Abstract
Background
Entomopathogenic fungi Metarhizium anisopliae and Beauveria bassiana isolates have been shown to infect and reduce the survival of mosquito vectors.
Methods
Here four different bioassays were conducted to study the effect of conidia concentration, co-formulation, exposure time and persistence of the isolates M. anisopliae ICIPE-30 and B. bassiana I93-925 on infection and survival rates of female Anopheles gambiae sensu stricto. Test concentrations and exposure times ranged between 1 × 107 - 4 × 1010 conidia m-2 and 15 min - 6 h. In co-formulations, 2 × 1010 conidia m-2 of both fungus isolates were mixed at ratios of 4:1, 2:1, 1:1,1:0, 0:1, 1:2 and 1:4. To determine persistence, mosquitoes were exposed to surfaces treated 1, 14 or 28 d previously, with conidia concentrations of 2 × 109, 2 × 1010 or 4 × 1010.
Results
Mosquito survival varied with conidia concentration; 2 × 1010 conidia m-2 was the concentration above which no further reductions in survival were detectable for both isolates of fungus. The survival of mosquitoes exposed to single and co-formulated treatments was similar and no synergistic or additive effects were observed. Mosquitoes were infected within 30 min and longer exposure times did not result in a more rapid killing effect. Fifteen min exposure still achieved considerable mortality rates (100% mortality by 14 d) of mosquitoes, but at lower speed than with 30 min exposure (100% mortality by 9 d). Conidia remained infective up to 28 d post-application but higher concentrations did not increase persistence.
Conclusion
Both fungus isolates are effective and persistent at low concentrations and short exposure times.
Background
The control tools currently deployed to reduce malaria transmission in Africa are early diagnosis and prompt treatment, insecticide-treated bed nets (ITNs) and indoor residual spraying (IRS) [1,2]. Although these strategies have delivered reductions in childhood disease [3,4], there remains a high incidence of malaria in many countries, with over 300 - 500 million infections and 1 million deaths each year [5]. The factors responsible for continuing transmission include the development of Plasmodium resistance to drugs [6], Anopheles resistance to insecticides [7,8], and socio-economic or cultural resistance to control measures [9]. Clearly, as effective as the current tools are, they are not sufficient on their own to eliminate malaria from intensely endemic regions [10]. Therefore, additional control tools are needed to combat this disease.
Laboratory and small-scale household studies have demonstrated a great potential to develop entomopathogenic fungi for field control of malaria vectors [11,12]. The fungi penetrate the mosquito cuticle through mechanical pressure and/or enzymatic degradation of major cuticle components [13,14]. Once inside the host, the fungi propagate, consuming nutrients and releasing metabolites resulting in mycosis and death [15]. In the laboratory, the entomopathogenic fungus, Beauveria bassiana was able to reduce the number of adult anopheline mosquitoes capable of transmitting malaria by a factor of approximately 80 [16], and one field study on a household scale has shown that Metarhizium anisopliae ICIPE-30 can cause a two-fold reduction in the life span of adult mosquitoes [17].
Before entomopathogenic fungi can be scaled-up for use in biological control programmes, it is essential to determine the optimum concentration of conidia (asexual spores shed at maturity) to apply, whether co-formulations offer any advantage over single isolate applications, the exposure time required for conidia to infect mosquitoes, and whether there is a relationship between concentration and persistence. The optimum concentration will be identified as the lowest concentration that is able to achieve the maximum reduction in survival time for each fungal species. Co-formulations could have a synergistic or additive effect due to the different life histories of the fungi involved, or inter-species interactions such as competition-altering activity. Determining the minimum exposure time for infection will guide in developing realistic field dissemination tools having pre-defined whether the intervention can target either host-seeking or resting mosquitoes or both. Examining persistence will allow re-application rates to be defined. Holistically, this information will provide a sound indication on the viability of this technology for field control of mosquitoes. The study was, therefore, designed to address these issues in assays of the fungal isolates M. anisopliae ICIPE-30 and B. bassiana I93-925 against adult Anopheles gambiae sensu stricto. These isolates were chosen because of their proven efficacy, availability in the market, and minimum risks to non-targeted organisms.
Methods
Mosquito rearing and maintenance
Anopheles gambiae s.s. mosquitoes were reared at the Ifakara Health Institute (IHI), Tanzania. The colony was established from a population caught near Njage village, 70 km from Ifakara, in 1996. Larval and adult stages of the mosquitoes were raised using methods based on those described by the Huho et al [18]. Bioassays were conducted using 3-6 d old non blood fed adult female mosquitoes. During all experiments, mosquitoes were supplied 9% glucose solution.
Fungal isolates, formulation and application
Two entomopathogenic fungi species were used in all bioassays: 1) Metarhizium anisopliae var. anisopliae isolate ICIPE-30, isolated originally in 1989 from the maize stalk borer, Busseola fusca (Lepidoptera, Noctuidae) in Western Kenya, and imported as dry conidia from Wageningen University, The Netherlands and 2) Beauveria bassiana isolate 193-825 (IMI 391510), isolated from a chrysomelid beetle (Coleoptera) in the USA and imported as dry conidia from the Commonwealth Scientific and Industrial Research Organization (CSIRO), Australia and Penn State University, USA. Before use each batch of conidia was checked for viability by inoculation on Sabouraud dextrose agar (SDA) plates, and only conidia with ≥ 85% germination were used in bioassays.
The conidia were formulated in oil for application. Oil protects conidia from adverse environmental conditions and facilitates adhesion to the insect cuticle. Initially, a fungal stock solution was prepared by suspending 1-2 g of conidia in 20 ml of highly refined mineral oil (Shell Ondina 917®, Shell, The Netherlands) or Enerpar (Enerpar M002®, BP Southern Africa Ltd). To homogenize the mixture it was shaken vigorously, vortexed for 25 sec and then sonicated (ultrasonic bath, Langford Electronics, UK) for 3 min. Dilutions of 1:10, 1:100 and 1:1000 were prepared in oil and the concentration of conidia was calculated using a Neubauer Haemocytometer (Hausser Scientific, USA) with the aid of a compound microscope (Leica ATC2000, USA) at 400× magnification. The solution was adjusted to the desired concentrations for application by diluting with mineral oil. Neither Ondina nor Enerpar oils had any negative effect on conidia germination or mosquito mortality (unpublished data). Unless otherwise stated, the conidia used during the bioassays were formulated in Ondina oil.
Mosquitoes were exposed to conidia applied to sheets of A4 printing paper within plastic exposure tubes (8.2 cm diameter × 12.5 cm height), closed with netting also treated with conidia. The paper and netting were treated using a hand-held pressure sprayer (Minijet®, SATA, Germany) operating at a constant pressure of 2 bars. The nozzle of the spray gun was held 50 cm away from, and at a right angle to, the application surface. A working solution of 23 ml containing the desired conidia concentration was applied to a 1 m2 area. Treated surfaces were left to dry for 24 h. To avoid cross-contamination, formulations of each fungal isolate were applied in separate rooms.
Bioassay procedures
30-50 adult An. gambiae s.s. mosquitoes were introduced to the exposure tubes and held for 6 h (unless stated otherwise in bioassay descriptions below), after which they were transferred to separate untreated cages (9 cm3) and maintained at 26-27°C and 85-95% relative humidity (RH) with access to 9% glucose solution ad libitum. Survival was monitored daily for a maximum of 28 d. Dead mosquitoes were collected individually and put onto moist filter paper in Petri dishes, sealed with parafilm, and incubated at 26-27°C and 85-95% RH for 3-4 d, after which they were examined for evidence of fungal sporulation basing on colour of their conidia. Metarhizium anisopliae yields green conidia, whereas, B. bassiana yields white conidia. Similar bioassay procedure was carried out for 30-50 mosquitoes in control groups, except that they were exposed to untreated surfaces. During all of the bioassays, four replicates were used for each experimental factor. Four different bioassays were conducted to study the effect of 1) concentration, 2) co-formulation 3) exposure time and 4) persistence on infection and survival of A. gambiae s.s.
Bioassay 1: concentration
Mosquitoes were exposed to six different concentrations of the two fungal isolates: (1 × 107, 2 × 108, 1 × 109, 2 × 109, 2 × 1010 and 4 × 1010 conidia m-2). Concentrations were chosen basing on their reported efficacy against mosquitoes and other arthropods, and likelihood of being cost effective.
Bioassay 2: co-infection with M. anisopliae and B. bassiana
Mosquitoes were exposed to co-formulations of 2 × 1010 conidia m-2of both fungus isolates mixed at ratios of 4:1, 2:1, 1:1, 1:0, 0:1, 1:2 and 1:4.
Bioassay 3: exposure time
Mosquitoes were exposed to 2 × 1010 conidia m-2 for four different lengths of time: 15 min, 30 min, 1 and 6 h. In a separate experiment two concentrations, 2 × 1010 and 4 × 1010 conidia m-2, formulated in Enerpar oil, were evaluated at 15 and 30 min exposure to determine whether concentration affected time required for infection.
Bioassay 4: persistence
The residual activity of conidia was determined by exposing mosquitoes to the same treated surfaces 1, 14 and 28 d post application. Treated surfaces were kept at 26-27°C and 85-95% RH in-between exposure rounds. Three different concentrations formulated in Enerpar oil (2 × 109, 2 × 1010 and 4 × 1010 conidia m-2).
Statistical analysis
Mosquito survival data were analysed by Kaplan-Meier pair wise comparison using SPSS version 15. Data were stratified by replicate and multiple chi-square pair-wise comparisons were used to examine the effect of treatment on mosquito survival. Survival curves were considered not statistically different at p > 0.05. A probit regression (R-package version 2.9.1) was used to determine the concentration of conidia required for 50% and 90% mortality 10 d after exposure (lethal concentration: LC50 and LC90, Bioassay 1).
Results
Bioassay 1: concentration
Concentrations of 2 × 109 conidia m-2 and above of both M. anisopliae ICIPE-30 and B. bassiana I93-825 resulted in 85-95% mortality of exposed mosquitoes after 10 d. This was higher than 36 ± 3.4% mortality recorded from untreated control after 10 d (Figure 1). The daily survival of mosquitoes exposed to any fungal concentration was significantly reduced compared to that of controls (P < 0.001; Figure 1; Table 1). The median survival time (MST ± SE) of control mosquitoes was 11 ± 0.06 d. The MST of fungus-exposed mosquitoes was similar for both isolates and ranged between 4.0 ± 0.09 d at 4 × 1010 conidia m-2 to 10 ± 0.32 d at 1 × 107 for M. anisopliae, and 4.0 ± 0.08 d at 4 × 1010 to 10 ± 0.467 d at 1 × 107 for B. bassiana. There was no difference between 2 × 1010 and 4 × 1010 for M. anisopliae (4.0 ± 0.11, 4.0 ± 0.09 d, X2 = 3.54, P = 0.07) or B. bassiana (4.0 ± 0.12, 4.0 ± 0.08 d, X2 = 3.56, P = 0.06; Table 1). As such, optimum reduction in survival was considered to be reached at a concentration of 2 × 1010 conidia m-2 (Figure 2). The concentrations of conidia that were modelled to result in 50% and 90% (LC90) mortality were 1.02 × 107 (LC50) and 9.77 × 108 conidia m-2 (LC90) M. anisopliae and 7.71 × 107 (LC50) and 2.66 × 109 conidia m-2 (LC90) for B. bassiana.
Figure 1.
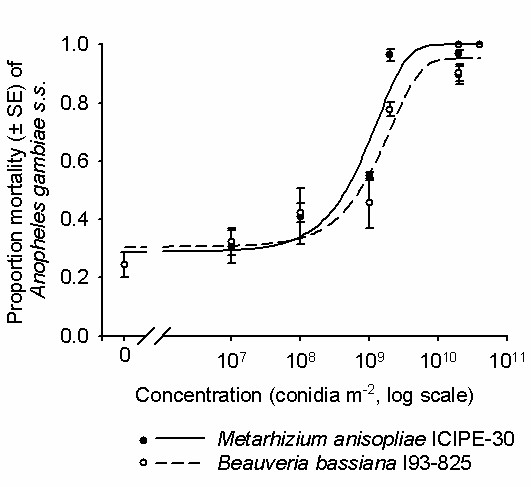
The percentage mortality of An. gambiae s.s. mosquitoes 3 - 6 d of age, 10 days post exposure to different concentrations of M. anisopliae ICIPE-30 and B. bassiana I93-825. Controls were not exposed to any fungus ('0' concentration). Mosquitoes were exposed to the treatments for 6 h. The sigmoidal models were fitted to the data using probit regression.
Table 1.
Kaplan Meier pair-wise comparisons of the median survival times (MST) of An. gambiae s.s. females exposed to different concentrations (1 × 107, 1 × 108, 1 × 109, 2 × 109, 2 × 1010 and 4 × 1010 conidia m-2) of M. anisopliae ICIPE-30 and B. bassiana I93-825 (IMI 391510) for 6 h.
| Concentrations | MST ± SE | 1 × 107 | 1 × 108 | 1 × 109 | 2 × 109 | 2 × 1010 | 4 × 1010 |
|---|---|---|---|---|---|---|---|
| M. anisopliae | |||||||
| Control | 11 ± 0.32 | χ2 = 15.12 | χ2 = 24.00 | χ2 = 50.7 | χ2 = 195.11 | χ2 = 239.68 | χ2 = 261.26 |
| p < 0.001* | p < 0.001* | p < 0.001* | p < 0.001* | p < 0.001* | P < 0.001* | ||
| 1 × 107 | 10 ± 0.32 | χ2 = 1.87 | χ2 = 14.46 | χ2 = 138.37 | χ2 = 197.25 | χ2 = 219.10 | |
| p = 0.17 | p < 0.001* | p < 0.001* | p < 0.001* | p <0.001* | |||
| 1 × 108 | 9 ± 0.37 | χ2 = 5.95 | χ2 = 98.86 | χ2 = 166.80 | χ2 = 188.48 | ||
| p <0.001* | p <0.001* | p < 0.001* | p < 0.001* | ||||
| 1 × 109 | 8 ± 0.43 | χ2 = 53.99 | χ2 = 119.57 | χ2 = 104.37 | |||
| p < 0.001* | p <0.001* | p < 0.001* | |||||
| 2 × 109 | 6 ± 0.15 | χ2 = 79.62 | χ2 = 104.37 | ||||
| p < 0.001* | p < 0.001* | ||||||
| 2 × 1010 | 4 ± 0.12 | χ2 = 3.54 | |||||
| p = 0.07 | |||||||
| B. bassiana | |||||||
| Control | 11 ± 0.32 | χ2 = 25.09 | χ2 = 47.61 | χ2 = 27.13 | χ2 = 109.37 | χ2 = 251.31 | χ2 = 251.60 |
| p < 0.001* | p < 0.001* | p < 0.001* | p < 0.001* | p <0.001* | p < 0.001* | ||
| 1 × 107 | 10 ± 0.47 | χ2 = 4.53 | χ2 = 0.319 | χ2 = 38.97 | χ2 = 171.18 p | χ2 = 181.16 | |
| p < 0.001* | p = 0.57 | p < 0.001* | <0.001* | p < 0.001* | |||
| 1 × 108 | 9 ± 0.23 | χ2 = 1.47 | χ = 21.40 | χ2 = 158.71 p | χ2 = 168.32 | ||
| p = 0.22 | p < 0.001* | <0.001* | p <0.001* | ||||
| 1 × 109 | 9 ± 0.58 | χ2 = 21.90 | χ2 = 112.07 p | χ2 = 114.26 | |||
| p < 0.001* | <0.001* | p < 0.001* | |||||
| 2 × 109 | 6 ± 0.17 | χ2 = 88.29 | χ2 = 130.05 | ||||
| p < 0.001* | p < 0.001* | ||||||
| 2 × 1010 | 4 ± 0.11 | χ2 = 3.56 | |||||
| p = 0.06 | |||||||
*Significant at p < 0.05
Figure 2.
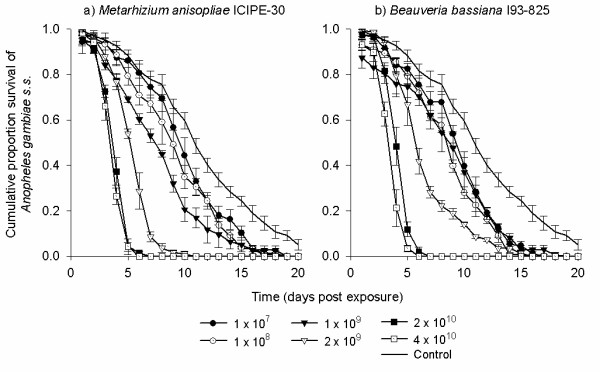
The survival of Anopheles gambiae s.s. females after 6 h exposure to different concentrations (1 × 107, 1 × 108, 1 × 109, 2 × 109, 2 × 1010 and 4 × 1010 conidia m-2) of a) Metarhizium anisopliae ICIPE-30 and b) Beauveria bassiana I93-825.
Bioassay 2: co-infection with M. anisopliae and B. bassiana
The survival of mosquitoes exposed to co-formulated conidia was significantly reduced compared with control mosquitoes (P < 0.001, Figure 3, Table 2). However, survival was not further reduced compared with the single fungus strain (P >0.001, Table 2). The MST of mosquitoes exposed to co-formulations and single species formulations ranged between 5 - 6 d, while that of untreated controls was 13 d.
Figure 3.
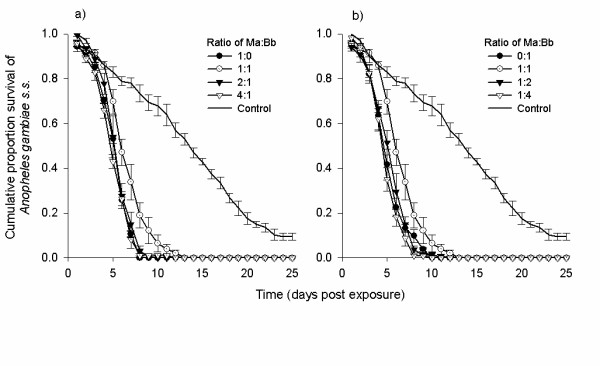
The survival of Anopheles gambiae s.s. females after exposure to 2 × 1010 conidia m-2 of co-formulated Metarhizium anisopliae ICIPE-30 and Beauveria bassiana I93-825 under laboratory conditions. The co-formulation was applied at different ratios of conidia (1:0, 1:1, 2:1 and 4:1) of M. anisopliae to B. bassiana.
Table 2.
Kaplan-Meier pair wise comparisons of the median survival times (MST) of An. gambiae s.s. females exposed to 2 × 1010 conidia m-2 of co-formulated Metarhizium anisopliae ICIPE-30 and Beauveria bassiana I93-825.
| Ratio of Ma:Bb | MST ± SE | 1:0 | 1:1 | 2:1 | 4:1 |
|---|---|---|---|---|---|
| Increasing ratio of M. anisopliae | |||||
| Control | 13 ± 0.73 | χ2 = 132.57 | χ2 = 109.63 | χ2 = 134.20 | χ2 = 121.23 |
| p < 0.001* | p < 0.001* | p < 0.001* | p < 0.001* | ||
| 1:0 | 6 ± 0.12 | χ2 = 0.15 | χ2 = 0.29 | χ2 = 1.37 | |
| p = 0.70 | p = 0.60 | p = 0.24 | |||
| 1:1 | 6 ± 0.21 | χ2 = 0.65 | χ2 = 2.59 | ||
| p = 0.34 | p = 0.07 | ||||
| 2:1 | 6 ± 0.16 | χ2 = 0.53 | |||
| p = 0.46 | |||||
| Increasing ratio of B. bassiana | |||||
| Control | 13 ± 0.73 | χ2 = 128.01 | χ2 = 109.63 | χ2 = 133.22 | χ2 = 114.34 |
| p < 0.001* | p < 0.001* | p < 0.001* | p < 0.001* | ||
| 0:1 | 5 ± 0.19 | χ2 = 1.63 | χ2 = 0.004 | χ2 = 0.29 | |
| p = 0.20 | p = 0.93 | p = 0.59 | |||
| 1:1 | 6 ± 0.21 | χ2 = 0.29 | χ2 = 3.10 | ||
| p = 0.59 | p = 0.08 | ||||
| 1:2 | 6 ± 0.19 | χ2 = 3.24 | |||
| p = 0.06 | |||||
Note: The co-formulation was applied at different ratios of conidia (1:0, 1:1, 2:1 and 4:1) of M. anisopliae to B. bassiana.
In all the co-formulation treatments, sporulation of both fungus species from the same cadaver was never observed. Metarhizium anisopliae sporulation only was observed in 78.17% (376/481) of the mosquito cadavers. No sporulation was observed on control mosquitoes.
Bioassay 3: exposure time
The exposure concentration used (2 × 1010) was selected on the basis of Bioassay 1 results. The ability of conidia of both fungi to kill and reduce the survival of mosquitoes was dependent on the length of exposure. The MST of mosquitoes after a 30 min exposure and above was significantly lower than that after 15 min for both M. anisopliae (P < 0.001) and B. bassiana (P < 0.001, Figure 4, Table 3). However, considerable mosquito mortality was still achieved with 15 min exposure (100% by 14 d), but at lower speed than with 30 min exposure (100% by 9 d). Nonetheless, 93.52% and 96.22% mortality was recorded when mosquitoes were exposed for 15 min to B. bassiana and M. anisopliae by 9 d. The MST for fungus-exposed mosquitoes ranged between 5 - 6 d. Survival of mosquitoes exposed to either M. anisopliae or B. bassiana for 15 min and above was significantly reduced as compared to controls (P < 0.001, Table 3).
Figure 4.
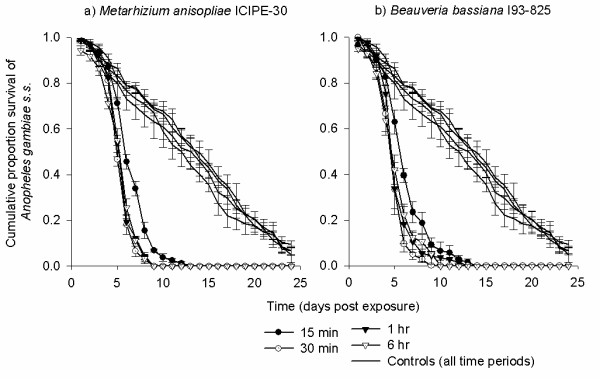
The survival of Anopheles gambiae s.s. females after exposure to 2 × 1010 conidia m-2 of a) Metarhizium anisopliae ICIPE-30 and b) Beauveria bassiana I93-825 for different times (15 min, 30 min, 1 h and 6 h).
Table 3.
Kaplan-Meier pair wise comparisons of the median survival times (MST) of An. gambiae s.s. females exposed to M. anisopliae ICIPE-30 and B. bassiana I93-825 (IMI 391510) for different exposure times (15 min, 30 min, 1 h and 6 h).
| Exposure time | MST ± SE | 15 min | 30 min | 1 h | 6 h |
|---|---|---|---|---|---|
| M. anisopliae | |||||
| Control | 13 ± 0.32 | χ2 = 124.1 | χ2 = 154.65 | χ2 = 149.59 | χ2 = 150.98 |
| p < 0.001* | p < 0.001* | p <0.001* | p < 0.001* | ||
| 15 min | 6 ± 0.32 | χ2 = 36.89 | χ2 = 26.07 | χ = 28.39 | |
| p < 0.001* | p <0.001* | p < 0.001* | |||
| 30 min | 5 ± 0.37 | χ2 = 1.15 | χ2 = 0.57 | ||
| p = 0.28 | p = 0.44 | ||||
| 1 h | 5 ± 0.43 | χ2 = 0.86 | |||
| p = 0.77 | |||||
| B. bassiana | |||||
| Control | 13 ± 0.32 | χ2 = 118.5 | χ2 = 97.98 | χ2 = 79.84 | χ2 = 103.81 |
| p < 0.001* | p < 0.001* | p < 0.001* | p < 0.001* | ||
| 15 min | 6 ± 0.17 | χ2 = 42.68 | χ2 = 16.28 | χ2 = 14.97 | |
| p < 0.001* | p < 0.001* | p < 0.001* | |||
| 30 min | 5 ± 0.11 | χ2 = 0.50, | χ2 = 0.29 | ||
| p = 0.23 | p = 0.59 | ||||
| 1 h | 5 ± 0.10 | χ2 = 0.06 | |||
| p = 0.81 | |||||
*Significant at p < 0.05
In separate bioassay where conidia concentrations of 2 × 1010 and 4 × 1010 were compared, at each exposure time; 15 and 30 min, the two concentrations equally reduced mosquito survival. This was observed for M. anisopliae (X2 = 0.63 - 2.92, P > 0.05) and B. bassiana (X2 = 0.76 - 5.23, P > 0.05), [see Additional file 1].
Bioassay 4: persistence
Overall, effect of M. anisopliae (P < 0.001) and B. bassiana (P < 0.001, Tables 4 and 5) on mosquito survival declined over time regardless of the conidia concentration used. For M. anisopliae, survival of mosquitoes exposed to each concentration 1 d post application were lower than survival of mosquitoes exposed to similar concentrations 14 and 28 d post application (P < 0.001). No difference, however, was observed for mosquitoes exposed to each concentration between 14 and 28 d post application (P >0.05, Table 4). For B. bassiana, survival of mosquitoes exposed to each concentration 1 d post application was lower than survival of mosquitoes exposed to same concentrations 28 d post application (P < 0.001). No difference however, was observed for mosquitoes exposed between 1 and 14 d, as well as 14 and 28 d post application (P > 0.05, Table 5). Concentration did not tend to influence the decline in conidia persistence (P > 0.05, Figure 5). The MSTs of mosquitoes exposed to 2 × 109 conidia m-2 of either isolate of fungus at all time intervals post application ranged between 11 - 13 d, while that of 2 × 1010 and 4 × 1010 ranged between 6 - 12 d. The survival of mosquitoes exposed to fungus was always lower than that of controls (P < 0.001, Tables 4 and 5). The MSTs of controls ranged between 15 - 16 d.
Table 4.
Kaplan-Meier pair wise comparisons of the median survival times (MST) of An. gambiae s.s. females exposed to surfaces 1, 14 and 28 d after treatment with different concentrations (2 × 109, 2 × 1010 and 4 × 1010 conidia m-2) of M. anisopliae ICIPE-30.
| Comparison of: | Different concentrations within same time post-application | The same concentration between different times post-application | ||||
|---|---|---|---|---|---|---|
| MST ± SE | 2 × 109 | 2 × 1010 | 4 × 1010 | 14 d | 28 | |
| 1 d | ||||||
| Control | 15 ± 0.95 | χ2 = 15.15 | χ2 = 43.34 | χ2 = 55.58 | χ2 = 0.08 | χ2 = 0.01 |
| p < 0.001* | p < 0.001* | p < 0.001* | p = 0.77 | p = 0.32 | ||
| 2 × 109 | 11 ± 0.51 | χ2 = 9.32 | χ2 = 15.44 | χ2 = 5.17 | χ2 = 4.10 | |
| p < 0.001* | p < 0.001* | p < 0.001* | p < 0.001* | |||
| 2 × 1010 | 6 ± 0.46 | χ2 = 0.001 | χ2 = 9.80 | χ2 = 8.95 | ||
| p = 0.98 | p < 0.001* | p < 0.001* | ||||
| 4 × 1010 | 8 ± 0.39 | χ2 = 14.74 | χ2 = 18.55 | |||
| p < 0.001* | p < 0.001* | |||||
| 14 d | ||||||
| Control | 16 ± 1.15 | χ2 = 30.48 | χ2 = 58.07 | χ = 49.02 | χ2 = 0.43 | |
| p < 0.001* | p < 0.001* | p < 0.001* | p = 0.54 | |||
| 2 × 109 | 12 ± 0.55 | χ2 = 6.59 | χ2 = 4.08 | χ2 = 0.06 | ||
| p < 0.001* | p < 0.001* | p = 0.80 | ||||
| 2 × 1010 | 10 ± 0.64 | χ2 = 0.21 | χ2 = 0.49 | |||
| p = 0.22 | p = 0.83 | |||||
| 4 × 1010 | 10 ± 0.45 | χ2 = 0.003 | ||||
| p = 0.96 | ||||||
| 28 d | ||||||
| Control | 16 ± 1.15 | χ2 = 35.94 | χ2 = 77.49 | χ2 = 62.29 | ||
| p < 0.001* | p < 0.001* | p < 0.001* | ||||
| 2 × 109 | 13 ± 0.33 | χ2 = 11.22 | χ2 = 5.76 | |||
| p < 0.001* | p < 0.001* | |||||
| 2 × 1010 | 11 ± 0.49 | χ2 = 0.56 | ||||
| p = 0.46 | ||||||
| 4 × 1010 | 11 ± 0.61 | |||||
*Significant at p < 0.05
Table 5.
Kaplan-Meier pair wise comparisons of the median survival times (MST) of An. gambiae s.s. females exposed to surfaces 1, 14 and 28 d after treatment with different concentrations (2 × 109, 2 × 1010 and 4 × 1010 conidia m-2) of B. bassiana I93-825.
| Comparison of: | Different concentrations within same time post-application | The same concentration between different times post-application | ||||
|---|---|---|---|---|---|---|
| MST ± SE | 2 × 109 | 2 × 1010 | 4 × 1010 | 14 d | 28 | |
| 1 d | ||||||
| Control | 15 ± 0.95 | χ2 = 5.73 | χ2 = 20.48 | χ2 = 16.50 | χ2 = 0.08 | χ2 = 0.01 |
| p < 0.001* | p < 0.001* | p < 0.001* | p = 0.77 | p = 0.32 | ||
| 2 × 109 | 11 ± 0.42 | χ2 = 7.03 | χ2 = 5.01 | χ2 = 0.61 | χ2 = 4.09 | |
| p < 0.001* | p < 0.001* | p = 0.44 | p < 0.001* | |||
| 2 × 1010 | 9 ± 0.55 | χ2 = 0.24 | χ2 = 1.74 | χ2 = 6.36 | ||
| p = 0.63 | p = 0.19 | p < 0.001* | ||||
| 4 × 1010 | 9 ± 0.79 | χ2 = 0.09 | χ2 = 6.03 | |||
| p = 0.77 | p < 0.001* | |||||
| 14 d | ||||||
| Control | 16 ± 1.15 | χ2 = 29.08 | χ2 = 52.46 | χ2 = 49.02 | χ2 = 0.43 | |
| p < 0.001* | p < 0.001* | p < 0.001* | p = 0.54 | |||
| 2 × 109 | 12 ± 0.44 | χ2 = 4.23 | χ2 = 7.04 | χ2 = 5.12 | ||
| p < 0.001* | p < 0.001* | p < 0.001* | ||||
| 2 × 1010 | 10 ± 0.67 | χ2 = 0.37 | χ2 = 1.79 | |||
| p = 0.54 | p = 0.18 | |||||
| 4 × 1010 | 10 ± 0.45 | χ2 = 0.40 | ||||
| p = 0.53 | ||||||
| 28 d | ||||||
| Control | 16 ± 1.15 | χ2 = 47.61 | χ2 = 34.67 | χ2 = 55.76 | ||
| p < 0.001* | p < 0.001* | p < 0.001* | ||||
| 2 × 109 | 13 ± 0.42 | χ2 = 7.56 | χ2 = 4.92 | |||
| p < 0.001* | p < 0.001* | |||||
| 2 × 1010 | 12 ± 0.54 | χ2 = 0.249 | ||||
| p = 0.11 | ||||||
| 4 × 1010 | 12 ± 0.64 | |||||
*Significant at p < 0.05
Figure 5.
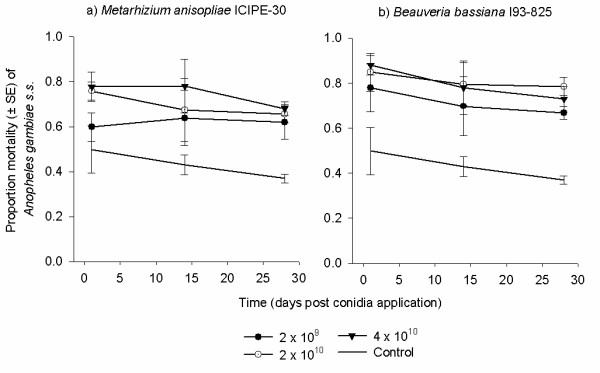
Percentage mortality of mosquitoes 12 d post exposure to different concentrations (2 × 109, 2 × 1010 and 4 × 1010 conidia m-2) of a) Metarhizium anisopliae ICIPE-30 and b) Beauveria bassiana I93-825 after storage of the treated materials for 1, 14 and 28 d. Treated materials were stored under 26°C-27°C temperature and 85-95% humidity before successive re-exposures.
Discussion
These experiments were designed to provide preparatory information necessary for the use of oil-formulated entomopathogenic fungi in field-based mosquito control. Due to the expense and logistics involved in applying any insecticidal agent on a large-scale, it is essential to first define the concentration and time required to infect and kill mosquitoes. Information concerning persistence is also needed to determine re-application rates.
Low conidia concentrations and short exposure times can result in small infective doses that can be countered by immune responses. Insect responses to entomopathogens involve melanization, encapsulation and phagocytosis of invading fungal blastospores [19], but it is likely that these responses can be overcome at high concentrations. In these experiments it was found that for both fungal species concentration was positively correlated with mortality, and that the maximum and most rapid reductions in mosquito survival were achieved at concentrations of 2 × 1010 conidia m-2 and above. With well standardized production systems, formulations, application methods and delivery tools, concentration of 2 × 1010 conidia m-2 can be operationally amenable. The efficacy of M. anisopliae in terms of mortality against An. gambiae s.s. was slightly, although not dramatically, higher than that recorded by Scholte et al [20], and these differences may be related to the use of mineral oil to formulate the conidia, instead of sunflower oil. Additionally, the quality of the conidia batch, the method of application and the target species can also impact on the efficacy of fungal applications. For example, in this study the efficacy of these two fungal species against An. gambiae s.s. was comparable, yet when a similar range of conidial concentrations were tested against Anopheles stephensi, B. bassiana was found to be much more effective than M. anisopliae [12]. Overall, a similar positive relationship between conidial concentration and mortality, as observed here, is also evident in the published literature [12,20,21]; it is difficult to directly compare studies because of differences in fungal isolate, oil formulation, target species, bioassay protocols and units used to express conidial concentration.
When M. anisopliae and B. bassiana were applied as a co-formulation against An. gambiae s.s., neither an additive nor synergistic effect was evident. Similar results have been found when entomopathogens were evaluated in combination against arthropods other than mosquitoes [22-25]. The initial interaction between the two fungal species occurs at the point of mosquito exposure. Even if conidia of both species adhere to the mosquito cuticle, a competitive advantage would be gained if one of the fungi was faster to invade and colonize the mosquito haemocoel. Following colonization, the successful fungus could prevent other fungi from becoming established by competitive exploitation, limiting resource availability, actively synthesizing and releasing inhibitory metabolites or stimulating host immune responses [26]. Considering that exposure to the co-formulation had no additive effect, it is likely that the activity of one fungal species was partially or completely redundant. The complete absence of co-sporulation and the predominance of M. anisopliae suggest a competitive advantage of M. anisopliae over B. bassiana. Additive [27] and synergistic [28,29] effects of co-infection have been recorded for other entomopathogens at sub-optimal temperature regimes. Thus the possibility remains that fluctuating temperature and or relative humidity either in the laboratory or field may affect co-formulations of M. anisopliae and B. bassiana.
The length of time required for conidia to infect and kill mosquitoes is an important consideration for developing dissemination tools for field use. The exposure times tested in the current study were selected to represent realistic exposure periods. Mosquitoes may spend up to 15 min trying to enter a bed net [30] and after blood-feeding may rest on a surface for up to 24 h [31], though in areas of high bed net coverage it is likely that mosquitoes spend on average less than six hours inside houses [32-34]. In this study it was found that exposure times as short as 15 and 30 min were sufficient for conidia of both M. anisopliae and B. bassiana to infect mosquitoes and reduce survival. Similarly, no effect of increasing exposure time beyond 5 minutes up to 6 h on infection rates was found when An. stephensi were exposed to 2 × 109 conidia m-2 of B. bassiana [12]. Increasing the exposure time beyond 6 h and/or concentration did increase mortality of An. stephensi [12] and other arthropods [35,36]. When the concentration tested against An. gambiae was increased, no effect of exposure time was observed, though only relatively short exposure times were tested (15 min - 6 h). However, longer exposure times (24 h, 48 h and continuous) of An. gambiae to M. anisopliae were tested by Scholte et al [20] and at high concentrations and no difference between the exposure times tested was observed. Formulations of either M. anisopliae or B. bassiana could, therefore, be used with dissemination tools/surfaces that target host seeking (short contact) as well as resting (long contact) mosquitoes.
Despite a general decline in the persistence M. anisopliae and B. bassiana against An. gambiae s.s., conidia were still pathogenic up to 28 d post application. During the current study, the conidia were stored under constant temperature (26°C-27°C) and RH (85-95%) thus it is unknown if fluctuating environmental conditions would affect the length of residual activity. A slight decline over time in the germination of conidia when applied in the field has been reported elsewhere [17], yet 63% of conidia remained viable after three weeks. Most importantly it was found that increasing the concentration of conidia did not increase the residual activity. Although the residual activity of fungi is short lived compared with traditional synthetic insecticides, it is comparable with other successful bio-insecticides such as Bacillus thuringiensis var. israelensis [37,38]. The MSTs values recorded during the persistence experiment for exposure immediately after drying (1 d) were lower than MSTs observed when similar concentrations were tested elsewhere in this study. The difference could have been due to variation in the quality of the conidia batch.
To be capable of transmitting malaria, a mosquito must survive for longer than the extrinsic incubation period of the pathogen, 9 - 14 d for Plasmodium spp [39]. This period is longer than the average mosquito life span and, therefore, malaria transmission can be attributed to a small fraction of the mosquito population. Daily survival is actually the most sensitive component of vectorial capacity [40,41] and thus control strategies that reduce vector age are highly desirable. The current study recorded large reductions in the daily survival of female An. gambiae s.s. when exposed to relatively high (2 × 1010 and 4 × 1010conidia m-2) concentrations of both M. anisopliae and B. bassiana. These concentrations were lower than that used by Scholte et al [17]. When mosquitoes were exposed to high conidial concentrations in this and other studies [11,16,17,20], 100% mortality was often achieved within 10 d. If these results can be replicated in the field this could lead to a considerable reduction in malaria transmission [42].
Conclusions
Of the few biological control tools targeting adult mosquitoes that are currently under development (including fungal, bacterial, viral and protozoan pathogens), entomopathogenic fungi are likely to be developed for programmatic use. Especially since fungus production and application all involve relatively simple infrastructures and processes, which could potentially be adopted in malaria endemic countries. An application of either M. anisopliae or B. bassiana at a concentration of 2 × 1010 conidia m-2 should be able to infect mosquitoes in a relatively short time (15 or 30 min) for up to one month after application. This concentration should provide a considerable safety margin for application error, exposure time and residual activity. However, there remains a need to test the fungi in large-scale field trials and to develop protocols to ensure simple and economical distribution and application in malaria endemic developing countries. Further developments to increase conidia persistence are still necessary in order to enhance the potential epidemiological impact of fungi on malaria transmission.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
LLM, TLR, MJK, DWL and MWM were directly involved in the data collection, analysis and drafting of the manuscript. BGJK and WT conceived the study, obtained funding for it and revised the manuscript before submission. All authors read and approved the final manuscript.
Supplementary Material
Survival curves of Anopheles gambiae s.s. fungus treated and non-treated surfaces. The survival of Anopheles gambiae s.s. females after exposure to 2 × 1010 and 4 × 1010 conidia m-2 of Metarhizium anisopliae ICIPE-30 and Beauveria bassiana I93-825 for different times (15 and 30 min).
Contributor Information
Ladslaus L Mnyone, Email: llaurent@ihi.or.tz.
Matthew J Kirby, Email: mkirby@ihi.or.tz.
Dickson W Lwetoijera, Email: dwilson@ihi.or.tz.
Monica W Mpingwa, Email: mmpingwa@ihi.or.tz.
Bart GJ Knols, Email: bart@malaria-world.com.
Willem Takken, Email: willem.takken@wur.nl.
Tanya L Russell, Email: trussell@ihi.or.tz.
Acknowledgements
We are grateful to the Addessium Foundation (Rotterdam, The Netherlands) for funding this research. We thank Jennifer Stevenson, Simon Blanford and Christian Luz for their useful comments and assistance, and Paulina Kasanga, Edgar Mbeyela and Emmanuel Simfukwe for technical assistance. We thank Frank van Breukelen (Wageningen University), Nina Jenkins and Mathew Thomas (CSIRO/Penn State University) for supplying the M. anisopliae and B. bassiana conidia.
References
- Rowland M, Mahmood P, Iqbal J, Carneiro I, Chavasse D. Indoor residual spraying with alphacypermethrin controls malaria in Pakistan: a community randomized trial. Trop Med Int Health. 2000;5:472–481. doi: 10.1046/j.1365-3156.2000.00581.x. [DOI] [PubMed] [Google Scholar]
- Sharp BL, Kleinschmidt I, Streat E, Maharaj R, Barnes KI, Durrheim DN, Ridl FC, Morris N, Seocharan I, Kunene S, La Grange JJP, Mthembu JD, Maartens F, Martin CL, Barreto A. Seven years of regional malaria control collaboration - Mozambique, South Africa and Swaziland. Am J Trop Med Hyg. 2007;76:42–47. [PMC free article] [PubMed] [Google Scholar]
- Abdulla S, Schellenberg R, Nathan R, Mukasa O, Marchant, Smith T, Tanner M, Lengeler C. Impact on malaria morbidity of a programme supplying insecticide treated nets in children aged under 2 years in Tanzania: Community cross sectional study. BMJ. 2001;322:270–271. doi: 10.1136/bmj.322.7281.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masanja H, De Savigny D, Smithson P, Schellenberg J, John T, Mbuya C. Child survival gains in Tanzania: analysis of data from demographic health surveys. Lancet. 2008;371:1276–1283. doi: 10.1016/S0140-6736(08)60562-0. [DOI] [PubMed] [Google Scholar]
- Snow RW, Guerra CA, Noor AM, Myint HY, Hay SI. The global distribution of clinical episodes of Plasmodium falciparum malaria. Science. 2005;434:214–217. doi: 10.1038/nature03342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talisuna AO, Bloland P, D'Allessandro U. History, dynamics, and public health importance of malaria parasite resistance. Clin Microbiol Review. 2004;17:235–254. doi: 10.1128/CMR.17.1.235-254.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hargreaves K, Koekemoer LL, Brooke BD, Hunt RH, Mthembu JD, Coetzee M. Anopheles funestus resistant to pyrethroid insecticides in South Africa. Med Vet Entomol. 2000;14:181–189. doi: 10.1046/j.1365-2915.2000.00234.x. [DOI] [PubMed] [Google Scholar]
- Etang J, Fondjo E, Chandre F, Morlais I, Brengues C, Nwane P, Chouaïbou M, Amadou D, Simard F. First report of the kdr mutations in the malaria vector Anopheles gambiae from Cameroon. Am J Trop Med Hyg. 2006;74:795–797. [PubMed] [Google Scholar]
- Greenwood BM, Bojang K, Whitty CJM, Targett GAT. Malaria. Lancet. 2005;365:1487–1498. doi: 10.1016/S0140-6736(05)66420-3. [DOI] [PubMed] [Google Scholar]
- Killeen GF, Mc Kenzie FE, Foy BD, Schieffelin C, Billingsley PF, Beier JC. A simplified model for predicting malaria entomological inoculation rates based on entomologic and parasitologic parameters relevant to control. Am J Trop Med Hyg. 2000;62:535–544. doi: 10.4269/ajtmh.2000.62.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farenhorst M, Farina D, Scholte EJ, Takken W, Hunt RH, Coetzee M, Knols BGJ. African water storage pots for the delivery of the entomopathogenic fungus Metarhizium anisopliae to the malaria vectors Anopheles gambiae s.s. and Anopheles funestus. Am J Trop Med Hyg. 2008;78:910–916. [PubMed] [Google Scholar]
- Stevenson JC. PhD Thesis. London School of Hygiene and Tropical Medicine, London; 2008. The use of fungi against adult malaria mosquitoes. [Google Scholar]
- Moore-Lendecker E. Fundamentals of the fungi. Prentice Hall, New Jersey; 1996. [Google Scholar]
- Gillespie JP, Bailey AM, Cobb B, Vilcinskas A. Fungi as elicitors of insect immune responses. Arch Insect Biochem Physiol. 2000;44:49–68. doi: 10.1002/1520-6327(200006)44:2<49::AID-ARCH1>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Clarkson JM, Charnley AK. New insights into the mechanism of fungal pathogenesis in insects. Trends Microbiol. 1996;4:197–203. doi: 10.1016/0966-842X(96)10022-6. [DOI] [PubMed] [Google Scholar]
- Blanford S, Chan BHK, Jenkins N, Sim D, Turner RJ, Read AF, Thomas MB. Fungal pathogen reduces potential for malaria transmission. Science. 2005;308:1638–1641. doi: 10.1126/science.1108423. [DOI] [PubMed] [Google Scholar]
- Scholte EJ, Ng'habi K, Kihonda J, Takken W, Paaijmans K, Abdulla S, Killeen GF, Knols BGJ. An entomopathogenic fungus for control of adult African malaria mosquitoes. Science. 2005;308:1641–1642. doi: 10.1126/science.1108639. [DOI] [PubMed] [Google Scholar]
- Huho BJ, Ng'habi KR, Killeen GF, Nkwengulila G, Knols BGJ, Ferguson HM. Nature beats nurture: a cast study of the physiological fitness of free-living and laboratory-reared male Anopheles gambiae s.l. J Exp Biol. 2007;210:2939–2947. doi: 10.1242/jeb.005033. [DOI] [PubMed] [Google Scholar]
- Bogus MI, Kedra E, Bania J, Szczepanik M, Czygier M, Jablonski P, Pasztaleniec A, Samborski J, Mazgajska J, Polanowski A. Different defense strategies of Dendrolimus pini, Galleria mellonella, and Calliphora vicina against fungal infection. J Insect Physiol. 2007;53:909–922. doi: 10.1016/j.jinsphys.2007.02.016. [DOI] [PubMed] [Google Scholar]
- Scholte EJ, Njiru BN, Smallegange RC, Takken W, Knols BG. Infection of malaria (Anopheles gambiae s.s.) and filariasis (Culex quinquefasciatus) vectors with the entomopathogenic fungus Metarhizium anisopliae. Malar J. 2003;2:29. doi: 10.1186/1475-2875-2-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas MB, Jenkins NE. Effects of temperature on growth of Metarhizium flavoviridae and virulence to the variegated grasshopper, Zonocerus variegatus. Mycol Res. 1997;101:1469–1474. doi: 10.1017/S0953756297004401. [DOI] [Google Scholar]
- Rao CUM, Devi KU, Khan PAA. Effect of combination treatment with entomopathogenic fungi Beauveria bassiana and Nomuraea rileyi (Hypocreales) on Spodoptera litura (Lepidoptera: Noctuidaeae) Biocontr Sci Tech. 2006;16:221–232. doi: 10.1080/09583150500335632. [DOI] [Google Scholar]
- Wang CS, Li ZZ, Butt TM. Molecular studies of co-formulated strains of the entomopathogenic fungus, Beauveria bassiana. J Invertebr Pathol. 2002;80:29–34. doi: 10.1016/S0022-2011(02)00045-9. [DOI] [PubMed] [Google Scholar]
- Leal-Bertioli SCM, Butt TM, Peberdy JF, Bertioli DJ. Genetic exchange in Metarhizium anisopliae strains co-infecting Phaedon cochleariae is revealed by molecular markers. Mycol Res. 2000;104:409–414. doi: 10.1017/S0953756299001549. [DOI] [Google Scholar]
- Maranga RO, Kaaya GP, Mueke JM, Hassanali A. Effects of combining the fungi Beauveria bassiana and Metarhizium anisopliae on the mortality of the tick Amblyoma variegatum (ixodae) in relation to seasonal changes. Mycopathologia. 2005;159:527–532. doi: 10.1007/s11046-005-3374-y. [DOI] [PubMed] [Google Scholar]
- Read AF, Taylor LH. The ecology of genetically diverse infections. Science. 2001;292:1099. doi: 10.1126/science.1059410. [DOI] [PubMed] [Google Scholar]
- Thomas MB, Watson EL, Valverde-Garcia P. Mixed infections and insect-pathogen interactions. Ecology Letters. 2003;6:183–188. doi: 10.1046/j.1461-0248.2003.00414.x. [DOI] [Google Scholar]
- Inglis GD, Duke GM, Kawchuk LM, Goettel MS. Influence of oscillating temperatures on the competitive infection and colonization of the migratory grasshopper by Beauveria bassiana and Metarhizium flavoviridae. Biol Control. 1999;14:111–120. doi: 10.1006/bcon.1998.0666. [DOI] [Google Scholar]
- Malakar R, Hajek AE, Burand JP. Within-host interaction of Lymantria dispar (Lepidoptera: Lymantriidae) Nucleopolyhedrosis virus and Entomophaga maimaga (Zygomycetes:Entomophorales) J Invertebr Pathol. 1999;73:91–100. doi: 10.1006/jipa.1998.4806. [DOI] [PubMed] [Google Scholar]
- Clements AN. The Biology of mosquitoes: Sensory reception and behaviour, London UK. 1992.
- Gillies MT. Studies in house leaving and outside resting of Anopheles gambiae Giles and Anopheles funestus Giles in East Africa. II. The exodus from houses and the house resting population. Bull Entomol Res. 1954;45:375–387. doi: 10.1017/S000748530002719X. [DOI] [Google Scholar]
- Mathenge EM, Gimnig JE, Kolczak M, Ombok M, Irungu LW, Hawley WA. Effect of permethrin-impregnated nets on exiting behavior, blood feeding success, and time of feeding of malaria mosquitoes (Diptera: Culicidae) in western Kenya. J Med Entomol. 2001;38:531–536. doi: 10.1603/0022-2585-38.4.531. [DOI] [PubMed] [Google Scholar]
- Lines JD, Myamba J, Curtis CF. Experimental hut trials of permethrin-impregnated mosquito nets and eave curtains against malaria vectors in Tanzania. Med Vet Entomol. 1987;1:37–51. doi: 10.1111/j.1365-2915.1987.tb00321.x. [DOI] [PubMed] [Google Scholar]
- Killeen GF, Tami A, Kihonda J, Oketch-Okumu FR, Kotas ME, Grundmann H, Kasigudi N, Ngonyani H, Mayagaya V, Nathan R, Abdulla S, Charlwood JD, Smith TA, Lengeler C. Cost-sharing strategies combining targeted public subsidies with private-sector delivery achieve high bed net coverage and reduced malaria transmission in Kilombero valley, southern Tanzania. BMC Infect Dis. 2007;7:7–121. doi: 10.1186/1471-2334-7-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniania NK. A laboratory technique for infecting adult tsetse with a fungal agent. Insect Sci Applic. 1994;15:421–426. [Google Scholar]
- Quesada-Moraga E, Carrasco-Daaz JA, Santiago-Ãlvarez C. Insecticidal and antifeedant activities of proteins secreted by entomopathogenic fungi against Spodoptera littoralis (Lep., Noctuidae) J Appl Entomol. 2006;130:442–453. doi: 10.1111/j.1439-0418.2006.01079.x. [DOI] [Google Scholar]
- Karch S, Manzambi ZA, Salaun JJ. Field trials with VectoLex (Bacillus sphaericus) and VectoBac (Bacillus thuringiensis (H-14)) against Anopheles gambiae and Culex quinquefasciatus breeding in Zaire. J Am Mosqu Control Assoc. 1991;7:176–179. [PubMed] [Google Scholar]
- Fillinger U, Knols BGJ, Becker N. Efficacy and efficiency of new Bacillus thuringiensis var. israelensis and Bacillus sphaericus formulations against Afrotropical anophelines in Western Kenya. Trop Med Int Health. 2003;8:37–47. doi: 10.1046/j.1365-3156.2003.00979.x. [DOI] [PubMed] [Google Scholar]
- Beier JC. Malaria parasite development in mosquitoes. Annu Rev Entomol. 1998;43:519–543. doi: 10.1146/annurev.ento.43.1.519. [DOI] [PubMed] [Google Scholar]
- Garrett-Jones C. Prognosis for interruption of malaria transmission through assessment of the mosquito's vectorial capacity. Nature. 1964;204:1173–1175. doi: 10.1038/2041173a0. [DOI] [PubMed] [Google Scholar]
- Miller DR, Weidhaas DE, Hall RC. Parameter sensitivity in insect population modeling. J Theoretical Biology. 1973;42:263–274. doi: 10.1016/0022-5193(73)90089-1. [DOI] [PubMed] [Google Scholar]
- Hancock PA, Thomas MB, Godfray HCJ. An age-structured model to evaluate the potential of novel malaria-control interventions: a case study of fungal biopesticide sprays. Proc R Soc London. 2008;Series B online:1–10. doi: 10.1098/rspb.2008.0689. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Survival curves of Anopheles gambiae s.s. fungus treated and non-treated surfaces. The survival of Anopheles gambiae s.s. females after exposure to 2 × 1010 and 4 × 1010 conidia m-2 of Metarhizium anisopliae ICIPE-30 and Beauveria bassiana I93-825 for different times (15 and 30 min).


