Abstract
Regulated activity of the retrograde molecular motor, cytoplasmic dynein, is crucial for multiple biological activities, and failure to regulate this activity can result in neuronal migration retardation or neuronal degeneration. The activity of dynein is controlled by the LIS1–Ndel1–Nde1 protein complex that participates in intracellular transport, mitosis, and neuronal migration. These biological processes are subject to tight multilevel modes of regulation. Palmitoylation is a reversible posttranslational lipid modification, which can dynamically regulate protein trafficking. We found that both Ndel1 and Nde1 undergo palmitoylation in vivo and in transfected cells by specific palmitoylation enzymes. Unpalmitoylated Ndel1 interacts better with dynein, whereas the interaction between Nde1 and cytoplasmic dynein is unaffected by palmitoylation. Furthermore, palmitoylated Ndel1 reduced cytoplasmic dynein activity as judged by Golgi distribution, VSVG and short microtubule trafficking, transport of endogenous Ndel1 and LIS1 from neurite tips to the cell body, retrograde trafficking of dynein puncta, and neuronal migration. Our findings indicate, to the best of our knowledge, for the first time that Ndel1 palmitoylation is a new mean for fine-tuning the activity of the retrograde motor cytoplasmic dynein.
Keywords: dynein, intracellular transport, Ndel1, neuronal migration, palmitoylation
Introduction
Regulated activity of the retrograde molecular motor, cytoplasmic dynein, is crucial for multiple biological activities ranging from mitosis to long-range neuronal transport. For example, mice lacking cytoplasmic dynein heavy chain exhibit early embryonic lethality (Harada et al, 1998), whereas abnormal neuronal transport is a common theme underlying pathogenesis of neurodegenerative diseases (for reviews, see Bruijn et al, 2004; Chevalier-Larsen and Holzbaur, 2006; Reiner et al, 2006; Stokin and Goldstein, 2006).
Dynein, a large multisubunit complex, belongs to one of the two families of microtubule motor proteins (for reviews, see Goldstein and Yang, 2000; Vallee et al, 2004). All dyneins contain the heavy chain, for ATPase and motor activities, and accessory subunits including light intermediate and light chains. The activity of dynein is regulated at multiple levels. For example, specific combinations of dynein isoforms may contribute to cargo specificity (Ha et al, 2008). Additional accessory proteins complexes, such as dynacin, allow dynein to bind a variety of cargoes, regulate dynein motor activity directly, and enhance dynein processivity (for reviews, see Schroer, 2004; Vallee et al, 2004). Finally, accumulating evidence suggest that the LIS1-containing protein complex is also involved in regulating cytoplasmic dynein motor activity. Deletions in the LIS1 gene result in a severe human neuronal migration deficit known as lissencephaly (Reiner et al, 1993; Lo Nigro et al, 1997; Pilz et al, 1998). Protein dosage in this locus is crucial because both decreased and increased LIS1 protein levels affect brain development both in humans and in mice (Reiner et al, 1993; Hirotsune et al, 1998; Cahana et al, 2001; Bi et al, 2009).
LIS1 has been found to interact with several subunits of dynein and dynactin (Faulkner et al, 2000; Sasaki et al, 2000; Smith et al, 2000; Tai et al, 2002), as well as with the microtubule plus end-binding protein CLIP-170 (Coquelle et al, 2002), suggesting that it modulates dynein activity in more than one way. LIS1 interacts tightly with the evolutionary conserved NUDE proteins (Efimov and Morris, 2000). In mammals there are two NudE homologs: Nde1 and its related paralog Ndel1. Nde1 interacts with LIS1, several centrosomal proteins, and dynein light and intermediate chains (Feng et al, 2000; Feng and Walsh, 2004; Hirohashi et al, 2006a, 2006b; Stehman et al, 2007). Ndel1 is found in complex with LIS1, and dynein heavy and intermediate chains (Sasaki et al, 2000; Niethammer et al, 2000a). LIS1, Ndel1, and Nde1 all participate in the dynein-mediated processes of intracellular transport (Liu et al, 2000; Sasaki et al, 2000; Smith et al, 2000; Niethammer et al, 2000b; Liang et al, 2004; Yamada et al, 2008), mitosis (Faulkner et al, 2000; Yan et al, 2003; Feng and Walsh, 2004; Tsai et al, 2005; Guo et al, 2006; Liang et al, 2007; Stehman et al, 2007; Vergnolle and Taylor, 2007), and neuronal migration (Hirotsune et al, 1998; Feng et al, 2000; Cahana et al, 2001; Feng and Walsh, 2004; Shu et al, 2004; Sasaki et al, 2005; Tsai et al, 2005, 2007; Grabham et al, 2007). These complex biological processes are subject to tight multilevel modes of regulation, in which reversible posttranslational modifications have an important role. For example, phosphorylation of Ndel1 is required for neuronal migration and function (Niethammer et al, 2000b; Toyo-oka et al, 2003; Taya et al, 2007), as well as for proper cell-cycle progression (Toyo-Oka et al, 2005; Mori et al, 2007).
In vitro studies have demonstrated that LIS1 can stimulate the ATPase activity of cytoplasmic dynein (Mesngon et al, 2006; Yamada et al, 2008). Furthermore, LIS1 suppressed the motility of cytoplasmic dynein on microtubules, whereas its interacting protein Ndel1 released the blocking effects of LIS1 (Yamada et al, 2008).
In vivo, injection of anti-LIS1 antibodies inhibited the anterograde transport of dynein to the tips of dorsal root ganglia neurons, thus suggesting that LIS1 may affect the activity of the anterograde motor kinesin in relation to dynein transport (Yamada et al, 2008).
Taking into consideration that reversible posttranslational modifications are likely to participate in the regulation of dynamic processes, we tested whether any of the LIS1–Ndel1–Nde1 complex proteins undergo palmitoylation. Palmitoylation is a reversible posttranslational modification, which has been shown to have an important role in the regulation of protein trafficking (Linder and Deschenes, 2007), and in the nervous system (Kang et al, 2008; for reviews, see Dunphy and Linder, 1998; Resh, 1999; El-Husseini Ael and Bredt, 2002; Washbourne, 2004). We found that both Ndel1 and Nde1 undergo palmitoylation on a conserved cysteine residue. We further identified the three palmitoylation enzymes DHHC2, DHHC3, and DHHC7, which are involved in this modification. Palmitoylation of Ndel1 reduced its interaction with cytoplasmic dynein resulting in reduced dynein activity measured by functional assays, including Golgi distribution, microtubule transport, ER-to-Golgi transport, transport to neurite tips, retrograde trafficking of dynein puncta in primary neurons, and aberrant pyramidal neuronal migration to the developing cortex. Our results suggest a new mode of regulating the activity of the molecular motor, cytoplasmic dynein.
Results
Nde1 and Ndel1 are palmitoylated proteins
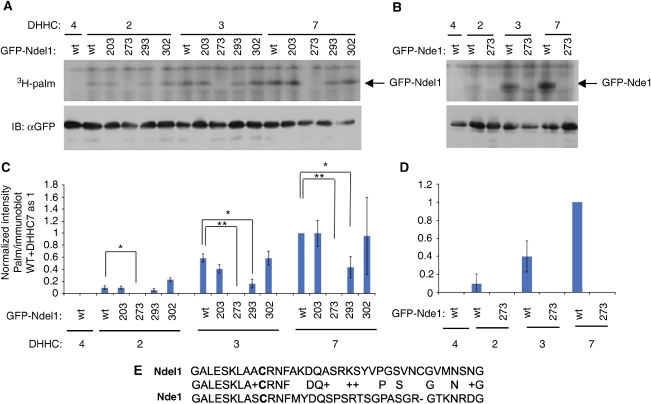
We tested whether Ndel1/Nde1 or LIS1 undergo palmitoylation. S-palmitoylation is a reversible posttranslational modification, which involves a thioester-bound addition of the fatty acid palmitate to cysteine residues (for review, see Linder and Deschenes, 2007). In mammals there are 23 known palmitoylating enzymes called palmitoyl-acyl transferases (PAT) (Fukata et al, 2004) that share in common an Asp-His-His-Cys (DHHC)-cysteine rich domain that is essential for the catalytic activity. The 23 PATs (DHHC 1–23) were transfected to HEK293 cells and assayed (Fukata et al, 2006) for their capability to palmitoylate Ndel1 or Nde1 (Figure 1A and B, Supplementary Figure S1 is a longer exposure of 1B). This assay involves metabolic labelling of tritiated-palmitate to HEK293 cells transfected with the enzymes and the tested proteins. The results indicated that both proteins are palmitoylated, and the enzymes that are capable of palmitoylating Ndel1 and Nde1 are DHHC2, DHHC3, and DHHC7. Minor palmitoylation was noted with DHHC21. LIS1 was not palmitoylated using the same assay (data not shown). We next explored whether Ndel1 and Nde1 are palmitoylated in vivo in primary cortical neurons using a sensitive assay (Drisdel and Green, 2004; Drisdel et al, 2006). This assay involves an exchange of the endogenous palmitate-linked group with a biotin-labelled reagent. This reaction requires hydroxylamine-mediated cleavage of the palmitoyl-thioester bond, followed by a specific labelling with a sulfhydryl-reactive biotinylated reagent that is later identified by avidin-HRP (known as ABE, acyl-biotinyl exchange method). Our results indicate that both Ndel1 and Nde1 are palmitoylated in vivo in mouse cortical neurons (Figure 1C and D). In both cases, avidin–HRP labelled immunoprecipitated only Ndel1 (Figure 1C) or Nde1 (Figure 1D) when hydroxylamine hydrolysed the thioester as a neutral pH base, but not when it was not added. It has been previously demonstrated that a band shift of PSD95 can be noted after the addition of hydroxylamine (Fukata et al, 2004; Figure 1E). We noted a similar band shift of Ndel1 (Figure 1F), thus suggesting that a noticeable proportion of Ndel1 population is palmitoylated in CAD cells. Determination of the half-life of GFP–Ndel1 palmitoylation in an inducible DHHC7 HEK293 cell line was conducted using metabolic labelling of the cells with 17-octadecynoic acid (17-ODYA) overnight, followed by a chase with palmitic acid (Figure 1G and H). The half-life of Ndel1 palmitoylation was estimated to be 1.75 h (R2=0.985). Ndel1 is a very stable protein and the protein half-life was estimated to be 24 h (Figure 1H and Supplementary data). In primary cortical neurons the half-life of Ndel1 palmitoylation was estimated to be 2.3 h, as determined by the relative reduction of the signal after incubation of the cortical cultures with the palmitate analogue 2-bromopalmitate (2-Br), which inhibits palmitoylation (Webb et al, 2000; Figure 1I and J).
Figure 1.
Ndel1 and Nde1 are palmitoylated proteins. (A, B) Radioactive palmitate was incorporated in HEK293 cells co-transfected with (A) wild-type GFP–Ndel1 or (B) GFP–Nde1 and each of the palmitoylation enzymes. (C, D) In vivo palmitoylation of Ndel1 and Nde1 using the ABE (acyl-biotinyl exchange) method. Brain proteins from P7 mice (C, D) were extracted in a buffer containing N-ethyl malamide. (C) Ndel1 or (D) Nde1 were immunoprecipitated using anti-Ndel1 or anti-Nde1 antibodies. Palmitate was removed by addition of hydroxylamine (controls without hydroxylamine). BMCC-Biotin binding to the free thioester group enabled detection of the palmitoylated population of proteins by avidin–HRP. The positions of palmitoylated Ndel1 or Nde1 are indicated by small arrows. The western blots using anti-Ndel1, anti-DIC, or Nde1 antibodies indicated the position of Ndel1 (C, top), and similar amount of immunoprecipitated DIC (C, bottom) or Nde1 (D, bottom) proteins. (E, F) A band shift is noted when (E) PSD95 from brain lysate or (F) Ndel1 from CAD cell lysate are separated on SDS–PAGE after addition of neutral pH hydroxylamine (without HA−, with HA+,). Short arrows indicate the position of the nontreated bands, whereas the longer arrows indicate the positions of the hydroxylamine-treated bands. (G–K) Determination of Ndel1 palmitoylation half-life. (G, H) GFP–Ndel1 transfected in DHHC7 HEK293 inducible cell line, was metabolically labelled with 17-ODYA, followed by chase of palmitic acid. Ndel1 was immunoprecipitated and subjected to click chemistry, separated by SDS–PAGE, in-gel fluorescence was monitored (top panel), and the fluorescence was removed after HA treatment (middle panel). Similar amounts of Ndel1 were immunoprecipitated (lower panel). (H) Palmitoylation and protein half-life linear regression. The in-gel fluorescence intensity was normalized according to the amount of immunoprecipitated Ndel1 and plotted (data points are triangles and the linear regression is solid black line). Ndel1 protein half-life data (described in supplementary data) was ploted as well (data points are squares ±s.e.m. and the linear regression is a dashed line). Ndel1 is a very stable protein with an estimated half-life of about 24 h; Ndel1 palmitoylation half-life is about 1.7 h. (I, J) Half-life in cortical neurons. Cortical neurons were cultured in the presence of 2-bromopalmitate (2-Br) and palmitoylation at the indicated time points were determined by the ABE method. (J) The relative intensities after normalization with intensity of the immunoprecipitated protein were plotted against time and the half-life line is indicated.
Next, the cysteine residue(s) undergoing S-palmitoylation were identified. Four Ndel1 cysteine residues were individually mutated (C203S, C273S, C293S, and C302S), and the mutated proteins were assayed for their capability to undergo palmitoylation (Figure 2A). The mouse Nde1 protein contains only one conserved cysteine residue, and it was mutated as well (C273S) (Figure 2B). The palmitoylation of both Ndel1 and Nde1 C273S mutant proteins was statistically significantly reduced using either DHHC2, DHHC3, or DHHC7, suggesting that this conserved site is the major site of palmitoylation on both proteins (Figure 2C and D). An alternative explanation will be that this site is required for palmitoylation of other sites. We conclude that both Ndel1 and Nde1 can be palmitoylated in vitro and in vivo by DHHC2, 3, and 7, and that C273 is important for palmitoylation.
Figure 2.
Identification of Ndel1 and Nde1 palmitoylated site. (A, B) Ndel1 (A) and Nde1 (B) are palmitoylated on cys 273 by DHHC2, 3, and 7. HEK293 cells co-transfected with wild-type GFP–Ndel1, or GFP–Nde1 or mutated on the indicated cysteines together with the indicated palmitoylation enzymes DHHC2, 3, and 7, were labelled with [3H]-palmitate. The cell lysates separated by SDS–PAGE were subjected to fluorography and expression levels of GFP–proteins were monitored by immunoblotting with an anti-GFP antibody. (C, D) The autoradiograms in (A, B) were subjected to quantification demonstrating that when Cys 273 is mutated, palmitoylation decreased. (E) Sequence comparison (BLAST) between Ndel1 and Nde1 of the amino acids surrounding Cys 273.
Palmitoylation of Ndel1 affects its interaction with cytoplasmic dynein
Palmitoylation may affect numerous processes, including protein–protein interactions. Our analysis depicted a single lipid modification that is not sufficient for membrane attachment (for review, see Resh, 2006). Indeed, Ndel1 palmitoylation did not grossly increase its relative proportion in the membrane as the relative proportion of wild type or C273S Ndel1 localization in the membrane or in the cytosol did not change after expression of the palmitoylation enzyme. Furthermore, the relative proportion of endogenous or transfected Ndel1 did not significantly change after expression of wild-type or mutated DHHC7 (Supplementary Figures S2 and S3). Nevertheless, we do not exclude the possibility of fine changes in the interaction of Ndel1 and some membranal subpopulations. Ndel1 is known to complex with LIS1, cytoplasmic dynein, and neurofilaments. The main palmitoylated cysteine in Ndel1 resides within the mapped interaction domain with cytoplasmic dynein in the C-terminal part of this protein (Sasaki et al, 2000), whereas the interaction with LIS1 has been localized to a different region within the coiled-coil domain (Derewenda et al, 2007; schematically presented in Figure 3A).
Figure 3.
The effect of Ndel1 or Nde1 palmitoylation on their interaction with dynein intermediate chain. (A) Schematic presentation of Ndel1 and its binding domains. The coiled-coil domain (amino acids 10–160, light grey) contains the mapped binding domain with LIS1 (amino acids 103–153, blue). The dynein-binding domain (amino acids 256–291, yellow) contains the palmitoylated site C273. (B) HEK293 cells were transiently transfected with GFP–Ndel1wt/C273S with or without HA–DHHC7. Ndel1 or DIC were immunoprecipitated. The interaction between Ndel1 and DIC is reduced when Ndel1 is palmitoylated (second lane from the left side). In the absence of the palmitoylation enzyme (DHHC7) or when Ndel1 cannot undergo palmitoylation (C273S), the interaction is increased. (C) Introduction of DHHC7 DN or Ndel1 C273S reduces Ndell palmitoylation as evident by 3H-palmitate incorporation. Similar amounts of the proteins were expressed evident by anti-GFP western blot. (D) Reduction in Ndel1 palmitoylaiton increases its interaction with DIC. More Ndel1 is co-immunoprecipitated with DIC after 2-Br treatment (upper row), similar amounts of DIC were immunoprecipitated and similar amount of Ndel1 were present in the lysates. Nevertheless, Ndel1 palmitoylation was decreased after 2-Br treatment evident by in gel fluorescence. The induction of DHHC7 in the HEK293 cell line was evident by western blot with the anti-HA tag. (E) The interaction between Nde1 and DIC is unaffected by palmitoylation. However, the interaction of mutant Nde1 (C273S) with DIC is diminished. The result is seen by both reciprocal immunoprecipitations: IP of DIC (top panel) and IP of Nde1 (bottom panel).
We used immunoprecipitation to test whether Ndel1 palmitoylation affects its interaction with cytoplasmic dynein or with LIS1 in transfected cells. Palmitoylated Ndel1 (co-expressed with its palmitoylation enzyme DHHC7) exhibited reduced interaction with the cytoplasmic dynein complex (Figure 3B) when dynein was co-immunoprecipitated with Ndel1. In contrast, the unpalmitoylatable C273S-mutated version did not exhibit the observed reduction in interaction with the dynein complex, confirming that this effect is due to palmitoylation of Ndel1. The interaction between Ndel1 and LIS1 was not affected by palmitoylation, or by the C273 point mutation. Using metabolic labelling and click chemistry, we could demonstrate that Ndel1 palmitoylation is reduced in the presence of DHHC7-DN or when C273 is mutated (Figure 3C). Similar reduction in the interaction between Ndel1 and DIC was noted when Ndel1 was co-immunoprecipitated with DIC. In this case palmitoylation of Ndel1 was reduced in HEK293 cells expressing inducible DHHC7 after treatment with the well-characterized inhibitor of palmitoylation 2-Br (Jennings et al, 2009; Figure 3D). Unlike Ndel1, Nde1 co-immunoprecipitation with the dynein complex was not affected by palmitoylation (Figure 3E). Similar amounts of immunoprecipitated Nde1 in the presence or absence of the palmitoylation enzyme DHHC7 were observed. Nevertheless, cys 273 has an important role in mediating the binding of Nde1 to cytoplasmic dynein, as Nde1 C273S exhibited a 5.6-fold reduced interaction with DIC (paired Student's t-test, P=0.0004) regardless of the presence of the palmitoylation enzyme (Figure 3E). The difference between the behaviour of Nde1 and Ndel1 in that respect may be due to slight differences in the sequence of the amino acids surrounding C273 (Figure 2E). Amino acids in that region may also contribute to the interaction with dynein. Structural analysis of the complexes involving Nde1 or Ndel1 and dynein are likely to provide better insight into this issue. The interaction between Nde1 and LIS1 also was not affected by palmitoylation, and not by introduction of the C273S mutation (data not shown). Therefore, despite the high sequence similarity (72.6%) between Ndel1 and Nde1, our results uncovered a clear functional difference between these two paralog proteins.
The idea that Nde1 and Ndel1 have non-overlapping functions in regard to palmitoylation-dependent dynein interactions, prompted us to carry out a phylogenetic analysis for Nde1 and Ndel1 from invertebrates and vertebrates (Supplementary Figure S4). The analysis revealed a clear distinction between the groups of Nde1 and Ndel1 proteins in vertebrates, whereas invertebrates (represented here by two different species of Drosophila) contain a single Nde1 protein. Interestingly, the possible divergence between the Protostomia (invertebrates) and the Deuterostomia (vertebrates) was estimated to occur 700–520 Mya (Erwin and Davidson, 2002) or between 580 and 520 Mya (Halanych, 2004). Therefore, it is likely that as sequences diverged, unique functionalities were adopted for the two proteins.
Palmitoylation is a reversible modification; therefore, a plausible hypothesis is that the differential interaction of palmitoylated versus unpalmitoylated Ndel1 with cytoplasmic dynein is a new switch mechanism to modulate the activity of the molecular motor. This hypothesis was tested directly by monitoring the activity of cytoplasmic dynein using several cellular assays using different cells. The levels of Ndel1 and the palmitoylation enzymes vary in the different cells (Supplementary Figure S5). Ndel1 is highly expressed in the rat brain and in CAD cells; it is expressed at moderate levels in NIH3T3 cells and at very low levels in COS7 cells and HEK293 cells. DHHC7 expression is very low in NIH3T3 cells and HEK293 cells, and is better expressed in CAD, COS7, and brain lysate, whereas DHHC3 is expressed at high levels in NIH3T3 cells. Therefore, in some cases, we needed to transfect both Ndel1 and the palmitoylation enzyme to observe an effect. We demonstrated that the Ndel1 is palmitoylated when it is co-transfected with the palmitoylation enzyme and its palmitoylation is significantly reduced when Ndel1 (C273S) was co-transfected with the wild-type enzyme or when wild-type Ndel1 was co-transfected with the DN-enzyme in HEK 293 cells and COS7 cells (Figure 3C and Supplementary Figure S6).
Cytoplasmic dynein and Golgi structure
Several lines of evidence suggest that the perinuclear position of the Golgi is driven by cytoplasmic dynein-mediated transport. In cells lacking cytoplasmic dynein heavy chain (DHC), the Golgi is dispersed (Harada et al, 1998) and injection of anti-DHC2 antibody also led to dispersion of the Golgi complex (Vaisberg et al, 1996). In addition, depletion of cytoplasmic dynein prevented the centrosomal localization of exogenously applied Golgi-derived vesicles in partially permeabilized cells (Corthesy-Theulaz et al, 1992). Finally, overexpression of the dynamitin subunit of the dynactin complex, which dissociates the dynein complex, also led to the dispersion of the Golgi complex among other cellular phenotypes (Burkhardt et al, 1997). Similarly, the activity of Ndel1 and LIS1 in regulating dynein-mediated perinuclear clustering of the Golgi apparatus was demonstrated in Lis1- and Ndel1-null mouse embryonic fibroblasts (Sasaki et al, 2005). In tissue culture cells, this activity has been demonstrated using either Ndel1 mutants defective in LIS1 or DHC binding or by the silencing of Ndel1 using siRNA (Liang et al, 2004).
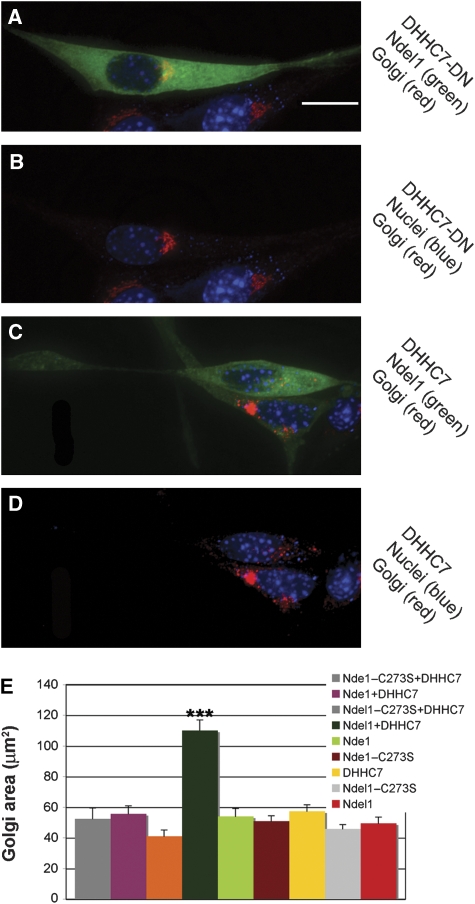
The compactness of the juxtanuclear Golgi complex was measured in NIH3T3 cells by immunostaining with anti-Mannosidase II (an integral Golgi enzyme) antibodies (Figure 4). Palmitoylated Ndel1 significantly induced Golgi dispersion (Figure 4C–E) without any obvious effects on the microtubule cytoskeleton (data not shown). In addition, a portion of Ndel1 clearly localized to the Golgi (Figure 4A and C). In contrast, when endogenous palmitoylation is reduced by expression of DHHC7-DN, the size of the Golgi is not changed (Figure 4A, B and E). Similarly, palmitoylated Nde1 or expression of individual components Ndel1, Nde1, or the palmitoylation enzyme DHHC7 alone had no effect on Golgi distribution (Figure 4E). These results are consistent with our previous findings, suggesting that strong interaction of unpalmitoylated Ndel1, but not Nde1, with cytoplasmic dynein is required for the perinuclear positioning of the Golgi.
Figure 4.
Palmitoylated Ndel1 reduces cytoplasmic dynein activity resulting in dispersed Golgi. NIH3T3 cells were transfected with all combinations of GFP–Ndel1 (wild type or C273S) with DHHC7 WT or C160S mutant (DHHC7-DN), or combinations of GFP–Nde1(wild type or C273S) and/or DHHC7 (wild type or DN). (A, B) When Ndel1 palmitoylation is low in the presence of DHHC7-DN, (B) the Golgi immunostained with anti-mannosidase II antibodies, is compact (A, merged picture). (C, D) When Ndel1 is palmitoylated (D), in the presence of DHHC7, the Golgi is more distributed (D, merge C). (E) The Golgi area in the indicated transfected cells was measured and subjected to statistical analysis. Co-transfection of Ndel1 and the palmitoylation enzyme DHHC7 increased the area of the Golgi (ANOVA, P<0.0001, n=21). Error bars indicate s.e.m. value. Size bar is 15 μm.
Transport of short microtubules mediated by cytoplasmic dynein
Transport to the cell periphery of short microtubule fragments synthesized at the centrosome is mediated by cytoplasmic dynein (Ahmad et al, 1998; Abal et al, 2002). The effect of Ndel1 palmitoylation status on this transport was assayed by following short microtubules nucleated at the centrosome in a ‘pulse-chase' regime. After treatment with nocodazole, which disassembled existing microtubules, nocodazole was washed away and cells were recovered for 3 min to allow reassembly of microtubules. Finally, cells were treated with low concentrations of vinblastine, which inhibits microtubule polymerization, but does not cause micortubule disassembly (Ahmad and Baas, 1995). Our results indicate that when Ndel1 is palmitoylated, less microtubule fragments reached the cell periphery (Supplementary Figure S7A–C, H). In addition, under these experimental conditions, substantial peri-surface fluorescence is evident for the Ndel1. Moreover, addition of unpalmitoylated Ndel1 to these cells enhanced cytoplasmic dynein activity, indicated by the marked increase in the level of microtubule fragments that reach the cell periphery (Shu et al, 2004; Supplementary Figure S7G). However, transfection of Nde1 did not affect this activity (Shu et al, 2004; Supplementary Figure S7I), neither in the presence of the palmitoylation enzyme, nor in case of cys 273 mutation (Supplementary Figure S7D–F, I). It is noteworthy that when C273 Ndel1 palmitoylation site is mutated, its activity did not differ from that of the unpalmitoylated wild-type Ndel1 (Supplementary Figure S7H). We concluded that palmitoylation of Ndel1 reduced the efficacy of dynein-mediated transport of short microtubules to the cell periphery.
Dynein-mediated VSVG transport
The viral glycoprotein VSVG is synthesized in the ER and then transported towards the Golgi complex along microtubules by using the activity of the retrograde motor cytoplasmic dynein (Presley et al, 1997). It has been reported that the temperature-sensitive fluorescent VSVG is rapidly translocated into the Golgi region after a 40–32°C temperature shift. Activation of cytoplasmic dynein is expected to result in a compact fluorescent VSVG localization during a shorter time period. This was clearly visualized in cells transfected with Ndel1 versus control cells (Supplementary Figure S8A–C versus D–F, and G). In this system, palmitoylation had also a clear effect on the activity of the retrograde motor (Supplementary Figure S9). When Ndel1 is palmitoylated, the rate in which the mutant VSVG protein is translocated to the Golgi is markedly reduced (Supplementary Figure S9A–C, and G). In contrast, Ndel1 mutated in the main palmitoylation site displayed a significant increase in VSVG translocation (Supplementary Figure S9G). In conclusion, palmitoylated Ndel1 reduced the activity of the retrograde motor protein cytoplasmic dynein, and thereby inhibited ER-to-Golgi mobilization of VSVG.
Transport to neurite tips and retrograde trafficking of dynein puncta
The localization of LIS1 and Ndel1 at the tips of neurites is determined by a balance between molecular motors working in different orientations: cytoplasmic dynein and kinesin (Taya et al, 2007). To test whether palmitoylation of Ndel1 affects the balance between cytoplasmic dynein and kinesin activities, we quantified the relative immunoreactivity fluorescence intensity of Ndel1, LIS1, and Nde1 in the neurite tips of differentiated CAD cells in comparison with the relative immunoreactivity fluorescence intensity found at the connection of the neurite to the cell body. Most differentiated CAD cells exhibited relatively higher intensities of LIS1 and Ndel1 at the neurite tips 57±0.07% (n=49) and 84±0.05% (n=39), respectively (Figure 5A, C and I). The ddition of the palmitoylation inhibitor 2-Br, significantly reduced the relative localization of LIS1 and Ndel1 at the neurite tips (8.5±3.3% (n=70, P<0.0001) and 62±7.3% (n=45, P=0.02) respectively, Figure 5B, D and I). Therefore, as expected, this treatment led to enhanced Ndel1 and LIS1 mobilization in the retrograde direction towards the cell body. In contrast, the localization of Nde1 at the tips was not altered after the inhibition of palmitoylation (data not shown).
Figure 5.
Ndel1 palmitoylation shifts the cellular localization of LIS1 and Ndel1 towards neurite tips. Below each of the neurite images, there is line histogram demonstrating the relative fluorescence intensity of the immunostained protein along the neurite. Differentiated CAD cells were treated with methanol (as a control) (A, C) or with 7.5 mM 2-Br (B, D) for 3.5 h, and then fixed and stained with anti-LIS1 antibodies (A, B) or anti-Ndel1 (C, D). WT or the dominant-negative form of GFP–DHHC7-transfected CAD cells were stained with anti-Ndel1 antibodies (E, F). WT or the C273S mutated form of GFP–Ndel1-transfected CAD cells were stained with anti-LIS1 antibodies (G, H). (I), The percentage of neurites exhibiting relatively higher intensity of fluorescence at the tip was plotted and subjected to Student's t-test, significance: *P<0.05, **P<0.01, ***P<0.005.
Overexpression of the dominant-negative form of GFP–DHHC7 significantly reduced the relative position of Ndel1 at the neurite tips, from 51±6.1% (n=68) to 27±6% (n=55, P=0.006) in comparison with overexpression of this enzyme (Figure 5E, F, and I). In agreement with previous results, the localization of Nde1 was not affected by the overexpression of either DHHC7 or DN-DHHC7 (the relative frequency of Nde1 at the tip was 71±7.3% (n=39) versus 55±7.7% (n=42)).
Next, we tested the effect of expressing the Ndel1 palmitoylation mutant on the localization of LIS1. In concord with the assays described above, the enhancement of cytoplasmic dynein activity by the palmitoylation mutant led to a reduction of the relative amount of LIS1 localized in neurite tips in comparison with wild-type Ndel1 (Figure 5G–I). In these cells, the relative proportion of cells expressing more LIS1 in the tips was decreased in a significant way from 53±7% (n=41) to 23±5% (n=52, P=0.002). Expression of either wild-type or the C273S mutant of Nde1 did not affect LIS1 localization at the tips of neurites in differentiated CAD cells (data not shown). We conclude that Ndel1 palmitoylation leads to reduced dynein activity that may disturb the balance between the activities of the plus and minus end motors, resulting in the accumulation of LIS1 and Ndel1 at neurite tips.
The aforementioned experiments provided us with indirect evidence regarding DIC activity. To monitor DIC activity directly, we transfected primary cerebellar neurons with DIC-1B–mCherry and either GFP–Ndel1 or GFP–Ndel1(C273S), and monitored the movements of the fluorescent dynein puncta within the axons (Ha et al, 2008). DIC puncta were tracked using the Imaris program (Supplementary Movies 1 and 2 are examples of DIC–mCherry puncta in neurons transfected with GFP–Ndel1 or GFP–Ndel1 (C273S), respectively). In each time frame, the position of the puncta were subtracted from the previous time frame and the relative orientation in relation to the cell body was determined (Figure 6A–D). We monitored DIC-1B–mCherry puncta over time and noticed that we could monitor them over more time frames. The processivity of DIC-1B–mCherry puncta increased in a significant manner when the unpalmitoylatable form of Ndel1 (C273S) was present; the number of points per track increased to 19.07±0.9, n=280 versus 16.12±0.7, n=314; P=0.0107 (Figure 6E). Thus, Ndel1 (C273S) resulted in dynein containing particles that moved during a longer time interval. Nevertheless, there was no change in the average retrograde velocity (Figure 6F).
Figure 6.
Unpalmitoylated Ndel1 increases the processivity of DIC–mCherry puncta retrograde tracks. Primary cerebellar neurons were transfected with either GFP–Ndel1 wild type or C273S mutant and DIC–mCherry. The red fluorescent puncta were tracked using Imaris software. (A–D) DIC–mCherry puncta were identified and their movements in the axon were tracked. In each time-lapse (here 4-s intervals are shown), the position of each puncta was compared with its previous position. Arrowheads mark trackable puncta. Retrograde and anterograde motility was defined according to the movement to or from the cell body. (E) The number of points in each retrograde track was plotted and the differences between neurons transfected with Ndel1 C273S and Ndel1 were significant P=0.0107. (F) Expression of Ndel1 C273S did not change the velocity in the retrograde direction.
Ndel1 palmitoylation and neuronal migration
The functional connection between cytoplasmic dynein and Ndel1 during neuronal migration is well documented (Sasaki et al, 2000, 2005; Niethammer et al, 2000b; Shu et al, 2004). Therefore, we investigated the expression pattern of the palmitoylation enzymes: DHHC2, DHHC3, and DHHC7 displayed in the genepaint database (http://www.genepaint.org) (Visel et al, 2004). No signal for the minor Ndel1 palmitoylation enzyme DHHC21 (see Figure 1) was noted in the developing embryo. DHHC2, DHHC3, and DHHC7 are expressed in several tissues (Supplementary Figure S10). Interestingly, a pronounced expression of DHHC7 was noted in the cortical plate (CP) of the developing brain cortex, whereas DHHC2 was stronger in the ventricular zone (VZ). The expression pattern of DHHC3 was relatively uniform. These results are consistent with the idea that DHHC7/3/2 can regulate the palmitoylation of Nde1/Ndel1 in the developing brain. To further pursue this idea, we tested whether Ndel1 palmitoylation affects neuronal migration in vivo using in utero electroporation (Figure 7). The expression of DHHC7-DN reduced the proportion of neurons that reached the superficial layers of the cortical plate in comparison with control (compare Figure 7E with J. The quantification of the relative proportion of cells in the different bins are shown in panel K). The expression of Ndel1, which activates cytoplasmic dynein, inhibited neuronal migration (Figure 7B, G and K). The expression of Ndel1 C273S, which may activate cytoplasmic dynein, indeed impaired neuronal migration (Figure 7C, H and K). Finally, the expected inhibition of the retrograde motor by DHHC7 expression caused a significant retardation of neuronal migration (Figure 7D, I and K). We, therefore, conclude that a tight balance of dynein activity is required for proper neuronal migration and that enhanced inhibition or, to a lesser extent, increased activation of the molecular motor impairs this process.
Figure 7.
Palmitoylation of Ndel1 affects neuronal migration. E14.5 embryos were electroporated in utero with plasmids expressing (left to right) (A, F) control; (B, G) Ndel1; (C, H) Ndel1 C273S; (D, I) DHHC7; or (E, J) DHHC7-DN lacking enzymatic activity together with a GFP-expressing plasmid. Brains were fixed, sectioned, and pictured 4 days after electroporation. (K) Sections from at least four different brains were analysed by counting the number of neurons found in six individual bins of equal sizes, and compared their relative percentage in each bin by ANOVA. Ndel1 (blue) differed significantly from the control (grey). DHHC7-DN (green) exhibited reduced migration in comparison with control migration (grey) with most neurons found in the superficial layer of the cortical plate. Expression of DHHC7 (red) significantly impaired neuronal migration, with a similar number of neurons found among different bins. Expression of Ndel1 C273S (yellow) inhibited the neuronal migration in a moderate but significant manner.
Next we tested the significance of reduction of each of the three palmitoylation enzymes in the developing brain. The efficacy of each of the designed shRNA sequences was verified in ectopically transfected HEK293 cells (Supplementary Figure S11). The efficacy of reducing endogenous DHHC3 was also tested using immunostaining of transfected primary neurons (Supplementary Figure S11). Knock down of each enzyme independently, reduced the proportion of neurons that reached the superficial layers of the cortical plate in comparison with control (Figure 8A–D). This reduction was statistically significant as tested by ANOVA (Figure 8F). The most pronounced effect of knock down of a single enzyme on radial migration was noted with reduced levels of DHHC7, although combined reduction of all three enzymes exhibited further reduction (Figure 8D–F). To further verify that these effects are indeed due to the reduction in the endogenous levels of the palmitoylating enzymes, silent mutations were introduced in DHHC7 allowing resistance to the corresponding shRNA (Supplementary Figure S11). The addition of this DHHC7-resistant form rescued the DHHC7 shRNA-induced migration phenotype (Figure 8G–I). The improvement in neuronal migration was significant as assayed by paired t-test (Figure 8I). In addition, enzyme specificity was explored by trying to rescue knock down of DHHC7 by DHHC3 or knock down of DHHC2 by DHHC7. In both cases, there was no significant improvement in neuronal migration. Collectively, these observations indicate that Ndel1 palmitoylation enzymes control proper neuronal migration during brain development.
Figure 8.
Reduction in Ndel1 palmitoylation enzymes inhibits neuronal migration. (A–E) E14.5 embryos were electroporated in utero with GFP expression plasmids and either (A) global control shRNA, (B) DHHC2 shRNA, (C) DHHC3 shRNA, (D) DHHC7 shRNA, or (E) a combination of DHHC2,3, and 7 shRNA. Brains were analysed 4 days after electroporation, and the position of GFP-labelled neurons was quantified in each of eight bins, in which bin 1 marked the most superficial area and bin 8 the area close to the ventricular zone. (F) The percentage of cells in each bin were calculated and compared by ANOVA. Each of the individual shRNA and the mixture of all three shRNA reduced the percentage of cells reaching the most superficial layer, (***P<0.001) (F, bin 1). The effect of DHHC7 and the mixture of all three shRNA was most obvious in bin 8 (**P<0.01, ***P<0.001) (F). Among the individual shRNA, the effect of DHHC7 was most pronounced. (G–I) The specificity of DHHC7 shRNA was verified by a rescue experiment. The percentage of neurons stalled in their migration route was significantly decreased when a DHHC7 expression plasmid harbouring silent mutations and resistant to the shRNA was introduced (*P=0.032) (compare G and H). (I) The results were subjected to paired t-test.
Discussion
Ndel1 and Nde1
Our results demonstrate, to the best of our knowledge, for the first time, palmitoylation on conserved cysteines of the two Nde paralogs. Significantly, in contrast to Ndel1 palmitoylation, which impinges on the activity of the motor itself, the palmitoylation of Nde1 (Ndel1's paralog) on the conserved cysteine had no effect on the measured dynein-mediated activities. Although the significance of Nde1 palmitoylation is a matter of future studies, the apparent divergent regulatory activities of the two similar proteins have an evolutionary basis reflected in their differential interaction with dynein subunits. Nde1 interacts with dynein light chain (Feng et al, 2000; Stehman et al, 2007) and DIC (Stehman et al, 2007). Ndel1 was also reported to interact with the intermediate chains, but also with light intermediate chains (Niethammer et al, 2000b) and cytoplasmic dynein heavy chain (Sasaki et al, 2000; Niethammer et al, 2000b), including two identified binding domains, one of which in the P1 loop (Sasaki et al, 2000). Similarly, the Nde paralogs also vary in their perspective roles in targeting of dynein to kinetochores (Vergnolle and Taylor, 2007).
A new mode of regulating cytoplasmic dynein
Our results indicate a new mean for regulating the activity of the molecular motor cytoplasmic dynein through the reversible palmitoylation of Ndel1. The regulation of the molecular motor is rather complex. Dynein heavy chain has at least two different regions involved in regulation of the ATPase enzymatic activity; the stalk and the C-terminal domain (for review, see Vallee and Hook, 2006). In addition, the transient interaction of dynein light chains (Makokha et al, 2002; Nyarko et al, 2004; Vallee et al, 2004), regulated by phosphorylation (Song et al, 2007), affects the structure of the dynein intermediate chain (Benison et al, 2006). The phosphorylation of the dynein intermediate chain also regulates its binding to dynactin (Vaughan et al, 2001) that in turn is involved in regulation of dynein processivity (Schroer, 2004). Furthermore, dyenin is regulated by interacting proteins such as dynactin (for reviews, see Schroer, 2004; Vallee et al, 2004). Interestingly, the palmitoylation of the dynein intermediate chain has been recently reported after a proteomics screen for neuronal palmitoylation substrates (Kang et al, 2008). However, the identity of the enzymes responsible for this modification and its functional significance is of yet unknown. The possibility that Ndel1 and cytoplasmic dynein are simultaneously co-modified will be a topic of future studies.
Palmitoylation is similar to phosphorylation in respect to its reversible modulation. Palmitoylation of Ndel1 affected dynein-mediated intracellular transport of several cargoes; it reduced the compactness of the Golgi that is redistributed in the cell during mitosis. In addition, transport of short microtubule fragments, and dynein-mediated transport of mutant VSVG was enhanced with overexpression of Ndel1, but was reduced when Ndel1 was palmitoylated. Furthermore, Ndel1 and LIS1 are also cargoes of cytoplasmic dynein, their intracellular localization is shifted towards the cell body when Ndel1 palmitoylation is reduced. There is no precedence in which palmitoylation of a dynein-interacting protein is known to affect its interaction with dynein and as such to affect dynein activity. Huntingtin undergoes palmitoylation by its interacting protein HIP14 (DHHC-17) (Huang et al, 2004), and this activity is important for its proper trafficking and function (Ohyama et al, 2007). In addition, Huntingtin interacts directly with dynein intermediate chain and is suggested to facilitate dynein-mediated vesicle motility (Caviston et al, 2007). In addition, it has been demonstrated that Huntingtin phosphorylation functions as a bimodular switch in regulating the direction of vesicle transport (Colin et al, 2008). Although a relationship between Huntingtin palmitoylation and dynein-mediated activity has not been demonstrated, it is attractive to speculate that this interaction may also be regulated by the status of Huntingtin palmitoylation. In another system, a different posttranslational modification of the RNA-binding protein, La, by sumoylation in sensory neurons was found to regulate the directionality of its transport (van Niekerk et al, 2007). Sumoylated La preferentially interacts with cytoplasmic dynein that transports it retrogradely, whereas sumoylation-incompetent La shows only anterograde transport. However, the La RNA chaperone protein has not been shown to affect dynein activity as observed in case of Ndel1. Importantly, S-nitrosylation of MAP1b was shown to be involved in axonal response to nitric oxide (Stroissnigg et al, 2007) and this modification resulted in a change in MAP1b-binding affinity to microtubules. In agreement with other data suggesting that MAP1b or other related proteins inhibit dynein activity (Ding et al, 2006; Jimenez-Mateos et al, 2006), a model has been proposed in which S-nitrosylated MAP1b consequently inhibits dynein activity, leading to axonal retraction. Thus suggesting that a signalling cascade initiated by nitric oxide results in inhibition of dynein activity.
It has been postulated that reversible palmitoylation has an important role in the nervous system (for reviews, see El-Husseini Ael and Bredt, 2002; Washbourne, 2004). Our results emphasize that Ndel1 palmitoylation is of great significance, particularly, in neuronal cells. Our in vivo treatments indicated that either reduced or increased Ndel1 palmitoylation resulted in impaired neuronal migration. The effect was much more pronounced when the molecular motor was inhibited. So far neuronal migration inhibition has been demonstrated in cases in which the activity of the molecular motor is reduced; reduction in dynein heavy chain, LIS1, (Tsai et al, 2007) Ndel1 (Shu et al, 2004), or overexpression of dynamitin, or dominant-negative LIS1 (Tsai et al, 2005). To the best of our knowledge, this is the first example demonstrating that tilting the balance of molecular motor activity results in neuronal migration retardation. Our experimental findings are summarized in the simplistic model, in which Ndel1 can be found in two states (Figure 9). Ndel1 can be found palmitoylated (red) after the activity of either DHHC7 or DHHC3, or depalmitoylated (yellow), by yet unknown depalmitoylation enzymes. In the depalmitoylated form, Ndel1 binds better to the retrograde motor and stimulates its activity. When Ndel1 is palmitoylated, the molecular motor works less effectively and transport is reduced. Our findings are likely to impact all known dynein-mediating activities, from mitosis of the single cells to long-range neuronal transport processes that are affected in cases of neuronal degeneration diseases.
Figure 9.
A schematic model demonstrating the effect of palmitoylation of Ndel1 on cytoplasmic dynein activity. The molecular motor, cytoplasmic dynein (blue) moves towards the minus ends of microtubules (light green). The large dynactin complex (orange) is attached to dynein. Ndel1 can be found either palmitoylated (red) after the activity of DHHC7 or DHHC3, or depalmitoylated (yellow). When Ndel1 is depalmitoylated it binds better to the motor domain of dynein and promotes its activity.
Materials and methods
Palmitate labelling in HEK293 cells
Palmitate labelling was performed as described previously (Fukata et al, 2004, 2006). Briefly, transfected HEK293 or COS7 cells were labelled with 0.25 mCi/ml [3H]palmitic acid (Perkin-Elmer) for 4 h. Cells were washed with PBS, scraped with SDS–PAGE sample buffer with 10 mM DTT, and boiled for 2 min. For fluorography, SDS–PAGE gels with protein samples were treated with Amplify (GE Healthcare) for 30 min, dried under vacuum, and exposed at −80°C.
Labelling of palmitoylated Ndel1 and Nde1 in cortical neurons
E15 cortices was dissected and cortical neurons were dissociated into MEM medium supplemented with 6% glucose, GlutaMAX, 5% horse serum, 5% fetal calf serum, B27, and gentamycin. Briefly, cells were homogenized in lysis buffer (PBS supplemented with 5 mM EDTA, 1% Triton X-100, 1 mM PMSF, 10 μg/μl aprotinin, 1 μg/μl pepstatin A and 10 μg/μl leupeptin, with 40 mM of N′-ethylmaleimide (E3876, Sigma)). The cell lysates were passed through a 21-gauge needle and left for 30 min on ice. The cell lysates were centrifuged at 4°C for 15 min at 13 000 r.p.m. The cleared supernatants were transferred to new Eppendorf tubes and then the tubes were rotated at 4°C overnight. Proteins were then immunoprecipitated with specific antibodies using A/G beads, after IP an additional blocking was conducted by incubating the beads for 1 h at RT in PBS supplemented with 40 mM of N′-ethylmaleimide. The beads were then washed with PBS followed by removal of palmitate by addition of 1 M hydroxylamine (pH 7.5) (55458, Fluka), whereas the control was incubated with PBS for 1 h at RT. After this incubation, the beads were washed thrice in PBS and then incubated with 320 μM EZ-Link BMCC-Biotin (21900, PIERCE). Proteins were separated by SDS–PAGE and blotted with ExtrAvidin-peroxidase (E2886, Sigma) or reacted with specific antibodies.
Palmitoylation half-life in mouse cortical neurons
E15 cortices was dissected and cortical neurons were dissociated into MEM medium supplemented with 6% glucose, GlutaMAX, 5% horse serum, 5% fetal calf serum, B27, and gentamycin. Neurons (∼5 × 106) were incubated at 37°C with 10 μM 2-Br for the indicated times. Cells were collected by centrifugation and washed once with ice cold PBS. Palmitoylation of Ndel1 was detected as described previously (Drisdel and Green, 2004; Drisdel et al, 2006), with some modifications. Briefly, cells were homogenized in lysis buffer (150 mM NaCl, 50 mM Tris (pH 7.4), 5 mM EDTA, 1% Triton X-100, 1 mM PMSF, 10 μg/μl aprotinin, 1 μg/μl pepstatin A and 10 μg/μl leupeptin) supplemented with 20 mM of N′-ethylmaleimide (E3876, Sigma). Proteins were precipitated by methanol–chloroform method and dissolved into 4% SDS, 50 mM Tris (pH 7.4), 5 mM EDTA with 10 mM N′-ethylmaleimide, followed by a 10-min incubation at 37°C with addition of lysis buffer with l mM N′-ethylmaleimide. Proteins were immunoprecipitated with specific antibodies. Palmitate was removed by addition of 1 M hydroxylamine (55458, Fluka), whereas the control was incubated with 1 M Tris–HCl (pH 7.4). Both the treated protein and the control were incubated with 100 μM EZ-Link BMCC-Biotin (21900, PIERCE). Proteins were separated by SDS–PAGE and blotted with ExtrAvidin-peroxidase (E2886, Sigma) or with specific antibodies. After blotting and exposure, the baseline was subtracted from the relative intensities and results were subjected to a linear regression analysis. The results fitted a linear regression model (r2=0.85). The calculated half-life was derived from the deduced line (presented in Figure 1H). Additional repetitions agreed with the calculated half-life.
Data regarding plasmids, antibodies, cell culture, transfections, immunoprecipitation, immunostaining, metabolic labelling using 17-ODYA and click chemistry, protein half-life, microtubule transport, and image analysis are detailed in the supplementary data.
Statistical analysis
The statistical analysis used included Student's t-test (parametric or non-parametric) in comparison of two populations using the InStat program. In case of multiple comparisons ANOVA analysis was used. Linear correlation curves were used for half-life determination. The later two analyses were conducted using Prism version 4 for Macintosh (GraphPad Software, San Diego, CA, USA, www.graphpad.com).
In utero electroporation
In utero electroporations were conducted as previously described (Sapir et al, 2008a, 2008b). Brains were fixed, sectioned, and pictured 4 days after electroporation. Sections from at least four different brains were analysed by counting the number of neurons found in indicated number of individual bins of equal sizes, and their relative percentage in each bin was compared by ANOVA. Pictures were taken from 60-μm thick vibrotome sections using Applied Precision DeltaVision microscopy. Animal protocols were approved by the Weizmann Institute IACUC.
Supplementary Material
Supplementary Movie 1
Supplementary Movie 2
Supplementary Figures S1–S11
Supplementary Data
Supplementary Figure
Review Process File
Acknowledgments
We thank Drs Kai Simons, Junken Aoki, Jan Faix, Anna S Akhmanova, Niels Galjart, Li-Huei Tsai, Kirsten J Verhey, Koty Hirschberg, and Michael Davidson for providing useful reagents; Talia Levy, Anna Kaplan, and Jehuda Melamed for technical assistance. The work has been supported (to OR) in part by the Israel Science Foundation (grant no. 270/04) and the Legacy Heritage Biomedical Program of the Israel Science Foundation (grant no. 1062/08); the National Institutes of Health grant (No. RO3TW007048); Foundation Jérôme Lejeune; a grant from the Paul Godfrey Research Foundation in Childrens' diseases; the Benoziyo Center for Neurological diseases; the Kekst Center; the Forcheimer Center; the Weizmann-Pasteur collaborative grant; and the David and Fela Shapell Family Center for Genetic Disorders Research. OR is an Incumbent of the Bernstein-Mason professorial chair of Neurochemistry.
References
- Abal M, Piel M, Bouckson-Castaing V, Mogensen M, Sibarita JB, Bornens M (2002) Microtubule release from the centrosome in migrating cells. J Cell Biol 159: 731–737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad FJ, Baas PW (1995) Microtubules released from the neuronal centrosome are transported into the axon. J Cell Sci 108 (Pt 8): 2761–2769 [DOI] [PubMed] [Google Scholar]
- Ahmad FJ, Echeverri CJ, Vallee RB, Baas PW (1998) Cytoplasmic dynein and dynactin are required for the transport of microtubules into the axon. J Cell Biol 140: 391–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benison G, Nyarko A, Barbar E (2006) Heteronuclear NMR identifies a nascent helix in intrinsically disordered dynein intermediate chain: implications for folding and dimerization. J Mol Biol 362: 1082–1093 [DOI] [PubMed] [Google Scholar]
- Bi W, Sapir T, Shchelochkov OA, Zhang F, Withers MA, Hunter JV, Levy T, Shinder V, Peiffer DA, Gunderson KL, Nezarati MM, Ann Shotts V, Amato SS, Savage SK, Harris DJ, Day-Salvatore DL, Horner M, Lu XY, Sahoo T, Yanagawa Y et al. (2009) Increased LIS1 expression affects human and mouse brain development. Nat Genet 41: 168–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruijn LI, Miller TM, Cleveland DW (2004) Unraveling the mechanisms involved in motor neuron degeneration in ALS. Annu Rev Neurosci 27: 723–749 [DOI] [PubMed] [Google Scholar]
- Burkhardt JK, Echeverri CJ, Nilsson T, Vallee RB (1997) Overexpression of the dynamitin (p50) subunit of the dynactin complex disrupts dynein-dependent maintenance of membrane organelle distribution. J Cell Biol 139: 469–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahana A, Escamez T, Nowakowski RS, Hayes NL, Giacobini M, von Holst A, Shmueli O, Sapir T, McConnell SK, Wurst W, Martinez S, Reiner O (2001) Targeted mutagenesis of Lis1 disrupts cortical development and LIS1 homodimerization. Proc Natl Acad Sci USA 98: 6429–6434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caviston JP, Ross JL, Antony SM, Tokito M, Holzbaur EL (2007) Huntingtin facilitates dynein/dynactin-mediated vesicle transport. Proc Natl Acad Sci USA 104: 10045–10050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevalier-Larsen E, Holzbaur EL (2006) Axonal transport and neurodegenerative disease. Biochim Biophys Acta 1762: 1094–1108 [DOI] [PubMed] [Google Scholar]
- Colin E, Zala D, Liot G, Rangone H, Borrell-Pages M, Li XJ, Saudou F, Humbert S (2008) Huntingtin phosphorylation acts as a molecular switch for anterograde/retrograde transport in neurons. EMBO J 27: 2124–2134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coquelle FM, Caspi M, Cordelieres FP, Dompierre JP, Dujardin DL, Koifman C, Martin P, Hoogenraad CC, Akhmanova A, Galjart N, De Mey JR, Reiner O (2002) LIS1, CLIP-170's key to the dynein/dynactin pathway. Mol Cell Biol 22: 3089–3102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corthesy-Theulaz I, Pauloin A, Pfeffer SR (1992) Cytoplasmic dynein participates in the centrosomal localization of the Golgi complex. J Cell Biol 118: 1333–1345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derewenda U, Tarricone C, Choi WC, Cooper DR, Lukasik S, Perrina F, Tripathy A, Kim MH, Cafiso DS, Musacchio A, Derewenda ZS (2007) The structure of the coiled-coil domain of ndel1 and the basis of its interaction with lis1, the causal protein of Miller–Dieker lissencephaly. Structure 15: 1467–1481 [DOI] [PubMed] [Google Scholar]
- Ding J, Allen E, Wang W, Valle A, Wu C, Nardine T, Cui B, Yi J, Taylor A, Jeon NL, Chu S, So Y, Vogel H, Tolwani R, Mobley W, Yang Y (2006) Gene targeting of GAN in mouse causes a toxic accumulation of microtubule-associated protein 8 and impaired retrograde axonal transport. Hum Mol Genet 15: 1451–1463 [DOI] [PubMed] [Google Scholar]
- Drisdel RC, Alexander JK, Sayeed A, Green WN (2006) Assays of protein palmitoylation. Methods 40: 127–134 [DOI] [PubMed] [Google Scholar]
- Drisdel RC, Green WN (2004) Labeling and quantifying sites of protein palmitoylation. BioTechniques 36: 276–285 [DOI] [PubMed] [Google Scholar]
- Dunphy JT, Linder ME (1998) Signalling functions of protein palmitoylation. Biochim Biophys Acta 1436: 245–261 [DOI] [PubMed] [Google Scholar]
- Efimov VP, Morris NR (2000) The LIS1-related NUDF protein of Aspergillus nidulans interacts with the coiled-coil domain of the NUDE/RO11 protein. J Cell Biol 150: 681–688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Husseini Ael D, Bredt DS (2002) Protein palmitoylation: a regulator of neuronal development and function. Nat Rev Neurosci 3: 791–802 [DOI] [PubMed] [Google Scholar]
- Erwin DH, Davidson EH (2002) The last common bilaterian ancestor. Development 129: 3021–3032 [DOI] [PubMed] [Google Scholar]
- Faulkner NE, Dujardin DL, Tai CY, Vaughan KT, O'Connell CB, Wang Y, Vallee RB (2000) A role for the lissencephaly gene LIS1 in mitosis and cytoplasmic dynein function. Nat Cell Biol 2: 784–791 [DOI] [PubMed] [Google Scholar]
- Feng Y, Olson EC, Stukenberg PT, Flanagan LA, Kirschner MW, Walsh CA (2000) LIS1 regulates CNS lamination by interacting with mNudE, a central component of the centrosome. Neuron 28: 665–679 [DOI] [PubMed] [Google Scholar]
- Feng Y, Walsh CA (2004) Mitotic spindle regulation by Nde1 controls cerebral cortical size. Neuron 44: 279–293 [DOI] [PubMed] [Google Scholar]
- Fukata M, Fukata Y, Adesnik H, Nicoll RA, Bredt DS (2004) Identification of PSD-95 palmitoylating enzymes. Neuron 44: 987–996 [DOI] [PubMed] [Google Scholar]
- Fukata Y, Iwanaga T, Fukata M (2006) Systematic screening for palmitoyl transferase activity of the DHHC protein family in mammalian cells. Methods 40: 177–182 [DOI] [PubMed] [Google Scholar]
- Goldstein LS, Yang Z (2000) Microtubule-based transport systems in neurons: the roles of kinesins and dyneins. Annu Rev Neurosci 23: 39–71 [DOI] [PubMed] [Google Scholar]
- Grabham PW, Seale GE, Bennecib M, Goldberg DJ, Vallee RB (2007) Cytoplasmic dynein and LIS1 are required for microtubule advance during growth cone remodeling and fast axonal outgrowth. J Neurosci 27: 5823–5834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo J, Yang Z, Song W, Chen Q, Wang F, Zhang Q, Zhu X (2006) Nudel contributes to microtubule anchoring at the mother centriole and is involved in both dynein-dependent and -independent centrosomal protein assembly. Mol Biol Cell 17: 680–689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha J, Lo KW, Myers KR, Carr TM, Humsi MK, Rasoul BA, Segal RA, Pfister KK (2008) A neuron-specific cytoplasmic dynein isoform preferentially transports TrkB signaling endosomes. J Cell Biol 181: 1027–1039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halanych KM (2004) The new view of animal phylogeny. Annu Rev Ecol Evol Syst 35: 229–256 [Google Scholar]
- Harada A, Takei Y, Kanai Y, Tanaka Y, Nonaka S, Hirokawa N (1998) Golgi vesiculation and lysosome dispersion in cells lacking cytoplasmic dynein. J Cell Biol 141: 51–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirohashi Y, Wang Q, Liu Q, Du X, Zhang H, Sato N, Greene MI (2006a) p78/MCRS1 forms a complex with centrosomal protein Nde1 and is essential for cell viability. Oncogene 25: 4937–4946 [DOI] [PubMed] [Google Scholar]
- Hirohashi Y, Wang Q, Liu Q, Li B, Du X, Zhang H, Furuuchi K, Masuda K, Sato N, Greene MI (2006b) Centrosomal proteins Nde1 and Su48 form a complex regulated by phosphorylation. Oncogene 25: 6048–6055 [DOI] [PubMed] [Google Scholar]
- Hirotsune S, Fleck MW, Gambello MJ, Bix GJ, Chen A, Clark GD, Ledbetter DH, McBain CJ, Wynshaw-Boris A (1998) Graded reduction of Pafah1b1 (Lis1) activity results in neuronal migration defects and early embryonic lethality. Nat Genet 19: 333–339 [DOI] [PubMed] [Google Scholar]
- Huang K, Yanai A, Kang R, Arstikaitis P, Singaraja RR, Metzler M, Mullard A, Haigh B, Gauthier-Campbell C, Gutekunst CA, Hayden MR, El-Husseini A (2004) Huntingtin-interacting protein HIP14 is a palmitoyl transferase involved in palmitoylation and trafficking of multiple neuronal proteins. Neuron 44: 977–986 [DOI] [PubMed] [Google Scholar]
- Jennings BC, Nadolski MJ, Ling Y, Baker MB, Harrison ML, Deschenes RJ, Linder ME (2009) 2-Bromopalmitate and 2-(2-hydroxy-5-nitro-benzylidene)-benzo[b]thiophen-3-one inhibit DHHC-mediated palmitoylation in vitro. J Lipid Res 50: 233–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez-Mateos EM, Gonzalez-Billault C, Dawson HN, Vitek MP, Avila J (2006) Role of MAP1B in axonal retrograde transport of mitochondria. Biochem J 397: 53–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang R, Wan J, Arstikaitis P, Takahashi H, Huang K, Bailey AO, Thompson JX, Roth AF, Drisdel RC, Mastro R, Green WN, Yates Iii JR, Davis NG, El-Husseini A (2008) Neural palmitoyl-proteomics reveals dynamic synaptic palmitoylation. Nature 456: 904–909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Y, Yu W, Li Y, Yang Z, Yan X, Huang Q, Zhu X (2004) Nudel functions in membrane traffic mainly through association with Lis1 and cytoplasmic dynein. J Cell Biol 164: 557–566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Y, Yu W, Li Y, Yu L, Zhang Q, Wang F, Yang Z, Du J, Huang Q, Yao X, Zhu X (2007) Nudel modulates kinetochore association and function of cytoplasmic dynein in M phase. Mol Biol Cell 18: 2656–2666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linder ME, Deschenes RJ (2007) Palmitoylation: policing protein stability and traffic. Nat Rev Mol Cell Biol 8: 74–84 [DOI] [PubMed] [Google Scholar]
- Liu Z, Steward R, Luo L (2000) Drosophila Lis1 is required for neuroblast proliferation, dendritic elaboration and axonal transport. Nat Cell Biol 2: 776–783 [DOI] [PubMed] [Google Scholar]
- Lo Nigro CL, Chong CS, Smith AC, Dobyns WB, Carrozzo R, Ledbetter DH (1997) Point mutations and an intragenic deletion in LIS1, the lissencephaly causative gene in isolated lissencephaly sequence and Miller–Dieker syndrome. Hum Mol Genet 6: 157–164 [DOI] [PubMed] [Google Scholar]
- Makokha M, Hare M, Li M, Hays T, Barbar E (2002) Interactions of cytoplasmic dynein light chains Tctex-1 and LC8 with the intermediate chain IC74. Biochemistry 41: 4302–4311 [DOI] [PubMed] [Google Scholar]
- Mesngon MT, Tarricone C, Hebbar S, Guillotte AM, Schmitt EW, Lanier L, Musacchio A, King SJ, Smith DS (2006) Regulation of cytoplasmic dynein ATPase by Lis1. J Neurosci 26: 2132–2139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori D, Yano Y, Toyo-oka K, Yoshida N, Yamada M, Muramatsu M, Zhang D, Saya H, Toyoshima YY, Kinoshita K, Wynshaw-Boris A, Hirotsune S (2007) NDEL1 phosphorylation by Aurora-A kinase is essential for centrosomal maturation, separation, and TACC3 recruitment. Mol Cell Biol 27: 352–367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niethammer M, Smith DS, Ayala R, Peng J, Ko J, Lee M-S, Morabito M, Tsai L-H (2000a) Nudel is a novel Cdk5 substrate that associates with Lis1 and cytoplasmic dynein. Neuron 28: 697–711 [DOI] [PubMed] [Google Scholar]
- Niethammer M, Smith DS, Ayala R, Peng J, Ko J, Lee MS, Morabito M, Tsai LH (2000b) NUDEL is a novel Cdk5 substrate that associates with LIS1 and cytoplasmic dynein. Neuron 28: 697–711 [DOI] [PubMed] [Google Scholar]
- Nyarko A, Hare M, Hays TS, Barbar E (2004) The intermediate chain of cytoplasmic dynein is partially disordered and gains structure upon binding to light-chain LC8. Biochemistry 43: 15595–15603 [DOI] [PubMed] [Google Scholar]
- Ohyama T, Verstreken P, Ly CV, Rosenmund T, Rajan A, Tien AC, Haueter C, Schulze KL, Bellen HJ (2007) Huntingtin-interacting protein 14, a palmitoyl transferase required for exocytosis and targeting of CSP to synaptic vesicles. J Cell Biol 179: 1481–1496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilz DT, Matsumoto N, Minnerath S, Mills P, Gleeson JG, Allen KM, Walsh CA, Barkovich AJ, Dobyns WB, Ledbetter DH, Ross ME (1998) LIS1 and XLIS (DCX) mutations cause most classical lissencephaly, but different patterns of malformation. Hum Mol Genet 7: 2029–2037 [DOI] [PubMed] [Google Scholar]
- Presley JF, Cole NB, Schroer TA, Hirschberg K, Zaal KJ, Lippincott-Schwartz J (1997) ER-to-Golgi transport visualized in living cells. Nature 389: 81–85 [DOI] [PubMed] [Google Scholar]
- Reiner O, Carrozzo R, Shen Y, Whenert M, Faustinella F, Dobyns WB, Caskey CT, Ledbetter DH (1993) Nature 364: 717–721 [DOI] [PubMed] [Google Scholar]
- Reiner O, Sapoznik S, Sapir T (2006) Lissencephaly 1 linking to multiple diseases: mental retardation, neurodegeneration, schizophrenia, male sterility, and more. Neuromolecular Med 8: 547–566 [DOI] [PubMed] [Google Scholar]
- Resh MD (1999) Fatty acylation of proteins: new insights into membrane targeting of myristoylated and palmitoylated proteins. Biochim Biophys Acta 1451: 1–16 [DOI] [PubMed] [Google Scholar]
- Resh MD (2006) Trafficking and signaling by fatty-acylated and prenylated proteins. Nat Chem Biol 2: 584–590 [DOI] [PubMed] [Google Scholar]
- Sapir T, Sapoznik S, Levy T, Finkelshtein D, Shmueli A, Timm T, Mandelkow EM, Reiner O (2008a) Accurate balance of the polarity kinase MARK2/Par-1 is required for proper cortical neuronal migration. J Neurosci 28: 5710–5720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapir T, Shmueli A, Levy T, Timm T, Elbaum M, Mandelkow EM, Reiner O (2008b) Antagonistic effects of doublecortin and MARK2/Par-1 in the developing cerebral cortex. J Neurosci 28: 13008–13013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki S, Mori D, Toyo-oka K, Chen A, Garrett-Beal L, Muramatsu M, Miyagawa S, Hiraiwa N, Yoshiki A, Wynshaw-Boris A, Hirotsune S (2005) Complete loss of Ndel1 results in neuronal migration defects and early embryonic lethality. Mol Cell Biol 25: 7812–7827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki S, Shionoya A, Ishida M, Gambello M, Yingling J, Wynshaw-Boris A, Hirotsune S (2000) A LIS1–NUDEL–cytoplasmic dynein heavy chain complex in the developing and adult nervous system. Neuron 28: 681–696 [DOI] [PubMed] [Google Scholar]
- Schroer TA (2004) Dynactin. Annu Rev Cell Dev Biol 20: 759–779 [DOI] [PubMed] [Google Scholar]
- Shu T, Ayala R, Nguyen MD, Xie Z, Gleeson JG, Tsai LH (2004) Ndel1 operates in a common pathway with LIS1 and cytoplasmic dynein to regulate cortical neuronal positioning. Neuron 44: 263–277 [DOI] [PubMed] [Google Scholar]
- Smith DS, Niethammer M, Ayala R, Zhou Y, Gambello MJ, Wynshaw-Boris A, Tsai LH (2000) Regulation of cytoplasmic dynein behaviour and microtubule organization by mammalian Lis1. Nat Cell Biol 2: 767–775 [DOI] [PubMed] [Google Scholar]
- Song Y, Benison G, Nyarko A, Hays TS, Barbar E (2007) Potential role for phosphorylation in differential regulation of the assembly of dynein light chains. J Biol Chem 282: 17272–17279 [DOI] [PubMed] [Google Scholar]
- Stehman SA, Chen Y, McKenney RJ, Vallee RB (2007) NudE and NudEL are required for mitotic progression and are involved in dynein recruitment to kinetochores. J Cell Biol 178: 583–594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokin GB, Goldstein LS (2006) Axonal transport and Alzheimer's disease. Annu Rev Biochem 75: 607–627 [DOI] [PubMed] [Google Scholar]
- Stroissnigg H, Trancikova A, Descovich L, Fuhrmann J, Kutschera W, Kostan J, Meixner A, Nothias F, Propst F (2007) S-nitrosylation of microtubule-associated protein 1B mediates nitric-oxide-induced axon retraction. Nat Cell Biol 9: 1035–1045 [DOI] [PubMed] [Google Scholar]
- Tai CY, Dujardin DL, Faulkner NE, Vallee RB (2002) Role of dynein, dynactin, and CLIP-170 interactions in LIS1 kinetochore function. J Cell Biol 156: 959–968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taya S, Shinoda T, Tsuboi D, Asaki J, Nagai K, Hikita T, Kuroda S, Kuroda K, Shimizu M, Hirotsune S, Iwamatsu A, Kaibuchi K (2007) DISC1 regulates the transport of the NUDEL–LIS1–14-3-3epsilon complex through kinesin-1. J Neurosci 27: 15–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyo-Oka K, Sasaki S, Yano Y, Mori D, Kobayashi T, Toyoshima YY, Tokuoka SM, Ishii S, Shimizu T, Muramatsu M, Hiraiwa N, Yoshiki A, Wynshaw-Boris A, Hirotsune S (2005) Recruitment of katanin p60 by phosphorylated NDEL1, an LIS1 interacting protein, is essential for mitotic cell division and neuronal migration. Hum Mol Genet 14: 3113–3128 [DOI] [PubMed] [Google Scholar]
- Toyo-oka K, Shionoya A, Gambello MJ, Cardoso C, Leventer R, Ward HL, Ayala R, Tsai LH, Dobyns W, Ledbetter D, Hirotsune S, Wynshaw-Boris A (2003) 14-3-3epsilon is important for neuronal migration by binding to NUDEL: a molecular explanation for Miller–Dieker syndrome. Nat Genet 34: 274–285 [DOI] [PubMed] [Google Scholar]
- Tsai JW, Bremner KH, Vallee RB (2007) Dual subcellular roles for LIS1 and dynein in radial neuronal migration in live brain tissue. Nat Neurosci 10: 970–979 [DOI] [PubMed] [Google Scholar]
- Tsai JW, Chen Y, Kriegstein AR, Vallee RB (2005) LIS1 RNA interference blocks neural stem cell division, morphogenesis, and motility at multiple stages. J Cell Biol 170: 935–945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaisberg EA, Grissom PM, McIntosh JR (1996) Mammalian cells express three distinct dynein heavy chains that are localized to different cytoplasmic organelles. J Cell Biol 133: 831–842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallee RB, Hook P (2006) Autoinhibitory and other autoregulatory elements within the dynein motor domain. J Struct Biol 156: 175–181 [DOI] [PubMed] [Google Scholar]
- Vallee RB, Williams JC, Varma D, Barnhart LE (2004) Dynein: an ancient motor protein involved in multiple modes of transport. J Neurobiol 58: 189–200 [DOI] [PubMed] [Google Scholar]
- van Niekerk EA, Willis DE, Chang JH, Reumann K, Heise T, Twiss JL (2007) Sumoylation in axons triggers retrograde transport of the RNA-binding protein La. Proc Natl Acad Sci USA 104: 12913–12918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan PS, Leszyk JD, Vaughan KT (2001) Cytoplasmic dynein intermediate chain phosphorylation regulates binding to dynactin. J Biol Chem 276: 26171–26179 [DOI] [PubMed] [Google Scholar]
- Vergnolle MA, Taylor SS (2007) Cenp-F links kinetochores to Ndel1–Nde1–Lis1–dynein microtubule motor complexes. Curr Biol 17: 1173–1179 [DOI] [PubMed] [Google Scholar]
- Visel A, Thaller C, Eichele G (2004) GenePaint.org: an atlas of gene expression patterns in the mouse embryo. Nucleic Acids Res 32: D552–D556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washbourne P (2004) Greasing transmission: palmitoylation at the synapse. Neuron 44: 901–902 [DOI] [PubMed] [Google Scholar]
- Webb Y, Hermida-Matsumoto L, Resh MD (2000) Inhibition of protein palmitoylation, raft localization, and T cell signaling by 2-bromopalmitate and polyunsaturated fatty acids. J Biol Chem 275: 261–270 [DOI] [PubMed] [Google Scholar]
- Yamada M, Toba S, Yoshida Y, Haratani K, Mori D, Yano Y, Mimori-Kiyosue Y, Nakamura T, Itoh K, Fushiki S, Setou M, Wynshaw-Boris A, Torisawa T, Toyoshima YY, Hirotsune S (2008) LIS1 and NDEL1 coordinate the plus-end-directed transport of cytoplasmic dynein. EMBO J 27: 2471–2483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan X, Li F, Liang Y, Shen Y, Zhao X, Huang Q, Zhu X (2003) Human Nudel and NudE as regulators of cytoplasmic dynein in poleward protein transport along the mitotic spindle. Mol Cell Biol 23: 1239–1250 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Movie 1
Supplementary Movie 2
Supplementary Figures S1–S11
Supplementary Data
Supplementary Figure
Review Process File