Abstract
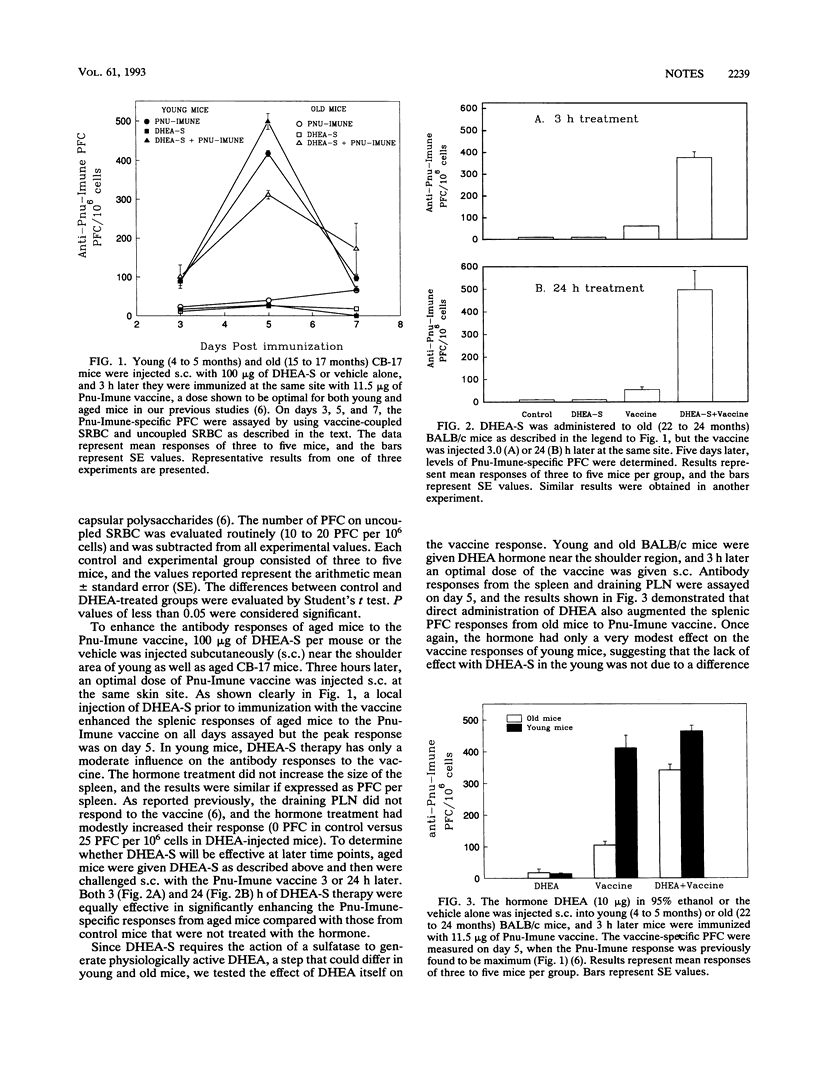
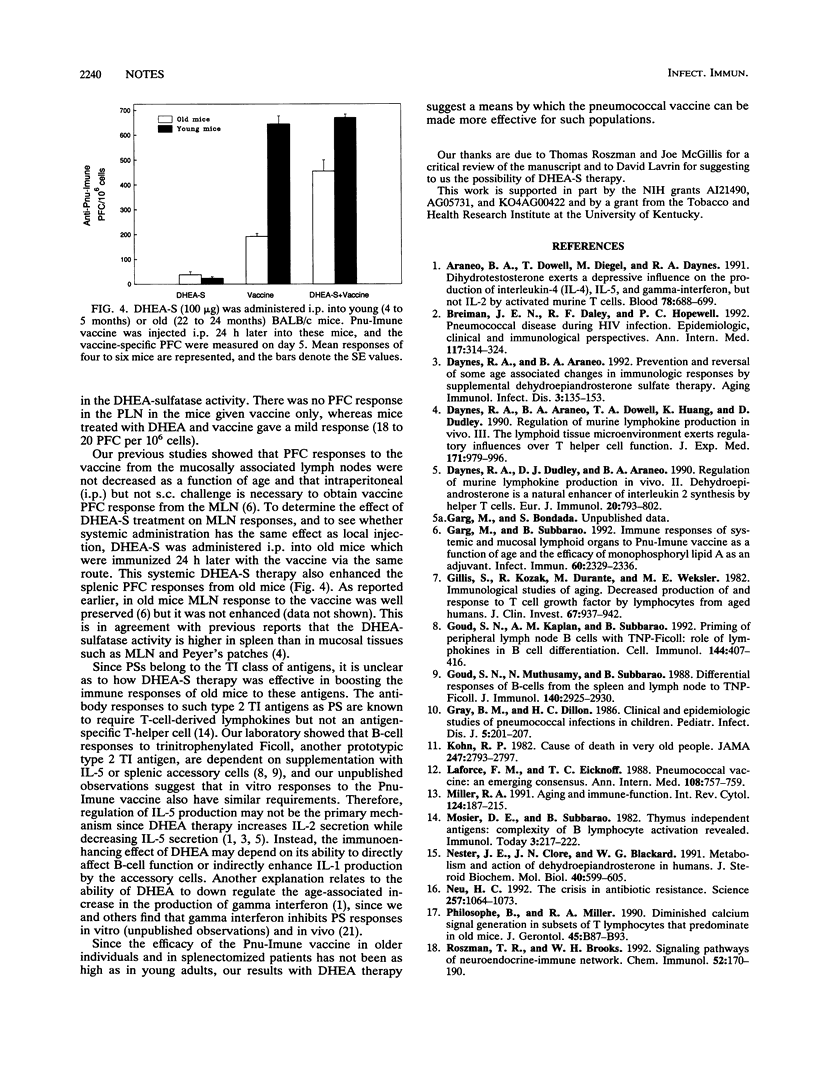
Recently, we reported that murine antibody responses to the 23-valent pneumococcal polysaccharide (Pnu-Imune) vaccine declined with age. Here we present data to support the concept that age-associated immune defects are not only due to intrinsic defects in immune cells but are also due to extrinsic factors emanating from the neuroendocrine system. We found that supplementation with dehydroepiandrosterone, a steroid hormone known to be reduced in the aged, corrects the immune deficiency of aged mice and significantly enhanced their splenic immune responses to the Pnu-Imune vaccine.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Araneo B. A., Dowell T., Diegel M., Daynes R. A. Dihydrotestosterone exerts a depressive influence on the production of interleukin-4 (IL-4), IL-5, and gamma-interferon, but not IL-2 by activated murine T cells. Blood. 1991 Aug 1;78(3):688–699. [PubMed] [Google Scholar]
- Daynes R. A., Araneo B. A., Dowell T. A., Huang K., Dudley D. Regulation of murine lymphokine production in vivo. III. The lymphoid tissue microenvironment exerts regulatory influences over T helper cell function. J Exp Med. 1990 Apr 1;171(4):979–996. doi: 10.1084/jem.171.4.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daynes R. A., Dudley D. J., Araneo B. A. Regulation of murine lymphokine production in vivo. II. Dehydroepiandrosterone is a natural enhancer of interleukin 2 synthesis by helper T cells. Eur J Immunol. 1990 Apr;20(4):793–802. doi: 10.1002/eji.1830200413. [DOI] [PubMed] [Google Scholar]
- Garg M., Subbarao B. Immune responses of systemic and mucosal lymphoid organs to Pnu-Imune vaccine as a function of age and the efficacy of monophosphoryl lipid A as an adjuvant. Infect Immun. 1992 Jun;60(6):2329–2336. doi: 10.1128/iai.60.6.2329-2336.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillis S., Kozak R., Durante M., Weksler M. E. Immunological studies of aging. Decreased production of and response to T cell growth factor by lymphocytes from aged humans. J Clin Invest. 1981 Apr;67(4):937–942. doi: 10.1172/JCI110143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goud S. N., Kaplan A. M., Subbarao B. Priming of peripheral lymph node B cells with TNP-Ficoll: role of lymphokines in B cell differentiation. Cell Immunol. 1992 Oct 15;144(2):407–416. doi: 10.1016/0008-8749(92)90255-n. [DOI] [PubMed] [Google Scholar]
- Goud S. N., Muthusamy N., Subbarao B. Differential responses of B cells from the spleen and lymph node to TNP-Ficoll. J Immunol. 1988 May 1;140(9):2925–2930. [PubMed] [Google Scholar]
- Gray B. M., Dillon H. C., Jr Clinical and epidemiologic studies of pneumococcal infection in children. Pediatr Infect Dis. 1986 Mar-Apr;5(2):201–207. doi: 10.1097/00006454-198603000-00009. [DOI] [PubMed] [Google Scholar]
- Janoff E. N., Breiman R. F., Daley C. L., Hopewell P. C. Pneumococcal disease during HIV infection. Epidemiologic, clinical, and immunologic perspectives. Ann Intern Med. 1992 Aug 15;117(4):314–324. doi: 10.7326/0003-4819-117-4-314. [DOI] [PubMed] [Google Scholar]
- Kohn R. R. Cause of death in very old people. JAMA. 1982 May 28;247(20):2793–2797. [PubMed] [Google Scholar]
- LaForce F. M., Eickhoff T. C. Pneumococcal vaccine: an emerging consensus. Ann Intern Med. 1988 May;108(5):757–759. doi: 10.7326/0003-4819-108-5-757. [DOI] [PubMed] [Google Scholar]
- Miller R. A. Aging and immune function. Int Rev Cytol. 1991;124:187–215. doi: 10.1016/s0074-7696(08)61527-2. [DOI] [PubMed] [Google Scholar]
- Nestler J. E., Clore J. N., Blackard W. G. Metabolism and actions of dehydroepiandrosterone in humans. J Steroid Biochem Mol Biol. 1991;40(4-6):599–605. doi: 10.1016/0960-0760(91)90282-a. [DOI] [PubMed] [Google Scholar]
- Neu H. C. The crisis in antibiotic resistance. Science. 1992 Aug 21;257(5073):1064–1073. doi: 10.1126/science.257.5073.1064. [DOI] [PubMed] [Google Scholar]
- Philosophe B., Miller R. A. Diminished calcium signal generation in subsets of T lymphocytes that predominate in old mice. J Gerontol. 1990 May;45(3):B87–B93. doi: 10.1093/geronj/45.3.b87. [DOI] [PubMed] [Google Scholar]
- Roszman T. L., Brooks W. H. Signaling pathways of the neuroendocrine-immune network. Chem Immunol. 1992;52:170–190. [PubMed] [Google Scholar]
- Sims R. V., Steinmann W. C., McConville J. H., King L. R., Zwick W. C., Schwartz J. S. The clinical effectiveness of pneumococcal vaccine in the elderly. Ann Intern Med. 1988 May;108(5):653–657. doi: 10.7326/0003-4819-108-5-653. [DOI] [PubMed] [Google Scholar]
- Subbarao B., Morris J., Kryscio R. J. Phenotypic and functional properties of B lymphocytes from aged mice. Mech Ageing Dev. 1990 Feb 15;51(3):223–241. doi: 10.1016/0047-6374(90)90073-o. [DOI] [PubMed] [Google Scholar]
- Taylor C. E., Fauntleroy M. B., Stashak P. W., Baker P. J. Antigen-specific suppressor T cells respond to recombinant interleukin-2 and other lymphokines. Infect Immun. 1991 Feb;59(2):575–579. doi: 10.1128/iai.59.2.575-579.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoman M. L., Weigle W. O. Lymphokines and aging: interleukin-2 production and activity in aged animals. J Immunol. 1981 Nov;127(5):2102–2106. [PubMed] [Google Scholar]


