Cuterebra species (Diptera: Oestridae) are an often-overlooked cause of central nervous system signs in cats with outdoor access during the summer months. Cuterebra species, of which there are 34 found throughout the Americas, are obligate parasites of rodents and lagomorphs (1,2–5). The female botfly may lay up to several thousand eggs, typically in groups of 5 to 15 per site, along rodent runs, near the opening to the burrow, or within the burrow, depending on the species (2,5). The eggs hatch approximately 1 wk later in response to a sudden rise in temperature, such as the hot breath of a passing host (3–5). Depending on environmental conditions, eggs may remain viable for 6 to 10 mo, resulting in late season infections (5). The moist larva sticks to the fur and achieves entry to the body via the mucous membranes of a natural orifice (2–4). The larva migrates extensively through the host, visiting the trachea, thoracic and abdominal cavities, before arriving at its preferred subcutaneous site (2–4). Here it feeds on local tissues, creating, 3 to 4 wk after initial infection, a local swelling (a warble) with a breathing/excretion pore, and molts through the various larval instar stages (2–4). Three to 8 wk after entering the host, the larva backs out through the pore, drops to the ground, and burrows into the soil to pupate and, possibly, overwinter (2–4). Emergence of the adult botfly from the pupa may vary from a month to several years later, starting in late spring (2–4). Given 1 to 2 wk for mating, 1 wk for egg maturation, and 3 to 4 wk for larval migration and warble development, the seasonal (midsummer through fall) occurrence of cuterebral myiasis becomes apparent (2).
Cats are an atypical host, and are speculated to acquire infection while exploring rodent and rabbit environs (2). It has been suggested that cats are not susceptible to rodent-infesting Cuterebra species, instead acquiring the lagomorph-infesting species (6). Cats in published case reports are young to middle-aged and, in Canada, they present in the months of July, August, and September (2,7–9). Clinical signs depend on the migration path of the larva. Most commonly, the cutaneous form is encountered — this is relatively benign and easily diagnosed and treated (2,10). There are case reports of feline ophthalmomyiasis (11–14). Similarly, Cuterebra has been reported in the trachea (15,16), pharynx (17), and upper respiratory tract (7,18). Intracranial cuterebral myiasis may be preceded by upper respiratory signs (2,7,19), with the larvae gaining access to the calvarium via the cribriform plate, middle ear, or other foraminae (7–9,20). Rarely, multiple larvae may be found in an individual animal (8). Larvae removed from their hosts do not pose a zoonotic threat (2,8,10).
Clinical signs associated with cerebrospinal cuterebriasis are a reflection of the neuroanatomical extent of the lesion(s) caused by the larva. As such, clinical signs may include acute status epilepticus, unilateral or bilateral central blindness, abnormal behavior, head pressing, anorexia, lethargy, disorientation, dementia, abnormal vocalization, circling, vestibular or other cranial nerve deficits, abnormal gait, postural reactions, or reflexes, and inappropriate responses to stimulation (2,7,8,12,19). Thus, lesion localization within the central nervous system may include any or all of the thalamocortex, brainstem, or spinal cord, depending on the larval path. The following 3 cases, seen recently at the Ontario Veterinary College, serve as good illustrations of the variety of clinical signs that may be observed in cats with presumptive or definitive cerebrospinal cuterebriasis.
Case 1
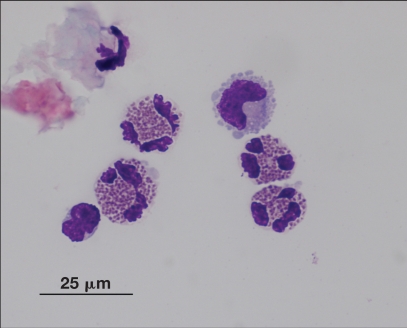
An intact, 10-month-old female, domestic shorthair cat presented with acute onset of lethargy, right-sided facial nerve paralysis and head tilt with horizontal nystagmus (fast phase to the left) after acute onset of fits of sneezing 1 wk earlier. An eosinophilic pleocytosis was found on cerebrospinal fluid analysis (Figure 1).
Figure 1.
Eosinophilic pleocytosis in the cerebrospinal fluid of a 10-month-old female intact cat with an acute onset of lethargy and right-side cranial nerves VII and VIII deficits. (Image courtesy of Dr. Pamela Baker)
Case 2
A 5-year-old male, castrated domestic shorthair cat, presented for right pelvic limb upper motor neuron weakness and coincident acute onset gagging and coughing. A Cuterebra larva, encysted within the wall of the oropharynx, was diagnosed via endoscopy, while the cerebrospinal fluid analysis was unremarkable.
Case 3
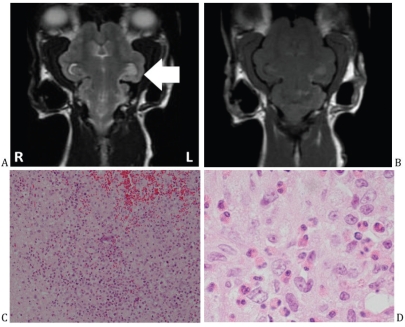
A 3-year-old female, spayed Persian cat, presented, after sneezing and nasal discharge 1 to 2 wk earlier, with acute onset focal and generalized seizures, progressing to obtundation. Magnetic resonance imaging (MRI) of the brain showed subtentorial and transforaminal brain herniation, with visible meandering tracks of parenchymal hyperintensity on T2-weighted images, and on post-contrast T1-weighted images, corresponding to eosinophilic track lesions on histopathology (Figure 2). This correlation between MRI and histopathology findings has not been reported in a cat.
Figure 2.
A — A T2 Fast Spin Echo-weighted magnetic resonance (MR) image in the dorsal plane of a 3-year-old female spayed Persian cat with acute onset seizures and depression after sneezing and nasal discharge in the preceding 1 to 2 wk. Hyperintensity of the parenchyma may be seen at the base of the left hemisphere, tracking caudally to the left rostral medulla and cerebellar peduncles. B — There was a corresponding enhancement on post-contrast T1 weighted-MR image. C — Histopathology of an involved portion of cerebellum from the same cat pictured in A. Hemorrhage and necrosis were found, surrounded by an eosinophilic inflammation. D — Detail of C showing eosinophilic inflammation surrounding the area of hemorrhage and necrosis.
As was seen in Case 2, the diagnosis is obvious if a Cuterebra larva is found (2,6,10). Inspection of the upper respiratory tract may identify a larva (2). Clinical suspicion of cerebrospinal cuterebriasis is raised when confronted with a history of upper respiratory signs 1 to 2 wk prior to onset of intracranial neurologic signs, as in Cases 1 and 3 (2,7,8,19). Abnormal body temperature has been reported (7). The hemogram may show a peripheral leukocytosis with eosinophilia (2,7). Globulin concentration may be increased on the serum biochemical profile (7). Cerebrospinal fluid (CSF) analysis may show a pleocytosis with eosinophils, neutrophils, or mononuclear cells, and an elevation in total protein (2). Computed tomography (CT) shows a mottled appearance to the brain, consistent with encephalitis (7,21). Magnetic resonance imaging may show linear regions of hypointensity on T1-weighted images, hyperintensity on T2-weighted images, and contrast enhancement, with the possibility of small areas of hemorrhage (21,22). Characteristic histopathologic features include parasite tracts, superficial laminar cerebrocortical necrosis, cerebral infarction, subependymal rarefaction and astrogliosis with or without ependymal cell loss, and subpial astrogliosis (7,9,20). Certain of these features, occurring within the brain parenchyma independent of the location of the larva or its track lesions, are similar to lesions reported for feline ischemic encephalopathy (FIE) (9,20).
This latter observation contributes to the argument that cerebrospinal cuterebriasis causes FIE. Additionally, where Cuterebra species are not geographically present, FIE is not reported (7,9). Furthermore, the seasons for occurrence and the clinical signs of the 2 diseases overlap (9). It is postulated that the migrating larva elaborates a biochemical mediator resulting in vascular spasm, for example, oxyhemoglobin and its derivatives from focal hemorrhage or a parasite-produced toxin, that circulates in the CSF bathing the cerebral arteries, thus inducing ischemic infarct (9). It has also been suggested, though not proven, that cuterebriasis may be a causative agent of feline idiopathic vestibular disease (7). This hypothesis is based upon similarities between seasonal occurrence and history of outdoor exposure in cats with idiopathic vestibular disease and cats with cuterebriasis. Confirmation of this hypothesis has been stymied by the high rate of recovery of cats with idiopathic vestibular disease, reducing the opportunities for postmortem examination (7).
Prognosis depends on lesion localization, clinical signs, and response to therapy. Animals may die within hours of onset of signs, sustain permanent neurological deficits, or recover to an acceptable level of function and quality of life (8). It is thought that cats with cuterebriasis resembling feline idiopathic vestibular disease will likely return to completely normal function (7).
There are no reported clinical trials for treatment of cerebrospinal cuterebriasis, possibly because of the difficulty in achieving antemortem diagnosis. Surgical removal of the offending larva has been suggested, but not reported (2). Ivermectin has been the therapy of choice in the majority of case reports (2,7,8,10,12). One report described the use of levamisole at 60 mg/d for 7 d (8). Ivermectin, at 0.1 mg/kg, is effective against Cuterebra species (2). Reported doses ranged from 0.2 mg/kg subcutaneously (10), 0.3 mg/kg subcutaneously every 48 h for 3 treatments (8), or 0.3 mg/kg orally every 14 d for 2 treatments (12) to 0.4 mg/kg subcutaneously every 24 h for 3 treatments (7). For presumptive cerebrospinal cuterebriasis, ivermectin may be given orally (2). Corticosteroids are recommended alongside ivermectin to prevent additional inflammatory damage during the treatment period (2,7,8). Doses range from prednisone [1 mg/kg, PO, q12h, for 3 wk, then q24h for 3 wk (2)] for cats with upper respiratory syndrome and a presumptive diagnosis to dexamethasone [0.1 mg/kg, IV, given at the same time as the ivermectin q24h for 3 treatments (7)]. Larval disruption has been associated with a Type I hypersensitivity-like reaction (2,7,8). Diphenhydramine pre-medication (4 mg/kg IM, 1 to 2 h prior to ivermectin administration) has been recommended to mitigate this possibility (7). Antibiotics (for example, enrofloxacin, 5 mg/kg, PO, q12h for 14 d) have also been administered in case of bacterial introduction during larval migration (7). A preventive program may be recommended (2), including owner education, lifestyle changes for cats with outdoor access, careful monitoring of “respiratory infections” in the late summer and early fall, and monthly administration of anthelmintics (such as, fipronil, imidacloprid, moxidectin, milbemycin oxime, selamectin, or ivermectin) (2,10).
In conclusion, cerebrospinal cuterebriasis represents a common neurological disease in cats presented during the summer months with acute to peracute onset of multiple cranial nerve deficits and other neurological signs often preceded by upper respiratory airway dysfunction. Early diagnosis and treatment may lead to complete recovery of function or at least an acceptable long-term quality of life.
Footnotes
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office ( hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
References
- 1.Sabrosky CW. Thomas Say Monograph. College Park, Md: Entomol Soc Am; 1986. North American Species of Cuterebra: The Rabbit and Rodent Bot Flies (Diptera: Cuterebridae) pp. 1–240. [Google Scholar]
- 2.Bowman DD, Hendrix CM, Lindsay DS, Barr SC. Feline Clinical Parasitology . Ames, Iowa: Iowa State Univ Pr; 2002. pp. 430–439. [Google Scholar]
- 3.Hunter DM, Webster JM. Determination of the migratory route of botfly larvae, Cuterebra grisea (Diptera: Cuterebridae) in deermice. Int J Parasitol. 1973;3:311–316. doi: 10.1016/0020-7519(73)90108-2. [DOI] [PubMed] [Google Scholar]
- 4.Baird CR. Bionomics of Cuterebra austeni (Diptera: Cuterebridae) and its association with Neotoma albigula (Rodentia: Cricetidae) in the southwestern United States. J Med Entomol. 1997;34:690–695. doi: 10.1093/jmedent/34.6.690. [DOI] [PubMed] [Google Scholar]
- 5.Catts EP. Biology of New World Bot Flies: Cuterebridae. Annu Rev Entomol. 1982;27:313–338. [Google Scholar]
- 6.Slansky F. Feline cuterebrosis caused by a lagomorph-infesting Cuterebra spp. larva. J Parasitol. 2007;93:959–961. doi: 10.1645/GE-1107R.1. [DOI] [PubMed] [Google Scholar]
- 7.Glass EN, Cornetta AM, deLahunta A, Center SA, Kent M. Clinical and clinicopathologic features in 11 cats with Cuterebra larvae myiasis of the central nervous system. J Vet Intern Med. 1998;12:365–368. doi: 10.1111/j.1939-1676.1998.tb02136.x. [DOI] [PubMed] [Google Scholar]
- 8.Hendrix CM, Cox NR, Clemons-Chevis CL, DiPinto MN, Sartin EA. Aberrant intracranial myiasis caused by larval cuterebra infection. Compend Contin Educ Pract Vet. 1989;11:550–562. [Google Scholar]
- 9.Williams KJ, Summers BA, deLahunta A. Cerebrospinal cuterebriasis in cats and its association with feline ischemic encephalopathy. Vet Pathol. 1998;35:330–343. doi: 10.1177/030098589803500502. [DOI] [PubMed] [Google Scholar]
- 10.Tilley LP, Smith FWK., Jr . Blackwell’s Five Minute Veterinary Consult Clinical Companion: Canine and Feline. Ames, Iowa: Blackwell Publ; 2007. pp. 324–325. [Google Scholar]
- 11.Johnson BW, Helper LC, Szajerski ME. Intraocular Cuterebra in a cat. J Am Vet Med Assoc. 1988;193:829–830. [PubMed] [Google Scholar]
- 12.Harris BP, Miller PE, Bloss JR, Pellitteri PJ. Ophthalmomyiasis interna anterior associated with Cuterebra spp. in a cat. J Am Vet Med Assoc. 2000;216:352–355. doi: 10.2460/javma.2000.216.352. [DOI] [PubMed] [Google Scholar]
- 13.Wyman M, Starkey R, Weisbrode S, Filko D, Grandstaff R, Ferrebee E. Ophthalmomyiasis (interna posterior) of the posterior segment and central nervous system myiasis: Cuterebra spp. in a cat. Vet Ophthalmol. 2005;8:77–80. doi: 10.1111/j.1463-5224.2005.00343.x. [DOI] [PubMed] [Google Scholar]
- 14.Stiles J, Rankin A. Ophthalmomyiasis interna anterior in a cat: Surgical resolution. Vet Ophthalmol. 2006;9:165–168. doi: 10.1111/j.1463-5224.2006.00461.x. [DOI] [PubMed] [Google Scholar]
- 15.Fitzgerald SD, Johnson CA, Peck EJ. A fatal case of intrathoracic cuterebriasis in a cat. J Am Anim Hosp Assoc. 1996;32:353–357. doi: 10.5326/15473317-32-4-353. [DOI] [PubMed] [Google Scholar]
- 16.Dvorak LD, Bay JD, Crouch DT, Corwin RM. Successful treatment of intratracheal cuterebrosis in two cats. J Am Anim Hosp Assoc. 2000;36:304–308. doi: 10.5326/15473317-36-4-304. [DOI] [PubMed] [Google Scholar]
- 17.Kazacos KR, Bright RM, Johnson KE, Anderson KL, Cantwell HD. Cuterebra sp. as a cause of pharyngeal myiasis in cats. J Am Anim Hosp Assoc. 1980;16:773–776. [Google Scholar]
- 18.Wolf AM. Cuterebra larva in the nasal passage of a kitten. Feline Pract. 1979;9:25–26. [Google Scholar]
- 19.Cook JR, Levesque DC, Nuehring LP. Intracranial cuterebral myiasis causing acute lateralizing meningoencephalitis in 2 cats. J Am Anim Hosp Assoc. 1985;21:279–284. [Google Scholar]
- 20.Summers BA, Cummings JF, deLahunta A. Veterinary Neuropathology. 1st ed. St Louis, Missouri: Mosby; 1995. pp. 242–244. [Google Scholar]
- 21.Thomas WB. Nonneoplastic disorders of the brain. Clin Tech Small Anim Pract. 1999;14:125–147. doi: 10.1016/S1096-2867(99)80030-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tieber LM, Axlund TW, Simpson ST, Hathcock JT. Survival of a suspected case of central nervous system cuterebrosis in a dog: Clinical and magnetic resonance imaging findings. J Am Anim Hosp Assoc. 2006;42:238–242. doi: 10.5326/0420238. [DOI] [PubMed] [Google Scholar]