Abstract
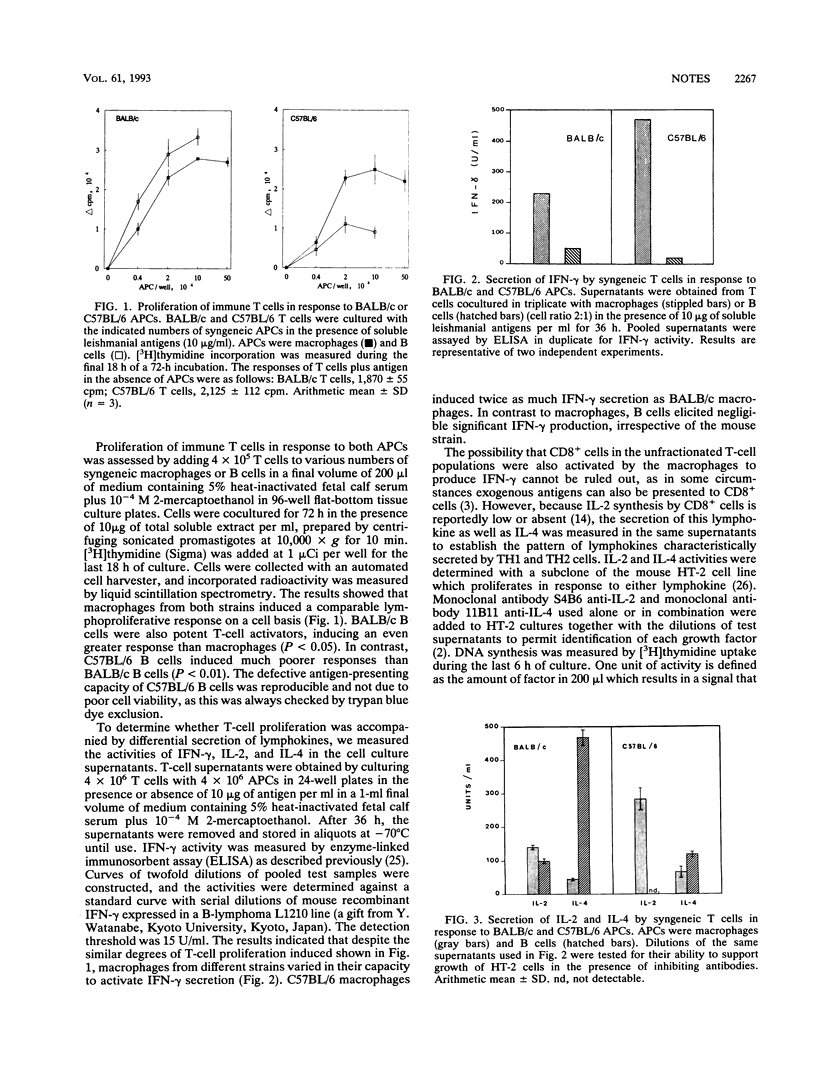
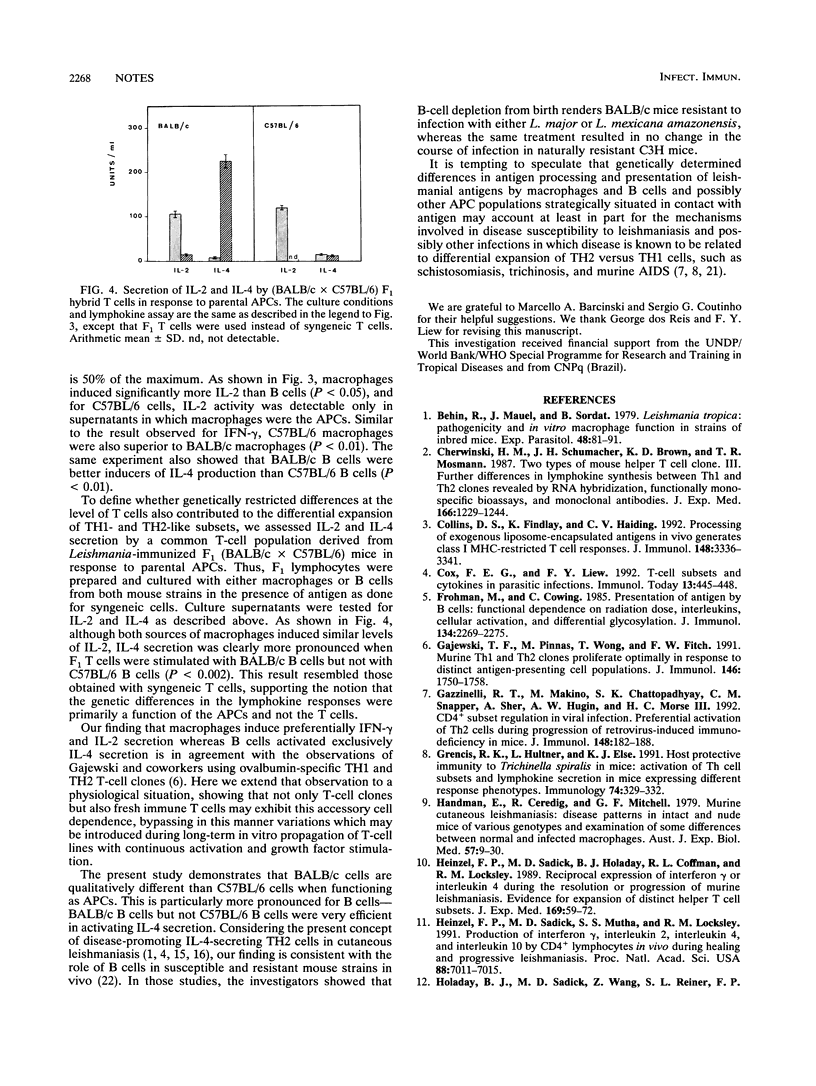
The role of antigen-presenting cells in the differential expansion of TH1 and TH2 T cells in murine leishmaniasis was investigated. In general, macrophages preferentially induced gamma interferon and interleukin-2 secretion by syngeneic Leishmania-specific T cells, whereas B cells were more efficient in activating interleukin 4 production. B cells from susceptible BALB/c mice were better in inducing TH2 responses than B cells from resistant C57BL/6 mice, whereas macrophages from C57BL/6 mice were superior to BALB/c macrophages in inducing TH1 responses.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Behin R., Mauel J., Sordat B. Leishmania tropica: pathogenicity and in vitro macrophage function in strains of inbred mice. Exp Parasitol. 1979 Aug;48(1):81–91. doi: 10.1016/0014-4894(79)90057-2. [DOI] [PubMed] [Google Scholar]
- Cherwinski H. M., Schumacher J. H., Brown K. D., Mosmann T. R. Two types of mouse helper T cell clone. III. Further differences in lymphokine synthesis between Th1 and Th2 clones revealed by RNA hybridization, functionally monospecific bioassays, and monoclonal antibodies. J Exp Med. 1987 Nov 1;166(5):1229–1244. doi: 10.1084/jem.166.5.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins D. S., Findlay K., Harding C. V. Processing of exogenous liposome-encapsulated antigens in vivo generates class I MHC-restricted T cell responses. J Immunol. 1992 Jun 1;148(11):3336–3341. [PubMed] [Google Scholar]
- Cox F. E., Liew F. Y. T-cell subsets and cytokines in parasitic infections. Immunol Today. 1992 Nov;13(11):445–448. doi: 10.1016/0167-5699(92)90072-F. [DOI] [PubMed] [Google Scholar]
- Frohman M., Cowing C. Presentation of antigen by B cells: functional dependence on radiation dose, interleukins, cellular activation, and differential glycosylation. J Immunol. 1985 Apr;134(4):2269–2275. [PubMed] [Google Scholar]
- Gajewski T. F., Pinnas M., Wong T., Fitch F. W. Murine Th1 and Th2 clones proliferate optimally in response to distinct antigen-presenting cell populations. J Immunol. 1991 Mar 15;146(6):1750–1758. [PubMed] [Google Scholar]
- Gazzinelli R. T., Makino M., Chattopadhyay S. K., Snapper C. M., Sher A., Hügin A. W., Morse H. C., 3rd CD4+ subset regulation in viral infection. Preferential activation of Th2 cells during progression of retrovirus-induced immunodeficiency in mice. J Immunol. 1992 Jan 1;148(1):182–188. [PubMed] [Google Scholar]
- Grencis R. K., Hültner L., Else K. J. Host protective immunity to Trichinella spiralis in mice: activation of Th cell subsets and lymphokine secretion in mice expressing different response phenotypes. Immunology. 1991 Oct;74(2):329–332. [PMC free article] [PubMed] [Google Scholar]
- Handman E., Ceredig R., Mitchell G. F. Murine cutaneous leishmaniasis: disease patterns in intact and nude mice of various genotypes and examination of some differences between normal and infected macrophages. Aust J Exp Biol Med Sci. 1979 Feb;57(1):9–29. doi: 10.1038/icb.1979.2. [DOI] [PubMed] [Google Scholar]
- Heinzel F. P., Sadick M. D., Holaday B. J., Coffman R. L., Locksley R. M. Reciprocal expression of interferon gamma or interleukin 4 during the resolution or progression of murine leishmaniasis. Evidence for expansion of distinct helper T cell subsets. J Exp Med. 1989 Jan 1;169(1):59–72. doi: 10.1084/jem.169.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinzel F. P., Sadick M. D., Mutha S. S., Locksley R. M. Production of interferon gamma, interleukin 2, interleukin 4, and interleukin 10 by CD4+ lymphocytes in vivo during healing and progressive murine leishmaniasis. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):7011–7015. doi: 10.1073/pnas.88.16.7011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holaday B. J., Sadick M. D., Wang Z. E., Reiner S. L., Heinzel F. P., Parslow T. G., Locksley R. M. Reconstitution of Leishmania immunity in severe combined immunodeficient mice using Th1- and Th2-like cell lines. J Immunol. 1991 Sep 1;147(5):1653–1658. [PubMed] [Google Scholar]
- Julius M. H., Simpson E., Herzenberg L. A. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973 Oct;3(10):645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- Kelso A., Glasebrook A. L. Secretion of interleukin 2, macrophage-activating factor, interferon, and colony-stimulating factor by alloreactive T lymphocyte clones. J Immunol. 1984 Jun;132(6):2924–2931. [PubMed] [Google Scholar]
- Liew F. Y. Functional heterogeneity of CD4+ T cells in leishmaniasis. Immunol Today. 1989 Feb;10(2):40–45. doi: 10.1016/0167-5699(89)90302-2. [DOI] [PubMed] [Google Scholar]
- Locksley R. M., Scott P. Helper T-cell subsets in mouse leishmaniasis: induction, expansion and effector function. Immunol Today. 1991 Mar;12(3):A58–A61. doi: 10.1016/S0167-5699(05)80017-9. [DOI] [PubMed] [Google Scholar]
- Ly I. A., Mishell R. I. Separation of mouse spleen cells by passage through columns of sephadex G-10. J Immunol Methods. 1974 Aug;5(3):239–247. doi: 10.1016/0022-1759(74)90108-2. [DOI] [PubMed] [Google Scholar]
- Mosmann T. R., Cherwinski H., Bond M. W., Giedlin M. A., Coffman R. L. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986 Apr 1;136(7):2348–2357. [PubMed] [Google Scholar]
- Nacy C. A., Fortier A. H., Pappas M. G., Henry R. R. Susceptibility of inbred mice to Leishmania tropica infection: correlation of susceptibility with in vitro defective macrophage microbicidal activities. Cell Immunol. 1983 Apr 15;77(2):298–307. doi: 10.1016/0008-8749(83)90030-8. [DOI] [PubMed] [Google Scholar]
- Pearce E. J., Caspar P., Grzych J. M., Lewis F. A., Sher A. Downregulation of Th1 cytokine production accompanies induction of Th2 responses by a parasitic helminth, Schistosoma mansoni. J Exp Med. 1991 Jan 1;173(1):159–166. doi: 10.1084/jem.173.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacks D. L., Scott P. A., Asofsky R., Sher F. A. Cutaneous leishmaniasis in anti-IgM-treated mice: enhanced resistance due to functional depletion of a B cell-dependent T cell involved in the suppressor pathway. J Immunol. 1984 Apr;132(4):2072–2077. [PubMed] [Google Scholar]
- Sadick M. D., Locksley R. M., Tubbs C., Raff H. V. Murine cutaneous leishmaniasis: resistance correlates with the capacity to generate interferon-gamma in response to Leishmania antigens in vitro. J Immunol. 1986 Jan;136(2):655–661. [PubMed] [Google Scholar]
- Scott P., Natovitz P., Coffman R. L., Pearce E., Sher A. Immunoregulation of cutaneous leishmaniasis. T cell lines that transfer protective immunity or exacerbation belong to different T helper subsets and respond to distinct parasite antigens. J Exp Med. 1988 Nov 1;168(5):1675–1684. doi: 10.1084/jem.168.5.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slade S. J., Langhorne J. Production of interferon-gamma during infection of mice with Plasmodium chabaudi chabaudi. Immunobiology. 1989 Oct;179(4-5):353–365. doi: 10.1016/S0171-2985(89)80041-5. [DOI] [PubMed] [Google Scholar]
- Watson J. Continuous proliferation of murine antigen-specific helper T lymphocytes in culture. J Exp Med. 1979 Dec 1;150(6):1510–1519. doi: 10.1084/jem.150.6.1510. [DOI] [PMC free article] [PubMed] [Google Scholar]



