Fig. 3c). As a control for these protein interactions, interactions between actin and PDGFRβ were also monitored. Upon stimulation with PDGF-BB, there was a significant increase in the amount of interactions between PDGFRβ and PI 3-kinase (p < 0.0001) in accordance with previously published data measured by co-immunoprecipitation (14). When imatinib-treated cells were stimulated with PDGF-BB, the amount of interactions was significantly reduced (p < 0.0001) although not to levels as low as for the unstimulated cells. The reduction in association between PDGFRβ and PI 3-kinase upon imatinib treatment is expected; the receptor must be phosphorylated at tyrosine 751 for PI 3-kinase to bind (15), and treating the cells with imatinib blocks the phosphorylation, reducing the possibilities for PI 3-kinase binding to the receptor. Interaction between PDGFRβ and actin is not known to be affected by PDGF-BB stimulation or tyrosine kinase inhibitor treatment, and accordingly we did not detect any significant changes by stimulation (p = 0.079) or imatinib treatment of stimulated cells (p = 0.22) compared with the unstimulated cells, although a small decrease was noted. This small decrease could be caused by proteins binding to the phosphorylated receptor, occluding the PDGFRβ-actin interaction, or the detection thereof by steric hindrance. In support of this theory is the fact that the amount of detected interactions between PDGFRβ and actin was slightly higher in the imatinib-treated stimulated cells compared with the untreated stimulated cells, although the increase was not significant (p = 0.38).
DISCUSSION
In this study, we have combined the benefits of in situ PLA with those of high content analysis techniques, creating a highly selective and sensitive medium-throughput assay that can be used to screen and validate drug compounds in genetically unmodified primary cells. We compared our assay with ordinary immunofluorescence staining, which is commonly used for drug screening in such cells, and in situ PLA showed far better suitability for drug compound screening purposes. The lesser difference between the inhibited and control cells in the IF experiments compared with the in situ PLA was probably due to the difference in both selectivity and sensitivity afforded by the two types of assays (16). Because the in situ PLA results are quantified by counting fluorescent rolling circle products, not by measuring total fluorescence intensity, they are much less affected by autofluorescence or bleaching of fluorophores compared with standard IF-based methods. In addition, even signals from single proximity probe binding events can be detected, ensuring the ability of the assay to detect proteins or PTMs of low abundance.
We show that our assay can be used to screen through a library of small molecule kinase inhibitors and identify inhibitors that specifically affect the PDGFR signaling pathway and that the assay can be used for measuring the dose response of such inhibitors with a large dynamic range and low variability. In addition, we show that the assay can be used to screen for drug-induced perturbations of protein-protein interactions, enabling drug screening of compounds that interfere with protein interactions, something which is difficult if not impossible to do in unmodified primary cells with current methods. Being able to screen for inhibitors of protein-protein interactions is considered of great importance in future drug discovery efforts because it would open up new interesting classes of potential targets to which drugs could be developed (17).
The usefulness of the assay includes the ability to validate compound hits from primary, high throughput cell-free screens in genetically unmodified cells such as primary cells or cells taken directly from patients with high sensitivity and selectivity and with a high signal to noise ratio. By using primary cells with an endogenous protein expression rather than cell lines with overexpressed fusion-tagged target proteins, drug compounds are studied in a much more natural cellular environment, ensuring drug target validation with a higher biological relevance. As has been pointed out (18), it is not the binding of a compound to a protein per se that is of interest; it is the effects that the binding of the compound has on the intricate macromolecular networks in the cells that matter. The ability to reveal changes in protein interactions or post-translational modifications in primary cells can provide direct insights into how drugs affect cellular processes and thus generate more accurate predictions of in vivo effects. Accordingly, we believe that in situ PLA should prove to be a useful tool for chemical genetic screens, drug discovery, and companion diagnostics, guiding the personalized selection of optimal treatment in clinical routine. In this study, we examined the effects of small molecule kinase inhibitors on PDGFRβ signaling, but the same setup could also be used to study other post-translational modifications and protein-protein interactions by using appropriate combinations of primary antibodies.
Acknowledgments
We thank Olink Bioscience for providing Duolink reagents. U. L. and J. J. are founders of Olink Bioscience, commercializing in situ PLA tests.
Footnotes
* This work was supported by grants from the Swedish Cancer Society, the Knut and Alice Wallenberg Foundation, the Swedish Research Council, the Beijer Foundation, the Uppsala Berzelii Center, and the European Union FP6 (Enlight).
1 The abbreviations used are:
- PTM
- post-translational modification
- IC50
- half-maximal inhibitory concentration
- IF
- immunofluorescence
- PDGF
- platelet-derived growth factor
- PDGFR
- platelet-derived growth factor receptor
- PLA
- proximity ligation assay
- RCA
- rolling circle amplification
- MEM
- minimum Eagle' medium
- FBS
- fetal bovine serum
- PI
- phosphatidylinositol
REFERENCES
- 1.An W. F., Tolliday N. J. (2009) Introduction: cell-based assays for high-throughput screening. Methods Mol. Biol. 486, 1–12 [DOI] [PubMed] [Google Scholar]
- 2.Lang P., Yeow K., Nichols A., Scheer A. (2006) Cellular imaging in drug discovery. Nat. Rev. Drug Discov. 5, 343–356 [DOI] [PubMed] [Google Scholar]
- 3.Krutzik P. O., Crane J. M., Clutter M. R., Nolan G. P. (2008) High-content single-cell drug screening with phosphospecific flow cytometry. Nat. Chem. Biol. 4, 132–142 [DOI] [PubMed] [Google Scholar]
- 4.Antipova A. A., Stockwell B. R., Golub T. R. (2008) Gene expression-based screening for inhibitors of PDGFR signaling. Genome Biol. 9R47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Butcher E. C., Berg E. L., Kunkel E. J. (2004) Systems biology in drug discovery. Nat. Biotechnol. 22, 1253–1259 [DOI] [PubMed] [Google Scholar]
- 6.Pradidarcheep W., Labruyère W. T., Dabhoiwala N. F., Lamers W. H. (2008) Lack of specificity of commercially available antisera: better specifications needed. J. Histochem. Cytochem. 56, 1099–1111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Szado T., Vanderheyden V., Parys J. B., De Smedt H., Rietdorf K., Kotelevets L., Chastre E., Khan F., Landegren U., Söderberg O., Bootman M. D., Roderick H. L. (2008) Phosphorylation of inositol 1,4,5-trisphosphate receptors by protein kinase B/Akt inhibits Ca2+ release and apoptosis. Proc. Natl. Acad. Sci. U.S.A 105, 2427–2432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Söderberg O., Gullberg M., Jarvius M., Ridderstråle K., Leuchowius K. J., Jarvius J., Wester K., Hydbring P., Bahram F., Larsson L. G., Landegren U. (2006) Direct observation of individual endogenous protein complexes in situ by proximity ligation. Nat. Methods 3, 995–1000 [DOI] [PubMed] [Google Scholar]
- 9.Jarvius M., Paulsson J., Weibrecht I., Leuchowius K. J., Andersson A. C., Wählby C., Gullberg M., Botling J., Sjöblom T., Markova B., Ostman A., Landegren U., Söderberg O. (2007) In situ detection of phosphorylated platelet-derived growth factor receptor beta using a generalized proximity ligation method. Mol. Cell. Proteomics 6, 1500–1509 [DOI] [PubMed] [Google Scholar]
- 10.Greenberg J. I., Shields D. J., Barillas S. G., Acevedo L. M., Murphy E., Huang J., Scheppke L., Stockmann C., Johnson R. S., Angle N., Cheresh D. A. (2008) A role for VEGF as a negative regulator of pericyte function and vessel maturation. Nature 456, 809–813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fuller B. G., Lampson M. A., Foley E. A., Rosasco-Nitcher S., Le K. V., Tobelmann P., Brautigan D. L., Stukenberg P. T., Kapoor T. M. (2008) Midzone activation of aurora B in anaphase produces an intracellular phosphorylation gradient. Nature 453, 1132–1136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang J. H., Chung T. D., Oldenburg K. R. (1999) A simple statistical parameter for use in evaluation and validation of high throughput screening assays. J. Biomol. Screen. 4, 67–73 [DOI] [PubMed] [Google Scholar]
- 13.Heldin C. H., Ostman A., Rönnstrand L. (1998) Signal transduction via platelet-derived growth factor receptors. Biochim. Biophys. Acta 1378, F79–F113 [DOI] [PubMed] [Google Scholar]
- 14.Coughlin S. R., Escobedo J. A., Williams L. T. (1989) Role of phosphatidylinositol kinase in PDGF receptor signal transduction. Science 243, 1191–1194 [DOI] [PubMed] [Google Scholar]
- 15.Kazlauskas A., Cooper J. A. (1990) Phosphorylation of the PDGF receptor beta subunit creates a tight binding site for phosphatidylinositol 3 kinase. EMBO J. 9, 3279–3286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leuchowius K. J., Weibrecht I., Landegren U., Gedda L., Söderberg O. (2009) Flow cytometric in situ proximity ligation analyses of protein interactions and post-translational modification of the epidermal growth factor receptor family. Cytometry A 75, 833–839 [DOI] [PubMed] [Google Scholar]
- 17.Mayr L. M., Fuerst P. (2008) The future of high-throughput screening. J. Biomol. Screen. 13, 443–448 [DOI] [PubMed] [Google Scholar]
- 18.Nolan G. P. (2007) What's wrong with drug screening today. Nat. Chem. Biol. 3, 187–191 [DOI] [PubMed] [Google Scholar]