Abstract
Chronic wasting disease (CWD), an important emerging prion disease of cervids, is readily transmitted by intracerebral or oral inoculation from deer-to-deer and elk-to-elk, suggesting the latter is a natural route of exposure. Studies of host range susceptibility to oral infection, particularly of those species found in habitats where CWD currently exists are imperative. This report describes the experimental transmission of CWD to red deer following oral inoculation with infectious CWD material of elk origin. At 18 to 20 months post-inoculation, mild to moderate neurological signs and weight loss were observed and animals were euthanized and tested using 3 conventional immunological assays. The data indicate that red deer are susceptible to oral challenge and that tissues currently used for CWD diagnosis show strong abnormal prion (PrPCWD) accumulation. Widespread peripheral PrPCWD deposition involves lymphoreticular tissues, endocrine tissues, and cardiac muscle and suggests a potential source of prion infectivity, a means of horizontal transmission and carrier state.
Résumé
Transmission orale expérimentale de la maladie du dépérissement chronique aux wapitis (Cervus elaphus elaphus) : Dépistage précoce et répartition de la protéine du prion résistante à la protéase aux derniers stades. La maladie du dépérissement chronique (MDC), une importante maladie à prions des cervidés qui est émergente, se transmet facilement par inoculation intracérébrale ou orale d’un cerf à un cerf et d’un wapiti à un wapiti, suggérant que cette dernière est une voie naturelle d’exposition. Des études sur l’ampleur de la susceptibilité de l’hôte à l’infection orale, particulièrement chez les espèces trouvées dans des habitats où se trouve la MDC, sont impératives. Ce rapport décrit la transmission expérimentale de la MDC aux wapitis après l’inoculation orale avec des produits infectants de MDC provenant des wapitis. De 18 à 20 mois après l’inoculation, des signes neurologiques de légers à modérés et la perte de poids ont été observés et les animaux ont été euthanasiés et testés à l’aide de 3 essais immunologiques conventionnels. Les données indiquent que les wapitis sont susceptibles aux infections défis orales et que les tissus actuellement utilisés pour le diagnostic de MDC montrent une accumulation anormalement élevée de prions (PrPCWD). Un dépôt périphérique généralisé de PrPCWD vise les tissus lymphoréticulaires, les tissus endocriniens et le muscle cardiaque et suggère une source potentielle d’infectivité des prions, un mode de transmission horizontale et un état de porteur.
(Traduit par Isabelle Vallières)
Introduction
Chronic wasting disease (CWD), a naturally occurring transmissible spongiform encephalopathy (TSE), is an enigmatic and contagious prion disease of North American cervids that has been described in both captive and free-ranging animals including mule deer (Odocoileus hemionus), Rocky Mountain elk (Cervus elaphus nelsoni), white-tailed deer (Odocoileus virginianus), and moose (Alces alces) (1). Chronic wasting disease shares many clinical and pathologic features with scrapie of sheep and goats, bovine spongiform encephalopathy (BSE), and transmissible mink encephalopathy (TME). However, CWD is unique among the animal TSEs because it seems more contagious than scrapie, BSE, or TME and is the only prion disease maintained in free-ranging populations. A key event in the pathogenesis of prion diseases, including CWD, is the conversion of a normal host-encoded membrane associated prion protein (PrPC) to the abnormal disease-associated isoform (PrPCWD) (2). It is generally believed that PrPCWD is the main or sole component of the infectious agent, and that the accumulation of PrPCWD within the central nervous system (CNS) is the primary event leading to neurodegeneration (3).
The emergence of CWD amongst the North American commercial cervid industry, the detection of CWD in free-ranging cervids over an increasingly wide geographic area, and the documented inter-species transmission between cervids, together, raise the possibility that CWD will spread across the continent, and perhaps beyond. Globally, farmed deer represent a significant alternative livestock industry with numbers exceeding 2 000 000 in New Zealand, 1 000 000 in China, 250 000 in the United States, 400 000 in Russia, and 100 000 in Canada (4). Red deer (Cervus elaphus elaphus), the Eurasian sub-species of elk, were imported into Canada for the farming of venison and antler in the late 1980’s and early 1990’s, and the captive cervid industry subsequently generated red deer/elk hybrids in order to increase the size of the red deer stock.
Among clinical CWD cases, the highest levels of prions are found in the brain and spinal cord, but other tissues, particularly those of the lymphoreticular system (LRS), exhibit substantial disease associated PrPCWD accumulation (5). Currently, BSE is the only animal TSE regarded as a zoonosis, whereas epidemiological evidence suggests that CWD has not been transmitted to humans (6,7). Recent reports, however, have demonstrated CWD infectivity in the skeletal muscle of experimentally infected mice (8), hamsters (9), and deer (10). Because of the presumed long incubation period of CWD, infectious prions may be present in the peripheral tissues for a considerable period of time before the onset of clinical signs. These findings have raised concerns over the potential human health risks that may be encountered by consuming CWD prion-contaminated meat (11) and highlight the need for a thorough determination of the distribution of CWD prions in peripheral tissues such as skeletal muscle.
The diagnostic hallmark of CWD infection is the detection of PrPCWD accumulation in the CNS and LRS of affected and preclinical animals. The medulla oblongata, at the level of the obex, and the retropharyngeal lymph node are the target tissues for CWD diagnosis of cervids. As a gold standard test, immunohistochemistry (IHC) is a sensitive and effective technique for detecting CWD in cervids (12) but is also a costly, labor-intensive, and time-consuming technique that requires skilled personnel. Laboratories conducting IHC may become overwhelmed by increasing demand to support large-scale CWD and other TSE surveys. To address these limitations, several rapid tests designed for large-volume sample screening have been developed including enzyme-linked immunosorbent assay (ELISA) and Western blotting (WB) (13). However, the ability of such rapid tests to detect PrPCWD in non-CNS and non-lymphoid tissues has not been well described.
Although there has yet to be a reported case of natural transmission to red deer, there is concern of the spread of CWD to this species and the potential for human exposure to cervid PrPCWD. This paper describes the extent and distribution of PrPCWD deposition in tissues from major organ systems of red deer after oral inoculation with infectious CWD brain material. Additional objectives were to evaluate lymphoid tissues from antemortem pre-clinical deer for PrPCWD deposition, to compare the performance of IHC and ELISA for the detection of PrPCWD in various CNS and non-CNS tissues, and to describe PrPCWD Western blot glycoform patterns from infected tissues. This knowledge will contribute to the understanding of CWD pathogenesis in red deer and help in establishing diagnostic methods for large-scale CWD surveillance. If CWD is found to occur naturally in the future, this will form the basis of consideration for limiting the spread of CWD from red deer and for the protection against any potential spread of CWD to humans.
Materials and methods
Experimental animals and CWD inoculum
Six 2-month-old captive red deer were obtained from herds free from CWD and randomly assigned to inoculated (n = 4) and control groups (n = 2). Inoculated deer were housed in a Biosafety Level 2 isolation barn at the Ottawa Laboratory Fallowfield, Canadian Food Inspection Agency (Ottawa, Ontario). All experimental protocols were approved by the institutional Animal Care Committee and were in accordance with humane animal treatment requirements of the Canadian Council on Animal Care.
Four red deer were orally inoculated with 5 mL of a tissue homogenate created from a pool of 4 CWD-positive (by IHC and ELISA) naturally infected captive Rocky Mountain elk. The inoculum was prepared from brain (n = 4) tissues that were placed in a mechanical grinder and a final homogenate concentration of 40% (w/v) was made with physiological saline resulting in an optical density for PrPCWD of 3.5 by ELISA (TeSeE; Bio-Rad Laboratories, Hercules, California, USA). The inoculum was administered using a small syringe inserted into the diastema of the oral cavity. The infected deer were monitored daily for clinical signs over the next 18 mo. Two non-infected (sham-inoculated) control deer were housed separately but under similar conditions to the CWD-inoculated deer.
Sample collection for pre-clinical detection of PrPCWD
At 214 d post-inoculation, samples of nictitating membrane and recto-anal mucosa associated lymphoid tissue were collected to assess the infection status of the deer and to evaluate the utility of such tissues for the pre-clinical detection of PrPCWD as previously described for sheep and other cervids (14,15).
Postmortem examination and tissue collection
When clinical signs of CWD (loss of appetite, loss of body condition, salivation, mild ataxia, weakness, dehydration, drooping of head and ears) became evident in 2 of the 4 inoculated deer, all animals were humanely euthanized with pentobarbital and subjected to a complete postmortem examination (585 d PI). Representative tissues from all major organ systems were collected including brain, cervical spinal cord (C1–C4), ganglia (dorsal root and trigeminal), peripheral nerves (brachial plexus, vagus, sciatic, facial, radial, and optic), digestive, respiratory, cardiovascular, endocrine, musculoskeletal (triceps brachii, trapezius, longissimus, semitendinosus, cardiac, lingual, masseter, and diaphragmatic muscles), urogenital, LRS (mesenteric, ileocecal, sublumbar, popliteal, pre-scapular, retropharyngeal, submandibular, parotid, ruminal, abomasal, hepatic, and pre-femoral lymph nodes), and integument tissue. Duplicate samples of tissue from adjacent areas were taken, one of which was fixed in 10% neutral buffered formalin and the other stored frozen at −70°C. The fresh brain was cut longitudinally, 1/2 being fixed in formalin and the remainder frozen at −70°C. The formalin fixed brain was cut into coronal sections, 2 to 4 mm wide. Eight brain areas per animal were selected for examination by light microscopy including the rostral cerebrum (frontal lobe), thalamus, midbrain (at 2 levels to include the superior colliculus and red nucleus), pons, medulla (at the level of the cerebellar peduncles and obex), and cerebellum at the vermis. Unfixed tissue from contralateral brain areas were sampled by ELISA and Western blot techniques.
Histopathology and immunohistochemistry (IHC)
The techniques used for histopathology and IHC were similar to those previously described (15,16). Tissues were embedded in paraffin blocks, and serial sections were cut at 5-μm intervals and mounted onto positively charged glass slides (Superfrost/plus; Fisher Scientific, Ottawa, Ontario). The sections were either stained with hematoxylin and eosin (HE) for the occurrence of spongiform degeneration (brain only), or subjected to IHC for the presence of PrPCWD. Prior to IHC, antigen retrieval was performed as follows: tissue sections were immersed in 96% formic acid (Mallinckrodt, Paris, Kentucky, USA) for 5 min, rinsed, and placed in 100 mM Tris buffer (pH 7.6) for 25 min at 121°C (liquid setting) and cooled for 30 min to room temperature. Subsequent steps of the IHC protocol were performed with an automated immunostainer (Ventana Medical Systems, Tucson, Arizona, USA). Sections were stained with primary monoclonal antibody (MAb) F99/97.6.1 (VMRD, Pullman, Washington, USA) for 30 min at 37°C, followed by a biotinylated anti-mouse secondary antibody, an alkaline phosphatase-streptavidin conjugate, substrate chromogen (fast red), and a hematoxylin counterstain with bluing (Ventana). The primary antibody was diluted to an IgG concentration of 5 μg/mL in diluent (Ventana) and placed in the refillable dispenser provided with the immunostainer. Positive and negative controls were included with each run.
Tissues that were positive by IHC had granular red chromogen deposits that were interpreted to be PrPCWD. The abundance of PrPCWD was subjectively graded on a scale of 0 to +++ based on examination at 20×, with 0 = no staining, + = mild, ++ = moderate, and +++ = strong. The intensity of the stain was bright red whether the scoring was+, ++, or +++. Since the dorsal motor nucleus of the vagus (DMNV) was the most consistently and severely affected nucleus with regard to the abundance of IHC positive material, this nucleus was graded as strong (+++) and all other sites were scored accordingly. Lymph nodes were considered positive if any of the follicles exhibited positive IHC.
ELISA and Western blot
PrPCWD purification and immunodetection by ELISA and Western blot were performed on unfixed tissue samples using commercial test kists (TeSeE ELISA and TeSeE Western blot; Bio-Rad) according to the manufacturer’s instructions, and as previously described (17). For the ELISA, the absorbance cutoff value for PrPCWD detection was calculated by adding a fixed value of 0.09 to the mean optical density of the negative controls (17). Western blot protein bands were identified, relative intensities quantified, and their molecular mass estimated with a Chemi Doc XRS (Bio-Rad) digital camera and the associated analysis software (Quantity One, version 4.6, Bio-Rad). Positive and negative control PrPCWD samples from CWD elk brain were run on all Western blots throughout the study. Samples were considered positive if the prion protein bands specific to the PK-resistant core of PrPCWD were detected.
PRNP genotyping
Genomic DNA from blood or tissue samples of experimental red deer was subject to PCR amplification and sequence analysis of the PRNP open reading frame as described for Rocky Mountain elk (18).
Results
Pre-clinical detection of PrPCWD
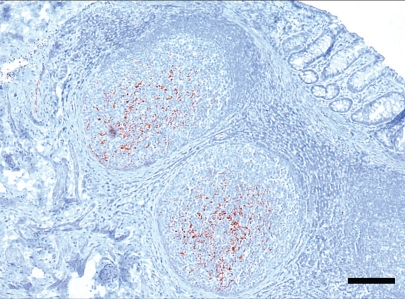
Samples of nictitating membrane collected at 214 d PI were negative by IHC and ELISA for all infected deer. Rectal lymphoid tissue collected at the same time was positive for PrPCWD by IHC (Figure 1) but negative by ELISA for all infected deer.
Figure 1.
Immunohistochemistry (IHC) detection of PrPCWD in rectal biopsy sample from a red deer (Cervus elaphus elaphus) 214 d after oral inoculation with infectious CWD material. Lymphoid follicles beneath the rectal epithelium have variable degrees of immunolabelling associated with tingible body macrophages and follicular dendritic cells. Monoclonal antibody F99/97.6.1 and hematoxylin counterstaining. Bar = 150 μm.
Clinical signs and postmortem examination
At 576 d PI, one of the inoculated deer (No. 103) began to show subtle variable clinical signs consistent with CWD. Abnormal neurological signs included ataxia, hyperexcitability, trembling, weakness, isolation, standing with lowered head, and hyper-salivation. The affected animal consumed reduced amounts of feed leading to a gradual loss of body condition. After a second infected deer (No. 105) began to show similar clinical signs at 583 d PI, all deer were euthanized and subject to postmortem examination. The primary gross lesions were emaciation accompanied by generalized absence or serous atrophy of subcutaneous and abdominal adipose tissue and yellow gelatinous bone marrow. All inoculated animals were otherwise normal and no lesions were found in control deer.
Histopathology
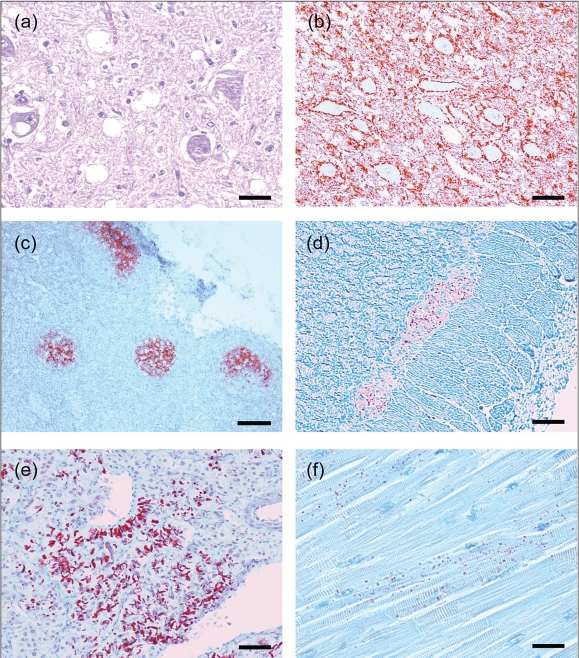
Spongiform encephalopathy was observed in the brain of the 4 infected deer and was characterized by severe and widespread microcavitation primarily of the grey matter, single or multiple intracytoplasmic vacuolation of neurons, neuronal degeneration and loss, mild astrocytic hypertrophy and hyperplasia, and the absence of any significant inflammatory response (Figure 2a). The severity of spongiform change varied in the grey matter of the medulla oblongata, pons, thalamus, and cerebral cortex. Specific areas consistently most severely affected included olfactory bulbs, thalamic nucleus, supraoptic nucleus, paraventricular nucleus, reticular formation, hypoglossal nucleus, parasympathetic nucleus nucleus of the vagus nerve, medial and lateral cuneatus nuclei, and nucleus of the spinal tract of the trigeminal nerve. No lesions suggestive of spongiform encephalopathy were seen in the control deer.
Figure 2.
Immunohistochemistry (IHC) detection of PrPCWD using MAb F99/97.6.1 in various tissues of orally inoculated red deer (Cervus elaphus elaphus) with CWD.
- Brain, medulla oblongata, obex, dorsal motor nucleus of vagus nucleus. There is moderate diffuse vacuolation of the neuropil and also occasional presence of vacuoles within neuronal perikarya. Hematoxylin & eosin. Bar = 30 μm.
- Brain, medulla oblongata, obex. There is extensive accumulation of PrPCWD (stained red) predominantly in the neuropil and with prominent perineuronal labelling. IHC. Bar = 70 μm.
- Tonsil. Detection of PrPCWD in tonsillar lymphoid follicles. IHC. Bar = 275 μm.
- Colon, myenteric plexus. IHC. Bar = 70 μm.
- Adrenal gland, medulla. IHC. Bar = 70 μm.
- Heart, ventricular septum. IHC. Bar = 40 μm.
Tissue distribution of PrPCWD by IHC and ELISA
The results of a comparative assessment of PrPCWD signal intensity obtained by IHC and the ELISA rapid test for various brain and spinal cord areas are shown in Table 1. Both tests detected high levels of PrPCWD from all areas of the brain and spinal cord. The pattern of staining was consistent amongst inoculated animals. The immunolabeling pattern consisted of coarse, granular, or particulate deposits of red chromogen. The labelled foci were located primarily in grey matter, but sometimes appeared in white matter, especially in the rostral cerebrum. The location of PrPCWD was often perineuronal, forming a ring around the cell (Figure 2b). Neuropil accumulations were of the fine particulate type, with some more coarse and linear deposits in severely affected areas. The most severely affected brain areas were the midbrain and medulla where foci were so numerous and large that they often formed large confluent areas. Granular deposits of PrPCWD within the cytoplasm of neurons were prominent, especially throughout the brainstem and thalamic nuclei. Intracellular deposits of PrPCWD were also noticeable in astrocytes and microglial cells in areas of heavy neuropil involvement. In the spinal cord, fine punctuate or coarse particulate neuropil-type accumulation of PrPCWD was greatest in the dorsal horns but at reduced levels as compared to the brain. The eyes of affected deer had consistent PrPCWD distribution by IHC in the optic nerve fibers, the layer of ganglion cells, and the inner and outer plexiform layers of the retina. The ELISA detected PrPCWD from the adjacently sampled ocular areas in 3 of 4 CWD infected deer.
Table 1.
PrPCWD distribution and comparative subjective assessment of the signal intensities of immunohistochemistry (IHC) and enzyme-linked immunosorbent assay (ELISA) from different brain and spinal cord regions from 4 orally inoculated CWD-positive red deer (Cervus elaphus elaphus)
| Animala ID | Spinal cordb |
Brainstem |
Cerebellum |
Midbrain |
Thalamus |
Cerebrum |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IHCc | ELISAd | IHC | ELISA | IHC | ELISA | IHC | ELISA | IHC | ELISA | IHC | ELISA | |
| 101 | +++ | 3.226 | +++ | >3.5 | +++ | 3.016 | +++ | > 3.5 | +++ | > 3.5 | ++ | 1.983 |
| 103a | +++ | 2.570 | +++ | 2.749 | +++ | 2.543 | ++ | 2.739 | ++ | 2.866 | +++ | 2.614 |
| 105a | +++ | 3.165 | +++ | 3.042 | +++ | 2.331 | +++ | 3.014 | +++ | 2.995 | +++ | 3.010 |
| 106 | +++ | 3.227 | +++ | > 3.5 | ++ | 1.362 | +++ | 3.434 | +++ | > 3.5 | ++ | 1.736 |
Animals no. 103 and 105 developed clinical signs at 576 and 583 d post-inoculation, respectively. All deer were subsequently euthanized and subjected to postmortem examination and PrPCWD testing.
Sample of cervical spinal cord (C1–C4).
PrPCWD was detected by IHC with MAb F99/97.6.6 with the scoring system (+ to +++) described in Materials and methods.
Optical density (OD) data from Bio-Rad Laboratories’ ELISA.
In addition to the CNS, PrPCWD was detected by IHC and ELISA in a variety of other tissues including lymphoid tissue, nerve ganglia (trigeminal and dorsal root), endocrine tissues (adrenal gland, pancreas, and pars nervosa of the pituitary gland), cardiac muscle, the myenteric plexus of most gastrointestinal tissues, and nasal mucosa (Table 2).
Table 2.
Comparison of immunohistochemical (IHC) staining and enzyme-linked immunosorbent assay (ELISA) detection of PrPCWD in selected tissues from 4 orally-inoculated CWD-positive red deer (Cervus elaphus elaphus)
| Detection of PrPCWD |
Detection of PrPCWD |
||||
|---|---|---|---|---|---|
| System, anatomical sites | ELISA | IHCa | System, anatomical sites | ELISA | IHC |
| Central nervous system | Pre-femoral LN | 1/4 | 3/4 | ||
| Eye | 3/4b | 4/4 | Popliteal LN | 4/4 | 3/4 |
| Cervical spinal cord (C1–C3): | 4/4 | 4/4 | Spleen | 3/4 | 3/4 |
| Medulla (at obex) | 4/4 | 4/4 | Tonsil | 3/4 | 4/4 |
| Rostral medulla | 4/4 | 4/4 | Digestive system | ||
| Midbrain | 4/4 | 4/4 | Submandibular salivary gland | 2/4 | 0/4 |
| Thalamus | 4/4 | 4/4 | Parotid salivary gland | 2/4 | 1/4 |
| Frontal cortex | 4/4 | 4/4 | Reticulum | 3/4 | 4/4 |
| Cerebellum/vermis | 4/4 | 4/4 | Rumen | 0/4 | 2/4 |
| Peripheral nervous system | Abomasum | 1/4 | 4/4 | ||
| Trigeminal ganglia | 1/4 | 4/4 | Duodenum | 2/3 | 3/3 |
| Dorsal root ganglia | 0/3c | 3/3 | Jejunum | 0/4 | 4/4 |
| Sympathetic trunk | 0/4 | 0/4 | Ileum | 4/4 | 4/4 |
| Vagus nerve | 0/4 | 0/4 | Proximal Colon | 3/4 | 4/4 |
| Sciatic nerve | 0/4 | 0/4 | Distal Colon | 1/4 | 4/4 |
| Radial nerve | 0/4 | 0/4 | Respiratory system | ||
| Facial nerve | 0/4 | 0/4 | Nasal mucosa | 3/3 | 2/4 |
| Brachial plexus | 0/4 | 0/4 | Lung | 0/4 | 0/4 |
| Lymphoreticular system | Endocrine system | ||||
| Rectal lymphoid tissue | 2/4 | 4/4 | Pancreas | 1/4 | 2/4 |
| Peyer’s Patches | 3/4 | 4/4 | Thyroid Gland | 0/4 | 1/4 |
| Retropharyngeal LN | 3/4 | 4/4 | Adrenal Gland | 3/3 | 3/3 |
| Parotid LN | 3/4 | 4/4 | Pituitary Gland | 4/4 | 4/4 |
| Submandibular LN | 3/4 | 4/4 | Musculoskeletal system | ||
| Pre-Scapular LN | 1/4 | 3/4 | Diaphragm | 0/4 | 0/4 |
| Hepatic LN | 3/4 | 4/4 | Masseter muscle | 0/4 | 0/4 |
| Ruminal LN | 3/3 | 3/4 | Triceps brachii muscle | 0/4 | 0/4 |
| Abomasal LN | 3/3 | 3/3 | Longissimus thoracis muscle | 0/4 | 0/4 |
| Mesenteric LN | 4/4 | 4/4 | Semitendinosus muscle | 0/4 | 0/4 |
| Ileocecal LN | 4/4 | 4/4 | |||
| Sublumbar LN | 2/3 | 3/3 | Heart | 2/4 | 4/4 |
PrPCWD was detected by IHC with MAb F99/97.6.6.
Results are expressed as the number of test positive animals over the total number of animals examined.
Some tissues were not available for examination.
LN = lymph node.
Chromogen staining consistent with PrPCWD was observed in the germinal centers of the tonsil, gut-associated lymphoid tissue (GALT), and all lymph nodes. The PrPCWD deposition was characterized by bright red granular material filling germinal centers of 50% to 95% of follicles (Figure 2c). The staining pattern was morphologically consistent with that of follicular dendritic cells within germinal centers of well-developed secondary follicles. Staining often occurred in clusters of adjacent follicles.
The ELISA results were in general agreement with IHC results, but were slightly less sensitive. Of 62 lymphoid tissues tested, ELISA and IHC classified 42 and 57 tissues as positive, respectively. PrPCWD was detected from each lymph node in at least 1 animal. For tissues reported to have potential preclinical CWD diagnostic utility, IHC detected PrPCWD in tonsil and rectal lymphoid tissue from all inoculated deer whereas ELISA classified 3 of 4 tonsil and 1 of 4 rectal lymphoid tissues as CWD positive.
Positive IHC and ELISA results were found in virtually all gastrointestinal tissues including the parotid and mandibular salivary glands, the myenteric plexus of rumen, abomasum, small intestine, ileum, and colon. Deposition of PrPCWD was located in the GALT with a pattern analogous to other lymphoid follicles. Perineuronal staining was present within the submucosal and myenteric plexuses (Figure 2d).
Positive IHC and ELISA results were found in several neuro-endocrine tissues including the pancreas, thyroid, adrenal, and pituitary glands. The pancreas of 2 of 4 CWD-positive deer contained coarse granular PrPCWD deposits confined to the islets of Langerhans. One of these deer was also classified as positive by ELISA. One animal had PrPCWD detected in the thyroid gland with deposits located along the apical surface of parafollicular cells. In the pituitary glands of all CWD-positive deer (IHC and ELISA), PrPCWD deposits were evident, primarily in the pars nervosa and intermedia. Similarly, PrPCWD was detected in the adrenal glands of 3 deer by IHC and ELISA. Staining was restricted to the medulla where large columnar cells appeared to have PrPCWD deposits intracellularly and associated with the cell surface (Figure 2e). The PrPCWD deposits were large and angular as opposed to small and granular as reported in other tissues.
The ventricular myocardium of all 4 infected deer showed PrPCWD deposition consisting of readily visible punctuate granules of chromogen scattered in apparently cell-associated fashion more prominent in longitudinal than in transverse cross-sections of cardiac myocytes. Groups of positive adjacent myocytes were surrounded by cells with no detectable stain (Figure 2f). Epicardial nerves and ganglia and subendocardial and myocardial Purkinje fibers lacked detectable PrPCWD staining. Cardiac muscle was also strongly positive by ELISA in 2 of 4 inoculated deer (Table 2).
Of the respiratory tissues, only the nasal mucosa was ELISA and IHC positive for PrPCWD. No PrPCWD was detected by IHC or ELISA in the following tissues: skeletal striated muscle, kidney, urinary bladder, ovary, uterus, and testis. In addition, no PrPCWD was detected in any tissue of control deer.
Western blot
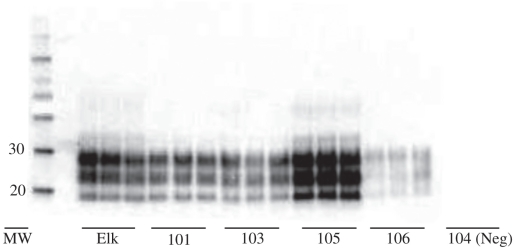
Western blot analysis was performed on selected samples including brainstem, skeletal muscle, cardiac muscle, adrenal gland, pancreas, thyroid gland, and nasal mucosa. For the brainstem, tissue was taken and homogenates prepared from the medulla contralateral to the section taken for IHC. The brainstem of all inoculated deer was positive by this method, showing the characteristic banding pattern of the TSEs (Figure 3). The profiles of 3 bands of proteinase K-resistant isoforms of PrPCWD, di-, mono-, and unglycosylated, were comparable in molecular weight and glycoform ratio to those of the original inoculum. The 2 control deer had no detectable PrPCWD in the brainstem by Western blot.
Figure 3.
Western blot analysis of the prion protein proteinase K-resistant core (PrPres of PrPCWD) from the homogenates of the medulla oblongata of orally inoculated red deer (Cervus elaphus elaphus). The positive banding pattern of 4 red deer samples (deer no. 101, 103, 105, and 106 in triplicate) is compared with that of a previously characterized positive control sample from a captive CWD-affected elk. Animals no. 103 and 105 developed clinical signs at 576 and 583 days post-inoculation respectively. All deer were subsequently euthanized and subject to post-mortem examination and PrPCWD testing. Protein molecular weight (MW) markers are shown on the left in kilodaltons. A negative control sample is shown on the right (deer no. 104).
The Western blot findings were consistent with IHC and ELISA for PrPCWD detection in selected extraneural tissues including cardiac muscle, pancreas, thyroid, and adrenal gland. For example, the adrenal glands, which demonstrated abundant PrPCWD by IHC and ELISA, also showed strong banding pattern signals by Western blot. In contrast, we were unable to demonstrate the presence of PrPCWD in the skeletal striated muscle of any deer.
PRNP genotype
The DNA sequence of the open reading frame of the PRNP gene from the 4 inoculated red deer in this study was similar to that reported previously for C. elaphus elaphus, with a single coding polymorphism (glutamic acid E and glutamine Q) at codon 226 (GenBank P67987 and AY748455) (19) with a synonymous change (GCC to GCT) at codon 106 on the allele encoding 226Q. Samples from all 4 animals encoded methionine at codon 132, glycine at codon 96, and serine at codon 225, sites associated with prion disease progression in Rocky Mountain elk, white-tailed deer, and mule deer, respectively. Red deer 105 was homozygous for 226E, red deer 106 was homozygous for 226Q, and red deer 101 and 103 were heterozygous for the non-synonymous (226EQ) and synonymous (106 gcc/gct) changes.
Discussion
Until recently, only 3 species, all members of the Family Cervidae, were known to be naturally susceptible to CWD, namely: mule deer, white-tailed deer, and Rocky Mountain elk. In 2005, however, a free-ranging moose (Alces alces) was found to be infected with CWD (20) and moose were shown to be susceptible to experimental CWD (21). Although a natural case CWD infection has yet to be reported for red deer, this study indicates that the potential for it to occur exists. This was expected given that elk and red deer are of the same genus and species (Cervus elaphus).
As with other TSEs, CWD is characterized by a long incubation period which, in deer, is seldom less than 18 mo. Although only subtle clinical signs of CWD were evident in 2 of 4 inoculated animals, the neurological lesions were quite obvious histologically for all 4 deer. The distribution and severity of these lesions were consistent with those previously reported for CWD in other cervid species (22,23) and qualitatively similar to those described for the other spongiform encephalopathies (24).
Immunohistochemistry has demonstrated the accumulation of CWD-specific prion protein in the brain, spinal cord, nerves, and ganglia of the peripheral nervous system, lymphoid tissues, and in endocrine tissues of clinical cases (25,26). This study demonstrates that red deer develop detectable PrPCWD after oral exposure to an inoculum containing CWD prions and provides knowledge about the tissue pattern of PrPCWD accumulation at the terminal stages of disease. The pattern of PrPCWD deposition in the brain was consistent with previous descriptions of naturally CWD-infected cervids (23,27). PrPCWD deposition was observed in all areas of spongiform encephalopathy, but in many areas where PrPCWD was present, spongiform degeneration was not found.
The PrPCWD was deposited in follicles of almost all lymphoid tissues, including those of the digestive tract, tonsil, and lymph nodes as has been shown to be the case for other CWD-infected cervids (23), and scrapie-infected sheep and goats (28,29). Sigurdson et al (30) demonstrated PrPCWD in tonsil and alimentary tract-associated lymphoid tissues of mule deer fawns as early as 42 d after they were orally inoculated with brain tissue from captive deer dying of CWD. The pre-clinical detection PrPCWD and the abundance of PrPCWD in the lymphoid tissues at postmortem in the present study suggest that lymphoid tissues of this species may play a role in the pathogenesis and transmission. Such disseminated lymphoid infection contrasts with some other TSEs, such as BSE, in which PrPBSE is detected only in the ileal Peyer’s patches or not at all (31). Similarly, recent experimental transmission of BSE to red deer found PrPBSE restricted to the central and peripheral nervous systems (32).
There is a strong correlation between the presence of PrPTSE and infectivity in prion diseases. Although the epidemiologic evidence strongly suggests that CWD is not transmissible to humans, this study and others suggest caution in this regard. The finding of PrPCWD in various organs, albeit in clinical CWD, suggests that humans who consume or handle meat from CWD-infected red deer may be at risk of exposure to CWD prions. This study found that red deer tissues other than nervous and lymphoid tissue can support CWD prion replication and accumulation. As a result, the consumption or handling of meat from CWD-infected red deer will put humans at risk of exposure to CWD prions. In spite of a well-documented species barrier, a cautious approach would involve preventing such tissues from entering the animal and human food chains. Future studies will require sensitive and quantitative techniques such as bioassays in transgenic mice that assess tissue infectivity and quantitative immunoassays adapted to PrPCWD detection in peripheral tissues.
The presence of PrPCWD in the left ventricular myocardium was probably due to dissemination by peripheral nerves, as this phenomenon has also been observed in CWD-infected mule deer, white-tailed deer, and elk (33,34). It is not known whether the prions that were detected in cardiac tissue were localized to nerve fibers or subcellular structures within myocytes.
Substantial deposits of PrPCWD were detected by IHC and ELISA in the adrenal medulla and pancreatic islet cells, and lesser amounts in the thyroid gland. Infectivity in the pancreas and adrenal medulla, which are innervated by splanchnic nerves and the vagus nerve, respectively, has been documented in natural and experimentally infected scrapie and CWD (25,35). PrP-containing islet cells and glandular cells of the adrenal medulla were often adjacent, which might suggest infection from a common nidus, such as innervation by a common nerve branch. Although PrPCWD has not been reported previously in the thyroid gland, the normal cellular isoform of prion protein (PrPC) has (36), indicative of the potential for conversion to PrPCWD. We also documented PrPCWD in the pars intermedia and nervosa of the pituitary in CWD-infected red deer. Potentially, PrPCWD could transit via nerve fibers from the hypothalamus to the pars nervosa, as the deer had abundant PrPCWD in the hypothalamus. Given the recent report demonstrating CWD infectivity of skeletal muscle tissue when inoculated into transgenic mice (10), the present study rigorously tested for PrPCWD deposits in the skeletal muscles of inoculated red deer. Importantly, no PrPCWD deposition was detected in any striated skeletal muscle using conventional methods. This finding corroborates similar negative observations in cervids made by others (26,33,37). Cardiac muscle differs from skeletal muscle in its structure, physiology, and innervation; one or more of these factors may underlie the difference in PrPCWD deposition between skeletal and cardiac muscle for cervids. Glatzel et al (38) proposed a positive correlation between the duration of disease and the detection of PrPCWD in muscle. A longer duration of clinical disease, therefore, may have increased the likelihood of detecting PrPCWD in muscle.
The exact mode of transmission of CWD in nature remains unclear but is believed to involve direct animal-to-animal contact or environmental contamination. As TSE agents are extremely resistant in the environment (39), oral exposure is the most plausible pathway by which the CWD prion may be introduced to deer in nature and represents a significant obstacle to eradication of CWD from either farmed or free-ranging cervid populations. The distribution of PrPCWD in gut-associated lymphoid tissues, salivary glands, and nasal mucosa in the red deer of this study suggests potential routes of PrPCWD shedding into the environment via fluids such as saliva or feces. However, this study did not identify the point at which an animal may become infectious during the course of infection. An improved understanding of the mechanisms of shedding and transmission will be important in the future management of CWD.
The general pattern of PrPCWD accumulation in cervids with CWD is characterized by relatively rapid and widespread involvement of lymphatic tissues, followed by progressive involvement of, and lesions in, central and peripheral nervous tissues, with involvement of a wider variety of tissues and organs as animals become terminally ill (34). Assuming the immunobiology of red deer is similar to that of other cervids and sheep, it seems probable that initial uptake and propagation of PrPCWD may occur in the lymphoid tissues draining the oral and intestinal mucosa (the retropharyngeal lymph nodes, tonsil, ileal Peyer’s patches, and ileocecal lymph nodes). The finding of PrPCWD in rectal lymphoid tissues as early as 7 months PI supports such a model for red deer.
The appearance of PrPCWD in myenteric and submucosal plexuses of the gastrointestinal tract and in brain and spinal cord is consistent with the hypothesis that autonomic innervations, particularly of enteric tissues, plays a role in the spread of PrPCWD from peripheral tissues to the CNS (25,40). The gastrointestinal tract receives parasympathetic vagal fibers from the DMNV and sympathetic nerve fibers from the spinal cord. PrPCWD was not detected either by ELISA, IHC or Western blot in various peripheral nerves including the brachial plexus, vagus nerve, sciatic nerve, radial nerve, and vagosympathetic trunk (data not shown). The inability to find PrPCWD in the vagus nerve was especially surprising given that this is the presumed primary path of transmission of PrPCWD to the DMNV of the brain (25). This observation, however, is consistent with others (23) and perhaps is due to concentrations below the threshold of detection by any of IHC, ELISA, or WB. PrPCWD was detected, however, in both trigeminal and dorsal root ganglia (Table 2) consistent with the ascending transmission hypothesis. Alternatively, other routes of PrPCWD transit, such as blood, cannot be excluded.
The Bio-Rad ELISA has been an effective diagnostic tool for screening obex or retropharyngeal lymph node tissues from deer and elk for evidence of CWD infection (17). The results presented here show that rapid tests are also able to detect PrPCWD from various other tissues of CWD-infected red deer. ELISA and WB rapid tests revealed essentially the same PrPCWD distribution pattern in tissues as was shown with IHC. However, ELISA generally classified fewer tissues as PrPCWD positive than did IHC. Discrepancies between ELISA and IHC tended to occur in tissues where PrPCWD deposition was sporadic or patchy such as heart, adrenal gland, salivary gland, and in preclinical rectal biopsies. The rapid tests require small samples of tissue to produce a homogenate for further analysis which may, as a result, contain few if any foci of PrPCWD deposition. By contrast, IHC allows for the examination of a large area of a tissue.
For cost-effective surveillance programs it is essential to identify the appropriate tissues for testing, and the present findings have implications for the screening methods for the detection of CWD in red deer. Currently, the obex and retropharyngeal lymph node are the tissues of choice when postmortem sampling for the diagnosis of CWD in cervids that are early in infection. Although additional examinations of field samples and animals at various stages of infection are required, this study supports the examination of brain and at least 1 lymphoid tissue to detect the disease at postmortem in most infected red deer. Both IHC and ELISA identified PrPCWD in the obex of all inoculated deer at postmortem, whereas IHC was more sensitive than ELISA for lymphoid tissues including the retropharyngeal lymph node and tonsil. When brainstem is not available (for example, autolysis), the data suggest that, for C. elaphus species, testing other available CNS areas including cervical spinal cord, thalamus, or cerebellum is preferred to testing lymphoid tissue alone.
The successful eradication of CWD from captive deer herds will require an accurate diagnostic test to identify infected animals during early pre-clinical stages of the disease. Recently, the early accumulation of PrPCWD in accessible peripheral lymphoid tissues such as the tonsil and rectum has become a potential pre-clinical antemortem indicator of disease (41,42). This study supports the use of these accessible lymphoid tissues for CWD surveillance of red deer, with IHC showing greater sensitivity than ELISA.
Differences in the apparent molecular weight and relative abundance of the 3 PrPCWD glycoforms (di-, mono-, and un-glycosylated) after they have been separated on polyacrylamide gels indicate differences between TSEs associated with different species as well as strain differences for particular TSEs (43,44). Western blot of the brainstem of all CWD-inoculated red deer demonstrated a glycoform banding pattern identical to that of the tested extraneural tissues and the inoculum prepared from previously characterized captive elk (Figure 3).
The primary sequence of the prion protein precursor is associated with prevalence, incubation time, and proteolytic cleavage sites in some cervid species exposed to CWD. A polymorphism (methionine M to leucine L) at PRNP codon 132 is associated with increased incubation time and a novel protease cleavage site in PrPCWD of Rocky Mountain elk with experimental disease and in a transgenic rodent model (45,46), although its association with CWD prevalence varies (47). A coding change was identified at codon 226, which encodes glutamic acid (E) in C. elaphus canadensis (48) and C. elaphus nelsoni (49) and glutamine (Q) in Hemionus spp. in North America. The site is polymorphic in red deer [(19) and unpublished data shown in GenBank AY748455] and all 3 diploid genotypes (EE, QQ, EQ) were represented in this study. There were no differences in Western blot profile or incubation time associated with these genotypes. If the effect of genotype on natural disease is similar to that observed in this study, the polymorphism is unlikely to affect disease progression or diagnostic testing.
In summary, this study demonstrates the potential for oral transmission of CWD to red deer and describes the pattern of PrPCWD accumulation for this species. The current surveillance testing regime for cervids would be expected to identify CWD-infected red deer should it occur in North America. These results confirm the usefulness of rapid tests such as ELISA but with generally slightly lower sensitivity when compared with IHC when testing tissues with patchy or sporadic PrPCWD deposition. The finding of PrPCWD in several extra-neural tissues including cardiac muscle and the endocrine system suggests that further investigation and monitoring of the potential transmissibility to other species including humans is warranted.
Acknowledgments
The authors acknowledge the assistance of the Animal Care Staff for animal handling at the Ottawa Laboratory Fallowfield. Excellent technical assistance for collecting, embedding, and cutting tissues was provided by S. Foster, D. Ghazi, and P. Shaffer. CVJ
Footnotes
Disclaimer: Names are necessary to report factually on available data; however, the CFIA neither guarantees nor warrants the standard of the products, and the use of the name by the CFIA implies no approval of the product to the exclusion of others that may also be suitable.
Reprints will not be available from the authors.
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office ( hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
Funding for this study was provided by the Canadian Food Inspection Agency.
References
- 1.Sigurdson CJ. A prion disease of cervids: Chronic wasting disease. Vet Res. 2008;39:41. doi: 10.1051/vetres:2008018. [DOI] [PubMed] [Google Scholar]
- 2.Prusiner SB. Novel proteinaceous infectious particles cause scrapie. Science. 1982;216:136–144. doi: 10.1126/science.6801762. [DOI] [PubMed] [Google Scholar]
- 3.Prusiner SB. Prions. Proc Natl Acad Sci U S A. 1998;95:13363–13383. doi: 10.1073/pnas.95.23.13363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Griffin JF, Mackintosh CG. Tuberculosis in deer: Perceptions, problems and progress. Vet J. 2000;160:202–219. doi: 10.1053/tvjl.2000.0514. [DOI] [PubMed] [Google Scholar]
- 5.Spraker TR, Zink RR, Cummings BA, Wild MA, Miller MW, O’Rourke KI. Comparison of histological lesions and immunohistochemical staining of proteinase-resistant prion protein in a naturally occurring spongiform encephalopathy of free-ranging mule deer (Odocoileus hemionus) with those of chronic wasting disease of captive mule deer. Vet Pathol. 2000;39:110–119. doi: 10.1354/vp.39-1-110. [DOI] [PubMed] [Google Scholar]
- 6.Belay ED, Maddox RA, Williams ES, Miller MW, Gambetti P, Schonberger LB. Chronic wasting disease and potential transmission to humans. Emerg Infect Dis. 2004;10:977–984. doi: 10.3201/eid1006.031082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kong Q, Huang S, Zou W, et al. Chronic wasting disease of elk: Transmissibility to humans examined by transgenic mouse models. J Neurosci. 2005;25:7944–7949. doi: 10.1523/JNEUROSCI.2467-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bartz JC, Kincaid AE, Bessen RA. Rapid prion neuroinvasion following tongue infection. J Virol. 2003;77:583–591. doi: 10.1128/JVI.77.1.583-591.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thomzig A, Kratzel C, Lenz G, Kruger D, Beekes M. Widespread PrPSc accumulation in muscles of hamsters orally infected with scrapie. EMBO Rep. 2003;4:530–533. doi: 10.1038/sj.embor.embor827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Angers RC, Browning SR, Seward TS, et al. Prions in skeletal muscles of deer with chronic wasting disease. Science. 2006;311:1117. doi: 10.1126/science.1122864. [DOI] [PubMed] [Google Scholar]
- 11.Bosque PJ, Ryou C, Telling G, et al. Prions in skeletal muscle. Proc Natl Acad Sci U S A. 2002;99:3812–3817. doi: 10.1073/pnas.052707499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spraker TR, O’Rourke KI, Balachandran A, et al. Validation of monoclonal antibody F99/97.6.1 for immunohistochemical staining of brain and tonsil in mule deer (Odocoileus hemionus) with chronic wasting disease. J Vet Diagn Invest. 2002;14:3–7. doi: 10.1177/104063870201400102. [DOI] [PubMed] [Google Scholar]
- 13.Gavier-Widen D, Stack MJ, Baron T, Balachandran A, Simmons M. Diagnosis of transmissible spongiform encephalopathies in animals: A review. J Vet Diagn Invest. 2005;17:509–527. doi: 10.1177/104063870501700601. [DOI] [PubMed] [Google Scholar]
- 14.O’Rourke KI, Baszler TV, Besser TE, et al. Preclinical diagnosis of scrapie by immunohistochemistry of third eyelid lymphoid tissue. J Clin Microbiol. 2000;38:3254–3259. doi: 10.1128/jcm.38.9.3254-3259.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wolfe LL, Spraker TR, Gonzalez L, et al. PrPCWD in rectal lymphoid tissue of deer (Odocoileus spp.) J Gen Virol. 2007;88:2078–2082. doi: 10.1099/vir.0.82342-0. [DOI] [PubMed] [Google Scholar]
- 16.Spraker TR, O’Rourke KI, Balachandran A, et al. Validation of monoclonal antibody F99/97.6.1 for immunohistochemical staining of brain and tonsil in mule deer (Odocoileus hemionus) with chronic wasting disease. J Vet Diagn Invest. 2002;14:3–7. doi: 10.1177/104063870201400102. [DOI] [PubMed] [Google Scholar]
- 17.Hibler CP, Wilson KL, Spraker TR, et al. Field validation and assessment of an enzyme-linked immunosorbent assay for detecting chronic wasting disease in mule deer (Odocoileus hemionus), white-tailed deer (Odocoileus virginianus), and Rocky Mountain elk (Cervus elaphus nelsoni) J Vet Diagn Invest. 2003;15:311–319. doi: 10.1177/104063870301500402. [DOI] [PubMed] [Google Scholar]
- 18.Spraker TR, Balachandran A, Zhuang D, O’Rourke KI. Variable patterns of distribution of PrP(CWD) in the obex and cranial lymphoid tissues of Rocky Mountain elk (Cervus elaphus nelsoni) with subclinical chronic wasting disease. Vet Rec. 2004;155:295–302. doi: 10.1136/vr.155.10.295. [DOI] [PubMed] [Google Scholar]
- 19.Kaluz S, Kaluzova M, Flint AP. Sequencing analysis of prion genes from red deer and camel. Gene. 1997;199:283–286. doi: 10.1016/s0378-1119(97)00382-x. [DOI] [PubMed] [Google Scholar]
- 20.Baeten LA, Powers BE, Jewell JE, Spraker TR, Miller MW. A natural case of chronic wasting disease in a free-ranging moose (Alces alces shirasi) J Wildl Dis. 2007;43:309–314. doi: 10.7589/0090-3558-43.2.309. [DOI] [PubMed] [Google Scholar]
- 21.Kreeger TJ, Montgomery DL, Jewell JE, Schultz W, Williams ES. Oral Transmission of chronic wasting disease in captive Shira’s moose. J Wildl Dis. 2006;42:640–645. doi: 10.7589/0090-3558-42.3.640. [DOI] [PubMed] [Google Scholar]
- 22.Williams ES, Young S. Neuropathology of chronic wasting disease of mule deer (Odocoileus hemionus) and elk (Cervus elaphus nelsoni) Vet Pathol. 1993;30:36–45. doi: 10.1177/030098589303000105. [DOI] [PubMed] [Google Scholar]
- 23.Spraker TR, Zink RR, Cummings BA, Wild MA, Miller MW, O’Rourke KI. Comparison of histological lesions and immunohistochemical staining of proteinase-resistant prion protein in a naturally occurring spongiform encephalopathy of free-ranging mule deer (Odocoileus hemionus) with those of chronic wasting disease of captive mule deer. Vet Pathol. 2002;39:110–119. doi: 10.1354/vp.39-1-110. [DOI] [PubMed] [Google Scholar]
- 24.Wells GA, Hancock RD, Cooley WA, Richards MS, Higgins RJ, David GP. Bovine spongiform encephalopathy: Diagnostic significance of vacuolar changes in selected nuclei of the medulla oblongata. Vet Rec. 1989;125:521–524. doi: 10.1136/vr.125.21.521. [DOI] [PubMed] [Google Scholar]
- 25.Sigurdson CJ, Spraker TR, Miller MW, Oesch B, Hoover EA. PrP(CWD) in the myenteric plexus, vagosympathetic trunk and endocrine glands of deer with chronic wasting disease. J Gen Virol. 2001;82:2327–2334. doi: 10.1099/0022-1317-82-10-2327. [DOI] [PubMed] [Google Scholar]
- 26.Spraker TR, Zink RR, Cummings BA, Sigurdson CJ, Miller MW, O’Rourke KI. Distribution of protease-resistant prion protein and spongiform encephalopathy in free-ranging mule deer (Odocoileus hemionus) with chronic wasting disease. Vet Pathol. 2002;39:546–556. doi: 10.1354/vp.39-5-546. [DOI] [PubMed] [Google Scholar]
- 27.Spraker TR, Miller MW, Williams ES, et al. Spongiform encephalopathy in free-ranging mule deer (Odocoileus hemionus), white-tailed deer (Odocoileus virginianus) and Rocky Mountain elk (Cervus elaphus nelsoni) in northcentral Colorado. J Wildl Dis. 1997;33:1–6. doi: 10.7589/0090-3558-33.1.1. [DOI] [PubMed] [Google Scholar]
- 28.Hadlow WJ, Kennedy RC, Race RE, Eklund CM. Virologic and neurohistologic findings in dairy goats affected with natural scrapie. Vet Pathol. 1980;17:187–199. doi: 10.1177/030098588001700207. [DOI] [PubMed] [Google Scholar]
- 29.Hadlow WJ, Kennedy RC, Race RE. Natural infection of Suffolk sheep with scrapie virus. J Infect Dis. 1982;146:657–664. doi: 10.1093/infdis/146.5.657. [DOI] [PubMed] [Google Scholar]
- 30.Sigurdson CJ, Williams ES, Miller MW, Spraker TR, O’Rourke KI, Hoover EA. Oral transmission and early lymphoid tropism of chronic wasting disease PrPres in mule deer fawns (Odocoileus hemionus) J Gen Virol. 1999;80:2757–2764. doi: 10.1099/0022-1317-80-10-2757. [DOI] [PubMed] [Google Scholar]
- 31.Wells GA, Hawkins SA, Green RB, et al. Preliminary observations on the pathogenesis of experimental bovine spongiform encephalopathy (BSE): An update. Vet Rec. 1998;142:103–106. doi: 10.1136/vr.142.5.103. [DOI] [PubMed] [Google Scholar]
- 32.Dagleish MP, Martin S, Steele P, et al. Experimental transmission of bovine spongiform encephalopathy to European red deer (Cervus elaphus elaphus) BMC Vet Res. 2008;4:17. doi: 10.1186/1746-6148-4-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jewell JE, Brown J, Kreeger T, Williams ES. Prion protein in cardiac muscle of elk (Cervus elaphus nelsoni) and white-tailed deer (Odocoileus virginianus) infected with chronic wasting disease. J Gen Virol. 2006;87:3443–3450. doi: 10.1099/vir.0.81777-0. [DOI] [PubMed] [Google Scholar]
- 34.Fox KA, Jewell JE, Williams ES, Miller MW. Patterns of PrPCWD accumulation during the course of chronic wasting disease infection in orally inoculated mule deer (Odocoileus hemionus) J Gen Virol. 2006;87:3451–3461. doi: 10.1099/vir.0.81999-0. [DOI] [PubMed] [Google Scholar]
- 35.Ye X, Carp RI, Yu Y, Kozielski R, Kozlowski P. Hyperplasia and hypertrophy of B cells in the islets of Langerhans in hamsters infected with the 139H strain of scrapie. J Comp Pathol. 1994;110:169–183. doi: 10.1016/s0021-9975(08)80188-0. [DOI] [PubMed] [Google Scholar]
- 36.Yamazaki K, Yamada E, Kanaji Y, et al. Stimulation of cellular prion protein expression by TSH in human thyrocytes. Biochem Biophys Res Commun. 2003;305:1034–1039. doi: 10.1016/s0006-291x(03)00801-5. [DOI] [PubMed] [Google Scholar]
- 37.Hamir AN, Miller JM, Cutlip RC. Failure to detect prion protein (PrPres) by immunohistochemistry in striated muscle tissues of animals experimentally inoculated with agents of transmissible spongiform encephalopathy. Vet Pathol. 2004;41:78–81. doi: 10.1354/vp.41-1-78. [DOI] [PubMed] [Google Scholar]
- 38.Glatzel M, Abela E, Maissen M, Aguzzi A. Extraneural pathologic prion protein in sporadic Creutzfeldt-Jakob disease. N Engl J Med. 2003;349:1812–1820. doi: 10.1056/NEJMoa030351. [DOI] [PubMed] [Google Scholar]
- 39.Georgsson G, Sigurdarson S, Brown P. Infectious agent of sheep scrapie may persist in the environment for at least 16 years. J Gen Virol. 2006;87:3737–3740. doi: 10.1099/vir.0.82011-0. [DOI] [PubMed] [Google Scholar]
- 40.Beekes M, Baldauf E, Diringer H. Sequential appearance and accumulation of pathognomonic markers in the central nervous system of hamsters orally infected with scrapie. J Gen Virol. 1996;77:1925–1934. doi: 10.1099/0022-1317-77-8-1925. [DOI] [PubMed] [Google Scholar]
- 41.Wild MA, Spraker TR, Sigurdson CJ, O’Rourke KI, Miller MW. Preclinical diagnosis of chronic wasting disease in captive mule deer (Odocoileus hemionus) and white-tailed deer (Odocoileus virginianus) using tonsillar biopsy. J Gen Virol. 2002;83:2629–2634. doi: 10.1099/0022-1317-83-10-2629. [DOI] [PubMed] [Google Scholar]
- 42.Gonzalez L, Horton R, Ramsay D, et al. Adaptation and evaluation of a rapid test for the diagnosis of sheep scrapie in samples of rectal mucosa. J Vet Diagn Invest. 2008;20:203–208. doi: 10.1177/104063870802000209. [DOI] [PubMed] [Google Scholar]
- 43.Baron TG, Madec JY, Calavas D. Similar signature of the prion protein in natural sheep scrapie and bovine spongiform encephalopathy-linked diseases. J Clin Microbiol. 1999;37:3701–3704. doi: 10.1128/jcm.37.11.3701-3704.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stack MJ, Chaplin MJ, Clark J. Differentiation of prion protein glycoforms from naturally occurring sheep scrapie, sheep-passaged scrapie strains (CH1641 and SSBP1), bovine spongiform encephalopathy (BSE) cases and Romney and Cheviot breed sheep experimentally inoculated with BSE using two monoclonal antibodies. Acta Neuropathol. 2002;104:279–286. doi: 10.1007/s00401-002-0556-2. [DOI] [PubMed] [Google Scholar]
- 45.O’Rourke KI, Spraker TR, Zhuang D, Greenlee JJ, Gidlewski TE, Hamir AN. Elk with a long incubation prion disease phenotype have a unique PrPd profile. Neuroreport. 2007;18:1935–1938. doi: 10.1097/WNR.0b013e3282f1ca2f. [DOI] [PubMed] [Google Scholar]
- 46.Green KM, Browning SR, Seward TS, et al. The elk PRNP codon 132 polymorphism controls cervid and scrapie prion propagation. J Gen Virol. 2008;89:598–608. doi: 10.1099/vir.0.83168-0. [DOI] [PubMed] [Google Scholar]
- 47.Perucchini M, Griffin K, Miller MW, Goldmann W. PrP genotypes of free-ranging wapiti (Cervus elaphus nelsoni) with chronic wasting disease. J Gen Virol. 2008;89:1324–1328. doi: 10.1099/vir.0.83424-0. [DOI] [PubMed] [Google Scholar]
- 48.Wopfner F, Weidenhofer G, Schneider R, et al. Analysis of 27 mammalian and 9 avian PrPs reveals high conservation of flexible regions of the prion protein. J Mol Biol. 1999;289:1163–1178. doi: 10.1006/jmbi.1999.2831. [DOI] [PubMed] [Google Scholar]
- 49.O’Rourke KI, Besser TE, Miller MW, et al. PrP genotypes of captive and free-ranging Rocky Mountain elk (Cervus elaphus nelsoni) with chronic wasting disease. J Gen Virol. 1999;80:2765–2769. doi: 10.1099/0022-1317-80-10-2765. [DOI] [PubMed] [Google Scholar]