Abstract
Breast cancer is the most frequent type of cancer of women in the western world. Antiestrogens, including Tamoxifen (OHT) and Faslodex (ICI), are widely used in the endocrine treatment of breast cancer. However, the majority of breast cancers are either resistant to endocrine therapy or eventually become unresponsive to antiestrogen therapy. Better understanding of the molecular mechnisms that govern tumour proliferation, is therefore needed to develop new therapies for the disease.
The Forkhead family of transcription factors plays an important role in regulating cell proliferation, cell death and differentiation.The estrogen receptor (ER) a positive breast carcinoma cell line MCF-7 and the ERa negative line MDA-MB-231 was used to study the potential regulation of the Forkhead member FOXM1 by ER. It was indicated that estrogen and ER regulate the expression of FOXM1 at the protein level.
Since Forkhead proteins play an important role in regulating cell proliferation, cell death and differentiation, this study helps to explain some of the functions of ER in tumourigensis, and the way these Forkhead proteins could be crucial targets for therapeutic strategies and/or markers for diagnosis and prognosis.
Keywords: Breast cancer, Forkhead, FOXM1, ER, FOXO3a
Introduction
Breast cancer is the leading female cancer in the western world. According to the World Health Organization, more than 1.2 million people will be diagnosed with breast cancer this year worldwide. Breast cancer incidence has risen 46% from 1979 to 20001. However, mortality rates have fallen by 28% from 1989 to 20022 . This decrease accounts for the fact that over the last 30 to 40 years, significant progress has been made in the diagnosis and treatment of breast cancer. Furthermore, dramatic efforts have been made to detect "risk factors" that will help to identify those women likely to develop breast cancer and the genetic factors that contribute to these risks and the subsequent development of the disease. These studies have led to investigations regarding the multiple causes of breast cancer and have generated efforts to prevent it.
There is strong evidence that the aetiology of breast cancer is the lifetime exposure of breast epithelium to steroid hormones.. The biological effects of estrogen are mediated through two different receptors, designated ERα and ERβ3. ERα and ERβ are encoded by different genes but are both members of the Nuclear Receptor (NR) superfamily of transcription factors4. The function of ERβ in breast cancer is not clear yet, so currently research is focused on ERα.
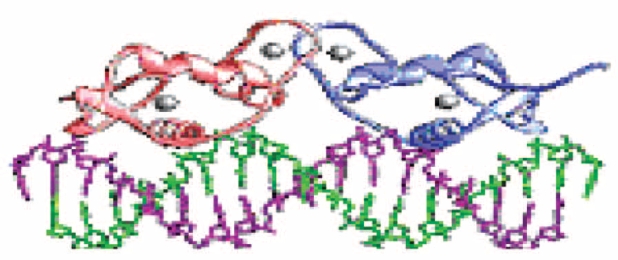
ERα has a NH2-terminal domain with a hormone independent transcriptional activation function (AF-1), a central DNA binding domain, and a COOH-terminal ligand-binding domain with a hormone-dependent tran- scriptional activation function (AF-2)5,6. Estrogen-bound ERαs bind as a homodimer to an estrogen responsive element (ERE) with their DNA-binding domain7.
ERα is mainly expressed in mammary luminal epithelial cells in human breast. Although only 15% to 25% of luminal epithelial cells express ERα in the normal resting breast3, studies have demonstrated that between 60% to 80% of human primary breast cancer cells express ERα. In the normal breast, ERα-expressing epithelial cells are largely non-dividing. However in malignancy, these ERα-positive cells are converted from a non-dividing state to an estrogen-dependent dividing state.
The mechanisms that contribute to this phenotype are still unclear3. A hypothesis is that estrogen may be stimulating the proliferation of ERα-positive epithelial cells in malignant breast tissues through regulation of estrogen-regulated genes.
Around 70% of ERα-positive breast cancer patients will respond to antiestrogen therapy such as tamoxifen; however, the remaining one-third will not respond to this type of therapy. Additionally, all breast tumours that do respond eventually, they become refractory to this form of treatment within 5 years. Resistance also limits the effectiveness of aromatase inhibitors and is associated with a more aggressive form of the disease3,8. This acquired resistance is a significant problem in breast cancer treatment.
New therapeutic strategies and the elucidation of regulatory mechanisms for the expression and function of ERs could provide useful information to predict the responsiveness to endocrine therapy and the prognosis. Moreover it is important to reveal roles of downstream target genes for ERs, which mediate the effects of estrogen on the proliferation of cancer cells, while these genes can be targeted to treat and diagnose estrogen-associated tumours.
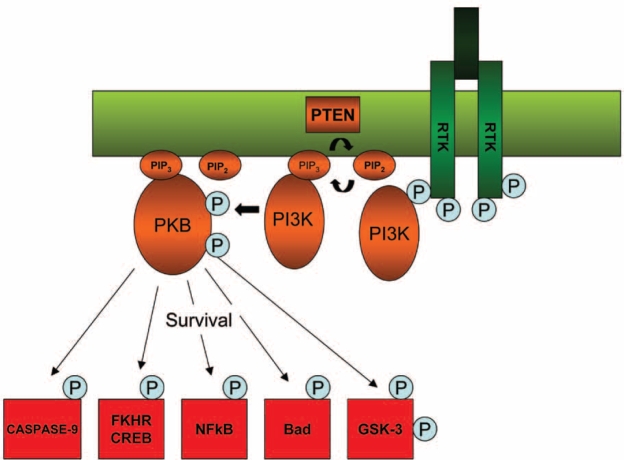
The ERs and the phosphatidylinositol 3-kinase (PI3K)/ protein kinase-B (PKB or Akt) signalling pathways are strongly implicated in the development of breast cancer. Deregulation of the PI3-K pathway has been demonstrated in many human breast cancers and has been linked to poor prognosis and endocrine therapy resistance. Alterations in the PI3-K signalling cascades can lead to changes in a number of cell functions that contribute to the transformed phenotype, including cell growth and proliferation, differentiation, cell sur vival, adhesion and cell motility6,9. The PKB (Akt) component of the PI3-K signalling pathway has been found to be a critical regulator of cell survival by phosphorylating a number of downstream proteins including the pro-apoptotic protein Bad, caspase-9 which is also involved in apoptosis, the Forkhead transcription factors (FKHR), Raf and p21-activated protein kinase10.
Recent work has identified as targets of PKB the three members of the forkhead box, group O subfamily forkhead transcription factors FOXO1, FOXO3a and FOXO4. Inactivation of the Forkhead transcription factors may play a major role in the control of cellular proliferation by the PI3-K pathway. FOXO1, FOXO3a and FOXO4 have been shown to inhibit cell cycle progression at the G1/S transition. Addiotionally, FOXM1 controls the transcriptional network of genes essential for progression through the cell cycle. It has been established as a key cell-cycle regulator of both the G1/S phase transition and progression into mitosis (G2/M).
As mentioned above, ligand-activated ER binds DNA and transactivates the promoters of estrogen target genes such as members of the forkhead transcription factor family. As FOXM1 is perhaps the most critical cell-cycle regulator among the forkhead transcription factor family, a link between ERα and transcriptional regulation of FOXM1 in breast carcinoma, would be very crucial and would provide a novel mechanism of estrogen action in hormone-responsive cells.
Materials and Methods
Maintenance of mammalian cell lines for the production of whole cell extracts with high salt buffer (HSB)
Human breast cancer cells were typically cultured at 37℃ in a humidified 10% (v/v) CO2 atmosphere and manipulations were generally carried out in a laminar flow hood.
The MCF-7 ER-positive human breast cancer cell line was used to carry out the experiments and was cultured routinely in Dr. Lams laboratory. Additionally, the MDA-MB-231 ER-negative human breast cancer cell line was used as a control. The cells were cultured in Dulbecco's Modified Eagle's Medium (DMEM) (Sigma) supplemented with 10% Foetal Calf Serum (FCS) (Globepharm Ltd.) or in DMEM without phenol red (GIBCO-BRL) supplemented with 10% Double Charcoal Stripped Foetal Calf Serum (DSS) (Globepharm Ltd.) when the effects of estrogen and antiestrogens were tested. The DMEM and phenol-free media were supplemented with a mixture of penicillin, streptomycin and L-glutamine (P/S/G) (Sigma).
When all the above cell lines were about 80% confluent, they were treated with antiestrogens and estrogen in 4 ml plates containing either DMEM plus 10% FCS and P/S/G or phenol-free DMEM plus 10 % DSS and P/S/G. Each plate was treated with 2x10-7 M OHT or 2x10-7 M ICI in DMEM plus 10% FCS plus P/S/G. Additionally, the above cell lines were treated with 10-8 M estradiol (E2) in phenol red-free DMEM plus 10% DSS and P/S/G. ICI and E2 were disolved in 100% ethanol and diluted in growth medium before use. The cells were then harvested from the plates at different time-points (0h, 4, 8h, 24h and 48h). To harvest the cells, first the media was washed away with Phosphate Buffered Saline (PBS) and then the cells were scrapped off in 160 µl HSB. and stored in 1.5 ml eppendorf tubes at -80℃. The cells were then frozen in dry ice and thawed in ice, three times and centrifuged at 13,000 x g for 10 minutes at 4℃.
SDS-PAGE
The protein gels were 7 % (w/v) acrylamide gels. First the running gel was poured to marked line (70%80% the way up of the small glass plate) and 2 ml of H2O (or Isopropanol) layered dropwise to give a levelled surface. The gel was left to polymerise for at least 30 minutes. Then, the water was removed, the stacking gel was poured and the combs were inserted. After 5 minutes the gel was topped up. Before assembling the gel tank, the wells were washed using a syringe containing running buffer .25 µg of protein samples plus 2X Laemmli loading dye buffer up tp equal volume; for each sample, were loaded on the gel and were run at 100 Volts 2.5 µl of rainbow ladder (Amersham) were loaded, in order to be able to estimate the size of the bands. The electrophoresis was performed in 1X running buffer.
Western blotting
The proteins were then transferred on to PROTRAN nitrocellulose transfer membrane for western blotting (Schleicher & Schuell). The transfer took place in 1X transfer buffer for at least 2 hours at 0.6 A.
All membranes were incubated in order to probe with primary antibodies used at various dilutions in 5 % BSA plus 0.005 % NaAzide, overnight at 4℃. The membranes were developed with ECL and exposed on hyperfilm to visualise the proteins.
Results
FOXM1 protein levels correlate with ERα's in cells treated with estradiol (E2).
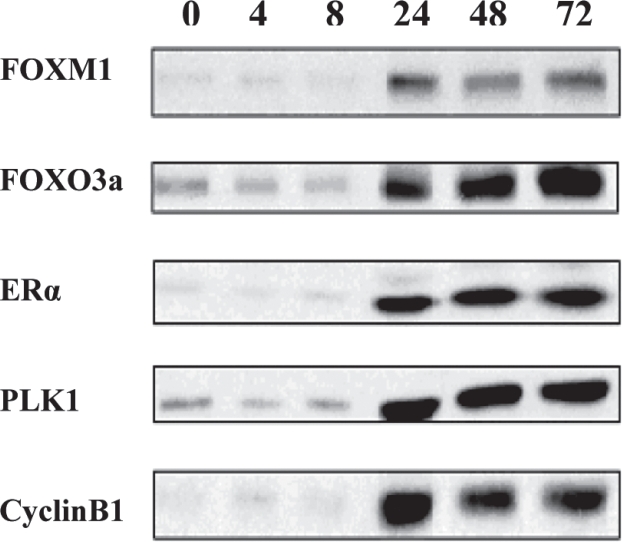
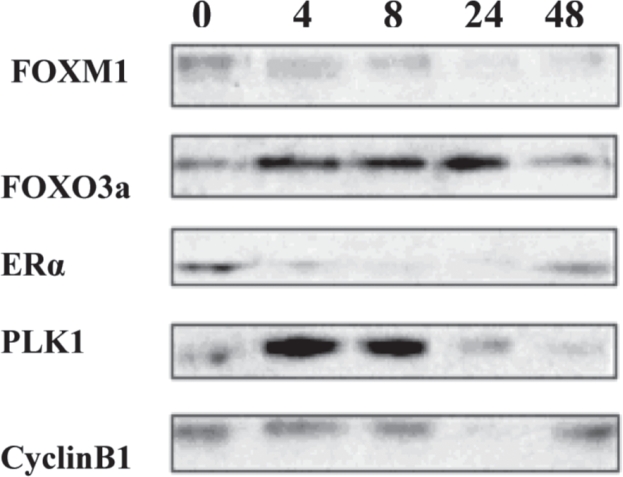
MCF-7 ER-positive breast cancer cells were harvested at different time points (0 to 72 hours), after estrogen (E2) treatment. The protein expression was studied by western blotting analysis, which showed clear correlation of FOXM1 protein levels with that of ERα. Upregulation of FOXM1 by ERα was observed at 24 hours after treatment with estrogen. ERα protein levels rose dramatically at 24 hours of treatment with E2 and the same expression pattern was observed for FOXM1 at 24 hours of treatment with E2. The efficiency of FOXM1 expression in MCF-7 cells, can be confirmed by the upregulation of PLK1 and Cyclin B1, which are known targets of FOXM111. Again, when MCF-7 cells were treated with E2, PLK1 and Cyclin B1 expression, had been shown to peak rapidly at 24 hours of treatment.
FOXO3a protein expression was also tested. FOXO3a is believed to regulate ERα protein expression in breast cancer cells12,13; thus, when breast cancer cells were treated with E2, it was expected for FOXO3a to show correlation of its protein levels with the ones of ERα. This observation is crucial as it supports the hypothesis that many Forkhead proteins regulate or are regulated by ERα.
FOXM1 protein levels correlate with ERα' in cells treated with the antiestrogen ICI.
The same ER-positive breast cancer cell line was treated with the pure antiestrogen ICI and harvested at dfferent times (0h. to 48h.). ERα expression was rapidly downregulated by the addition of ICI.
FOXM1 expression was also downregulated, mirroring the same pattern of expression as ERα. The above result clearly indicates that the expression of FOXM1 follows that of ERα, indicating that ERα regulates FOXM1 expression.
Next the expression of the two downstream targets of FOXM1, Cyclin B1 and PLK1 was investigated. Cyclin B1 and PLK1 were observed to follow the expected FOXM1-dependent expression pattern, being totally downregulated when after 8 hours of treatment with ICI.
FOXM1 protein levels show a different response in MDA -MB-231 ER-negative breast cancer cells.
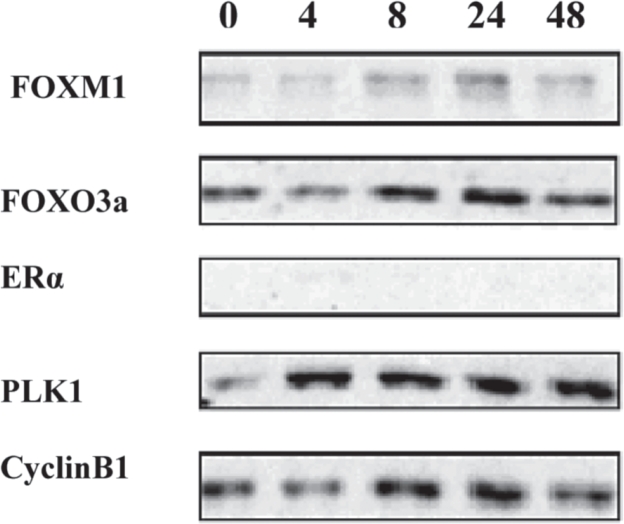
The MDA-MB-231 ER-negative breast cancer cell line was used as a control, as it does not express any ERα. As expected, ERα protein was totally absent and FOXM1 protein levels showed a different response to ICI treatment, compared to the ER-positive cell lines.
PLK1 and Cyclin B1, especially when the cells were treated with antiestrogen, showed a more stable expression, similar to that of FOXM1.
FOXO3a also showed a similar expression pattern as the other proteins. All the above results indicate that FOXM1 protein expression correlates with ERα 's in breast cancer cells treated with estrogen and antiestrogenic agents.
Discussion
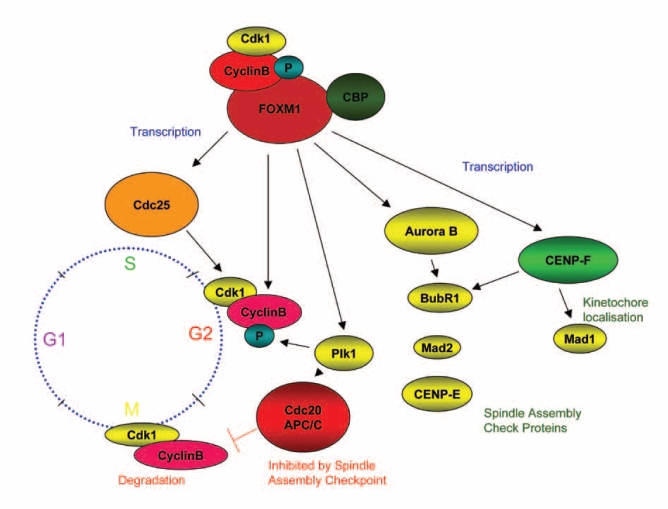
FOXM1 is known to be a critical cell cycle regulator, regulating the transcription of cell-cycle genes essential for the G1/S and most importantly the G2/M cell cycle progression. FOXM1 regulates the transcription of PLK1 and cyclin B1 which are important for cell prolifearion at G2/M11. ERα protein upregulation by estrogen was observed to correlate with FOXM1 protein upregulation and subsequently PLK1 and Cyclin B1 protein upregulation. Whereas ERα protein downregulation by antiestrogens correlated with decreased FOXM1 protein levels and subsequently PLK1 and Cyclin B1 protein levels. FOXM1-/- cells have been shown being unable to restore PLK1 and Cyclin B1 levels when induced with other FOXOs and it was unable for these cells to overcome the mitotic entry defect. The FOXM1 transcription factor has also been found to control the expression of genes that are involved both in the mitotic spindle assembly checkpoint and in cytokinesis. FOXM1 is therefore demonstrated to be involved in the proliferation of normal and breast cancer cells as an important transcription factor and is very likely to mediate cellular transformation into cancer cells.
Another Forkhead protein which has been shown to regulate ERα expression is FOXO3a. It was observed that FOXO3a levels correlated with ERα protein levels in ER-positive breast cancer cell lines. FOXO3a was upregulated when ER-positive cells were treated with E2 and downregulated when treated with ICI. Furthermore, FOXO3a showed a more stable, ER-inependent expression in MDA-MB-231 ER-negative breast cancer cells. It has been shown that FOXO3a can be regulated by the PI3-K signalling pathway. Nuclear receptors, including ER, have been shown to be able to crosstalk with the PI3-K/FOXOs signalling pathways through non-genomic activity. However, my data show that ERα can induce PI3-K signalling directly through regulation of FOXOs in breast cancer cells.
These data provide an important mechanism of estrogen regulation through the modulation of forkhead transcription factors. The above findings, describe an important link between cell surface signalling mechanisms that act through the PI3-K signalling pathway and nuclear hormone receptors. This cooperation between ERα and the FKHR transcription factors is important not only to understand how ER regulates the FKHR transcription factors directly but also how it can regulate them together with other proteins implicated in breast cancer, indirectly.
Additionally, the ERα cell cycle proteins c-myc and cyclin D1, have been found to play a role in estrogen-induced proliferation. It would be important to study if and how these proteins may regulate or be regulated by proteins of the Forkhead transcription family. It has already been shown that cyclin D1 is a target of FOXOs14. Furthermore, it would be essential to correlate the BRCA with the ERα expression and how these two may interact with FKHR to regulate the G2/M cell cycle transition. BRCA1 (BReast-CAncer susceptibility gene 1) and BRCA2 are tumour suppressor genes, the mutant phenotypes of which predispose to breast and ovarian cancers. Research has shown that BRCA proteins are involved in cellular processes such as cell cycle and maintenance of chromosomal stability. FOXM1 is also a regulator of these cellular processes, indicating that these two may be transregulated in a way. New data has also shown that BRCAs transcriptionally regulate some genes involved in DNA repair, the cell cycle, and apoptosis. Many of these functions have been found to be mediated by a large number of cellular proteins that interact with BRCAs. The Forkhead transcription factors play a major role in these functions too. FOXO3a is involved in apoptosis and FOXM1 is involved in cell cycle and DNA damage control. It should be assessed if these two types of proteins interact in any way. As, the reasons why mutations in BRCA genes lead to the development of breast and ovarian cancers are not clearly understood, understanding the precise molecular mechnisms of action could improve diagnosis and treatment of these diseases.
FOXM1 is a very important cell cycle regulator implicated in cell proliferation through the G1/S and G2/M progression. For this reason, FOXM1 might be a potential therapeutic target to inhibit proliferative expansion of breast tumour cells and prevent transformation into metastatic breast cancer cells. Additionally, FOXM1 could also assesed as a therapeutic target, together with other Forkhead proteins, to overcome the issue of endocrine-treatment resistance in antiestrogenis agents such as Tamoxifen (OHT). Moreover, as the Forkhead transcription factors analysed seem to regulate or be regulated by ERα, they could be used potentially as markers of the diagnosis or the prognosis of breast cancer.
Figure 1. Molecular structure of ER homodimer bound on DNA via its DNA-binding domains.
Figure 2. The PI3-K/PKB signalling pathway contributing in cell survival, growth and proliferation, cell differentiation and motility. Caspase-9, Forkhead transcription factors(FKHR), c-AMP responsive element binding protein(CREB), Nuclear factor kB(NFkB), Bad and Glycogen synthase kinase 3(GSK-3), are some of the dowstrean targets of the PI3-K signalling pathway that contribute to the various functions of the cascade.
Figure 3. The FOXM1 transcription factor regulates transcription of cell-cycle genes essential for G1/S and G2/M progression, chromosomal segregation and cytokinesis.
Figure 4. MCF-7 ER-positive breast cancer cells treated with E2 up tp 72 hours. FOXM1, FOXO3a, ERα, PLK1 and cyclin B1 protein levels were analysed by Western blotting.
Figure 5. MCF-7 ER-positive breast cancer cells treated with ICI up to 48 hours. FOXM1, FOXO3a, ERα, PLK1 and cyclin B1 protein levels were analysed by Western blotting.
Figure 6. MDA-MB-231 ER-negative breast cancer cells treated with ICI up tp 48 hours. FOXM1, FOXO3a, ERα, PLK1 and cyclin B1 protein levels were analysed by Western blotting.
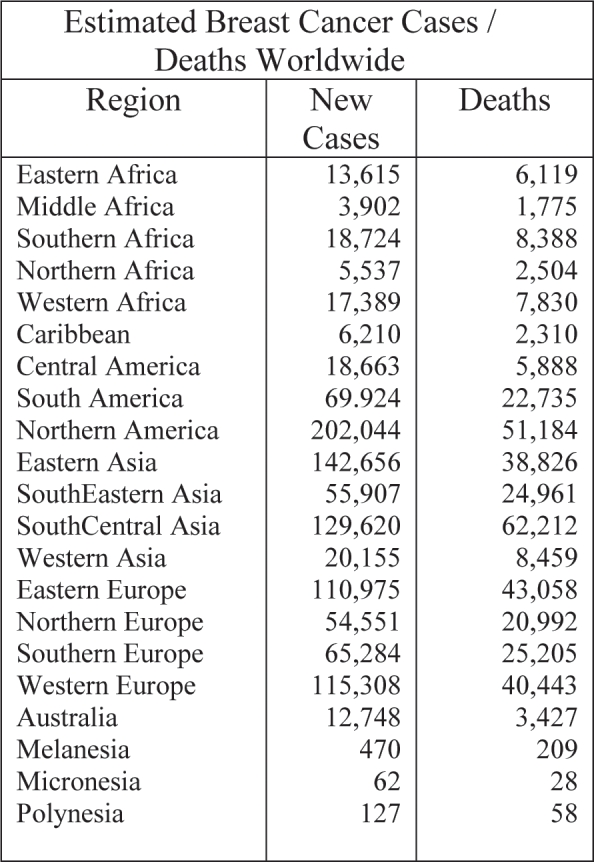
Table 1. Estimated breast cancer new cases and deaths for the year 2000, worldwide. In Southern Europe (Greece), 65,284 new cases are estimated each year.
References
- 1.Geert Kops JPL, Medema Rene H, Glassford Janet, et al. Control of cell cycle exit and entry by protein kinase B-regulated forkhead transcription factors. MCB. 2002:2025–2036. doi: 10.1128/MCB.22.7.2025-2036.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. www.cancerresearchuk.org.
- 3.Simak Ali, Coombes R Charles. Endocrine responsive breast cancer and strategies for combating resistance. Nature Reviews Cancer. 2002;2:101–115. doi: 10.1038/nrc721. [DOI] [PubMed] [Google Scholar]
- 4.Hall JM, Couse JF, Korach KS. The multifaceted mechanisms of estradiol abd estrogen receptor signalling. Journal of Biological Chemistry. 2001;276:36869–36872. doi: 10.1074/jbc.R100029200. [DOI] [PubMed] [Google Scholar]
- 5.Mangelsdorf DJ, Thummel C, Beato M, Herrlich P, et al. The nuclear receptor superfamily: the second decade. Cell. 1995;83:835–839. doi: 10.1016/0092-8674(95)90199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roymans D, Slegers H. Phosphatidylinositol 3-kinases in tumour progression. Eur. J. Biochem. 2001;268:487498. doi: 10.1046/j.1432-1327.2001.01936.x. [DOI] [PubMed] [Google Scholar]
- 7.Weigel NL. Steroid hormone receptors and their regulation by phosphorylation. Biochem. J. 1996;319(3):657–667. doi: 10.1042/bj3190657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Murphy MJ., Jr Molecular Action and clinical relevance of aromatase inhibitors. Oncologist. 1998;3:129–130. [PubMed] [Google Scholar]
- 9.Leevers SJ, Vanhaesebroeck B, Waterfield MD. Signalling through phosphoinositide 3-kinases: the lipids take centre stage. Curr. Opin. Cell Biol. 1999;11:219–225. doi: 10.1016/s0955-0674(99)80029-5. [DOI] [PubMed] [Google Scholar]
- 10.Klaus Kaestner H, Knochel Walter, Martinez Daniel E. Unified nomenclature for the winged helix/forkhead transcription forkhead transcription factors. Genes and Development. 2000;14:142–146. [PubMed] [Google Scholar]
- 11.Robert Costa H. FOXM1 dances with mitosis. Nature Cell Biology. 2005;7:108–110. doi: 10.1038/ncb0205-108. [DOI] [PubMed] [Google Scholar]
- 12.Shangqin Guo, Sonenshein Gail E. Forkhead Box Transcription Factor FOXO3a Regulates Estrogen Receptor Alpha Expression and Is Repressed by the Her-2/neu/PI3-K/Akt Signalling Pathway. MCB. 2004;24:8681–8690. doi: 10.1128/MCB.24.19.8681-8690.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. www.chemie.uni-kl.de.
- 14.Kazuhiro Ikeda, Inoue Satoshi. Estrogen receptors and their downstream targets in cancer. Arch. Histol. Cytol. 2004;67:435–442. doi: 10.1679/aohc.67.435. [DOI] [PubMed] [Google Scholar]