Abstract
New cancer treatments pose a substantial financial burden on health-care systems, insurers, patients, and society. Cost–utility analyses (CUAs) of cancer-related interventions have received increased attention in the medical literature and are being used to inform reimbursement decisions in many health-care systems. We identified and reviewed 242 cancer-related CUAs published through 2007 and included in the Tufts Medical Center Cost-Effectiveness Analysis Registry (www.cearegistry.org). Leading cancer types studied were breast (36% of studies), colorectal (12%), and hematologic cancers (10%). Studies have examined interventions for tertiary prevention (73% of studies), secondary prevention (19%), and primary prevention (8%). We present league tables by disease categories that consist of a description of the intervention, its comparator, the target population, and the incremental cost-effectiveness ratio. The median reported incremental cost-effectiveness ratios (in 2008 US $) were $27 000 for breast cancer, $22 000 for colorectal cancer, $34 500 for prostate cancer, $32 000 for lung cancer, and $48 000 for hematologic cancers. The results highlight the many opportunities for efficient investment in cancer care across different cancer types and interventions and the many investments that are inefficient. Because we found only modest improvement in the quality of studies, we suggest that journals provide specific guidance for reporting CUA and assure that authors adhere to guidelines for conducting and reporting economic evaluations.
Innovative interventions in cancer prevention, diagnosis, and treatment may improve patients’ survival and quality of life, but such improvements may come at a substantial economic cost. The cost of cancer treatment has risen dramatically in recent years and has created financial burdens for both patients and third-party payers (1–7). In the United States, for example, the National Institutes of Health estimated that the overall cost of cancer care in 2007 was $219.2 billion: direct medical care costs accounted for $89.0 billion of this total, $18.2 billion was attributed to indirect morbidity costs (ie, costs of lost productivity because of illness), and the remainder to indirect mortality costs (ie, cost of lost productivity because of premature death) (8). Jönsson and Wilking (9) found that the direct cost for cancer care in 19 selected European countries in 2004 was approximately €57 billion (US $71 billion). Recently, a study by Yabroff et al. suggested that the value of life lost from all cancer deaths in the year 2000 was $960.6 billion, and this value is projected to be $1472.5 billion in 2020 (10). This projected increase is because of increasing life expectancy and to the expected growth and aging of the US population.
The economic impact of cancer-related interventions has received increased attention in the medical literature and in the popular media because of the very high cost of many newer cancer drugs and treatment protocols (3,4,7,11–13). The debate has focused not only on the costs of treatments but also on their relatively modest benefits. Many new interventions in oncology, such as those targeted at patients with metastatic disease, produce relatively small gains in life expectancy or quality of life in relation to existing treatments. Therefore, it has become crucial to understand the potential costs and benefits of each intervention to determine whether they provide good value.
Economic evaluations of health-care interventions have become more common in the medical and health economics literature and are increasingly being used to inform reimbursement and coverage decisions in Australia, Canada, the United Kingdom, and other European countries (14–17), although results from economic evaluations have not traditionally been used in the United States for these purposes (18–20). Because reimbursement and coverage decisions influence patient care, it is important for both decision makers and medical practitioners to be able to adequately interpret the design, the results, and the conclusions of economic evaluations.
Cost-effectiveness analysis (CEA) provides a standard well-accepted methodological technique for judging whether a medical service provides “good value for money.” The approach has emerged as an important tool for evaluating the impact of a wide variety of health interventions. A cost–utility analysis (CUA) is a type of CEA in which benefits are measured in terms of quality-adjusted life-years (QALYs) to allow comparison of the relative efficiency of health-care interventions across a spectrum of conditions. The main elements of CUAs and their application and interpretation in oncology are described in greater detail elsewhere (21,22). Also, we briefly describe the main elements that should be addressed when designing, performing, and reporting findings from a CUA in an appendix (Supplementary Material, available online).
CUAs have the potential to inform coverage decisions and patient care if they are of high quality and use standard recommended methods. Nearly a decade ago, we published an overview of cost–utility assessments in oncology, which examined the literature published before 1998 (23). In this article, we have described and synthesized published analyses of cancer-related care, examined the number and quality of such analyses over time and related factors, and summarized the resulting standardized cost–utility ratios.
Methods
We analyzed data from the Tufts Medical Center Cost-Effectiveness Analysis Registry (www.cearegistry.org), a database with detailed information on 1677 CEAs published in the peer-reviewed medical and economic literature through 2007. Our methodology for searching the literature and extracting information, which is described in more detail elsewhere (24), involved searching MEDLINE by the keywords, QALYs, quality-adjusted, and cost–utility analysis and then retrieving English-language publications that contained an original cost per QALY estimate. Our review included all studies that pertained to prevention, screening, and treatment of cancer. We excluded review, editorial, or methodological articles; CEAs that measured health effects in units other than QALYs; and articles in languages other than English.
We used a standard data auditing form to review each CUA for clarity, completeness, and health economic methodological quality. The form was developed based on a variety of sources, including the “checklist” for reporting reference-case CUAs recommended by the Panel on Cost-Effectiveness in Health and Medicine as well as other published guidelines (25,26). Two readers with advanced training in decision analysis and CUA independently read each article and then convened for a consensus review to resolve discrepancies. Readers received a detailed set of instructions to help ensure that they interpreted items uniformly. Readers were not masked to the identity of the authors or the journal where the study was published. We collected data on a wide variety of elements related to study origin, methods, and reporting of results. For each CUA, descriptive characteristics collected included year of publication, country of origin, intervention type, publication journal, and study funding source. Methodological and analytic characteristics included the study perspective, discounting of future costs and life-years, whether the economic data were collected alongside a clinical trial, and the type of sensitivity analysis performed (ie, univariate, multivariable, or probabilistic). We assigned each study a quality score based on subjective assessment of the overall study quality on a Likert scale from 1 (low) to 7 (high). The quality score was calculated as the mean of the evaluations from two readers who considered the rigor of the methodology, the quality of the presentation, and the potential value of the study to decision makers. Studies were summarized and tabulated in three phases (CUAs published through 1997, CUAs published in 1998 through 2001, and CUAs published in 2002 through 2007), which reflect the different data collection phases in the CEA Registry.
We arbitrarily classified journals as “high volume” (those which published 10 or more CUAs over the review period, ie, the Journal of Clinical Oncology, Cancer, Pharmacoeconomics, International Journal of Radiation Oncology Biology and Physics, and Breast Cancer Research and Treatment) and “low volume” (those that published fewer than 10 CUAs, eg, Annals of Oncology, British Journal of Cancer, European Journal of Cancer, Journal of the National Cancer Institute, Journal of the American Medical Association, and others) and compared the quality of studies in these groups of journals.
The CUAs of cancer-related interventions that were included in our review were conducted in numerous countries using different currencies for a period of almost 20 years. To allow comparisons across countries, all non-US currencies were converted into US dollars using the appropriate foreign exchange factor for the relevant year, and all ratios were inflated to 2008 US dollars using the Consumer Price Index. However, because changes in relative and absolute treatment costs and their associated benefits can substantially alter cost per QALY over time, we also have presented the original cost-effectiveness ratios converted to US dollars without adjusting for inflation. Finally, we constructed a league table consisting of a description of the intervention, its comparator, the target population, the incremental cost-effectiveness ratio (ICER), and the study rating. A cost-effectiveness league table is a listing of health interventions ranked by their ICER presented in terms of cost per QALYs gained (25).
Studies that pertained to cancer were grouped into nine broad subcategories by the type of cancer that was treated or prevented: breast cancer, colorectal cancer, cervical cancer, gastrointestinal and hepatocellular cancers, hematologic cancers, lung cancer, melanoma, prostate cancer, and other cancers. Because some CUAs compared several interventions and included scenarios specific to patient subgroups or settings, each study may have contributed more than one ICER.
We used t tests and analysis of variance to determine differences in study quality scores. All analyses were conducted using SPSS 15.0 (SPSS, Inc, Chicago, IL) and SAS 9.1 software (SAS, Cary, NC); a P value less than .05 determined statistical significance for all comparisons. All statistical tests were two-sided.
Results
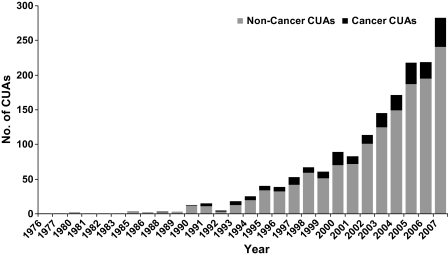
We identified 242 original cancer-related CUAs in the Tufts Medical Center Cost-Effectiveness Analysis Registry (www.cearegistry.org). The rate at which CUAs are published has risen markedly over time, and the annual average number of cancer-related CUAs has increased from seven per year between 1988 and 2001 to 25 per year between 2002 and 2007. Overall, 14% of the studies in the CEA Registry pertained to cancer, and this proportion did not change substantially over time (Figure 1). The most frequent cancers studied were breast cancer (36% of studies), colorectal cancer (12%), and hematologic cancers (10%). Studies have pertained to the United States (50% of studies), followed by the United Kingdom (11%), Canada (8%), and the Netherlands (7%) (Table 1). Most studies have examined interventions for tertiary prevention (ie, chemotherapy and postdiagnosis interventions; 73%), followed by secondary prevention (ie, cancer screening; 19%) and primary prevention (eg, vaccination; 8%). Pharmaceuticals comprised the largest category of interventions that were analyzed among published CUAs (53%), followed by medical procedures (18%) and screening strategies (16%). Approximately 28% of studies were funded by industry, 42% were funded solely by nonindustry sources (ie, government, foundations, and health-care organizations), and 28% did not disclose their funding source (Table 1). Nearly half of all studies used a lifetime time horizon, most discounted both costs and QALYs, and most used univariate or multivariable sensitivity analyses and threshold values to interpret study results, presenting a correct incremental analysis. However, only one-quarter of studies were taken from a societal perspective, and only few analyses were conducted alongside a clinical trial (Table 2).
Figure 1.
Growth of the cost–utility literature over time. The rate at which cost–utility analyses (CUAs) are published in the medical and health economic literature has risen markedly over time. Overall, 14% of the studies in the Tufts Medical Center Cost-Effectiveness Analysis Registry pertained to cancer, and this proportion did not change substantially over time.
Table 1.
Characteristics of the cancer cost–utility analysis literature
| Characteristic | Number of studies (total N = 242) | Percentage of studies |
| Country of study* | ||
| United States | 120 | 49.6 |
| United Kingdom | 26 | 10.7 |
| Canada | 20 | 8.3 |
| The Netherlands | 16 | 6.6 |
| Norway | 9 | 3.7 |
| Australia | 8 | 3.3 |
| France | 8 | 3.3 |
| Sweden | 7 | 2.9 |
| Italy | 6 | 2.5 |
| Other | 27 | 11.2 |
| Intervention type* | ||
| Pharmaceuticals | 129 | 53.3 |
| Medical procedure | 43 | 17.8 |
| Screening | 38 | 15.7 |
| Diagnostic | 36 | 14.9 |
| Surgical | 32 | 13.2 |
| Other | 24 | 9.9 |
| Prevention stage† | ||
| Primary | 19 | 7.9 |
| Secondary | 46 | 19.0 |
| Tertiary | 177 | 73.1 |
| Study theme* | ||
| Women | 75 | 31.0 |
| Public health | 22 | 9.1 |
| Men | 22 | 9.1 |
| Children | 3 | 1.2 |
| Elderly | 8 | 3.3 |
| None/not stated | 127 | 52.5 |
| Sponsorship | ||
| Industry | 52 | 21.5 |
| Nonindustry | 101 | 41.7 |
| Industry and nonindustry | 16 | 6.6 |
| None | 6 | 2.5 |
| Not disclosed | 67 | 27.7 |
| Journal | ||
| Journal of Clinical Oncology | 22 | 9.1 |
| Cancer | 18 | 7.4 |
| Pharmacoeconomics | 15 | 6.2 |
| International Journal of Radiation Oncology Biology and Physics | 12 | 5.0 |
| Breast Cancer Research and Treatment | 11 | 4.5 |
| Annals of Oncology | 8 | 3.3 |
| British Journal of Cancer | 8 | 3.3 |
| European Journal of Cancer | 7 | 2.9 |
| Journal of the National Cancer Institute | 7 | 2.9 |
| Journal of the American Medical Association | 7 | 2.9 |
| Other | 127 | 52.5 |
| Study quality | ||
| Mean (SD) | 4.26 (1.08) | |
Non-exclusive.
These terms are defined as follows: primary prevention = measures to prevent onset of condition (eg, vaccination); secondary prevention = measures to identify and treat asymptomatic persons with risk factors or preclinical disease (eg, screening); tertiary prevention = interventions to limit disability after harm has occurred (eg, chemotherapy).
Table 2.
Methods and quality of the cost–utility analysis literature*
| Characteristic | Number of studies (total N = 242) | Percentage of studies |
| Clear presentation of | ||
| The relevant intervention | 236 | 97.5 |
| The comparator | 233 | 96.3 |
| The target population | 236 | 97.5 |
| Time horizon | ||
| Lifetime | 113 | 46.7 |
| Other | 90 | 37.2 |
| Not stated | 39 | 16.1 |
| Study perspective | ||
| Societal | 56 | 23.1 |
| Health-care payer | 183 | 75.6 |
| Not stated/could not determine | 3 | 1.2 |
| Discounting | ||
| Costs only | 11 | 4.5 |
| QALYs only | 4 | 1.7 |
| Both costs and QALYs | 168 | 69.4 |
| Not needed | 23 | 9.5 |
| No/could not determine | 36 | 14.9 |
| Clinical trial based economic analysis | ||
| Yes | 31 | 12.8 |
| No | 209 | 86.4 |
| Could not be determined | 2 | 0.8 |
| Incremental analysis | ||
| Correct | 184 | 76.0 |
| Incorrect | 54 | 22.3 |
| Not reported | 4 | 1.7 |
| Sensitivity analysis† | ||
| Univariate or multivariable | 219 | 90.5 |
| Probabilistic | 69 | 28.5 |
| Other/unknown | 7 | 2.9 |
| Not performed | 14 | 5.8 |
| Presentation of cost-effectiveness acceptability curve | ||
| Yes | 39 | 16.1 |
| No | 203 | 83.9 |
| Use of threshold value to interpret study results | ||
| Yes | 151 | 62.4 |
| No | 91 | 37.6 |
| Data source for utility weights (n = 1171) | ||
| Primary only | 260 | 22.2 |
| Secondary only | 655 | 55.9 |
| Both primary and secondary | 50 | 4.3 |
| Could not be determined/unknown | 206 | 17.6 |
QALY = quality-adjusted life-years.
Non-exclusive.
In general, adherence to recommended methods for conducting and reporting CEA results (eg, applying a societal perspective, discounting both costs and QALYs, providing a clear presentation of the intervention, comparator and the target population; 25,26) was high and has somewhat improved over time. During 2002–2007, almost all studies clearly presented the relevant intervention, the comparator, and the target population. The proportion of studies that correctly calculated ICERs increased from 48% before 1998 to 84% after 2001. Most studies performed a sensitivity analysis to explore uncertainties in cost-effectiveness results, and the proportion of studies that presented a probabilistic sensitivity analysis increased from zero during 1976–1997 to 44% during 2002–2007 (Table 3). It is also noteworthy that more than two-thirds of the utility weights used for the analyses came from published sources and were not directly elicited and reported as part of the described economic evaluation.
Table 3.
Changes over time in methods used in cost–utility analyses*
| Characteristic | 1976–1997 (n = 42) | 1998–2001 (n = 48) | 2002–2007 (N = 152) |
| Clear presentation of | |||
| The relevant intervention, % | 100.0 | 91.7 | 98.7 |
| The comparator, % | 95.2 | 91.7 | 98.0 |
| The target population, % | 100.0 | 91.7 | 98.7 |
| Time horizon, % | |||
| Lifetime | 57.1 | 45.8 | 44.1 |
| Other | 26.2 | 31.3 | 42.1 |
| Not stated | 16.7 | 22.9 | 13.8 |
| Time horizon stated, % | 83.3 | 77.1 | 86.2 |
| Study perspective, % | |||
| Societal | 21.4 | 29.2 | 22.7 |
| Health-care payer | 76.2 | 70.8 | 77.0 |
| Discounting, % | |||
| Costs only | 11.9 | 6.3 | 2.0 |
| QALYs only | 0.0 | 4.2 | 1.3 |
| Both costs and QALYs | 54.8 | 62.5 | 75.7 |
| Not needed | 9.5 | 12.5 | 8.6 |
| Any discounting, % | 76.2 | 85.4 | 87.5 |
| Clinical trial based economic analysis, % | 14.3 | 4.2 | 15.1 |
| Correct incremental analysis, % | 47.6 | 75.0 | 84.2 |
| Sensitivity analysis†, % | |||
| Univariate or multivariable | 83.3 | 93.8 | 91.5 |
| Probabilistic | 0.0 | 4.2 | 44.1 |
| Other/unknown | 0.0 | 0.0 | 4.6 |
| Not performed | 16.7 | 6.3 | 2.6 |
| Any sensitivity analysis, % | 83.3 | 93.8 | 96.1 |
| Presentation of cost-effectiveness acceptability curve, % | 0.0 | 0.0 | 25.7 |
| Use of threshold value to interpret study results, % | 33.3 | 58.3 | 71.7 |
QALY = quality-adjusted life-years.
Non-exclusive.
Although the field has improved in terms of adherence to methodological guidelines, we did not observe a statistically significant change on our subjective quality score (on a 1–7 scale). The mean quality score for all studies was 4.26 and was 4.0 for studies published during 1976–1997, 4.3 for studies published during 1998–2001, and 4.3 for studies published during 2002–2007. The quality of industry-sponsored studies was similar to that of studies sponsored by other organizations and of studies for which the sponsorship source could not be determined. The average quality of CUAs published in “high volume” journals (ie, J Clin Oncol, Pharmacoeconomics, Cancer, Int J Radiat Oncol Biol Phys) as a group was higher compared with “low volume” journals as a group (mean score = 4.48 vs 4.16, difference = 0.32, 95% confidence interval = 0.03 to 0.61, P = .028). Among “high volume” journals, the average paper quality was highest in J Clin Oncol (mean score = 5.0), followed by Breast Cancer Res Treat (mean score = 4.7), Pharmacoeconomics (mean score = 4.5), Cancer (mean score = 4.1), and Int J Radiat Oncol Biol Phys (mean score = 3.9).
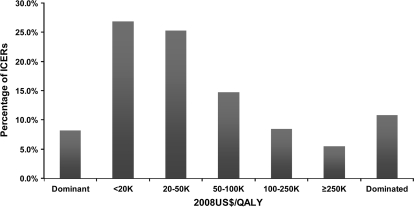
Overall, the 242 analyses presented 636 ICERs (of which 120 CUAs presented more than one ratio). A league table and description of these ratios by main cancer sites is presented in Supplementary Table 2 (available online). The median reported cost-effectiveness ratios (in 2008 US $) were $27 000 for breast cancer, $22 000 for colorectal cancer, $32 000 for lung cancer, and $48 000 for hematologic cancers (Table 4). When the distribution of ICERs found in our study (in 2008 US $) was examined, 8.2% of the interventions were reported to be both cost-saving and more effective (dominant) and 52.2% were reported to have an ICER of less than $50 000 per QALY gained. The ICER was greater than $100 000 per QALY gained in 14.0% of interventions examined, and interventions were cost-increasing and less effective (dominated) in 10.8% of analyses (Figure 2). This distribution of cost-effectiveness ratios is similar to the distribution found in other studies, which have examined measures used for preventive medicine and other disease areas (27–29).
Table 4.
Number of studies and incremental cost-effectiveness ratios (ICERs) by main cancer types*
| Type of cancer | No. of studies (ratio) | Median ICER (2008 US $/QALY) |
| Breast | 86 (226) | 27 000 |
| Hematologic | 24 (37) | 48 000 |
| Colorectal | 29 (62) | 22 000 |
| Lung | 18 (61) | 32 000 |
| Prostate | 22 (42) | 34 500 |
| Gastrointestinal and hepatocellular | 12 (38) | 45 500 |
QALY = quality-adjusted life-years.
Figure 2.
Distribution of published incremental cost-effectiveness ratios (ICERs) in cancer studies. 8.2% of the cancer-related interventions were reported to be both cost-saving and more effective (dominant), and 52.2% were reported to have an ICER of less than $50 000 per quality-adjusted life-year (QALY) gained. The ICER was greater than $100 000 per QALY gained in 14.0% of interventions examined, and interventions were cost-increasing and less effective (dominated) in 10.8% of analyses.
Discussion
We critically reviewed all published cancer-related CUAs and determined their characteristics for more than a 20-year period. The rapid increase in the number of published CUAs is perhaps not surprising because payers worldwide now require data from economic evaluations to inform and support reimbursement decisions (5,14–16,29,30). It is important, however, to understand whether CUAs are improving in quality and whether researchers are following published guidelines for conducting economic evaluations and reporting their results (25,26). We found, among cancer-related CUAs, good adherence to methodological standards in the field, as well as evidence that reporting practices have been improving over time. It is encouraging that almost all studies in recent years clearly state the framework of the analysis (intervention, comparator, and target population) and perform a sensitivity analysis to examine the robustness of the cost-effectiveness results. Still, there is room for improvement because almost 15% of the studies still did not state the time horizon of the analysis and 13% did not discount costs or QALYs. We found that the quality of the studies published in journals that were more experienced with cost-effectiveness research was substantially higher than those in less-experienced journals. These findings, which were similar to our findings in another review (31), were not surprising because these journals may have acquired expertise in economic evaluations and may apply a more rigorous review process. Some of these journals (eg, Pharmacoeconomics, Annals of Internal Medicine) provide additional guidance and/or a checklist for reporting cost-effectiveness results.
The median cost-effectiveness ratios and the distributions of cost-effectiveness ratios in our study are similar to those found in other fields of health care (27–29). These findings are somewhat surprising, given that many observers suggest that many new anticancer drugs do not present good value for money and thus have unfavorable cost-effectiveness ratios (5,14,15). There are three possible explanations for our findings. First, because our analysis reflects only studies that were published through 2007, it is possible that studies that examined the cost-effectiveness of new and expensive biological drugs were published only in 2008 and thereafter, beyond the time frame of our study. Indeed, economic evaluations of some of the most expensive drugs (eg, cetuximab, bevacizumab) have been published only in 2007, although they were available on the market several years earlier (32,33). Economic evaluations of other drugs (eg, sunitinib, sorafenib) were only recently or not yet published. Second, the distribution of ratios for cancer-related interventions that is described in our study may reflect the true distribution of cost-effectiveness ratios for health-care interventions, and thus, economically unattractive interventions may not have been brought to market (27). Finally, as in other areas in medicine, the cancer-related cost-effectiveness literature may have been subject to publication bias, and both selective conduct of cost-effectiveness studies and underreporting of unfavorable cost-effectiveness results may be a problem, particularly for pharmaceutical industry–funded studies (27). Indeed, an examination of industry submissions to the National Institute for Clinical Excellence (NICE) in the United Kingdom suggested that, in many cases, industry estimates were substantially lower (ie, more favorable) than the ICERs determined by NICE (5).
Faced with limited health-care budget and the rising costs of health care, many countries are using economic analyses to inform coverage decisions and they have frequently decided to limit patients’ access to new and expensive drugs (5,14,15,17,34). Either the absence of cost-effectiveness evidence or unfavorable cost-effectiveness results were often cited as the reasons for not recommending the use of a drug (5,14,17). Many of these decisions, however, were controversial, so the insurance coverage of anticancer drug costs has gained attention both in the scientific literature and in the news media (12–14,35).
Acknowledging the unique circumstances of end-of-life care, several countries have adopted special mechanisms for coverage of cancer drugs or more flexible reimbursement criteria (14,15,17). For example, a Canadian study recently suggested that cancer drugs are adopted at the highest threshold of acceptability (15). Even more recently, following a public debate over the coverage of four expensive drugs for treating metastatic renal cell carcinoma and after a brief consultation, the National Institute for Clinical Excellence (NICE) in the United Kingdom outlined a new approach to end-of-life drugs starting January 2009 (5). To qualify for the new approach, drugs must meet four specific criteria: that a limited number of patients can benefit from the drug, the patients must have short life expectancy, the drug must extend life expectancy, and there can be no alternative treatment available within the National Health Service. When all of these conditions are met, NICE’s appraisal committee may consider “the impact of giving greater weight to QALYs achieved in the later stages of terminal diseases, using the assumption that the extended survival period is experienced at the full quality of life anticipated for a healthy individual of the same age” (http://www.nice.org.uk/). This innovative approach will result in an ICER that may fall outside the current threshold range used to determine value for money in the United Kingdom. It may provide some measures of flexibility and responsiveness to political realities and citizens’ preferences.
We hope that our results can help US policy makers as they struggle to incorporate cost-effectiveness considerations into the American health-care system. In the United States, payers such as the Medicare program and others have not used CEA explicitly, and evidence suggests that cancer treatments with high (unfavorable) cost-effectiveness ratios have been covered by insurers (2,18,36). Still, the momentum may be building for CEAs and ratios such as those presented in this article may become more important in the future (20).
Our study has several limitations. First, our review only includes CEAs using the QALY metric. Other economic evaluations of cancer-related intervention may have used other outcome measures as cost per life-year gained or cost per one year of progression-free survival. Second, our review of the cost-effectiveness literature was limited to English-language peer-reviewed publications indexed in MEDLINE. We did not include, for example, health technology assessment reports, such as those generated by the National Institute for Clinical Excellence (NICE) in the United Kingdom or other health technology assessment agencies. Third, it should be noted that readers were not blinded to articles’ journals and authors, which may have influenced results. This lack of blinding may be a potential source of bias, particularly in our subjective assessment of quality scores. Fourth, we did not evaluate the merits of clinical or modeling assumptions included in analyses nor were we able to assess the quality of the data collected in studies conducted alongside clinical trials. Lastly, the cost-effectiveness ratios we present are not static because changes in the costs of the interventions and the associated benefits since the study was published can substantially alter their cost per QALY.
In summary, the large and rapidly growing cost–utility literature yields opportunities to use the results from these analyses to better allocate scarce resources devoted to health care. The use of economic evaluation to guide reimbursement decisions and medical practice will most likely continue to increase. Decision makers will have to deliberate on how other criteria (eg, values, preferences, patient affordability) will be incorporated in these decisions and whether priority should be given to anticancer interventions, specifically those targeted at patients with terminal illness.
Funding
National Cancer Institute contract No. HHSN261200800748P to the Center for the Evaluation of Value and Risk in Health at Tufts Medical Center, Boston, Massachusetts.
Supplementary Material
Footnotes
The sponsor helped to define the scope of this project but was not involved in the study design, data collection, data analysis, interpretation of the data, drafting the manuscript, and the decision to submit the manuscript for publication.
We would like to thank the following individuals for reviewing articles for the CEA Registry:
▪ Kathy Bungay, PharmD, MS
▪ Michael Cangelosi, MPH, MA
▪ Natalie Carvalho, MPH
▪ Amit Chhabra, MD, MPH
▪ Maki Kamae, MD, MPH
▪ Lisa Meckley, PhD
▪ Chizanya Mpinja, MS
▪ Mkaya Mwamburi, MD, PhD
▪ Hansel Otero, MD
▪ Ipek Özer Stillman, MS
▪ Jenny Palmer, MS
▪ Ankur Pandya, MPH
▪ Corey Probst, BA
▪ Lien Quach, MD, MPH
▪ Manu Sondhi, MD, MBA, MS
▪ DeeDee Tobias, MS
▪ Zheng-Yi Zhou, MS.
We would like to thank Dr Robin Yabroff and Dr Bryce Reeve for their helpful comments on an earlier version of this manuscript.
References
- 1.Bach PB. Costs of cancer care: a view from the centers for Medicare and Medicaid services. J Clin Oncol. 2007;25(2):187–190. doi: 10.1200/JCO.2006.08.6116. [DOI] [PubMed] [Google Scholar]
- 2.Bach PB. Limits on Medicare's ability to control rising spending on cancer drugs. N Engl J Med. 2009;360(6):626–633. doi: 10.1056/NEJMhpr0807774. [DOI] [PubMed] [Google Scholar]
- 3.Drummond MF, Mason AR. European perspective on the costs and cost-effectiveness of cancer therapies. J Clin Oncol. 2007;25(2):191–195. doi: 10.1200/JCO.2006.07.8956. [DOI] [PubMed] [Google Scholar]
- 4.Meropol NJ, Schulman KA. Cost of cancer care: issues and implications. J Clin Oncol. 2007;25(2):180–186. doi: 10.1200/JCO.2006.09.6081. [DOI] [PubMed] [Google Scholar]
- 5.Raftery J. NICE and the challenge of cancer drugs. BMJ. 2009;338 doi: 10.1136/bmj.b67. b67. [DOI] [PubMed] [Google Scholar]
- 6.Schrag D. The price tag on progress—chemotherapy for colorectal cancer. N Engl J Med. 2004;351(4):317–319. doi: 10.1056/NEJMp048143. [DOI] [PubMed] [Google Scholar]
- 7.Fojo T, Grady C. How much is life worth? Cetuximab, non-small cell lung cancer, and the $440 billion question. J Natl Cancer Inst. 2009;101(15):1044–1048. doi: 10.1093/jnci/djp177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.American Cancer Society. Cancer Facts & Figures 2008. http://www.cancer.org/downloads/STT/2008CAFFfinalsecured.pdf. [Google Scholar]
- 9.Jönsson B, Wilking N. A global comparison regarding patient access to cancer drugs: the burden and cost of cancer. Ann Oncol. 2007;18(suppl3) doi: 10.1093/annonc/mdm095. iii8–iii22. [DOI] [PubMed] [Google Scholar]
- 10.Yabroff KR, Bradley CJ, Mariotto AB, Brown ML, Feuer EJ. Estimates and projections of value of life lost from cancer deaths in the United States. J Natl Cancer Inst. 2008;100(24):1755–1762. doi: 10.1093/jnci/djn383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yabroff KR, Warren JL, Brown ML. Costs of cancer care in the USA: a descriptive review. Nat Clin Pract Oncol. 2007;4(11):643–656. doi: 10.1038/ncponc0978. [DOI] [PubMed] [Google Scholar]
- 12.Berenson A. Cancer drugs offer hope, but at huge expense. New York Times. July 12, 2005. [PubMed] [Google Scholar]
- 13.Gardiner H. The evidence gap: British balance benefit vs. cost of latest drugs. New York Times. December 2, 2008. [Google Scholar]
- 14.Mason AR, Drummond MF. Public funding of new cancer drugs: is NICE getting nastier? Eur J Cancer. 2009;45(7):1188–1192. doi: 10.1016/j.ejca.2008.11.040. [DOI] [PubMed] [Google Scholar]
- 15.Rocchi A, Menon D, Verma S, Miller E. The role of economic evidence in Canadian oncology reimbursement decision-making: to lambda and beyond. Value Health. 2008;11(4):771–783. doi: 10.1111/j.1524-4733.2007.00298.x. [DOI] [PubMed] [Google Scholar]
- 16.Cairns J. Providing guidance to the NHS: the Scottish Medicines Consortium and the National Institute for Clinical Excellence compared. Health Policy. 2006;76(2):134–143. doi: 10.1016/j.healthpol.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 17.Raftery JP. Paying for costly pharmaceuticals: regulation of new drugs in Australia, England and New Zealand. Med J Aust. 2008;188(1):26–28. doi: 10.5694/j.1326-5377.2008.tb01500.x. [DOI] [PubMed] [Google Scholar]
- 18.Neumann PJ, Rosen AB, Weinstein MC. Medicare and cost-effectiveness analysis. N Engl J Med. 2005;353(14):1516–1522. doi: 10.1056/NEJMsb050564. [DOI] [PubMed] [Google Scholar]
- 19.Neumann PJ. Using Cost-Effectiveness Analysis to Improve Health Care. New York, NY: Oxford University Press; 2005. [Google Scholar]
- 20.Neumann PJ, Greenberg D. Is the United Stated ready for QALYs? Health Affairs. 2009;28(5):1366–1371. doi: 10.1377/hlthaff.28.5.1366. [DOI] [PubMed] [Google Scholar]
- 21.Grusenmeyer PA, Wong YN. Interpreting the economic literature in oncology. J Clin Oncol. 2007;25(2):196–202. doi: 10.1200/JCO.2006.09.0738. [DOI] [PubMed] [Google Scholar]
- 22.Shih YC, Halpern MT. Economic evaluations of medical care interventions for cancer patients: how, why, and what does it mean? CA Cancer J Clin. 2008;58(4):231–244. doi: 10.3322/ca.2008.0008. [DOI] [PubMed] [Google Scholar]
- 23.Earle CC, Chapman RH, Baker CS, et al. Systematic overview of cost-utility assessments in oncology. J Clin Oncol. 2000;18(18):3302–3317. doi: 10.1200/JCO.2000.18.18.3302. [DOI] [PubMed] [Google Scholar]
- 24.Neumann PJ, Greenberg D, Olchanski NV, Stone PW, Rosen AB. Growth and quality of the cost-utility literature, 1976–2001. Value Health. 2005;8(1):3–9. doi: 10.1111/j.1524-4733.2005.04010.x. [DOI] [PubMed] [Google Scholar]
- 25.Drummond M, Sculpher M, Torrance G, O'Brien BJ, Stoddart GL. Methods for the Economic Evaluation of Health Care Programmes. 3rd ed. New York, NY: Oxford University Press; 2005. [Google Scholar]
- 26.Gold MR, Siegel JE, Russell LB, Weinstein MC. Cost-Effectiveness in Health and Medicine. New York, NY: Oxford University Press; 1996. [Google Scholar]
- 27.Bell CM, Urbach DR, Ray JG, et al. Bias in published cost effectiveness studies: systematic review. BMJ. 2006;332(7543):699–703. doi: 10.1136/bmj.38737.607558.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cohen JT, Neumann PJ, Weinstein MC. Does preventive care save money? Health economics and the presidential candidates. N Engl J Med. 2008;358(7):661–663. doi: 10.1056/NEJMp0708558. [DOI] [PubMed] [Google Scholar]
- 29.Dalziel K, Segal L, Mortimer D. Review of Australian health economic evaluation—245 interventions: what can we say about cost effectiveness? Cost Eff Resour Alloc. 2008;6:9. doi: 10.1186/1478-7547-6-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tengs TO. Cost-effectiveness versus cost-utility analysis of interventions for cancer: does adjusting for health-related quality of life really matter? Value Health. 2004;7(1):70–78. doi: 10.1111/j.1524-4733.2004.71246.x. [DOI] [PubMed] [Google Scholar]
- 31.Otero HJ, Rybicki FJ, Greenberg D, Neumann PJ. Twenty years of cost-effectiveness analysis in medical imaging: are we improving? Radiology. 2008;249(3):917–925. doi: 10.1148/radiol.2493080237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Starling N, Tilden D, White J, Cunningham D. Cost-effectiveness analysis of cetuximab/irinotecan vs active/best supportive care for the treatment of metastatic colorectal cancer patients who have failed previous chemotherapy treatment. Br J Cancer. 2007;96(2):206–212. doi: 10.1038/sj.bjc.6603561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tappenden P, Jones R, Paisley S, Carroll C. The cost-effectiveness of bevacizumab in the first-line treatment of metastatic colorectal cancer in England and Wales. Eur J Cancer. 2007;43(17):2487–2494. doi: 10.1016/j.ejca.2007.08.017. [DOI] [PubMed] [Google Scholar]
- 34.Shemer J. Year 2006 update of the National List of Health Services—an endless process. Isr Med Assoc J. 2006;8(9):646–648. [PubMed] [Google Scholar]
- 35.O'Dowd A. Watchdog set to reject four drugs for kidney cancer on the NHS. BMJ. 2008;337 doi: 10.1136/bmj.a1262. a1262. [DOI] [PubMed] [Google Scholar]
- 36.Tunis SR. Why Medicare has not established criteria for coverage decisions. N Engl J Med. 2004;350(21):2196–2198. doi: 10.1056/NEJMe048091. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.