Abstract
Wnt5a regulates multiple intracellular signalling cascades, but how Wnt5a determines the specificity of these pathways is not well understood. This study examined whether the internalization of Wnt receptors affects the ability of Wnt5a to regulate its signalling pathways. Wnt5a activated Rac in the β-catenin-independent pathway, and Frizzled2 (Fz2) and Ror1 or Ror2 were required for this action. Fz2 was internalized through a clathrin-mediated route in response to Wnt5a, and inhibition of clathrin-dependent internalization suppressed the ability of Wnt5a to activate Rac. As another action of Wnt5a, it inhibited Wnt3a-dependent lipoprotein receptor-related protein 6 (LRP6) phosphorylation and β-catenin accumulation. Wnt3a-dependent phosphorylation of LRP6 was enhanced in Wnt5a knockout embryonic fibroblasts. Fz2 was also required for the Wnt3a-dependent accumulation of β-catenin, and Wnt5a competed with Wnt3a for binding to Fz2 in vitro and in intact cells, thereby inhibiting the β-catenin pathway. This inhibitory action of Wnt5a was not affected by the impairment of clathrin-dependent internalization. These results suggest that Wnt5a regulates distinct pathways through receptor internalization-dependent and -independent mechanisms.
Keywords: β-catenin, endocytosis, Frizzled2, Rac, Wnt5a
Introduction
Wnt proteins constitute a large family of secreted ligands that control developmental processes in animals (Logan and Nusse, 2004). In mice and humans, there are 19 Wnt members, which exhibit unique expression patterns and distinct functions in development. Wnts control various cellular functions including proliferation, differentiation, apoptosis, survival, migration, and polarity, by regulating multiple intracellular signalling cascades. In humans and mice, the 10 members of the Frizzled (Fz) seven transmembrane receptor family have been identified as Wnt receptors (Wang et al, 2006). In addition to Fz proteins, single-pass transmembrane proteins, such as low-density lipoprotein receptor-related protein 5 (LRP5), LRP6, receptor tyrosine kinase-like orphan receptor 1 (Ror1), Ror2, and atypical tyrosine kinase receptor (Ryk), have been shown to act as Wnt receptors (Gordon and Nusse, 2006; Fukuda et al, 2008; Green et al, 2008; Kikuchi et al, 2009).
The intracellular signalling pathway activated by Wnts was originally identified as a β-catenin-dependent signalling pathway that is highly conserved among various species (He et al, 2004; Kikuchi et al, 2009). In the β-catenin pathway, the binding of Wnts, such as Wnt1 and Wnt3a, to a cell surface receptor complex consisting of Fz and LRP5 or LRP6 induces the stabilization of cytoplasmic β-catenin and its entry into the nucleus, where it activates the transcription factor T-cell factor (Tcf) or lymphoid enhancer factor (Lef) and stimulates the transcription of target genes. Some Wnts, such as Wnt5a and Wnt11, activate β-catenin-independent pathways that modulate cell movement and polarity (Veeman et al, 2003; Kikuchi and Yamamoto, 2008). The β-catenin-independent pathways include multiple signalling cascades such as Rac/Jun N-terminal kinase (JNK), Rho/Rho-associated kinase (Rho-kinase), Ca2+/protein kinase C, and Ca2+/calmodulin-dependent protein kinase II.
Wnt5a is representative of the Wnt proteins that activate the β-catenin-independent pathway, although the activation mechanism has not yet been clarified (Veeman et al, 2003; Kikuchi and Yamamoto, 2008). Many studies on signal transduction and membrane trafficking have suggested that the sorting of signalling molecules and their receptors to different membrane-bound compartments has a critical function in regulating signalling (Conner and Schmid, 2003). β-Arrestin mediates the internalization of the seven transmembrane receptor family in clathrin-coated pits by binding to clathrin, μ2-adaptin of adaptor protein-2 (AP-2), and other elements of the endocytic machinery (Lefkowitz and Shenoy, 2005). Wnt5a triggered the endocytosis of Fz4, which was mediated by the recruitment of Dvl2 and β-arrestin2 to the plasma membrane in HEK293 cells (Chen et al, 2003). Wnt5a also induced the internalization of Fz5 (Kurayoshi et al, 2007). Dvl2 interacted with AP-2, which also bound to clathrin, and this interaction was required for the internalization of Fz4 (Yu et al, 2007). In addition, reduction of β-arrestin2 levels in Xenopus embryos led to defects in convergent extension, which is believed to be mediated by the β-catenin-independent pathway (Kim and Han, 2007; Bryja et al, 2008). Therefore, it is possible to speculate that Wnt5a might induce the internalization of its receptors through a clathrin-mediated route, thereby activating the β-catenin-independent pathway. However, there is no direct evidence for this possibility at present.
Another Wnt5a action is to inhibit the β-catenin pathway. An early experiment in Xenopus embryos showed that coexpression of XWnt5a with XWnt8 reduces the ability of XWnt8 to induce a secondary axis (Torres et al, 1996). Several possible mechanisms have been proposed to explain this inhibition. Wnt5a was reported to inhibit the transcriptional activity of Tcf downstream of β-catenin by the phosphorylation of Tcf-4 by Nemo-like kinase (NLK) (Ishitani et al, 2003). However, it was also reported that Wnt5a inhibits Tcf independently of the NLK pathway (Smit et al, 2004). Another possible mechanism is that Wnt5a induced the downregulation of β-catenin through the expression of Siah2, which acts as an E3 ubiquitin ligase for β-catenin (Topol et al, 2003). Thus, the inhibitory mechanism of the β-catenin pathway by Wnt5a remains to be clarified. In addition, it is unclear whether the internalization of receptors is involved in the Wnt5a-dependent inhibition.
In this study, it was found that Wnt5a-dependent activation of Rac requires clathrin-mediated internalization of Fz2. It was also shown that Wnt5a inhibits the β-catenin pathway by competing with Wnt3a for binding to Fz2, and that the impairment of clathrin-mediated internalization does not affect this Wnt5a inhibitory action. These results suggest that Wnt5a regulates distinct pathways through the receptor internalization-dependent and -independent mechanisms.
Results
Fz2 is involved in the activation of both β-catenin-dependent and -independent pathways
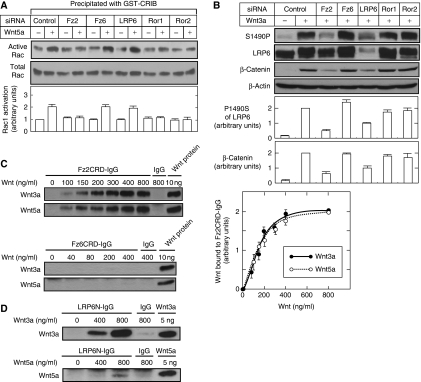
To identify the actions of Wnts, purified Wnt3a and Wnt5a proteins were used in all of the following experiments. First, it was determined whether which receptors mediate distinct Wnt signalling pathways. As Wnt receptors, HeLaS3 cells expressed mRNAs of Fz2, Fz5, and Fz6 and also mRNAs of LRP6, Ror1, and Ror2 (Supplementary Figure S1A and data not shown). These receptors, except for Fz5, were knocked down using small interference RNAs (siRNAs) (Supplementary Figure S1B), because Fz5 mRNA was reduced to only 50% even though 10 different siRNAs or their combinations were used to target Fz5. Individual siRNA did not show off-target effects on the expression of other receptors (Supplementary Figure S1B). Therefore, Fz2, Fz6, LRP6, Ror1, and Ror2 were analysed in this study.
Among the β-catenin-independent pathways, the activation of Rac was observed clearly in HeLaS3, CHO, and L cells when they were stimulated with purified Wnt5a (Figure 1A; Supplementary Figure S2A). However, it was hard to detect the activation of JNK and Rho by Wnt5a and that of Rac by Wnt3a in these cells (Supplementary Figure S2B–D). Knockdown of Fz2, Ror1, and Ror2 but not that of Fz6 or LRP6 suppressed the Wnt5a-dependent Rac activation in HeLaS3 cells (Figure 1A). Wnt3a, but not Wnt5a, induced the phosphorylation of LRP6 and the accumulation of β-catenin in HeLaS3, CHO, and L cells (Yamamoto et al, 2006) (Figures 1B and 4B). Knockdown of Fz2 and LRP6 in HeLaS3 cells suppressed the Wnt3a-dependent phosphorylation of LRP6 and accumulation of β-catenin, but knockdown of Fz6, Ror1, or Ror2 did not (Figure 1B).
Figure 1.
Fz2 mediates both β-catenin-dependent and -independent pathways. (A) After HeLaS3 cells transfected with the indicated siRNAs were treated with 50 ng/ml Wnt5a for 1 h, the cells were incubated with GST-CRIB immobilized on glutathione-Sepharose. The total lysates (total Rac) and precipitates (active Rac) were probed with anti-Rac1 antibody. The signals of active Rac were quantified using NIH Image and expressed as arbitrary units as compared with the signal intensity in control cells without Wnt5a stimulation. The results shown are means±s.e. from four independent experiments. (B) HeLaS3 cells transfected with the indicated siRNAs were treated with 100 ng/ml Wnt3a for 1 h. The lysates were probed with the indicated antibodies. S1490P, anti-phospho-S1490 LRP6 antibody. β-Actin was used as a loading control. The signals of β-catenin and S1490P were quantified using NIH Image and expressed as arbitrary units as compared with the signal intensities in control cells with Wnt3a stimulation. The results shown are means±s.e. from four independent experiments. (C) Left panel, the indicated concentrations of Wnt3a or Wnt5a proteins were incubated with 0.5 nM (25 ng/ml) of FzCRD-IgG proteins or 0.5 nM (17.5 ng/ml) control IgG protein for 2 h. The precipitates were probed with anti-Wnt3a and anti-Wnt5a antibodies. Right panel, the signals of Wnt3a or Wnt5a bound to Fz2CRD-IgG were quantified using NIH Image and expressed as arbitrary units. Wnt3a or Wnt5a (10 ng) was loaded into the right-hand lane as a control. (D) The indicated concentrations of Wnt3a or Wnt5a were incubated with 2 nM (400 ng/ml) LRP6N-IgG protein or 2 nM (70 ng/ml) control IgG protein for 2 h. The precipitates were probed with anti-Wnt3a and anti-Wnt5a antibodies.
To perform the ligand-receptor binding assay, the cysteine-rich domain (CRD) of Fz or the extracellular domain of LRP6 (LRP6N) fused to IgG (Fz2CRD-IgG, Fz6CRD-IgG, and LRP6N-IgG) were expressed in HEK293 cultured medium, and proteins were purified using protein A-Sepharose. Consistent with the results in Figure 1A and B, Wnt5a and Wnt3a bound to the CRD of Fz2 with Kd values of 2.3 nM (90 ng/ml)±0.7 nM and 2.8 nM (112 ng/ml)±0.9 nM, respectively, and neither of them bound to the CRD of Fz6 (Figure 1C). Wnt5a showed a weak-binding activity with LRP6N as compared with Wnt3a (Figure 1D). In contrast, Wnt5a, but not Wnt3a, has been reported to bind to Ror2 (Oishi et al, 2003). Thus, it is likely that Fz2 is involved in the activation of both β-catenin-dependent and -independent pathways.
Clathrin-dependent internalization of receptors is required for Wnt5a-dependent Rac activation
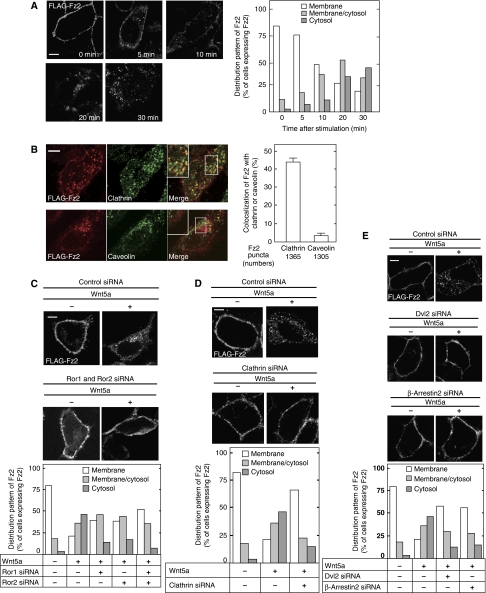
To examine whether the internalization of Fz2 is necessary for the activation of Wnt signalling pathways, the endocytic route of Fz2 was analysed. It has been reported that Wnt5a induces the internalization of Fz4 and Fz5 (Chen et al, 2003; Kurayoshi et al, 2007). Wnt5a also induced the internalization of FLAG-tagged Fz2 (FLAG-Fz2) in HeLaS3 cells (Figure 2A). Approximately 45% of the internalized Fz2 was colocalized with clathrin but not with caveolin in HeLaS3 cells (Figure 2B). Knockdown of either Ror1 or Ror2 suppressed Wnt5a-dependent internalization of Fz2, and knockdown of both Ror1 and Ror2 further inhibited it (Figure 2C; Supplementary Figure S3A). Knockdown of clathrin (Supplementary Figure S4) or treatments of the cells with monodansylcadaverine (MDC) or chlorpromazine, which suppressed the clathrin-dependent internalization of receptors, also inhibited the Wnt5a-induced internalization of Fz2 (Figure 2D; Supplementary Figure S3B). Although it has been reported that the protein levels of Dvl are reduced in the cells treated with hyperosmotic sucrose and potassium depletion that suppress the clathrin-dependent internalization (Bryja et al, 2007a), clathrin siRNA, MDC, or chlorpromazine did not affect Dvl levels (Supplementary Figure S5). In addition, knockdown of Dvl2 and β-arrestin2 also suppressed the Wnt5a-dependent internalization of Fz2 (Figure 2E; Supplementary Figure S4). These results suggest that Wnt5a induces the internalization of Fz2 through a clathrin-mediated endocytic route and that Ror1, Ror2, Dvl2, and β-arrestin have a function in this step.
Figure 2.
Wnt5a induces the internalization of Fz2 through a clathrin-mediated route. (A) HeLaS3 cells expressing FLAG-Fz2 were treated with 100 ng/ml Wnt5a for the indicated periods of time. Left panel, confocal images; right panel, quantification of internalized FLAG-Fz2. The results shown are means of four independent experiments. (B) Left panel, HeLaS3 cells expressing FLAG-Fz2 were treated with Wnt5a for 30 min, and then the cells were stained with anti-FLAG and anti-clathrin or anti-caveolin antibodies. In the merged images, FLAG-Fz2 is shown in red and clathrin or caveolin is in green. Colocalization of FLAG-Fz2 and clathrin appears as yellow. Right panel, the percentages of Fz2 colocalized with clathrin- or caveolin-positive vesicles were quantified. The results shown are means±s.e. from three independent experiments. Approximately 43% of 1365 Fz2 puncta were colocalized with clathrin-positive vesicles. (C) HeLaS3 cells expressing FLAG-Fz2 were transfected with siRNA for Ror1 or/and Ror2, and then the cells were treated with Wnt5a for 30 min. (D) HeLaS3 cells expressing FLAG-Fz2 were transfected with siRNA for clathrin, and then the cells were treated with Wnt5a for 30 min. (E) HeLaS3 cells expressing FLAG-Fz2 were transfected with siRNA for Dvl2 or β-arrestin2, and then the cells were treated with Wnt5a for 30 min. Scale bars, 5 μm.
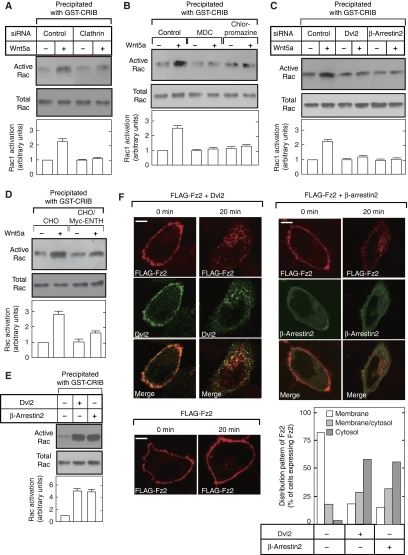
The knockdown of clathrin in HeLaS3 cells, but not that of caveolin, inhibited the Wnt5a-dependent Rac activation (Figure 3A; Supplementary Figures S4 and S6A). Treatment of the cells with MDC or chlorpromazine also inhibited Wnt5a-dependent Rac activation (Figure 3B), whereas that with Nystatin, which disrupts the lipid raft containing caveolin, did not (Supplementary Figure S6B). In addition, Wnt5a failed to activate Rac in Dvl2 or β-arrestin2 knockdown cells (Figure 3C). It has been shown that Epsin is involved in the clathrin-dependent internalization of receptors for trasnferrin, EGF, and insulin, and that clathrin-dependent internalization of these receptors is inhibited in cells expressing the ENTH domain of Epsin (Nakashima et al, 1999; Itoh et al, 2001). As expected, the Wnt5a-dependent activation of Rac was inhibited in CHO cells expressing the ENTH domain (Figure 3D). These results suggest that the clathrin-dependent internalization is required for Wnt5a-dependent activation of Rac.
Figure 3.
Clathrin-dependent internalization of Fz2 is required for Rac activation. (A) HeLaS3 cells transfected with control or clathrin siRNA were treated with 50 ng/ml Wnt5a for 1 h, and then the cells were subjected to the Rac activation assay. The results shown are means±s.e. from four independent experiments. (B) HeLaS3 cells pretreated with MDC or chlorpromazine were incubated with Wnt5a for 1 h. (C) HeLaS3 cells transfected with the indicated siRNAs were treated with Wnt5a for 1 h. (D) CHO cells or CHO cells expressing Myc-ENTH (CHO/Myc-ENTH) were treated with Wnt5a for 1 h. (E) HEK293T cells expressing HA-Dvl2 or β-arrestin2-GFP were subjected to the Rac activation assay. (F) After HeLaS3 cells expressing FLAG-Fz2 with HA-Dvl2 or β-arrestin2-GFP were kept for 30 min at 4°C and further incubated with anti-FLAG antibody for 1 h at 4°C, the cells were incubated for 20 min at 37°C without Wnt5a stimulation. In the merged images, FLAG-Fz2 is shown in red and HA-Dvl2 or β-arrestin2-GFP is in green. Colocalization of FLAG-Fz2 and HA-Dvl2 appears as yellow. Top panel, confocal images; bottom panel, quantification of internalized FLAG-Fz2 after incubation for 20 min at 37°C. The results shown are means of four independent experiments. Scale bar, 5 μm.
Consistent with the results from mouse embryonic fibroblasts (MEFs) (Bryja et al, 2008), the overexpression of β-arrestin2 in HEK293T cells activated Rac to the same extent as with Dvl2 (Figure 3E). Whether the activation of Rac by Dvl2 or β-arrestin2 is related with the internalization of Fz2 without Wnt5a stimulation was examined. After HeLas3 cells expressing Fz2 was kept at 4°C, internalization was initiated by the shift to 37°C. When Fz2 was expressed alone, Fz2 was localized to the cell surface membrane after 20-min incubation at 37°C (Figure 3F). When Fz2 and Dvl2 were coexpressed, Dvl2 was observed at the cell surface membrane at 4°C, and the expression of Dvl2 induced the internalization of Fz2 at 37°C (Figure 3F). Moreover, the internalized Fz2 was colocalized with Dvl2 (Figure 3F). The expression of β-arrestin2 also induced the internalization of Fz2 without Wnt5a stimulation although β-arrestin2 was distributed throughout the cytoplasm (Figure 3F). Dvl2- and β-arrestin2-induced internalization of Fz2 was observed even in the presence of secreted Fz-related protein 2 (sFRP2), which binds to Wnt proteins and blocks Wnt signalling (Kawano and Kypta, 2003) (Supplementary Figure S7A). It is notable that Dvl2-induced Rac activation was observed in Fz2 knockdown cells, whereas β-arrestin2-induced Rac activation was lost by knockdown of Fz2 (Supplementary Figure S7B). Taken together, these results suggest that the clathrin-dependent internalization of Fz2 has a function in Wnt5a-dependent activation of Rac, and it is likely that overexpressed Dvl2 by itself has another way to activate Rac.
Wnt5a inhibits Wnt3a-dependent accumulation of β-catenin
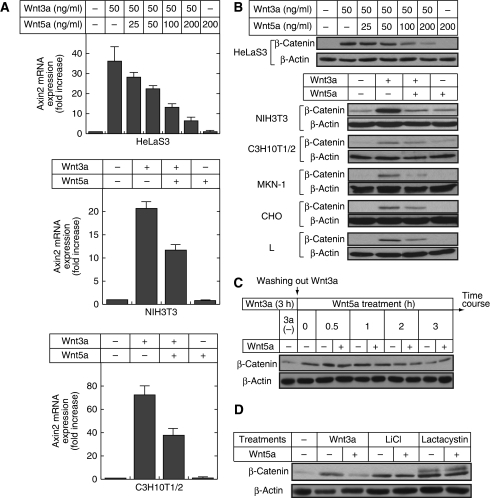
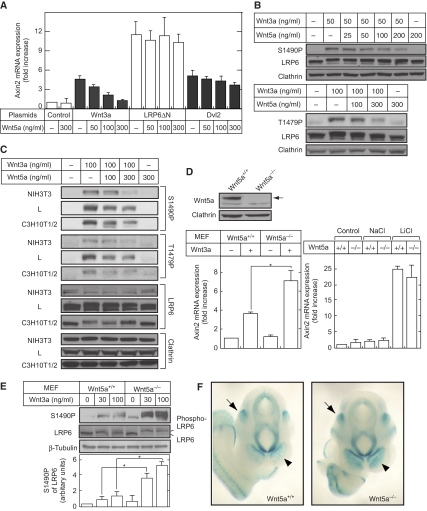
One of the earliest observations about Wnt5a was that it has an ability to inhibit the β-catenin pathway. As the effects of Wnt5a were analysed using artificial reporter gene assays in earlier studies (Ishitani et al, 2003; Mikels and Nusse, 2006), this study investigated the inhibitory mechanism of the β-catenin pathway by Wnt5a by measuring target gene expression, β-catenin stability, and the phosphorylation of LRP6 at endogenous levels. Among target genes such as c-Myc, cyclinD1, Dkk1, and Axin2 in the β-catenin pathway, Wnt3a increased Axin2 mRNA levels greatly in HeLaS3, NIH3T3, and C3H10T1/2 cells (Figure 4A). Wnt5a did indeed suppress Wnt3a-dependent increases in Axin2 mRNA levels in these cells, and it also inhibited the Wnt3a-dependent β-catenin accumulation (Figure 4A and B). Furthermore, the inhibition of β-catenin accumulation by Wnt5a was observed in MKN-1, CHO, and L cells (Figure 4B), suggesting that Wnt5a is able to suppress the β-catenin pathway by inhibiting the accumulation of β-catenin.
Figure 4.
Wnt5a inhibits Wnt3a-dependent accumulation of β-catenin. (A) Top panel, HeLaS3 cells were treated with 50 ng/ml Wnt3a in the presence of the indicated concentrations of Wnt5a for 12 h. Total RNA was extracted from these cells, and semi-quantitative RT–PCR analyses for Axin2 mRNA expression were performed. Middle and bottom panels, NIH3T3 or C3H10T1/2 cells were treated with 20 ng/ml Wnt3a and/or 100 ng/ml Wnt5a for 12 h. The results are expressed as fold increase compared with that without Wnt3a stimulation, and indicate means±s.e. from three independent experiments. (B) Top panel, HeLaS3 cells were treated with 50 ng/ml Wnt3a in the presence of the indicated concentrations of Wnt5a for 3 h. The lysates were probed with anti-β-catenin and anti-β-actin antibodies. β-Actin was used as a loading control. Bottom panel, NIH3T3, C3H10T1/2, MKN-1, CHO, and L cells were treated with 50 ng/ml Wnt3a with or without 300 ng/ml Wnt5a for 3 h. (C) After stimulation with 20 ng/ml Wnt3a for 3 h, HeLaS3 cells were washed with PBS, and then treated with 100 ng/ml Wnt5a for the indicated periods of time. (D) HeLaS3 cells were treated with 20 ng/ml Wnt3a, 10 mM LiCl, or 10 μM lactacystin for 3 h with or without 100 ng/ml Wnt5a.
Wnt5a has been reported to induce the expression of Siah2, which functions as an E3 ubiquitin ligase to degrade β-catenin, in HEK293 cells (Topol et al, 2003). However, in our hand Wnt5a did not increase Siah1 or Siah2 mRNA levels in HEK293 or HeLaS3 cells (data not shown). Although Axin2 is also known to degrade β-catenin and acts as a negative regulator of the β-catenin signalling (Yamamoto et al, 1998; Lustig et al, 2002), Wnt5a alone did not increase Axin2 mRNA levels in contrast to Wnt3a (Figure 4A). After HeLaS3 cells were treated with Wnt3a for 3 h, under conditions where β-catenin accumulated maximally, Wnt3a was removed from the culture medium. The β-catenin levels reduced gradually in a time-dependent manner, but the addition of Wnt5a did not affect the β-catenin levels (Figure 4C). Furthermore, the accumulation of β-catenin following LiCl treatment, which acts as a GSK-3 inhibitor, or lactacystin, which functions as a proteasome inhibitor, was not suppressed by Wnt5a (Figure 4D). These results suggest that Wnt5a does not induce the degradation of β-catenin but is involved in the regulation of β-catenin stabilization upstream of β-catenin.
Wnt5a inhibits Wnt3a-dependent phosphorylation of LRP6
Although Wnt5a suppressed the increases in Axin2 mRNA levels induced by overexpression of Wnt3a, it did not affect the increases due to LRP6ΔN, a constitutively active form of LRP6, or Dvl2 in HeLaS3 cells (Figure 5A). Wnt3a induced the phosphorylation of LRP6 at Ser1490 and Thr1479 (Davidson et al, 2005; Zeng et al, 2005), and Wnt5a inhibited it in HeLaS3 cells (Figure 5B). These findings were confirmed in NIH3T3, L, and C3H10T1/2 cells (Figure 5C), suggesting that Wnt5a affects the β-catenin pathway at the receptor level.
Figure 5.
Wnt5a inhibits Wnt3a-dependent phosphorylation of LRP6. (A) HeLaS3 cells expressing Wnt3a, LRP6ΔN-GFP, or FLAG-Dvl2 were treated with the indicated concentrations of Wnt5a, and semi-quantitative RT–PCR analyses for Axin2 mRNA expression were performed. The results are expressed as fold increase as compared with the Axin2 mRNA expression level in control cells, and indicate means±s.e. from three independent experiments. (B) HeLaS3 cells were treated with 50 or 100 ng/ml Wnt3a in the presence of the indicated concentrations of Wnt5a for 1 h. The lysates were probed with the indicated antibodies. S1490P, anti-phospho-S1490 LRP6 antibody; T1479P, anti-phospho-T1479 LRP6 antibody. Clathrin was used as a loading control. (C) NIH3T3, L, and C3H10T1/2 cells were treated with 100 ng/ml Wnt3a in the presence of the indicated concentrations of Wnt5a for 1 h. (D) Top panels, lysates of MEFs isolated from wild-type embryos (Wnt5a+/+) or Wnt5a knockout embryos (Wnt5a−/−) were probed with anti-Wnt5a/b and anti-clathrin antibodies. Clathrin was used as a loading control. Bottom panels, Wnt5a+/+ MEFs or Wnt5a−/− MEFs were treated with 50 ng/ml Wnt3a for 9 h (left panel) or with 10 mM LiCl for 9 h (right panel), and semi-quantitative RT–PCR analyses of Axin2 mRNA expression were performed. The results are expressed as fold increases as compared with the Axin2 mRNA expression levels in Wnt5a+/+ MEFs without stimulation, and indicate means±s.e. from three independent experiments. *P<0.05. (E) Wnt5a+/+ MEFs or Wnt5a−/− MEFs were treated with the indicated concentrations of Wnt3a for 1 h. The lysates were probed with indicated antibodies. The results shown are representative of four different pairs of Wnt5a−/− and the littermate Wnt5a+/+ mice. The signals of S1490P were quantified using NIH Image. The results are expressed as arbitrary units as compared with the signal intensity from Wnt5a+/+ MEFs with 100 ng/ml Wnt3a stimulation and are shown as means±s.e. from four independent experiments. *P<0.05. (F) Wnt5a+/+ and Wnt5a−/− embryos were stained with X-gal to detect BATlacZ reporter activity at E10.5. Embryos are shown in anterior view. In the telencephalon, β-galactosidase activity of Wnt5a−/− embryo was stronger than that of Wnt5a+/+ (arrowheads). The staining in otic vesicles (arrows) and other regions showed similar staining intensity between Wnt5a+/+ and Wnt5a−/− embryos.
To further examine the loss of function of Wnt5a, MEFs were prepared from Wnt5a knockout (Wnt5a−/−) mice (Figure 5D). Wnt3a-dependent increases in Axin2 mRNA levels were enhanced in Wnt5a−/− MEFs as compared with wild-type (Wnt5a+/+) MEFs (Figure 5D). However, increases in the levels of Axin2 mRNA following LiCl treatment were similar between Wnt5a−/− and Wnt5a+/+ MEFs (Figure 5D). Furthermore, the Wnt3a-dependent phosphorylation of LRP6 at Ser1490 was enhanced in Wnt5a−/− MEFs (Figure 5E). These results support the findings that Wnt5a acts on the receptor to inhibit the β-catenin pathway.
The in vivo activity of the β-catenin pathway was assessed directly in the Wnt5a−/− embryos using BATlacZ transgenic mice, in which LacZ expression is under the control of eight Tcf/Lef-binding sites (Maretto et al, 2003; Nakaya et al, 2005). It has been reported that the Wnt5a mRNA is expressed in the fronted nasal process, telencephalon, diencephalons, mesencephalon, limb buds, genital primordial, and tailbud at 9.5–12.5 d.p.c (Takada et al, 1994; Yamaguchi et al, 1999). BATlacZ expression was clearly detected in the telencephalon, diencephalons, limb buds, and tailbud in Wnt5a+/+ embryos at 10.5 d.p.c., which was consistent with the earlier observation (Maretto et al, 2003). Ectopic LacZ staining was enhanced on the dorsal side of the telencephalon of Wnt5a−/−/BATlacZ mice (Figure 5F, arrowheads), whereas otic vesicles (Figure 5F, arrows) and other regions including the bronchial arches, limb buds, and somites (data not shown) were stained at similar levels between Wnt5a+/+ and Wnt5a−/− embryos. Therefore, these observations suggest that the activity of the β-catenin pathway was enhanced at least in the telencephalon in vivo by removing Wnt5a.
Wnt5a competes with Wnt3a for binding to Fz2
It has been reported that overexpression of Ror2 mediates Wnt5a-dependent inhibition of the β-catenin pathway in L cells (Mikels and Nusse, 2006). However, knockdown of Ror2 and/or Ror1 in HeLaS3 cells did not affect the Wnt5a-induced inhibition of β-catenin accumulation and Axin2 mRNA expression (Figure 6A). It has been proposed that Wnt3a induces the phosphorylation of LRP6 by CK1γ in a Dvl-dependent manner, and the recruitment of Axin further enhances the phosphorylation of the PPPSP motifs by GSK-3 (Bilic et al, 2007; Zeng et al, 2008). The present results also raised the possibility that Wnt5a inhibits CK1γ phosphorylation of LRP6. However, Wnt5a could not affect the phosphorylation of LRP6, which was induced by overexpression of CK1γ (Figure 6B).
Figure 6.
Wnt5a competes with Wnt3a for binding to Frizzled2. (A) HeLaS3 cells transfected with Ror1 and/or Ror2 siRNA were treated with 20 ng/ml Wnt3a with or without 100 ng/ml Wnt5a for 3 h to detect the β-catenin accumulation and for 12 h to examine Axin2 mRNA levels. The lysates were probed with the indicated antibodies. β-Actin was used as a loading control. The results of Axin2 expression are expressed as fold increase compared with that without Wnt3a stimulation, and indicate means±s.e. from three independent experiments. (B) HeLaS3 cells expressing Myc-XWnt8-Fz5 fusion or GFP-CK1γ were treated with 300 ng/ml Wnt5a. Control cells were treated with 100 ng/ml Wnt3a with or without 300 ng/ml Wnt5a. (C) Wnt3a (200 ng/ml) was incubated with 0.5 nM Fz2CRD-IgG proteins for 2 h in the presence of the indicated concentrations of Wnt5a. The precipitates were probed with anti-Wnt3a and anti-Wnt5a antibodies. The signals of Wnt3a bound to Fz2CRD-IgG were quantified using NIH Image and expressed as arbitrary units. The results shown are representative of four independent experiments. (D) HeLaS3 cells expressing FLAG-Fz2 were incubated with 300 ng/ml Wnt3a for 1 h in the presence of the indicated concentrations of Wnt5a, and then cell lysates were immunoprecipitated with anti-FLAG antibody. The precipitates were probed with anti-Wnt3a, anti-Wnt5a, and anti-FLAG antibodies. The signals of Wnt3a bound to FLAG-Fz2 were quantified using NIH Image and expressed as arbitrary units. The results shown are representative of three independent experiments. (E) HeLaS3 cells transfected with control or clathrin siRNA were treated with 100 ng/ml Wnt3a with or without 300 ng/ml Wnt5a for 1 h. The lysates were probed with the indicated antibodies. The signals of β-catenin and S1490P were quantified using NIH Image. The results are expressed as arbitrary units compared with the signal intensities in control cells with Wnt3a stimulation, and indicate means±s.e. from four independent experiments. (F) CHO cells or CHO cells expressing Myc-ENTH were treated with 100 ng/ml Wnt3a with or without 300 ng/ml Wnt5a for 1 h. The lysates were probed with the indicated antibodies. The signals of β-catenin and S1490P were quantified using NIH Image.
As shown in Figure 1, Wnt3a and Wnt5a bound to CRD of Fz2 with a similar affinity. Therefore, one possibility for the inhibitory mechanism of the β-catenin pathway by Wnt5a is that Wnt5a may inhibit the binding of Wnt3a to Fz2. Wnt5a indeed competed with Wnt3a for binding to the CRD of Fz2 in vitro (Figure 6C). Wnt3a bound to Fz2 expressed in HeLaS3 cells, and Wnt5a inhibited it (Figure 6D). Consistent with these results, Wnt5a could not inhibit the phosphorylation of LRP6 induced by the fusion construct of Wnt8 and Fz5, which activates the β-catenin pathway (Figure 6B) (Holmen et al, 2005).
Another possibility is that Wnt5a may remove Fz2 by internalizing it, thereby suppressing the Wnt3a-dependent activation of the β-catenin pathway. However, the manipulations to inhibit clathrin-dependent receptor internalization did not affect the ability of Wnt5a to inhibit the Wnt3a-dependent phosphorylation and accumulation of β-catenin (Figure 6E and F; Supplementary Figure S8). Therefore, the clathrin-dependent internalization of receptors is not necessary for the action of Wnt5a to suppress the β-catenin pathway. Taken together, these results suggest that Wnt5a suppresses the β-catenin pathway by inhibiting the binding of Wnt3a to Fzs on the cell surface membranes.
Discussion
Wnt5a is involved in various cellular functions through the regulation of multiple signalling pathways (Veeman et al, 2003; Kikuchi and Yamamoto, 2008). This study showed that Wnt5a activates the β-catenin-independent pathway and inhibits the β-catenin pathway by binding to Fz2, at least in HeLaS3 cells. To activate Rac, Wnt5a internalized Fz2 probably with Ror1 or Ror2 through the clathrin-mediated route, whereas Wnt5a competed with Wnt3a for binding to Fz2 to inhibit the β-catenin pathway. These results suggest that one Wnt ligand can function in two distinct pathways by binding to the same receptor through different mechanisms.
The mechanism by which Wnt5a activates the Rac pathway
β-Arrestin is critical for mediating the internalization of the receptors in clathrin-coated pits by binding to clathrin, AP-2, and other elements of the endocytic machinery (Lefkowitz and Shenoy, 2005). It has been reported that the mutation of β-arrestin2 and Dvl2, which are not able to bind to clathrin and AP-2, or antisense morpholinos for β-arrestin2 cause defects of convergent extension movement in Xenopus embryos (Kim and Han, 2007; Yu et al, 2007; Bryja et al, 2008). In addition, the expression of Dvl2 mutants that do not bind to AP-2 prevented Wnt5a-dependent internalization of Fz4 in HEK293 cells (Yu et al, 2007). These results suggest that the clathrin-dependent endocytic route is involved in the convergent extension movement, which is probably regulated by the β-catenin-independent pathway.
In this study, it was shown that the clathrin-mediated internalization of Wnt receptors, at least Fz2, is involved in Wnt5a-dependent Rac activation. This conclusion was based on the following findings. First, Wnt5a induced the internalization of Fz2 in a clathrin-mediated route. Second, the impairment of clathrin-dependent internalization of receptors inhibited Wnt5a-induced Rac activation. Third, the expression of β-arrestin2 or Dvl2 induced the internalization of Fz2 and activation of Rac. Ror1 and Ror2 are known to mediate the β-catenin-independent pathway (Green et al, 2008). Consistent with this, knockdown of Ror1 and/or Ror2 reduced Wnt5a-dependent activation of Rac and internalization of Fz2. It was hard to see the internalization of Ror2, because the expression of Ror2 induces morphological changes when Wnt5a was added to the cells (data not shown). The caveolin-dependent internalization of LRP6 and Fz5 in response to Wnt3a has been demonstrated to be necessary for activation of the β-catenin pathway (Yamamoto et al, 2006, 2008). Therefore, by the analogy with the case of Wnt3a and LRP6, Ror1 or Ror2 may be internalized in response to Wnt5a. It is intriguing to speculate that the different endocytic routes in response to specific Wnts determine the activation of either the β-catenin-dependent or -independent pathway in addition to the combination of Fz and a single-pass transmembrane receptor such as LRP6, Ror1, or Ror2 with Wnts.
How the internalization of Wnt receptors triggers the activation of the pathway is unclear. It is known that β-arrestin serves as a scaffold linking seven transmembrane receptors to other signalling proteins, such as the src-family kinases and members of the mitogen-activated protein kinase cascade (Perry and Lefkowitz, 2002). It is possible that the clathrin-dependent internalization of Wnt5a receptors induces the recruitment of GDP/GTP exchange factor of Rac to Dvl through β-arrestin, thereby activating Rac. The Wnt3a-dependent accumulation of β-catenin was inhibited in β-arrestin2 knockout MEFs, and β-arrestin2 bound to Axin that functions in the degradation of β-catenin (Bryja et al, 2007b). Therefore, β-arrestin may act as a scaffold protein to regulate both the β-catenin-dependent and -independent pathways. It has also been reported that clathrin- and Rab5-mediated endocytic route is required for the activation of Rac induced by epidermal growth factor and hepatocyte growth factor (Palamidessi et al, 2008). In this scenario, Rac is activated on early endosomes, where Tiam1 (one of GDP/GTP exchange factor of Rac) is recruited. It is intriguing to speculate that Wnt5a receptors traffic to early endosomes in a Rab5-dependent manner and then Rac is activated.
The mechanism by which Wnt5a inhibits the β-catenin pathway
It was shown that the activity of the β-catenin pathway is enhanced in the telencephalon in Wnt5a−/− mouse embryos. These findings were consistent with the observations that β-catenin is accumulated in the limb buds of Wnt5a−/− embryos and in pipetail (Wnt5a) knockout zebrafish embryos (Topol et al, 2003; Westfall et al, 2003). Therefore, Wnt5a suppresses the β-catenin pathway in vivo. Concerning the inhibitory mechanism by Wnt5a, it has already been reported that Wnt5a inhibits the β-catenin pathway by suppressing the formation of β-catenin and Tcf complex or by inducing Siah2-dependent β-catenin degradation (Ishitani et al, 2003; Topol et al, 2003). These results suggested that Wnt5a acts on β-catenin itself or downstream of β-catenin.
However, this study showed that Wnt5a inhibits the Wnt3a-dependent phosphorylation of LRP6, accumulation of β-catenin, and increases in Axin2 mRNA at endogenous levels in several cultured cell lines. It was also shown that Wnt5a affects neither LRP6ΔN- or Dvl2-dependent Axin2 mRNA increases nor LiCl- or lactacystin-induced β-catenin accumulation at least in HeLaS3 cells. Furthermore, Wnt3a-dependent LRP6 phosphorylation was enhanced in Wnt5a−/− MEFs. These results suggest that Wnt5a acts upstream of β-catenin, probably at the receptor level. Wnt5a competed with Wnt3a for binding to both the CRD of Fz2 in vitro and the full length of Fz2 expressed in intact cells. Fz2 was required for the Wnt3a-dependent phosphorylation of LRP6 and accumulation of β-catenin. In addition, the clathrin-dependent internalization was not required for the inhibitory action of Wnt5a. Taken together, Wnt5a could inhibit the β-catenin pathway by titrating Fz2 from Wnt3a activating the β-catenin pathway on the cell surface membrane.
The discrepancy between earlier observations and the present results in the inhibitory mechanism by Wnt5a may be due to assay conditions. The results in earlier studies were obtained from experiments with the transfection of Wnt ligands and/or reporter gene constructs such as TOPFLASH. We showed earlier that Wnt5a inhibits the transcriptional activity of Tcf downstream of β-catenin in HEK293 cells (Kurayoshi et al, 2007) as reported by other groups (Ishitani et al, 2003; Mikels and Nusse, 2006). Knockdown of clathrin suppressed Wnt5a-dependent inhibition of Tcf-4 activity activated by a constitutively active form of β-catenin (SA-β-cat) (Supplementary Figure S9). Thus, some intracellular signal transduction pathways activated by Wnt5a may inhibit the transcription activated by β-catenin in the nucleus of HEK293 cells, and this inhibition may require the clathrin-dependent internalization. Therefore, the inhibitory mechanism by Wnt5a may depend on the cell type. At present, it is conceivable that Wnt5a can interfere with different steps of the β-catenin pathway.
The mechanism by which Wnt determines the specificity of signalling pathway
How do Wnt3a and Wnt5a regulate different pathways by binding to the same receptor such as Fz2? The distribution of receptors in the lipid raft or non-lipid raft microdomains could be important for the selective regulation of the signalling pathways by different Wnts. For instance, Fz2, Fz5, and LRP6 were located to both the lipid raft and non-lipid raft domains (Yamamoto et al, 2006) (Supplementary Figure S10).
When cells expressing FLAG-Fz2 alone were stimulated with Wnt3a, FLAG-Fz2 was internalized and colocalized with clathrin mainly (Supplementary Figure S11A). However, when cells coexpressing FLAG-Fz2 and LRP6-GFP were stimulated with Wnt3a, the internalized receptor complex was colocalized with caveolin mainly (Supplementary Figure S11B). Therefore, Wnt3a may induce the internalization of Fz2 through a clathrin-mediated pathway probably with Ror1 or Ror2, and when both Fz2 and LRP6 are activated by Wnt3a, the complex may be internalized through the caveolin-mediated pathway. These results support our data that Wnt3a activates both the β-catenin and Rho (PCP) pathways (Kishida et al, 2004) and are consistent with our earlier observations using FLAG-Fz5 (Yamamoto et al, 2006). In contrast, when cells coexpressing FLAG-Fz2 and LRP6-GFP were stimulated with Wnt5a, FLAG-Fz2 was internalized and colocalized with clathrin mainly, whereas LRP6-GFP remained on the cell surface membrane (Supplementary Figure S12). In addition, it is notable that the numbers of Fz2 puncta internalized by Wnt5a decreased when Fz2 and LRP6 were coexpressed as compared with that when Fz2 was expressed alone (see Figure 2B). This suggests that the coexpression of LRP6 causes unknown effects to lead to the retention of Fz2 on the cell surface membrane when the cells were stimulated with Wnt5a that had low affinity for LRP6. The overexpression of LRP6 might influence the circumstance of Fz2 and affect the Wnt5a-mediated internalization of Fz2 in the non-lipid raft. It is unlikely that Wnt5a induces the internalization of LRP6 with Fz2 through a caveolin-mediated route at least in HeLaS3 cells. Taken together, it is conceivable that the endocytic routes of and the signalling pathways activated by Fz2 are determined by the combination of distinct Wnt ligands and single-pass transmembrane receptors.
Although the experiments with Fz5 knockdown could not be performed due to poor efficiency in this study, it was reported that Fz5 functions as a receptor for both Wnt3a and Wnt5a (He et al, 1997; Kurayoshi et al, 2007; Pan et al, 2008). Wnt3a and Wnt5a also had similar affinities for CRDs of Fz5 and Fz2 in vitro (Supplementary Figure S13). Wnt3a still induced the phosphorylation of LRP6 in Fz2 knockdown cells, suggesting that other Fzs including Fz5 act as receptors for Wnt3a/β-catenin signalling. Knockdown of Fz2 suppressed Wnt5a-dependent Rac activation completely in HeLaS3 cells. Therefore, it is unlikely that Fz5 mediates Wnt5a-dependent Rac activation in this cell line. It might be possible that Fz5 functions depending on the ligand and signalling context.
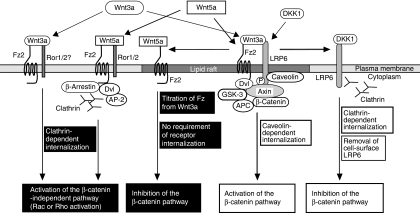
The possible model to explain how Wnt determines the specificity of signalling pathway is as follows (Figure 7). When Wnt3a interacts with Fz and LRP6 simultaneously in the lipid raft microdomain, it activates the β-catenin pathway through the caveolin-mediated internalization route (Yamamoto et al, 2006). When Wnt3a binds to Fz in the non-lipid raft, Wnt3a may activate the β-catenin-independent pathway through the clathrin-dependent internalization route probably with Ror1 or Ror2. Dkk1 removes LRP6 from the lipid raft to the non-lipid raft, and internalizes LRP6 through a clathrin-dependent route, thereby inhibiting the β-catenin pathway (Yamamoto et al, 2008). When Wnt5a acts on Fz and Ror1 or Ror2 in the non-lipid raft domain, it may induce the clathrin-dependent internalization of Fz with Ror1 or Ror2, thereby activating the β-catenin-independent pathway. When Wnt5a acts on Fz that can activate the β-catenin pathway in the lipid raft domain, it may compete with Wnt3a, thereby inhibiting the β-catenin pathway. Thus, Wnt5a and Wnt3a may regulate the β-catenin-dependent and -independent pathways by differences in the specificity and in the internalization of receptors.
Figure 7.
Possible mechanism by which Wnt determines the specificity of signalling pathway. When Wnt3a interacts with Fz and LRP6 simultaneously in the lipid raft microdomain, it activated the β-catenin pathway through the caveolin-mediated internalization route. Wnt3a may activate the β-catenin-independent pathway through the clathrin-dependent internalization route. Dkk1 removes LRP6 from the lipid raft to the non-lipid raft, and internalized LRP6 through a clathrin-dependent route, thereby inhibiting the β-catenin pathway. When Wnt5a acts on Fz and Ror1 or Ror2 in the non-lipid raft domain, it may induce the clathrin-dependent internalization of Fz probably with Ror1 or Ror2, thereby activating the β-catenin-independent pathway. When Wnt5a acts on Fz that can activate the β-catenin pathway in the lipid raft domain, it may compete with Wnt3a, thereby inhibiting the β-catenin pathway. Black boxes indicate Wnt5a actions, which were revealed in this study.
The following observations are different from our results, and these issues should be addressed. It has been reported that Wnt5a induces axis duplication in Xenopus embryos overexpressing Fz5 (He et al, 1997) and stabilized β-catenin in HEK293 cells overexpressing Fz4 and LRP5 (Mikels and Nusse, 2006), suggesting that Wnt5a can activate the β-catenin pathways. However, as far as we examined in 10 different cultured cells expressing at least one of Fz2, Fz4, or Fz5 and LRP6 endogenously, the stabilization of β-catenin by Wnt5a was not observed. In addition, although Wnt5a bound to Fz2 with an affinity similar to Wnt3a, it showed less affinity to LRP6 than Wnt3a. Therefore, it is conceivable that Wnt5a is able to activate the β-catenin pathway under conditions of the overexpression of certain receptors. It has also been shown that Wnt3a conditioned medium and overexpression of Wnt3a activate Rac in ST2 and HEK293 cells (Habas et al, 2003; Wu et al, 2008). Therefore, whether Wnt3a activates Rac may be dependent on cell types. Alternatively, other factor(s) in addition to Wnt3a might be necessary for the activation of Rac. Furthermore, another model is that LRP6 inhibits the β-catenin-independent pathway by binding to Wnt5a (Bryja et al, 2009). It was also shown that the phenotypes by deletion of LRP6 in mice and Xenopus embryos are rescued by additional deletion of Wnt5a. These are interesting findings, but at present it is difficult to conclude that these phenotypes are due to LRP6-dependent suppression of the β-catenin-independent pathway. Further studies will be necessary for the understandings of selective activation mechanisms of Wnt signalling pathways.
Materials and methods
Rac activation assay
Activation of Rac was assayed using glutathione-S-transferase (GST)-CRIB. After HeLaS3, CHO, HEK293T, or L cells (60-mm diameter dish) were cultured in serum-free medium for 36 h, the cells were stimulated with 50 ng/ml Wnt5a for 1 h. Cells were lysed in 200 μl of buffer A (20 mM Tris–HCl [pH 7.5], 150 mM NaCl, 1 mM dithiothreitol, and protease inhibitors (1 mM phenylmethylsulfonylfluoride, 1 μg/ml leupeptin, and 1 μg/ml aprotinin)) containing 10 mM MgCl2, 1% Triton-X100, 0.1% SDS, and 20 μg of GST-CRIB. After the lysates were centrifuged at 20 000 g for 10 min, the supernatants were incubated with glutathione-Sepharose (20 μl each) for 2 h at 4°C. Glutathione-Sepharose was precipitated by the centrifugation, and the bound proteins were probed with the anti-Rac1 antibody.
Receptor binding assay
Conditioned media from HEK293 cells expressing rFz2CRD-IgG, mFz6CRD-IgG, or hLRP6N-IgG was incubated with protein A-Sepharose for 2 h. Protein A-Sepharose was collected by centrifugation, and the precipitates were washed with PBS containing 0.5% 3-[(3-cholamidopropyl) dimethylammonio] propanesulfonic acid (CHAPS) three times. When the binding activity of the Wnt proteins to LRP6 was measured, Wnt3a or Wnt5a was incubated with 2 nM hLRP6N-IgG or control IgG in 400 μl of DH10 supplemented containing 10% FBS for 2 h on ice. When the binding activity of Wnt proteins to Fzs was measured, Wnt3a or Wnt5a was incubated with 0.5 nM rFz2CRD-IgG and mFz6CRD-IgG for 2 h on ice in 400 μl of PBS containing 0.5% CHAPS for 2 h on ice. Then, hLRP6N-IgG and FzCRD-IgG were collected by centrifugation followed by washing with PBS containing 0.1% CHAPS three times. The precipitates of binding assay were analysed by SDS–PAGE and then were probed with anti-Wnt3a and anti-Wnt5a antibodies.
When the binding activity of the Wnt proteins to Fz2 expressed in intact cells was measured, HeLaS3 cells expressing FLAG-Fz2 were treated with 300 ng/ml Wnt3a in the presence of 150–600 ng/ml Wnt5a for 1 h at 4°C. The cells were lysed in NP-40 buffer (20 mM Tris–HCl [pH 8.0], 137 mM NaCl, 10% glycerol, 1% NP-40, and protease inhibitors) and centrifuged at 20 000 g for 10 min. The supernatants were incubated with anti-FLAG M2 agarose (Sigma-Aldrich, Steinheim, Germany) for 1.5 h on ice. Then FLAG-Fz2 was collected by centrifugation followed by washing with PBS containing 0.1% CHAPS three times. The precipitates of the binding assay are analysed by SDS–PAGE and then probed with anti-Wnt3a, anti-Wnt5a, and anti-FLAG antibodies.
Internalization of FLAG-Fz2
HeLaS3 cells were seeded onto 18-mm glass coverslips coated with poly-D-lysine (Sigma, St Louis, MA), and pCS2/FLAG-rFz2 was transfected into the cells using Lipofectamine 2000. At 24 h after transfection, the cells were incubated with ice-cold binding medium (DMEM containing 20 mM Hepes-NaOH [pH 7.5] and 0.1% bovine serum albumin (BSA)) for 30 min and further incubated with 100 ng/ml Wnt5a in the presence of 500 ng/ml anti-FLAG antibody for 1 h at 4°C. After unbound Wnt5a and anti-FLAG antibody were removed by washing with cold PBS three times, internalization was initiated by adding warm DMEM medium and the dishes were transferred to a heated chamber (37°C, 5% CO2) for 5–30 min. After the cells were washed three times with cold PBS to stop endocytosis, the cells were fixed for 15 min in PBS containing 4% (w/v) paraformaldehyde (PFA) and then permeabilized with PBS containing 0.2% (w/v) Triton X-100 and 2 mg/ml BSA for 20 min. In Figures 2 and 3, the cells were viewed directly using a confocal microscope (LSM510, Carl-Zeiss, Jena, Germany) to observe β-arrestin2-GFP or were also stained with anti-clathrin, anti-caveolin, or anti-HA antibody. The cells were probed with Alexa 488- or 546-conjugated anti-mouse Ig or anti-rabbit Ig (Molecular Probes, Eugene, OR) and viewed using a confocal microscope (LSM510, Carl-Zeiss, Jena, Germany) to observe FLAG-Fz2, clathrin, caveolin, or HA-Dvl2. When necessary, the HeLaS3 cells were pretreated with 50 μM MDC, 10 μg/ml chlorpromazine, 25 μg/ml Nystatin for 30 min at 37°C before Wnt5a stimulation.
Quantification of signals in fixed cells
To quantify the distribution of FLAG-Fz2, their localization was classified into three types with regard to the distribution of these proteins and the number of puncta in the cytosol. The ‘membrane' type showed clear localization to the cell surface, with a few puncta in the cytosol. The ‘membrane/puncta' type showed both localization of puncta to the cell surface and puncta in the cytosol. The ‘puncta' type showed the disappearance of the cell surface distribution, with >20 puncta in the cytosol. More than 100 cells were evaluated in each experiment.
Mice
The mice used in this study were maintained under the guidelines of the Institute of Laboratory Animal Science, Hiroshima University. All mice were C57BL/6J background. The morning of the appearance of a vaginal plug was considered as embryonic day 0.5. MEFs were prepared from E13.5 embryos using a 3T3 protocol (Todaro and Green, 1963) and were maintained in DMEM supplemented with 10% FBS.
X-gal staining
The β-galactosidase assay was carried out as described (Nakaya et al, 2005). E10.5 embryos derived from crosses of Wnt5a+/− with Wnt5a+/− BATlacZ mice were dissected in PBS. Embryos were fixed in 4% PFA in PBS for 1 h at 4°C, and stained with 5-bromo-4-chloro-3-indolyl-β-D-galactopyranoside (X-Gal) staining solution (5 mM potassium ferricyanide, 5 mM potassium ferrocyanide, 2 mM MgCl2, 0.02% NP-40, 0.1% X-gal in PBS) for 30 min at 37°C. After staining, embryos were washed with PBS three times and post-fixed with 4% PFA in PBS. Photos of stained embryos were taken using a Leica MZ6 camera equipped with an OLYMPUS DP20 digital camera system.
Statistical analysis
The experiments were performed at least three times and the results were expressed as means±s.e. Statistical analysis was performed using StatView software (SAS Institute Inc.). Differences between the data were tested for statistical significance by the t-test. P-values <0.05 were considered statistically significant.
Others
Western blotting data were representative of at least four independent experiments.
Supplementary Material
Supplementary Information
Review Process File
Acknowledgments
We are grateful to Drs X He, R Habas, RJ Lefkowitz, BO Williams, K Kaibuchi, M Negishi, C Niehrs, S Takada, G Yamada, and T Tsukiyama for donating mice, antibodies, and plasmids. We thank all of our laboratory members, especially Drs A Inoue, S Matsumoto, and K Fumoto for helpful discussions. This work was supported by Grants-in-Aid for Scientific Research and for Scientific Research on Priority Areas from the Ministry of Education, Science, and Culture, Japan (2007, 2008, 2009).
Footnotes
The authors declare that they have no conflict of interest.
References
- Bilic J, Huang YL, Davidson G, Zimmermann T, Cruciat CM, Bienz M, Niehrs C (2007) Wnt induces LRP6 signalosomes and promotes dishevelled-dependent LRP6 phosphorylation. Science 316: 1619–1622 [DOI] [PubMed] [Google Scholar]
- Bryja V, Andersson ER, Schambony A, Esner M, Bryjova L, Biris KK, Hall AC, Kraft B, Cajanek L, Yamaguchi TP, Buckingham M, Arenas E (2009) The extracellular domain of Lrp5/6 inhibits noncanonical Wnt signaling in vivo. Mol Biol Cell 20: 924–936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryja V, Cajanek L, Grahn A, Schulte G (2007a) Inhibition of endocytosis blocks Wnt signalling to β-catenin by promoting dishevelled degradation. Acta Physiol (Oxf) 190: 55–61 [DOI] [PubMed] [Google Scholar]
- Bryja V, Gradl D, Schambony A, Arenas E, Schulte G (2007b) β-arrestin is a necessary component of Wnt/β-catenin signaling in vitro and in vivo. Proc Natl Acad Sci USA 104: 6690–6695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryja V, Schambony A, Cajanek L, Dominguez I, Arenas E, Schulte G (2008) β-Arrestin and casein kinase 1/2 define distinct branches of non-canonical WNT signalling pathways. EMBO Rep 9: 1244–1250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, ten Berge D, Brown J, Ahn S, Hu LA, Miller WE, Caron MG, Barak LS, Nusse R, Lefkowitz RJ (2003) Dishevelled 2 recruits β-arrestin 2 to mediate Wnt5A-stimulated endocytosis of Frizzled 4. Science 301: 1391–1394 [DOI] [PubMed] [Google Scholar]
- Conner SD, Schmid SL (2003) Regulated portals of entry into the cell. Nature 422: 37–44 [DOI] [PubMed] [Google Scholar]
- Davidson G, Wu W, Shen J, Bilic J, Fenger U, Stannek P, Glinka A, Niehrs C (2005) Casein kinase 1γ couples Wnt receptor activation to cytoplasmic signal transduction. Nature 438: 867–872 [DOI] [PubMed] [Google Scholar]
- Fukuda T, Chen L, Endo T, Tang L, Lu D, Castro JE, Widhopf GF II, Rassenti LZ, Cantwell MJ, Prussak CE, Carson DA, Kipps TJ (2008) Antisera induced by infusions of autologous Ad-CD154-leukemia B cells identify ROR1 as an oncofetal antigen and receptor for Wnt5a. Proc Natl Acad Sci USA 105: 3047–3052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon MD, Nusse R (2006) Wnt signaling: multiple pathways, multiple receptors, and multiple transcription factors. J Biol Chem 281: 22429–22433 [DOI] [PubMed] [Google Scholar]
- Green JL, Kuntz SG, Sternberg PW (2008) Ror receptor tyrosine kinases: orphans no more. Trends Cell Biol 18: 536–544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habas R, Dawid IB, He X (2003) Coactivation of Rac and Rho by Wnt/Frizzled signaling is required for vertebrate gastrulation. Genes Dev 17: 295–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X, Saint-Jeannet JP, Wang Y, Nathans J, Dawid I, Varmus H (1997) A member of the Frizzled protein family mediating axis induction by Wnt-5A. Science 275: 1652–1654 [DOI] [PubMed] [Google Scholar]
- He X, Semënov M, Tamai K, Zeng X (2004) LDL receptor-related proteins 5 and 6 in Wnt/β-catenin signaling: arrows point the way. Development 131: 1663–1677 [DOI] [PubMed] [Google Scholar]
- Holmen SL, Robertson SA, Zylstra CR, Williams BO (2005) Wnt-independent activation of β-catenin mediated by a Dkk1-Fz5 fusion protein. Biochem Biophys Res Commun 328: 533–539 [DOI] [PubMed] [Google Scholar]
- Ishitani T, Kishida S, Hyodo-Miura J, Ueno N, Yasuda J, Waterman M, Shibuya H, Moon RT, Ninomiya-Tsuji J, Matsumoto K (2003) The TAK1-NLK mitogen-activated protein kinase cascade functions in the Wnt-5a/Ca2+ pathway to antagonize Wnt/β-catenin signaling. Mol Cell Biol 23: 131–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh T, Koshiba S, Kigawa T, Kikuchi A, Yokoyama S, Takenawa T (2001) Role of the ENTH domain in phosphatidylinositol-4,5-bisphosphate binding and endocytosis. Science 291: 1047–1051 [DOI] [PubMed] [Google Scholar]
- Kawano Y, Kypta R (2003) Secreted antagonists of the Wnt signalling pathway. J Cell Sci 116: 2627–2634 [DOI] [PubMed] [Google Scholar]
- Kikuchi A, Yamamoto H (2008) Tumor formation due to abnormalities in the β-catenin-independent pathway of Wnt signaling. Cancer Sci 99: 202–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi A, Yamamoto H, Sato A (2009) Selective activation mechanisms of Wnt signaling pathways. Trends Cell Biol 19: 119–129 [DOI] [PubMed] [Google Scholar]
- Kim GH, Han JK (2007) Essential role for β-arrestin 2 in the regulation of Xenopus convergent extension movements. EMBO J 26: 2513–2526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishida S, Yamamoto H, Kikuchi A (2004) Wnt-3a and Dvl induce neurite retraction by activating Rho-associated kinase. Mol Cell Biol 24: 4487–4501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurayoshi M, Yamamoto H, Izumi S, Kikuchi A (2007) Post-translational palmitoylation and glycosylation of Wnt-5a are necessary for its signalling. Biochem J 402: 515–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefkowitz RJ, Shenoy SK (2005) Transduction of receptor signals by β-arrestins. Science 308: 512–517 [DOI] [PubMed] [Google Scholar]
- Logan CY, Nusse R (2004) The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol 20: 781–810 [DOI] [PubMed] [Google Scholar]
- Lustig B, Jerchow B, Sachs M, Weiler S, Pietsch T, Karsten U, van de Wetering M, Clevers H, Schlag PM, Birchmeier W, Behrens J (2002) Negative feedback loop of Wnt signaling through upregulation of conductin/axin2 in colorectal and liver tumors. Mol Cell Biol 22: 1184–1193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maretto S, Cordenonsi M, Dupont S, Braghetta P, Broccoli V, Hassan AB, Volpin D, Bressan GM, Piccolo S (2003) Mapping Wnt/β-catenin signaling during mouse development and in colorectal tumors. Proc Natl Acad Sci USA 100: 3299–3304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikels AJ, Nusse R (2006) Purified Wnt5a protein activates or inhibits β-catenin-TCF signaling depending on receptor context. PLoS Biol 4: 570–582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashima S, Morinaka K, Koyama S, Ikeda M, Kishida M, Okawa K, Iwamatsu A, Kishida S, Kikuchi A (1999) Small G ptrotein Ral and its downstream molecules regulate endocytosis of EGF and insulin receptors. EMBO J 18: 3629–3642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakaya MA, Biris K, Tsukiyama T, Jaime S, Rawls JA, Yamaguchi TP (2005) Wnt3a links left-right determination with segmentation and anteroposterior axis elongation. Development 132: 5425–5436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oishi I, Suzuki H, Onishi N, Takada R, Kani S, Ohkawara B, Koshida I, Suzuki K, Yamada G, Schwabe GC, Mundlos S, Shibuya H, Takada S, Minami Y (2003) The receptor tyrosine kinase Ror2 is involved in non-canonical Wnt5a/JNK signalling pathway. Genes Cells 8: 645–654 [DOI] [PubMed] [Google Scholar]
- Palamidessi A, Frittoli E, Garre M, Faretta M, Mione M, Testa I, Diaspro A, Lanzetti L, Scita G, Di Fiore PP (2008) Endocytic trafficking of Rac is required for the spatial restriction of signaling in cell migration. Cell 134: 135–147 [DOI] [PubMed] [Google Scholar]
- Pan W, Choi SC, Wang H, Qin Y, Volpicelli-Daley L, Swan L, Lucast L, Khoo C, Zhang X, Li L, Abrams CS, Sokol SY, Wu D (2008) Wnt3a-mediated formation of phosphatidylinositol 4,5-bisphosphate regulates LRP6 phosphorylation. Science 321: 1350–1353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry SJ, Lefkowitz RJ (2002) Arresting developments in heptahelical receptor signaling and regulation. Trends Cell Biol 12: 130–138 [DOI] [PubMed] [Google Scholar]
- Smit L, Baas A, Kuipers J, Korswagen H, van de Wetering M, Clevers H (2004) Wnt activates the Tak1/Nemo-like kinase pathway. J Biol Chem 279: 17232–17240 [DOI] [PubMed] [Google Scholar]
- Takada S, Stark KL, Shea MJ, Vassileva G, McMahon JA, McMahon AP (1994) Wnt-3a regulates somite and tailbud formation in the mouse embryo. Genes Dev 8: 174–189 [DOI] [PubMed] [Google Scholar]
- Todaro GJ, Green H (1963) Quantitative studies of the growth of mouse embryo cells in culture and their development into established lines. J Cell Biol 17: 299–313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topol L, Jiang X, Choi H, Garrett-Beal L, Carolan PJ, Yang Y (2003) Wnt-5a inhibits the canonical Wnt pathway by promoting GSK-3-independent β-catenin degradation. J Cell Biol 162: 899–908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres MA, Yang-Snyder JA, Purcell SM, DeMarais AA, McGrew LL, Moon RT (1996) Activities of the Wnt-1 class of secreted signaling factors are antagonized by the Wnt-5A class and by a dominant negative cadherin in early Xenopus development. J Cell Biol 133: 1123–1137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veeman MT, Axelrod JD, Moon RT (2003) A second canon. Functions and mechanisms of β-catenin-independent Wnt signaling. Dev Cell 5: 367–377 [DOI] [PubMed] [Google Scholar]
- Wang HY, Liu T, Malbon CC (2006) Structure-function analysis of Frizzleds. Cell Signal 18: 934–941 [DOI] [PubMed] [Google Scholar]
- Westfall TA, Brimeyer R, Twedt J, Gladon J, Olberding A, Furutani-Seiki M, Slusarski DC (2003) Wnt-5/pipetail functions in vertebrate axis formation as a negative regulator of Wnt/β-catenin activity. J Cell Biol 162: 889–898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Tu X, Joeng KS, Hilton MJ, Williams DA, Long F (2008) Rac1 activation controls nuclear localization of β-catenin during canonical Wnt signaling. Cell 133: 340–353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi TP, Bradley A, McMahon AP, Jones S (1999) A Wnt5a pathway underlies outgrowth of multiple structures in the vertebrate embryo. Development 126: 1211–1223 [DOI] [PubMed] [Google Scholar]
- Yamamoto H, Kishida S, Uochi T, Ikeda S, Koyama S, Asashima M, Kikuchi A (1998) Axil, a member of the Axin family, interacts with both glycogen synthase kinase-3β and β-catenin and inhibits axis formation of Xenopus embryos. Mol Cell Biol 18: 2867–2875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto H, Komekado H, Kikuchi A (2006) Caveolin is necessary for Wnt-3a-dependent internalization of LRP6 and accumulation of β-catenin. Dev Cell 11: 213–223 [DOI] [PubMed] [Google Scholar]
- Yamamoto H, Sakane H, Yamamoto H, Michiue T, Kikuchi A (2008) Wnt3a and Dkk1 regulate distinct internalization pathways of LRP6 to tune the activation of β-catenin signaling. Dev Cell 15: 37–48 [DOI] [PubMed] [Google Scholar]
- Yu A, Rual JF, Tamai K, Harada Y, Vidal M, He X, Kirchhausen T (2007) Association of dishevelled with the clathrin AP-2 adaptor is required for frizzled endocytosis and planar cell polarity signaling. Dev Cell 12: 129–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng X, Huang H, Tamai K, Zhang X, Harada Y, Yokota C, Almeida K, Wang J, Doble B, Woodgett J, Wynshaw-Boris A, Hsieh JC, He X (2008) Initiation of Wnt signaling: control of Wnt coreceptor Lrp6 phosphorylation/activation via frizzled, dishevelled and axin functions. Development 135: 367–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng X, Tamai K, Doble B, Li S, Huang H, Habas R, Okamura H, Woodgett J, He X (2005) A dual-kinase mechanism for Wnt co-receptor phosphorylation and activation. Nature 438: 873–877 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information
Review Process File