Abstract
Conformational activation increases the affinity of integrins to their ligands. On ligand binding, further changes in integrin conformation elicit cellular signalling. Unlike any of the natural ligands of α2β1 integrin, human echovirus 1 (EV1) seemed to bind more avidly a ‘closed' than an activated ‘open' form of the α2I domain. Furthermore, a mutation E336A in the α2 subunit, which inactivated α2β1 as a collagen receptor, enhanced α2β1 binding to EV1. Thus, EV1 seems to recognize an inactive integrin, and not even the virus binding could trigger the conformational activation of α2β1. This was supported by the fact that the integrin clustering by EV1 did not activate the p38 MAP kinase pathway, a signalling pathway that was shown to be dependent on E336-related conformational changes in α2β1. Furthermore, the mutation E336A did neither prevent EV1 induced and α2β1 mediated protein kinase C activation nor EV1 internalization. Thus, in its entry strategy EV1 seems to rely on the activation of signalling pathways that are dependent on α2β1 clustering, but do not require the conformational regulation of the receptor.
Keywords: echovirus 1, integrins, p38 MAPK, signalling, virus entry
Introduction
Adhesion receptors of the integrin family are known to anchor most cell types to the surrounding matrix. Several intracellular pathogens also bind to integrins to gain entry to the cell. Integrins are optimal virus receptors for several reasons. They are abundantly expressed on the cell surface and they have relatively low affinity for their natural ligands. In addition, integrins are connected to signalling proteins that may trigger endocytotic pathways. Activation of integrin-mediated signalling is considered to be an essential mechanism for the internalization of viruses. In this process they may mimic the natural ligands.
The initial step in virus infection is binding of the virus particle to a specific receptor on the cell surface. Many adenoviruses (Wickham et al, 1993), coxsackievirus A9 (Chang et al, 1989; Roivainen et al, 1991, 1994; Williams et al, 2004), human parechovirus 1 (Hyypiä et al, 1992; Stanway et al, 1994; Joki-Korpela et al, 2001), foot-and-mouth disease virus (Fox et al, 1989; Jackson et al, 1997) and Kaposi's sarcoma-associated herpesvirus (KSHV/HHV-8; Akula et al, 2002; Veettil et al, 2008) have surface proteins harbouring an arginine–glycine–aspartic acid (RGD) motif, a well-known recognition sequence for a subset of integrins (Ruoslahti and Pierschbacher, 1987). Integrin α2β1, a collagen receptor, binds to human echovirus 1 (EV1; Bergelson et al, 1992) and rotavirus (Zárate et al, 2000) in an RGD-independent manner.
Recent investigations have unveiled many essential facts concerning the structural basis of integrin signalling (for reviews, see Springer and Wang, 2004; Arnaout et al, 2005). Inactivated integrins are proposed to take a bent conformation. Activating ‘inside-out' signals, such as talin or kindlin binding to β-integrin cytoplasmic domain, can trigger a conformational change leading to the extension of integrin ectodomain (for review, see Moser et al, 2009). Natural ligand binding to a site formed by the inserted domain of the β-subunit (βI domain) and the β-propeller domain of the α-subunit triggers a conformational change in the βI domain, leading to the separation of the α- and β-leg regions (Xiao et al, 2004). This results further in the dissociation of α- and β-cytoplasmic domains, allowing activation of intracellular signalling protein binding to the integrins. Multivalent ligands can, in addition to conformational changes, induce integrin cluster formation. Integrin-binding viruses have been thought to act in a manner similar to natural multivalent ligands. However, the results reported here indicate that EV1 diverges from all previously studied α2β1 integrin ligands.
Integrin α2β1, as well as the three other human collagen receptor integrins (α1β1, α10β1 and α11β1) and the five leukocyte integrins (αLβ2, αMβ2, αXβ2, αDβ2 and αEβ7), belong to a structurally distinct subgroup of integrins. These nine α-subunits have a ligand-binding αI domain, homologous to the βI domain found in all the integrin β-subunits. The ‘I' domains or ‘inserted' domains are also called ‘A' domains on the basis of their structural similarity to von Willebrand factor A domains (Arnaout et al, 2005). The αL and αM integrin αI domains can assume a closed, an intermediate or an open conformation (Shimaoka et al, 2003a), whereas in the α1I and α2I domains the intermediate form may not exist (Jin et al, 2004), suggesting that ligand binding to the latter domains triggers a change from the closed to the open conformation (Emsley et al, 2000). The open conformation is also detected in activated integrins before ligand binding, and it may represent a high avidity state of the αI domain. In a recombinant α2I domain, the alteration from the closed to the open conformation can be induced by the gain-of-function mutation E318W (Aquilina et al, 2002). All natural ligands, including different collagen and laminin subtypes, have shown better binding to the open α2I domain when compared with the closed domain form (Aquilina et al, 2002; Tulla et al, 2008).
The key mechanism involved in signalling by the αMβ2 and αLβ2 integrins seems to act through a glutamate residue, located close to the C-terminus of the α7 helix in the αI domain (E310 in αL and E320 in αM), which acts as an intrinsic ligand for the β2I domain and participates in the conformational activation of the integrin receptor (Alonso et al, 2002; Shimaoka et al, 2003a; Yang et al, 2004). In αLβ2 integrin mutation E310A has been reported to push the equilibrium between the bent and extended conformations towards the bent conformation (Salas et al, 2004). In the α2 integrin, glutamate 336 (E336) in the α7 helix of the αI domain seems to have a similar role (Connors et al, 2007), as mutation E336A affects the activation of α2β1 and the regulation of α2I domain conformation (Connors et al, 2007). Thus α2β1 integrin that harbours E336A mutation is in the bent rather than extended conformation. We report here that EV1, unlike any currently known extracellular matrix ligand, favours binding to the closed α2I domain and inactive α2β1 integrin. Furthermore, the activation of protein kinase Cα (PKCα) and the EV1 entry pathway are independent of E336.
We have also studied structural requirements of α2β1 signalling through p38 mitogen-activated protein kinase (MAPK) pathway, a signalling pathway strongly linked to α2β1 integrin (Ivaska et al, 1999; Ravanti et al, 1999; Xu et al, 2001; Bix et al, 2004; Mazharian et al, 2005). Our results indicate that clustering, mediated either by collagen or antibodies, leads to rapid and transient activation of p38 MAPK. We also demonstrate that E336 in the α2I domain is a key determinant in the α2β1-mediated activation of p38. Thus, the activation of p38 pathway can be considered as an indicator of E336-dependent conformational change in α2β1 integrin. However, the clustering of α2β1 integrin by EV1 did not significantly activate the p38 pathway during the early stage of infection. Thus, our results demonstrate that there seem to be fundamental differences in the mechanisms of EV1 action when compared with the natural ligands of α2β1 integrin. EV1 seems to gain its entry by activation of signalling pathways that are dependent on α2β1 clustering, but do not require conformational activation of the integrin.
Results
EV1 binds to the closed conformation of the α2I domain
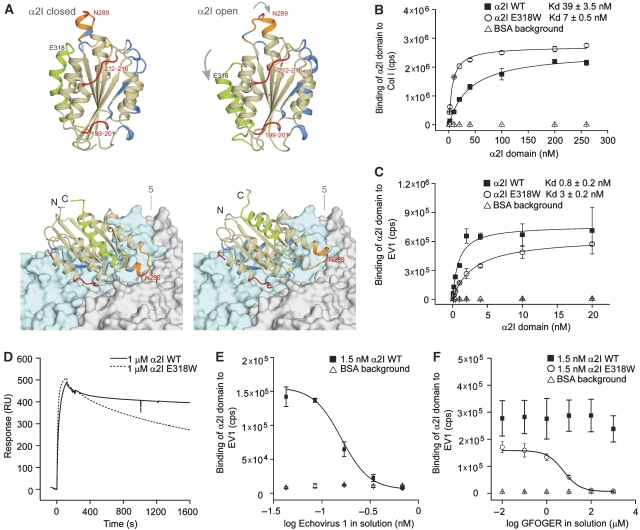
To compare the possible interactions of the open and closed conformations of α2I with EV1, we constructed a model of the α2I (open)–EV1 complex by superimposing the open-form crystal structure on our previously published model of the α2I (closed)–EV1 complex. The model is based on a cryoelectron microscopy (cryo-EM) structure of the complex, into which the high-resolution crystal structures of EV1 and the closed form of α2I have been fitted (Xing et al, 2004). EV1 is known to bind in a metal ion-independent manner (Bergelson et al, 1993) at a binding site different from the MIDAS (King et al, 1997). The site has also been mapped by mutagenesis (King et al, 1997; Dickeson et al, 1999) to the side-face of the αI domain as opposed to the MIDAS location on top of the domain (Figure 1A). The comparison of the open and closed conformations shows that most of the region participating in virus binding on the αI domain surface is not involved in the closed–open conformational alteration and remains essentially unchanged (Figure 1A). The α7 helix that undergoes a large movement is located on the opposite side of the αI domain, compared with the virus-binding surface. Most of the loop between the β7 strand and the α6 helix, including the αC helix in the closed conformation, is also not in close contact with EV1. Thus, the structural model can be considered to be compatible with EV1 binding to either the closed or the open conformation of the α2I domain.
Figure 1.
EV1 prefers the closed conformation of the α2I domain. (A) The crystal structure of the α2I domain in the closed (left) and the open conformations (right). Both conformations are also shown docked onto the surface of EV1 on the basis of the structure of the α2I–EV1 complex determined by cryo-EM (bottom). The surfaces of two protomers of the EV1 capsid are shown, one coloured light blue and the other light grey. The fivefold symmetry axis of the icosahedral capsid is labelled ‘5'. The regions in α2I that undergo the most extensive conformational changes (grey arrows) are the α7 helix (green), the βE-α6 loop (orange; includes the αC helix in the closed conformation) and the MIDAS (the metal ion in yellow). The position of amino-acid E318 is indicated. Residues that have been shown by mutagenesis to affect EV1 binding are coloured red (residues 199–201, 212–216 and 289), and the residues that are within 4.0 Å of the virus structure in the model of the complex are coloured blue. Most of the regions of the α2I involved in the conformational changes are not in close contact with the virus. Microtitre plates were coated with (B) collagen I (Col I) or (C) EV1. Delfia® Diluent II (BSA; Δ) was used as a background control. The GST fusion proteins, containing wild-type α2I (▪) or high affinity mutant α2I E318W (O) domains, were allowed to react with the ligand for 1 h in the presence of 2 mM MgCl2. Bound αI domains were detected with Eu3+-labelled GST antibody, and the signal was measured using time-resolved fluorescence. Data are presented as mean values±s.d. of triplicate measurements. Approximate Kd values for αI domain binding were obtained by fitting the binding data for αI domain concentrations series to a Michaelis–Menten form equation. (D) BIAcore analyses of the interactions between 1 μM α2I WT (solid line) or α2I E318W (dashed line) with immobilized EV1 are presented as overlaid sensograms. After a short (120 s) association phase, the measurement of the dissociation was continued for 25 min. (E, F) Binding of α2I WT (▪) and α2I E318W (O) to immobilized EV1 was competed with increasing concentrations of soluble (E) EV1 (0.04–0.7 nM) or (F) triple helical GFOGER peptide (0.01–1000 μM) that mimic a high-affinity integrin-binding site on collagen by using Eu3+-based solid phase binding assay described above (B, C). Results were fitted to the model representing a dose–response curve with variable Hill slope.
Previously, residue Asn289 in the αC helix of α2I has been shown by mutagenesis to be required for EV1 binding (Dickeson et al, 1999). Here, in the modelled complexes (Figure 1A), this amino acid is in contact with the virus, but it is located at the periphery of the α2I–EV1 interface and part of the residue is exposed to solvent. Asn289 is in the middle of a segment that undergoes an extensive structural rearrangement upon the α2I change from the closed to open form, and therefore, it is possible to hypothesize that EV1 binding to Asn289 may affect the avidity or may even activate a conformational effect in the αI domain (Xing et al, 2004).
The introduction of the mutation E318W into α2I has previously been shown to lead to a shift from the closed to the high affinity state open conformation (Aquilina et al, 2002; Tulla et al, 2008). Glu318 is located in helix α7, and it is not in contact with EV1 (Figure 1A). Here, the binding of human recombinant α2I domains (α2I WT and α2I E318W) to EV1 was tested in a solid phase binding assay using microtitre plates coated with either the virus or collagen I as a control. The results were fitted to a Michaelis–Menten form equation and approximate Kd values were determined to quantify α2I domain binding (Figures 1B and C). As expected, the mutation E318W increased α2I domain binding to collagen I from Kd≈39±3.5 nM (α2I WT) to Kd≈7±0.5 nM (α2I E318W; Figure 1B). Wild-type α2I bound tighter to EV1 than to collagen I in accordance with our previously published results (Xing et al, 2004). Surprisingly, the approximate Kd for α2IE318W domain binding to EV1 seemed to be weaker (Kd≈3±0.2 nM) than the binding of wild-type α2I (Kd≈0.8±0.2 nM; Figure 1C). Thus, the requirements for EV1 binding seem to differ from all previously tested α2β1 integrin ligands that have been shown to favour the open α2I conformation.
To further study the virus–receptor interaction additional experiments were performed. For BIAcore surface plasmon resonance measurements, EV1 was covalently coupled on the surface of the sensor chip. The measurements indicated that the association of both α2I WT and α2I E318W to immobilized EV1 was very fast (Figure 1D). Importantly, the dissociation of α2I WT was remarkably slower when compared with α2E318W (Figure 1D), which partially explains the tighter binding of α2I WT to EV1 seen in solid phase binding assays.
We also used BIAcore to perform kinetic titration series, in which samples were injected sequentially without a regeneration step. The results failed to fit 1:1 binding model (Karlsson et al, 2006), indicating that more than one kind of binding site exist in EV1 (data not shown). To make further estimates of the stoichiometry of the virus binding to α2I, increasing concentrations (0.04–0.7 nM) of soluble EV1 were allowed to compete with 1.5 nM α2I WT in binding to immobilized EV1 in a solid phase binding assay (Figure 1E). In two independent experiments, 0.17–0.34 nM EV1 seemed to block α2I binding to immobilized EV1. On the basis of calculations using estimated Mr of virion (5.65 × 106) and α2I domain–GST fusion (49 500), the results indicated that in these conditions 7–15% of 60 putative integrin-binding sites per virion were occupied.
On the basis of molecular modelling, we have previously proposed that collagen and EV1 cannot concomitantly bind to α2I domain (Xing et al, 2004). To test this experimentally, a soluble triple helical GFOGER peptide, which mimics a high-affinity integrin-binding site in collagen (Knight et al, 1998), was used in the competition study. Interestingly, the collagenous peptide (0.01–1000 μM) effectively inhibited the binding of open α2I (E318W) to EV1 (Figure 1F), but could not compete with the binding of closed α2I (WT) to EV1 within the concentration range used. The result suggests that in the presence of collagen, EV1 significantly benefits from its preference for inactive α2β1.
To test the phenomenon at the cellular level, Chinese hamster ovary (CHO) cells expressing either full-length α2WT or α2E318W were allowed to attach to immobilized collagen I, EV1 or BSA for 15 min. CHO cells do not naturally express integrin-type collagen receptors (Nykvist et al, 2000). Adherent cells were detected using WST-1 reagent. In agreement with the experiments performed at the α2I domain level, integrin activation significantly (P<0.001) increased cell adhesion to collagen I (Figure 2). At the same time the activation decreased cell adhesion to EV1 (P<0.05; Figure 2). Thus, EV1 seemed to bind better to the closed than open α2I conformation and not only at the α2I domain level but also when the tests are performed using full-length α2 integrins expressed on cell surface.
Figure 2.
Integrin activation decreased EV1 binding to cellular α2β1. CHO-α2 and CHO-α2E318W cells were allowed to adhere to immobilized EV1 or collagen I for 15 min. Adhered cells were detected using WST-1 reagent. Integrin activation significantly (P<0.001) increased cell adhesion to collagen I. At the same time, EV1 seemed to favour CHO-α2WT cells (P<0.05). Mean values±s.d. of eight parallel measurements are shown. Statistical significances were determined by two-tailed Student's t-test.
EV1 favours inactive α2β1 integrin
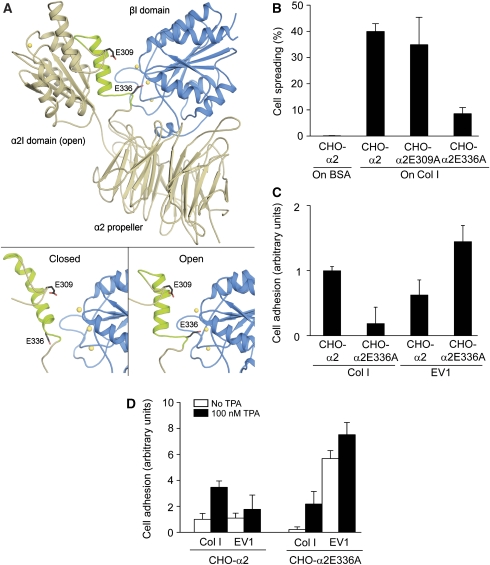
To study further the structural requirements of EV1 binding to α2β1 integrin, we constructed a conformationally inactive integrin and expressed it on cell surface. We have used molecular modelling to assess, whether the α2I domain could interact with the β1 subunit in the same manner as αLI and αMI interact with the β2 subunit (Alonso et al, 2002; Shimaoka et al, 2003b; Yang et al, 2004; Arnaout et al, 2005). Our model suggests that when the α2I domain is in the open conformation, E336 could interact with the metal ion of MIDAS of the β1I domain (Connors et al, 2007; Figure 3A). The model predicts that collagen binding to the α2I domain will most probably induce a conformational change in the β1I domain that leads to the separation of the integrin leg regions, as has been described for other integrins (Alonso et al, 2002; Kim et al, 2003; Yang et al, 2004). In addition to E336 at the C-terminal end of helix α7, E309 in the loop preceding helix α7 was chosen as a target for mutagenesis (Figure 3A).
Figure 3.
EV1 favours inactive α2β1 integrin. (A) A structural model of the human α2β1 integrin head region was built based on the crystal structures of the αVβ3 integrin and the α2I domain. (A; bottom, left) Modelling indicates that the closed conformation of the α2I domain does not form specific contacts with the β1I domain (blue). (A; bottom, right) However, when the α2I domain adopts an open conformation, E336 at the C-terminal end of helix α7 is positioned in such a way that it could coordinate to the metal ion (yellow) of the MIDAS in the β1I domain and act as an intrinsic ligand. E309 does not seem to participate in the process. (B) In a cell spreading assay, CHO-α2 and CHO-α2E309A cells attached and spread on a collagen I (Col I) matrix in 120 min, whereas α2E336A mutation caused a dramatic decrease in the spreading. Cells were not able to attach to or spread on BSA, which was used as a negative control. (C) When CHO-α2 and CHO-α2E336A cells were allowed to adhere to immobilized EV1 or collagen I for 15 min, the E336A mutation seemed to decrease α2-mediated cell adhesion to collagen I. Interestingly, EV1 seemed to favour CHO-α2E336A cells. Mean values±s.d. of four parallel measurements are shown (B, C). (D) However, at 15 min, CHO-α2 and CHO-α2E336A cell adhesion to both collagen I and EV1 was significantly increased in the presence of integrin cluster-inducing TPA (100 nM; black columns). Mean values±s.d. of four parallel measurements are shown.
Wild-type α2 and mutant α2 integrins, containing either E336A or E309A, were expressed in CHO cells. Equal expression levels of the mutant α2 integrins were confirmed by flow cytometry (Supplementary Figure S1). In addition, cell lines were metabolically labelled with 35S-methionine/cysteine, and the α2β1 complex was immunoprecipitated with α2 antibodies under conditions that maintained the subunit interactions. Analysis by SDS–PAGE confirmed that the expression levels of the mutant integrins were practically equal. Furthermore, the mutations seemed to have no effect on the stability of the α2β1 heterodimer (data not shown). When tested by a spreading assay on collagen I, CHO-α2E309A cells did not significantly differ from CHO-α2 cells expressing the wild-type α2 subunit (Figure 3B). Cells carrying the point mutation, E336A, in their α2 subunit were able to attach to collagen I but their spreading was delayed. Similar results were obtained when the CHO-α2 and CHO-α2E336A cells were allowed to attach to an immobilized collagen I for 15 min and adherent cells were detected with WST-1 reagent (Figure 3C). The results suggest that the mechanism of α2β1 action would closely resemble that of αLβ2 integrin (Yang et al, 2004): the communication of the conserved residue E336 in α2 with the metal ion at MIDAS of the β1I domain may induce the high-affinity conformation of the α2I domain. Importantly, when CHO-α2 and CHO-α2E336A binding to EV1 was analysed in the adhesion assay, EV1 seemed to favour the inactive state of α2β1 (Figure 3C). Thus, the results with the E336A variant confirmed the idea that EV1 binds to inactive rather than active integrins. Furthermore, the corresponding mutation in αL integrin has been reported to push the equilibrium between the bent and extended conformations towards the bent conformation (Salas et al, 2004). Therefore, the data propose that EV1 may bind to bent rather than extended integrins.
We have previously shown that activation of PKC by TPA induces both ligand-independent macroaggregation of α2β1 integrins and conformational activation of α2I, whereas the E336A mutation prevents the change in conformation but not receptor clustering (Connors et al, 2007). When TPA-treated (100 nM) cells were allowed to attach to collagen I for 15 min, both CHO-α2 and CHO-α2E336A cell adhesions were increased (Figure 3D). Similarly, TPA increased binding of EV1 to the α2WT and the α2E336A mutant cells. A previous study has also shown that TPA can increase EV1 binding to α2β1 integrin (Bergelson et al, 1993). Our observations indicate that the formation of α2β1 clusters before ligand binding, rather than the conformational activation of the integrin, may explain the increased integrin avidity to EV1.
Activation of p38 after α2β1 integrin clustering by collagen I requires E336-dependent conformational changes in the integrin
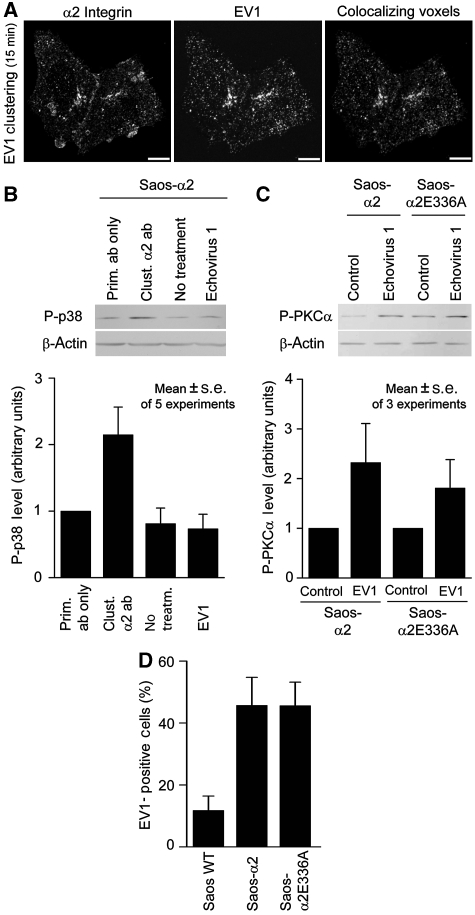
Next we tested the structural requirements of α2β1 signalling after integrin clustering. The formation of α2β1 integrin clusters after treatment with α2 subunit-specific primary antibodies and clustering secondary antibodies was imaged using confocal microscopy. At 15 min, clear integrin clusters appeared when primary and secondary antibodies were used together (Figure 4A). Collagen binding to α2β1 has been reported to lead to specific activation of the p38α MAPK signalling pathway (Ivaska et al, 1999; Ravanti et al, 1999; Xu et al, 2001; Bix et al, 2004; Mazharian et al, 2005). To analyse the effect of α2β1 cluster formation on p38 activation, Saos-α2 and CHO-α2 cells were treated with the antibodies and p38 phosphorylation was analysed by immunoblotting. Antibody-induced clustering caused a rapid and transient phosphorylation of p38 at 15 min in both cell lines (Figures 4B and C). The transient nature of p38 activation (Figure 4B) is most probably because of the fact that antibody-generated α2β1 clusters are rapidly internalized (Upla et al, 2004). To confirm the observation, we also analysed the phosphorylation of p38 by a flow cytometry-based method and a similar activation of p38 in CHO-α2 cells was detected (Figure 4D). It was also obvious that neither the α2 integrin-specific antibody, nor the secondary antibody alone affected p38 phosphorylation (Figure 4D).
Figure 4.
Clustering of α2β1 integrins induces a rapid transient phosphorylation of p38. (A, bottom) Volume renderings of confocal image data show that secondary antibodies (goat anti-mouse IgG) were able to induce integrin clustering in Saos-α2 cells treated with α2 primary antibody (Alexa Fluor 555-conjugated 16B4). (A, top) Without secondary antibodies, no clustering occurs. In both cases, the same living cell was imaged at 0- and 15-min time points. (B) When p38 phosphorylation (P-p38) induced by the antibody (16B4 and anti-mouse IgG)-mediated α2β1 clustering was analysed in Saos-α2 cells at successive time points, a rapid and transient p38 phosphorylation, peaking at 15 min, was obvious. (C) Similarly, antibody-mediated clustering caused p38 activation even in CHO-α2 cells in 15 min. Representative immunoblots of one experiment (B, C) and statistical analyses of scanned blot images of one (C) or five (B) independent experiments are shown. Mean levels of phosphorylated p38 (P-p38)±s.d. relative to total p38 or β-actin levels are shown. (D) In addition to immunoblotting, flow cytometric analysis of Saos-α2 cells showed that neither α2-specific primary nor secondary antibody alone caused p38 activation, whereas the combination of the antibodies elicited p38 phosphorylation. For analysis, cells were treated with clustering antibodies for 15 min, fixed with isopropanol, permeabilized with methanol and stained with Alexa Fluor 488-conjugated phospho-p38 antibody.
To analyse the effect of inactivating mutation E336A on α2β1 integrin signalling, we performed two series of experiments. CHO-α2 and CHO-α2E336A cells were exposed either to collagen I or fibronectin immobilized on cell culture plates (Figure 5A and B). When p38 phosphorylation was measured by immunoblotting, it seemed that CHO-α2 cells contained higher phospho-p38 levels on collagen I than on fibronectin (Figure 5A). However, in the CHO-α2E336A cells, p38 was not phosphorylated on collagen I, whereas its activation was clear on fibronectin (Figure 5A). Alternatively, α2 antibodies were used to cluster integrins (Figure 5B and C). In both CHO-α2 (Figure 5B) and Saos-α2 (Figure 5C) cells, p38 activation was obvious, whereas the E336A mutation inhibited the effect. On the basis of these data, in the following experiments, we used p38 activation as an indicator of E336-dependent conformational change in α2β1 integrin.
Figure 5.
Activation of p38 after α2β1 clustering by antibodies or natural ligands requires E336-dependent conformational changes in α2 subunit. (A) CHO-α2 and CHO-α2E336A cells were sub-cultured onto collagen I (Col I) or fibronectin-coated cell culture plates. After o/n incubation, cells were lysed and p38 phosphorylation (P-p38) was detected by immunoblotting. CHO-α2 cells showed higher p38 activation on collagen I than on fibronectin. Fibronectin seemed to activate p38 even in CHO-α2E336A cells; however, the E336A mutation inhibited α2β1 integrin-mediated p38 activation induced by collagen I. (B, C) Similarly, E336A caused a dramatic decrease in p38 activation after antibody-mediated α2 clustering both in (B) CHO-α2E336A and (C) Saos-α2E336A cells, whereas the p38 phosphorylation was induced in CHO and Saos cells expressing α2WT. Immunoblots and quantified data from representative experiments are shown. The mean level of p38 activation is shown relative to (A) total p38 or (B, C) β-actin levels.
EV1 entry is independent of E336-mediated conformational activation of α2β1
In addition to clustering antibodies, a similar relocation of α2β1 integrins to macroaggregates on the Saos-α2 cell surface was induced by EV1 after 15 min (Figure 6A). Both EV1 and antibody treatments caused similar cluster formation even in Saos-α2E336A cells (data not shown).
Figure 6.
α2β1 clustering, not the activation of p38 or E336-mediated conformational activation of the receptor, is required for the EV1 entry. (A) In Saos-α2 cells EV 1 induced cluster formation of α2 integrins in 15 min as indicated by the co-localization image (scale bar, 10 μm). Manders' co-localization coefficients are 0.52 for integrin and 0.51 for EV1 and P=1.0. (B) A 15-min EV1 clustering does not, however, induce p38 activation similar to that seen in antibody-mediated clustering in Saos-α2 cells (C). In both Saos-α2 and Saos-α2E336A cells, 30-min EV1 clustering induced a clear PKCα activation. (B, C) An immunoblot and quantified data (mean±s.e.) of five or three representative experiments are shown. Untreated cells or cells treated with α2 primary antibody (16B4) only were used as controls. (D) When the ability of EV1 to infect Saos-α2 and Saos-α2E336A was studied, both EV1 and α2β1 were labelled, and EV1-positive cells were manually calculated from confocal microscopy images. The E336A mutation introduced into α2 subunit seemed not to prevent EV1 entry into Saos cells. Data shown are mean values±s.d. of EV1-positive cells (%).
We have shown above that p38 activation after antibody-mediated clustering requires E336-dependent conformational changes in the integrin. This indicates that the clusters of extended α2β1 integrins activate different signalling pathways when compared with bent integrins. Accordingly, α2β1 clustering caused by EV1 did not induce p38 activation (Figure 6B), suggesting that binding of the virus to α2β1 does not lead to the activation of integrin conformation. Previously, EV1 entry has been shown to require PKCα activation (Upla et al, 2004). After 30-min EV1 clustering, both Saos-α2 and Saos-α2E366A cells showed similar PKCα activation when analysed by immunoblotting using phospho-specific antibody (Figure 6C). Finally, we imaged the ability of EV1 to infect cells that express mutant α2E336A integrins using confocal microscopy. Similarly to PKCα activation, even EV1 infection was observed both in Saos-α2 and Saos-α2E336A cells (Figure 6D). The results indicate that EV1 neither activates α2β1 in the same manner as the natural ligands, nor is dependent on these activation mechanisms during its cell-entry process.
EV1 may bind to integrins that are in the bent conformation
Our observations indicate that EV1 can bind to α2β1 integrins that have closed α2I domains, and also to integrins that are unable to effectively interact with natural ligands as a consequence of E336A mutation. Furthermore, the integrins harbouring E336A mutations may take the bent conformation. To test this further, we allowed CHO-α2 cells to adhere to collagen I or EV1 for 15 min in the presence of 2 mM ethylenediamine tetraacetate (EDTA). EDTA prevented cell adhesion to collagen I, but not to EV1 (Supplementary Figure S2A). The result replicates an old observation that EV1 can bind to α2β1 integrin in the absence of divalent cations (Bergelson et al, 1993) and reflects the fact that EV1 binding, unlike collagen binding, is independent of Mg2+ ion. However, the data also support the hypothesis that EV1 binds to the bent rather than extended α2β1 integrin, as more recent observations, based on activation-dependent antibodies, indicate that EDTA keeps integrins in the bent conformation (Xie et al, 2004). Furthermore, we could show that 2 mM EDTA also prevents p38 activation after α2β1 clustering, supporting the essential role of extended conformation in the process (Supplementary Figure S2B).
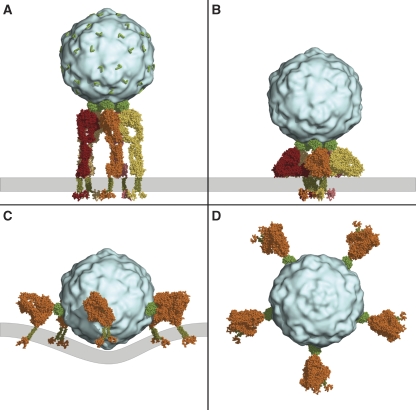
To further test the hypothesis that EV1 binds to the bent α2β1 integrin, we constructed models of the α2β1 heterodimer bound to EV1 in the bent and the extended conformation (Figure 7). The icosahedral EV1 capsid consists of 60 identical copies of the capsid protomer. Five protomers are arranged symmetrically in a pentameric structure around a fivefold symmetry axis at each of the 12 vertices of the icosahedron. Each protomer contains one binding site for the α2I domain. We have previously published a model in which the α2β1 ectodomain in the extended conformation can occupy all of the five sites in one pentamer on the EV1 surface without steric hindrance (Xing et al, 2004; Figure 7A). In the case of the bent conformation, a much wider receptor structure must be accommodated close to the virus surface. However, the linker segments connecting the α2I domain to the propeller domain and the ectodomain to the transmembrane helices are long and flexible enough to allow the heterodimer to adopt a large range of different conformations. We constructed a realistic model of a bent heterodimer that can fit into adjacent binding sites in one EV1 capsid pentamer (Figure 7B). However, the five integrins are necessarily packed very closely together and against the membrane in this arrangement, and the orientation of the α2I domain relative to the rest of the ectodomain is substantially different from that in the extended conformation model. An alternative model, in which integrins, in the bent conformation, are located at larger distances from each other around the virus surface, can also be constructed (Figure 7C and D). In this case, the α2β1 heterodimers are bound to protomers in different, adjacent pentamers. The transmembrane helices of the five heterodimers point roughly in the same direction, but the model requires the cell membrane to be curved around the viral particle. The illustrated models represent idealized cases in which each binding site of a symmetric set is occupied. On the basis of the results shown in Figure 1E, up to 10 out of 60 putative integrin-binding sites can be concomitantly occupied. This stoichiometry supports the models presented in Figures 7C and D rather than the model in Figure 7B.
Figure 7.
Structural models of the binding of α2β1 integrin clusters to EV1. (A) Five α2β1 heterodimers (different shades of orange and red) in the extended conformation are shown bound to the five EV1 capsid (light blue) protomers around one fivefold symmetry axis. α2I domains are drawn in green and the transmembrane helices in dark green. The position of the membrane is marked with a grey bar. The position and orientation of the unoccupied α2I domain-binding sites on the virus surface (one in each of the 60 protomers) is marked with green L-shaped lines. (B) Five α2β1 heterodimers in the bent conformation are shown bound to five protomers (forming a pentamer) around a fivefold symmetry axis. This arrangement results in the five integrins packing very closely together and against the membrane, but it may still be sterically feasible. The α2I domain and the transmembrane helices are connected to the rest of the integrin structure by highly flexible linkers that may allow a bent conformation that can fit in the neighbouring binding sites of one pentamer. (C) Five α2β1 heterodimers in the bent conformation, each bound to one protomer in five adjacent pentamers around the EV1 capsid. A grey line marks the position of the plasma membrane. The transmembrane helices of the five integrins are positioned such that the membrane would be required to be substantially curved around the virus. The same arrangement, looking down an axis perpendicular to the membrane, is shown in (D). In this pentagonal arrangement, the distance from the intracellular end of one transmembrane helix to corresponding part in the proximal, neighbouring integrin is approximately 320 Å and to a distal integrin is approximately 500 Å.
Discussion
EV1 is a human pathogen, which belongs to the Picornaviridae family of RNA viruses and causes meningoencephalitis, carditis and rashes, as well as mild respiratory and enteric diseases (Grist et al, 1978). Earlier studies have shown that EV1 binds to α2β1 on the cell surface and that the life cycle of this virus is critically dependent on the receptor (Bergelson et al, 1992, 1993). This has been evident in experiments performed on human cell lines, such as Saos-2 osteosarcoma cells that are α2β1 integrin negative and resistant to EV1 infection, but can act as EV1 host cells after transfection with α2 integrin cDNA (Marjomäki et al, 2002). Despite the facts that EV1 cannot infect mouse α2β1 integrin-expressing cell lines and that mouse integrin α2I domain does not bind to EV1 (Bergelson et al, 1994; Zhang and Racaniello, 1997), a transgenic mouse harbouring human α2 integrin is susceptible to the infection (Hughes et al, 2003). Taken together, the data suggest that high-affinity recognition of a cellular receptor is an essential step in the EV1 infection.
In addition to receptor binding, activation of an entry pathway is an essential process in the life cycle of several viruses. Many viruses that use members of the integrin family as their cellular receptors are internalized in clathrin-coated pits and later found in endosomes (Wickham et al, 1993, Joki-Korpela et al, 2001; Jin et al, 2002; O'Donnell et al, 2005). However, EV1 forms an exception, as, inside the host cell, virus particles seem to accumulate in caveolin-1-positive structures (Marjomäki et al, 2002). On the host cell surface, some EV1 particles can be detected inside caveolae (Marjomäki et al, 2002), but other entry mechanisms may be more important (Pietiäinen et al, 2004). We have recently proposed that EV1 is internalized through a clathrin- and caveolin-independent mechanism that resembles macropinocytosis (Karjalainen et al, 2008). After binding to α2β1 integrin, EV1 has been shown to activate PKCα and inhibition of this signalling pathway also blocks the entry process (Upla et al, 2004). Other integrin-binding viruses are also known to activate various signalling proteins. For example, binding of HHV-8 to α3β1 integrin activates the focal adhesion kinase immediately downstream in the outside-in signalling pathway mediated by integrins, this leads to further activation of several signalling molecules (Krishnan et al, 2006). In addition, the endocytosis of human adenoviruses that recognize αV integrins is dependent on the activation of phosphoinositide-3-OH kinase (Li et al, 1998). New approaches, such as the use of siRNA silencing, will most probably cast new light on the complex signalling pathways involved in virus internalization (Pelkmans et al, 2005).
In general, viruses are regarded as mimicking natural integrin ligands in their receptor-binding mechanism, as well as in the activation of cellular signalling. Human adenovirus type 2 (Ad2) is a good example. Cryo-EM has shown that the integrin-binding RGD protrusion of the Ad2 penton base protein is highly mobile (Stewart et al, 1997), in the same manner as the RGD motif in fibronectin (Main et al, 1992). When the structure of soluble recombinant integrin αVβ5 in complex with Ad2 was determined by cryo-EM, the results suggested that the precise spatial arrangement of five RGD protrusions on the penton base promotes integrin clustering and the signalling events required for virus internalization (Chiu et al, 1999).
When compared with Ad2, our experiments using EV1 and α2β1 integrin give a very different idea about the virus–integrin interaction. In αI domain-containing integrins, the binding of a natural ligand, such as collagen, triggers a change from the closed to the open αI domain conformation (Emsley et al, 2000). We tested EV1 binding to α2I domain harbouring the E318W mutation, which allows the integrin α2I domain to adopt the open high-affinity conformation. In contrast, the wild-type α2I domain has a salt bridge between residues R288 and E318, stabilizing the closed low-affinity state (Aquilina et al, 2002; Tulla et al, 2008). Unexpectedly, we noticed that EV1, unlike any other α2β1 ligand, favours the closed α2I domain over the open state. The result was confirmed using CHO cells expressing full-length wild-type α2 or α2E318W integrin. To further study the phenomenon in cell culture, we used cells transfected to express α2E336A mutant integrin. We have previously shown that α2β1 integrin avidity to collagen I is regulated by two synergistic mechanisms: first, an α2 E336-dependent switch to the open α2I conformation; second, an α2E336-independent mechanism associated with receptor aggregation (Connors et al, 2007). Previous studies have also indicated that mutation of the residue corresponding to α2E336 in αM (E320; Alonso et al, 2002) and αL (E310; Huth et al, 2000) may completely inactivate the integrin. In agreement with the previous observations (Connors et al, 2007), CHO-α2E336A cells could attach to collagen I but their spreading was significantly delayed. CHO-α2 cells were also able to adhere to immobilized collagen I better than the mutant CHO-α2E336A cells. Despite the fact that the mutation E336A in the α2 subunit prevents the conformational activation of α2β1 integrin and decreases cell adhesion to collagen I, the mutation increased adhesion to EV1. Thus, experiments using recombinant αI domains and cell lines harbouring integrin mutations led to the conclusion that EV1, unlike the natural ligands of α2β1 integrin, has evolved to recognize the non-activated integrin conformation. These observations, together with the fact that EV1 binding to α2β1 integrin also takes place in the absence of divalent cations, suggest that EV1 may also bind to integrins in the bent conformation. The integrin ectodomain is highly flexible, and it may be possible to accommodate α2β1 heterodimers in the bent conformation at adjacent binding sites in the pentamer of the EV1 capsid, even though this leads to very close packing of the integrins. Our calculations on the basis of molar ratios of α2I domain binding to a concentration series of EV1 suggest a stoichiometry in which up to 10 out of 60 putative integrin-binding sites can be occupied at the same time. This supports another model in which bent α2β1 heterodimers are bound at non-adjacent sites around the virus surface, assuming that the plasma membrane is curved around the viral particle. In this case, the intracellular domains of the integrins would be located about 320 Å apart, possibly forming a ring-like adhesion site. As integrin signalling is mediated by proteins that bind to intracellular domains of integrins, the unique architecture of EV1-dependent integrin clusters may govern the entry mechanism and life cycle of EV1.
On the basis of our structural model of the α2I–EV1 complex, we could not exclude the possibility that EV1 binding to the α2I domain could induce a conformational change from the closed to open form. Therefore, we selected an indirect way to test whether the conformational change in α2β1 integrin takes place after EV1 binding. So far, we have been unable to use direct methods to monitor integrin conformation due to technical problems. Previously, the tail separation of the αLβ2 heterodimer has been demonstrated by using integrins with fluorescent protein tags fused to their intracellular domains (Kim et al, 2003). However, we have not succeeded in expressing tagged α2 subunits in the cells that we used in these experiments. The indirect approach was based on the previous observation that α2β1 integrin specifically activates p38 MAPK (Ivaska et al, 1999). Here, we could show that antibody-mediated clustering of α2β1 integrin causes a rapid but transient phosphorylation of p38. However, when the E336A mutation is introduced into the α2 subunit, the activation of p38 is not achieved. Thus, clustering itself is not sufficient for p38 activation but the conformational modulation of α2β1 is also required. The observation also allowed us to use p38 phosphorylation as an indicator of the conformational activation of α2β1 integrin after EV1-mediated clustering. Importantly, EV1-induced clustering did not evoke p38 activation at an early stage of infection, strongly suggesting that after virus binding α2β1 does not undergo a change to similar conformation that takes place after clustering induced by either antibodies or natural ligands. In previous studies, p38 activation has been studied some hours after infection and p38 seems to be phosphorylated when EV1 replication has started (Huttunen et al, 1998). The E336A mutation in the α2 subunit did neither affect EV1 induced activation of PKCα, which has been previously linked to EV1 internalization (Upla et al, 2004), nor the ability of EV1 to infect cells. Thus, the results propose that EV1 does not activate its entry pathway by direct mimicry of natural ligands, but it has evolved to take the advantage of integrin biology in a unique manner.
To conclude, our observations during the early steps of the EV1 life cycle suggest that the virus binds first to inactive, bent α2β1 integrins and clusters them without triggering a conformational activation of the receptor. Thus, EV1 seems to activate its own entry by simply clustering α2β1 integrins. PKCα has been suggested to be one part of the entry machinery (Upla et al, 2004), and our new observations also indicate that PKCα activation by EV1 is not dependent on the E336-mediated conformational changes in α2β1. Previous observations have shown that the deletion of the α2 cytoplasmic domain (Kawakuchi et al, 1994) or its replacement with the α1 cytoplasmic domain (Marjomäki et al, 2002) has no effect on EV1 entry. PKCα may interact with α2β1 integrin through β1 cytoplasmic domain (Connors et al, 2007), whereas the activation of p38 is dependent on the α2 cytoplasmic domain (Ivaska et al, 1999). Thus, EV1 also provides a unique tool to discriminate between the effects of clustering and conformational activation in integrin signalling, as well as between α2- and β1-mediated signalling.
Materials and methods
EV1 and cell cultures
EV1 (Farouk strain, ATCC) was propagated in GMK cells and purified in sucrose gradients as previously described (Abraham and Colonno, 1984). Saos-2 (ATCC) and CHO cells (ATCC) were cultured and stably transfected with human α2 or with the α2E336A, α2E309A mutation in the pAWneo2 expression vector as described (Ivaska et al, 1999; Connors et al, 2007). A point mutation E318W was introduced into human α2 cDNA in a pcDNA 3.1 expression vector (Invitrogen) using QuikChange mutagenesis kit (Stratagene) according to the manufacturer's instructions. The construct was stably expressed in CHO cells using the previously described method (Connors et al, 2007).
Molecular modelling
Structural models of human α2β1 headpiece domains were based on the crystal structures on the αVβ3 and the α2I domain as described (Connors et al, 2007). The model of the α2I (closed)–EV1 complex, based on cryo-EM data and the crystal structures of EV1 (Filman et al, 1998; 1EV1) and α2I domain in the closed conformation (Emsley et al, 1997; 1AOX), was constructed earlier by Xing et al (2004). The EV1 complex with the open conformation was built by superimposing on this model the open form I domain structure (1DZI; Emsley et al, 2000). To model clusters of α2β1 bound to EV1, a comparative model of the entire α2β1 integrin heterodimer in the bent conformation was built with Modeller 9.6 (Marti-Renom et al, 2000) using the crystal structures of both αVβ3 (1JV2; Xiong et al, 2001) and αIIbβ3 (Zhu et al, 2008; 3FCS) as templates. The crystal structure of α2I in the closed conformation (1AOX) was added. The orientation of the I domain was modelled manually, constrained by the distance of α2E336 to the βI domain MIDAS site. The extended conformation of the dimer was modelled by moving the domains by hand to match published electron micrographs. Molecular graphics in Figures 1A, 3A and 7 were created using PyMOL v1.1 (DeLano, 2002).
Solid phase binding assay
The α2I domain was generated by PCR using human integrin α2 cDNA as a template (Ivaska et al, 1999). E318W point mutation was introduced into the α2I domain as described previously (Tulla et al, 2008). Both α2I WT and α2I E318W were produced as GST fusion protein as previously described (Tulla et al, 2001). Microtitre plates (Costar) were coated with collagen I (20 μg/cm2; PureCol, INAMED Biomaterials) or with similar concentrations of EV1 o/n at 4°C. In the competition studies, 1.5 nM α2I domain was allowed to attach to immobilized EV1 in the presence of 0.04–0.7 nM EV1 or 0.01–1000 μM GFOGER collagen peptide (Auspep, Australia) synthesized as previously described (Knight et al, 2000). Otherwise the assay was carried out essentially as previously described (Jokinen et al, 2004).
BIAcore analysis
Measurements were performed using surface plasmon resonance on a BIAcore-X (GE Healthcare Bio-Sciences AB, Uppsala, Sweden). EV1 was covalently coupled through primary amine groups to the dextran matrix of a CM5 sensor chip (GE Healthcare Bio-Sciences AB, Uppsala, Sweden) using sodium formate (pH 3.0) according to Lea et al (1998). Bound EV1 levels were adjusted to about 2000 resonance units (RU). The 1 μM α2I WT and α2I E318W were passed over the chip at flow rate 30 μl/min at 25°C. HEPES-buffered saline (10 mM HEPES (pH 7.4), 150 mM NaCl, 3.4 mM EDTA, and 0.005% surfactant P20; GE Healthcare Bio-Sciences AB, Uppsala, Sweden) was used as a running buffer throughout.
Cell spreading and adhesion assay
For the assays, microtitre plates (Costar) were coated with collagen I (20 μg/cm2), EV1 or 1% BSA o/n at 4°C. The spreading assay was performed as described previously (Jokinen et al, 2004). In the adhesion assay, non-specific binding sites were blocked with 1% BSA in PBS for 1 h at 37°C before addition of 2 × 105 cells per well. When indicated, cells were pre-treated with 100 nM 12-O-tetradecanoylphorbol-13-acetate (TPA; Calbiochem) in serum-free media for 10 min. EtOH was added to control cells. Cells were allowed to attach for 15 min at 37°C in the presence of 2 mM MgCl2 or 2 mM EDTA. Wells were then washed and adherent cells were detected using tetrazolium salt WST-1 reagent (Roche) according to manufacturer's instructions. The absorbance was measured at 450 nm (Labsystems Multiscan Plus).
Confocal microscopy imaging of integrin clustering
Saos-α2 cells were cultured as described above, but on chambered cover glasses with CO2-independent medium (Sigma). For antibody clustering, cells were incubated with Alexa-555-conjugated mAb against α2 integrin (16B4, Serotec) for 15 min at 37°C. The secondary antibody (goat anti-mouse IgG, Molecular Probes) was added to induce clustering. Three-dimensional image stacks of the same cell were obtained with a laser scanning confocal fluorescence microscope (Carl Zeiss, Axiovert 100 M with LSM510), before and 15 min after adding the secondary antibody. For the negative control no secondary antibody was added. Alternatively, Saos-α2 cells were incubated with EV1 for 15 min at 37°C, samples were fixed with 4% PFA for 20 min at RT, and stained for EV1 and α2 integrin. To detect EV1, rabbit anti-EV1 (Marjomäki et al, 2002) and Alexa-555-conjugated secondary antibodies (Molecular Probes) were used. Integrin α2 was stained with mouse mAb 16B4 (Serotec) and Alexa-488-conjugated secondary antibody (Molecular Probes). Three-dimensional ray cast opacity volume renderings and co-localization analyses (automatic thresholding after background subtraction, Costes P-value calculation with 100 iterations) of selected image stacks were performed using BioImageXD software (Kankaanpää et al, 2006).
Measurement of EV1 infection
For the measurement of EV1 infection, Saos-WT, Saos-α2 and Saos-α2E336A cells were incubated with EV1 for 2–6 h and fixed with 3% paraformaldehyde for 20 min at RT. Triton X-100 (0.1%)-permeabilized cells were stained for EV1 and α2 integrin as mentioned above. Finally, cells were observed under confocal microscope (Carl Zeiss Axiovert 100 M with LSM510), and the percentage of EV1-positive cells was determined by counting manually.
p38 and PKCα activation induced by integrin clustering
Cells incubated with 0.1% FCS in DMEM o/n, were treated with integrin α2 antibody (16B4, Serotec) for 15 min at 37°C, followed by 15–120-min secondary antibody treatment (anti-mouse IgG, DAKO) at 37°C. Alternatively, cells were incubated with EV1 for 15 min (P-p38) or 30 min (P-PKCα) at 37°C. When indicated, clustering was induced in the presence of 2 mM EDTA. Finally, cells were collected and analysed by immunoblotting as described below.
p38 activation on collagen I and fibronectin-coated plates
Cell culture plates were coated with collagen I or with human plasma fibronectin (Chemicon International) 20 μg/cm2 in PBS o/n at 4°C. Before addition of cells, the coated wells were washed with PBS and blocked with 0.1% BSA in PBS for 1 h at 37°C. Cells were allowed to attach in serum-free αMEM at 37°C, 5% CO2 o/n. Finally, cells were collected and the samples were immunoblotted as described below.
Analysis of kinase activation
Cells were lysed in Laemmli SDS–PAGE sample buffer and the sonicated samples were separated on a 10% acrylamide SDS–PAGE gel and electroblotted onto a Hybond ECL membrane (Amersham, UK). Membranes were probed for activated p38 as previously described (Ivaska et al, 1999) using a phospho-specific p38 antibody (T180/Y182; Cell Signaling Technology or Zymed) and antibodies against total p38 (Cell Signaling Technology or Zymed) or β-actin (I-19, Santa Cruz). To measure PKCα activation, phospho-specific antibody (Millipore) was used. The intensity of bands was quantified by densitometry using a Microcomputer Imaging Device version M5plus (Imaging Research). Alternatively, p38 activation was analysed with Flow Cytometric analysis using Alexa Fluor 488-conjugated P-p38 (T180/Y182) mouse mAb according to manufacturer's instructions (Cell Signaling Technology). The Cyflogic 1.1.1. (CyFlo, Turku, Finland) software was used for the analysis of the cytometry data.
Supplementary Material
Supplementary Figure S1
Supplementary Figure S2
Figure for Referees
Supplementary Figure S1 Legend
Supplementary Figure S2 Legend
Review Process File
Acknowledgments
We thank Petri Susi and Ritva Kajander for providing the virus, Jouko Sandholm and Perttu Terho for cell sorting and Maria Tuominen for technical assistance. This study was funded by the Academy of Finland, the Technology Development Center of Finland, the Sigrid Jusélius Foundation, the Finnish Cancer Association, the Finnish Cultural Foundation, the Foundation of Åbo Akademi University's Center of Excellence Program, the Tor, Joe and Pentti Borgs Memorial Fund, and the National Graduate School of Informational and Structural Biology. Development of BioImageXD software and studies on integrin trafficking have been supported by EU 7th framework program as part of the Metafight project.
Footnotes
The authors declare that they have no conflict of interest.
References
- Abraham G, Colonno RJ (1984) Many rhinovirus serotypes share the same cellular receptor. J Virol 51: 340–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akula SM, Pramod NP, Wang FZ, Chandran B (2002) Integrin α3β1 (CD49c/29) is a cellular receptor for Kaposi's sarcoma-associated herpesvirus (KSHV/HHV-8) entry into the target cells. Cell 108: 407–419 [DOI] [PubMed] [Google Scholar]
- Alonso JL, Essafi M, Xiong JP, Stehle T, Arnaout MA (2002) Does the integrin αA domain act as a ligand for its βA domain? Curr Biol 12: 340–342 [DOI] [PubMed] [Google Scholar]
- Aquilina A, Korda M, Bergelson JM, Humphries MJ, Farndale RW, Tuckwell D (2002) A novel gain-of function mutation of the integrin α2 vWFA domain. Eur J Biochem 269: 1136–1144 [DOI] [PubMed] [Google Scholar]
- Arnaout MA, Mahalingam B, Xiong JP (2005) Integrin structure, allostery, and bidirectional signaling. Annu Rev Cell Dev Biol 21: 381–410 [DOI] [PubMed] [Google Scholar]
- Bergelson JM, Chan BM, Finberg RW, Hemler M (1993) The integrin VLA-2 binds echovirus 1 and extracellular matrix ligands by different mechanisms. J Clin Invest 92: 232–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergelson JM, Shepley MP, Chan ME, Hemler ME, Finberg RW (1992) Identification of the integrin VLA-2 as a receptor for echovirus 1. Science 255: 1718–1720 [DOI] [PubMed] [Google Scholar]
- Bergelson JM, St John NF, Kawaguchi S, Pasqualini R, Berdichevsky F, Hemler M, Finberg RW (1994) The I domain is essential for echovirus interaction with VLA-2. Cell Adhes Commun 2: 455–464 [DOI] [PubMed] [Google Scholar]
- Bix G, Fu J, Gonzalez EM, Macro L, Barker A, Campbell S, Zutter MM, Santoro SA, Kim JK, Hook M, Reed CC, Iozzo RV (2004) Endorepellin causes endothelial cell disassembly of actin cytoskeleton and focal adhesions through α2β1 integrin. J Cell Biol 166: 97–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang KH, Auvinen P, Hyypiä T, Stanway G (1989) The nucleotide sequence of coxsackievirus A9—implications for receptor binding and enterovirus classification. J Gen Virol 70: 3269–3280 [DOI] [PubMed] [Google Scholar]
- Chiu CY, Mathias P, Nemerow GR, Stewart PL (1999) Structure of adenovirus complexed with its internalization receptor, αVβ5 integrin. J Virol 3: 6759–6768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connors WL, Jokinen J, White DJ, Puranen JS, Kankaanpää P, Upla P, Tulla M, Johnson MS, Heino J (2007) Two synergistic activation mechanisms of α2β1 integrin-mediated collagen binding. J Biol Chem 282: 14675–14683 [DOI] [PubMed] [Google Scholar]
- DeLano WL (2002) The PyMOL Molecular Graphics System. San Carlos, CA, USA: DeLano Scientific http://www.pymol.org [Google Scholar]
- Dickeson SK, Mathis NL, Rahman M, Bergelson JM, Santoro SA (1999) Determinants of ligand binding specificity of the α1β1 integrins. J Biol Chem 274: 32182–32191 [DOI] [PubMed] [Google Scholar]
- Emsley J, King SL, Bergelson JM, Liddington RC (1997) Crystal structure of the I domain from integrin α2β1. J Biol Chem 272: 28512–28517 [DOI] [PubMed] [Google Scholar]
- Emsley J, Knight CG, Farndale RW, Barnes MJ, Liddington RC (2000) Structural basis of collagen recognition by integrin α2β1. Cell 101: 47–56 [DOI] [PubMed] [Google Scholar]
- Filman DJ, Wien MW, Cunningham JA, Bergelson JM, Hogle JM (1998) Structure determination of echovirus 1. Acta Crystallogr 54: 1261–1272 [DOI] [PubMed] [Google Scholar]
- Fox G, Parry NR, Barnett PV, McGinn B, Rowlands DJ, Brown F (1989) The cell attachment site on foot-and-mouth disease virus includes the amino acid sequence RGD (arginine-glycine-aspartatic acid). J Gen Virol 70: 625–637 [DOI] [PubMed] [Google Scholar]
- Grist NR, Bell EJ, Assaad F (1978) Enteroviruses in human disease. Prog Med Virol 24: 114–157 [PubMed] [Google Scholar]
- Hughes SA, Thaker HM, Racaniello VR (2003) Transgenic mouse model for echovirus myocarditis and paralysis. Proc Natl Acad Sci USA 100: 15906–15911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huth JR, Olejniczak ET, Mendoza R, Liang H, Harris EA, Lupher ML, Wilson AE Jr, Fesik SW, Staunton DE (2000) NMR and mutagenesis evidence for an I domain allosteric site that regulates lymphocyte function-associated antigen 1 ligand binding. Proc Natl Acad Sci USA 97: 5231–5236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttunen P, Hyypiä T, Vihinen P, Nissinen L, Heino J (1998) Echovirus 1 infection induces both stress- and growth-activated mitogen-activated protein kinase pathways and regulates the transcription of cellular immediate-early genes. Virology 250: 85–93 [DOI] [PubMed] [Google Scholar]
- Hyypiä T, Horsnell C, Maaronen M, Khan M, Kalkkinen N, Auvinen P, Kinnunen L, Stanway G (1992) A distinct picornavirus group identified by sequence analysis. Proc Natl Acad Sci USA 89: 8847–8851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivaska J, Reunanen H, Westermarck J, Koivisto L, Kähäri V-M, Heino J (1999) Integrin α2β1 mediates isoform-specific activation of p38 and upregulation of collagen gene transcription by a mechanism involving the α2 cytoplasmic tail. J Cell Biol 147: 401–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson T, Sharma A, Ghazaleh RA, Blakemore WE, Ellard FM, Simmons DL, Newman JW, Stuart DI, King AM (1997) Arginine-glycine-aspartatic acid-specific binding by foot-and-mouth disease viruses to the purified integrin αVβ3 in vitro. J Virol 71: 8357–8361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin M, Andricioaei I, Springer TA (2004) Conversion between three conformational states of integrin I domains with a C-terminal pull spring studied with molecular dynamics. Structure 12: 2137–2147 [DOI] [PubMed] [Google Scholar]
- Jin M, Park J, Lee S, Park B, Shin J, Song KJ, Ahn TI, Hwang SY, Ahn BY, Ahn K (2002) Hantaan virus enters cells by clathrin-dependent receptor-mediated endocytosis. Virology 294: 60–69 [DOI] [PubMed] [Google Scholar]
- Joki-Korpela P, Marjomäki V, Krogerus C, Heino J, Hyypiä T (2001) Entry of human parechovirus 1. J Virol 75: 1958–1967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jokinen J, Dadu E, Nykvist P, Käpylä J, White DJ, Ivaska J, Vehviläinen P, Reunanen H, Larjava H, Häkkinen L, Heino J (2004) Integrin-mediated cell adhesion to type I collagen fibrils. J Biol Chem 279: 31956–31963 [DOI] [PubMed] [Google Scholar]
- Kankaanpää P, Pahajoki K, Marjomäki V, Heino J, White DJ (2006) BioImageXD—free open source software for analysis and visualization of multidimensional biomedical images. (http://www.bioimagexd.net)
- Karjalainen M, Kakkonen E, Upla P, Paloranta H, Kankaanpää P, Liberali P, Renkema GH, Hyypiä T, Heino J, Marjomäki V (2008) A Raft-derived, Pak1-regulated entry participates in α2β1 integrin-dependent sorting to caveosomes. Mol Biol Cell 19: 2857–2869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson R, Phinikoula SK, Nordin H, Pol E, Myszka DG (2006) Analyzing a kinetic titration series using affinity biosensors. Anal Biochem 349: 136–147 [DOI] [PubMed] [Google Scholar]
- Kawakuchi S, Bergelson JM, Finberg RW, Hemler M (1994) Integrin α cytoplasmic domain deletion effects: loss of adhesivity activity parallels ligand-independent recruitment into focal adhesions. Mol Biol Cell 5: 977–988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M, Carman CV, Springer TA (2003) Bidirectional transmembrane signaling by cytoplasmic domain separation in integrins. Science 301: 1720–1725 [DOI] [PubMed] [Google Scholar]
- King SL, Kamata T, Cunningham JA, Emsley J, Liddington RC, Takada Y, Bergelson JM (1997) Echovirus 1 interaction with the human very late antigen-2 (integrin α2β1) I domain. Identification of two independent virus contact sites distinct from the metal ion-dependent adhesion site. J Biol Chem 272: 28518–28522 [DOI] [PubMed] [Google Scholar]
- Knight CG, Morton LF, Onley DJ, Peachey AR, Messent AJ, Smethurst PA, Tuckwell DS, Farndale RW, Barnes MJ (1998) Identification in collagen type I of an integrin α2β1-binding site containing an essential GER sequence. J Biol Chem 273: 33287–33294 [DOI] [PubMed] [Google Scholar]
- Knight CG, Morton LF, Peachey AR, Tuckwell DS, Farndale RW, Barnes MJ (2000) The collagen-binding A-domains of integrins α1β1 and α2β1 recognize the same specific amino acid sequence, GFOGER, in native (triple-helical) collagens. J Biol Chem 273: 35–40 [DOI] [PubMed] [Google Scholar]
- Krishnan HH, Sharma-Walia N, Streblow DN, Naranatt PP, Chandran B (2006) Focal adhesion kinase is critical for entry of Kaposi's sarcoma-associated herpesvirus into target cells. J Virol 80: 1167–1180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lea SM, Powell RM, McKee T, Evans DJ, Brown D, Stuart DI, van der Merwe PA (1998) Determination of the affinity and kinetic constants for the interaction between the human virus echovirus 11 and its cellular receptor, CD55. J Biol Chem 273: 30443–30447 [DOI] [PubMed] [Google Scholar]
- Li E, Stupack D, Klemke R, Cheresh DA, Nemerow GR (1998) Adenovirus endocytosis via αv integrins requires phosphoinositide-3-OH kinase. J Virol 72: 2055–2061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Main AL, Harvey TS, Baron M, Boyd J, Campbell ID (1992) The three-dimensional structure of the tenth type III module of fibronectin: an insight into RGD-mediated interactions. Cell 71: 671–678 [DOI] [PubMed] [Google Scholar]
- Marjomäki V, Pietiäinen V, Matilainen H, Upla P, Ivaska J, Nissinen L, Reunanen H, Huttunen P, Hyypiä T, Heino J (2002) Internalization of echovirus 1 in caveolae. J Virol 76: 1856–1865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marti-Renom MA, Stuart A, Fiser A, Sánchez R, Melo F, Sali A (2000) Comparative protein structure modeling of genes and genomes. Annu Rev Biophys Biomol Struct 29: 291–325 [DOI] [PubMed] [Google Scholar]
- Mazharian A, Roger S, Maurice P, Berrou E, Popoff MR, Hoylaerts MF, Fauvel-Lafeve F, Bonnefoy A, Bryckaert M (2005) Differential involvement of ERK2 and p38 in platelet adhesion to collagen. J Biol Chem 280: 26002–26010 [DOI] [PubMed] [Google Scholar]
- Moser M, Legate KR, Zent R, Fässler R (2009) The tail of integrins, talin, and kindlins. Science 324: 895–899 [DOI] [PubMed] [Google Scholar]
- Nykvist P, Tu H, Ivaska J, Käpylä J, Pihlajaniemi T, Heino J (2000) Distinct recognition of collagen subtypes by α1β1 and α2β1 integrins. α1β1 mediates cell adhesion to type XIII collagen. J Biol Chem 275: 8255–8261 [DOI] [PubMed] [Google Scholar]
- O'Donnell V, LaRocco M, Duque H, Baxt B (2005) Analysis of foot-and-mouth disease virus internalization events in cultured cells. J Virol 79: 8506–8518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelkmans L, Fava E, Grabner H, Hannus M, Habermann B, Krausz E, Zerial M (2005) Genome-wide analysis of human kinases in clathrin- and caveolae/raft-mediated endocytosis. Nature 436: 78–86 [DOI] [PubMed] [Google Scholar]
- Pietiäinen V, Marjomäki V, Upla P, Pelkmans L, Helenius A, Hyypiä T (2004) Echovirus 1 endocytosis into caveosomes requires lipid rafts, dynamin II, and signaling events. Mol Biol Cell 15: 4911–4925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravanti L, Heino J, López-Otín C, Kähäri V-M (1999) Induction of collagenase-3 (MMP-13) expression in human skin fibroblasts by three-dimensional collagen is mediated by p38 mitogen-activated protein kinase. J Biol Chem 274: 2446–2455 [DOI] [PubMed] [Google Scholar]
- Roivainen M, Hyypiä T, Piirainen L, Kalkkinen N, Stanway G, Hovi T (1991) RGD-dependent entry of coxsackievirus A9 into host cells and its bypass after cleavage of VP1 by intestinal proteases. J Virol 65: 4735–4740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roivainen ML, Piirainen T, Hovi I, Virtanen T, Riikonen J, Heino J, Hyypiä T (1994) Entry of coxsackievirus A9 into host cells: specific interactions with αVβ3, the vitronectin receptor. Virology 203: 357–365 [DOI] [PubMed] [Google Scholar]
- Ruoslahti E, Pierschbacher MD (1987) New perspectives in cell adhesion: RGD and integrins. Science 238: 491–493 [DOI] [PubMed] [Google Scholar]
- Salas A, Shimaoka M, Kogan AN, Harwood C, von Andrian UH, Springer TA (2004) Rolling adhesion through an extended conformation of integrin αLβ2 and relation to αI and βI-like domain interaction. Immunity 20: 393–406 [DOI] [PubMed] [Google Scholar]
- Shimaoka M, Salas A, Yang W, Weitz-Schmidt G, Springer TA (2003b) Small molecular integrin antagonists that bind to the β2 subunit I-like domain and activate signals in one direction and block them in the other. Immunity 19: 391–402 [DOI] [PubMed] [Google Scholar]
- Shimaoka M, Xiao T, Liu J-H, Yang Y, Dong Y, Jun C-D, McCormack A, Zhang R, Joachimiak A, Takagi J, Wang J-H, Springer T (2003a) Structures of the αL I domain and its complex with ICAM-1 reveal a shape-shifting pathway for integrin regulation. Cell 112: 99–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer TA, Wang JH (2004) The three-dimensional structure of integrins and their ligands, and conformational regulation of cell adhesion. Adv Protein Chem 68: 29–63 [DOI] [PubMed] [Google Scholar]
- Stanway G, Kalkkinen N, Roivainen M, Ghazi F, Khan M, Smyth M, Meurman O, Hyypiä T (1994) Molecular and biological characteristics of echovirus 22—A representative of a new picornavirus group. J Virol 68: 8232–8238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart PL, Chiu CY, Huang S, Muir T, Zhao Y, Chait B, Mathias P, Nemerow GR (1997) Cryo-EM visualization of an exposed RGD epitope on adenovirus that escapes antibody neutralization. EMBO J 16: 1189–1198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulla M, Lahti M, Puranen JS, Brandt AM, Käpylä J, Domogatskaya A, Salminen TA, Tryggvason K, Johnson MS, Heino J (2008) Effects of conformational activation of integrin α1I and α2I domains on selective recognition of laminin and collagen subtypes. Exp Cell Res 314: 1734–1743 [DOI] [PubMed] [Google Scholar]
- Tulla M, Pentikäinen OT, Viitasalo T, Käpylä J, Impola U, Nykvist P, Nissinen L, Johnson MS, Heino J (2001) Selective binding of collagen subtypes by integrin alpha 1I, alpha 2I, and alpha 10I domains. J Biol Chem 276: 48206–48212 [DOI] [PubMed] [Google Scholar]
- Upla P, Marjomäki V, Kankaanpää P, Ivaska J, Hyypiä T, Van Der Goot FG, Heino J (2004) Clustering induces a lateral redistribution of α2β1 integrin from membrane rafts to caveolae and subsequent protein kinase C-dependent internalization. Mol Biol Cell 15: 625–636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veettil MV, Sadagopan S, Sharma-Walia N, Wang FZ, Raghu H, Varga L, Chandran B (2008) Kaposi's sarcoma associated Herpesvirus (KSHV/HHV-8) forms a multi-molecular complex of integrins (αVβ5, αVβ3 and α3β1) and CD98-xCT during infection of human dermal microvascular endothelial (HMVEC-d) cells and CD98-xCT is essential for post-entry stage of infection. J Virol 82: 12126–12144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickham RE, Mathias P, Cheresh D, Nemerow G (1993) Integrins αVβ3 and αVβ5 promote adenovirus internalization but not virus attachment. Cell 73: 309–319 [DOI] [PubMed] [Google Scholar]
- Williams CH, Kajander T, Hyypiä T, Jackson T, Sheppard D, Stanway G (2004) Integrin αVβ6 is an RGD-dependent receptor for coxsackievirus A9. J Virol 78: 6967–6973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao T, Takagi J, Coller BS, Wang J-H, Springer TA (2004) Structural basis for allostery in integrins and binding to fibrinogen-mimetic therapeutics. Nature 432: 99–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie C, Shimaoka M, Xiao T, Schwab P, Klickstein LB, Springer TA (2004) The integrin α-subunit leg extends at the Ca2+-dependent epitope in the thight/genu interface upon activation. Proc Natl Acad Sci USA 101: 15422–15427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing L, Huhtala M, Pietiäinen V, Käpylä J, Vuorinen K, Marjomäki V, Heino J, Johnson M, Hyypiä T, Cheng RH (2004) Structural and functional analysis of integrin α2I domain interaction with echovirus 1. J Biol Chem 279: 11632–11638 [DOI] [PubMed] [Google Scholar]
- Xiong JP, Stehle T, Diefenbach B, Zhang R, Dunker R, Scott DL, Joachimiak A, Goodman SL, Arnaout MA (2001) Crystal structure of the extracellular segment of integrin αVβ3. Science 294: 339–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Clark RA, Parks WC (2001) p38 mitogen-activated kinase is a bidirectional regulator of human fibroblast collagenase-1 induction by three-dimensional collagen lattices. Biochem J 355: 437–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W, Shimaoka M, Salas A, Takagi J, Springer TA (2004) Intersubunit signal transmission in integrins by a receptor-like interaction with a pull spring. Proc Natl Acad Sci USA 101: 2906–2911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zárate S, Espinosa R, Romero P, Guerrero CA, Arias CF, López S (2000) Integrin α2β1 mediates the cell attachment of the rotavirus neuraminidase-resistant variant nar3. Virology 278: 50–54 [DOI] [PubMed] [Google Scholar]
- Zhang S, Racaniello VR (1997) Persistent echovirus infection of mouse cells expressing the viral receptor VLA-2. Virology 235: 293–301 [DOI] [PubMed] [Google Scholar]
- Zhu J, Luo BH, Xiao T, Zhang C, Nishida N, Springer TA (2008) Structure of a complete integrin ectodomain in a physiologic resting state and activation and deactivation by applied forces. Mol Cell 32: 849–861 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure S1
Supplementary Figure S2
Figure for Referees
Supplementary Figure S1 Legend
Supplementary Figure S2 Legend
Review Process File