Abstract
Background. Preeclampsia (PE) is a pregnancy complication usually of the third trimester. The clinical manifestations are hypertension and proteinuria with or without edema. Its pathogenesis is characterized by generalized vasoconstriction and endothelial dysfunction. The aim of this study was to evaluate the diagnostic value of the Doppler ultrasound examination of the renal interlobar vessels in pregnancy complicated with preeclampsia in the context of the theory about the increased vessel resistance in this pregnancy disorder.
Methods. Fifty two women aged 16-38, ( mean age 23±9.54) streamed into three groups were examined by abdominal ultrasound in Triplex regime. The first group included 18 pregnant with PE, the second 21 women in the third trimester of normal pregnancy, and the third group 13 non-pregnant women. In all 3 groups the renal volume (RV) and parenchyma volume (PV) were determined by conventional ultrasound (CU). The doppler waveform analysis (DWA) of the interlobar renal arteries (IRA) gave the following indices: resistance index (RI), pulsatility index (PI), peak systolic velocity (Vmax) and end diastolic velocity (Vmin).
Results. RV and PV in the PE group were significantly higher then those of the healthy pregnant women: RV: 231.4±58 to 187±45, p<0.05 and for PV: 200±11 to 130±6.78, p< 0.05. Such a difference was not found out for the healthy pregnant women compared to the non-pregnant ones. No significant differences of the examined doppler indices were foundin the three groups.
Conclusion. Although the arteriolar vasoconstriction and the tissue hypoxia are leading in the PE pathogenesis, the DWA of the IRA in PE pregnancy does not differ from those of the healthy pregnant and nonpregnant women. As one of the most frequently used imaging technique Doppler Ultrasound (DU) of the renal IRA and DWA of these vessels shows no diagnostic advantage as compared to the renal CU in pregnancy complicated with PE.
Keywords: preeclampsia, pregnancy, vasoconstriction, Conventional and Doppler ultrasound
Introduction
Clinical findings of preeclampsia (PE) which usually occurs in the third trimester are hypertension -diastolic blood pressure more then 15 mmHg as compared to the values measured at the beginning of pregnancy or more than 90 mmHg, proteinuria > 0,3 g/24 with or without edema. The frequency of this disorder is 3-14%. PE pathogenesis has not been completely clarified. In normal pregnancy cytotrophoblast infiltrates the decidual spiral arteries which are branches of the uterine artery leading to their transformation into "sac-like" vessels. In PE these arteries remain narrow and extremely sensitive to vasoconstictive substances. The placenta ischemia, due to a possible immune conflict, as an abnormal inflammatory response lead to the release of cytokines, increased production of growth factors- insulin- like growth factor (ILGF), epidermal growth factor (EGF) and macroph-age-colony stimulating factor (M-CSF). The causse of the generalized arteriolar constriction and the increased vascular resistance is the accumulation of vasoconstrictive peptides (endothelin, angiotensin II, thromboxan A2) which activates the coagulation system as well as a deficit of vasodilatators (nitric oxide, prostacyclin). One of the possible mechanisms for endothelial dysfunction and increased production of vasopressors is abnormal oxidative stress with antioxidants deficit1. A lot of clinical markers are known to lead to the early diagnosis of PE and to have predictive value: high levels of uric acid, leptin, fibronectin, placenta growth factor, homocystein, the increased serum concentration of the intercellular adhesive molecules - (ICAM-1) and the decreased one of the vascular adhesive molecules (VCAM-1) as well as the decrease of the Vit C2–4.
DU examination of the uterine vessels shows a typical change of the Doppler waveform in the PE, which is a result of the increased vessel resistance2.
The kidney morphological changes in PE are defined as "endotheliosis". The aim of this study is to define what is the role of the renal DU in pregnancy complicated with PE and to find out wheather there is a typical change of spectral Doppler wave analysis and measured indices of the IRA in the light of the theory of the increased arteriolar constriction in PE.
Material and methods
Fifty two women (average age 23±9.54) streamed into three groups were examined. The first group included 18 third trimester pregnant women, (average age 25.7) with PE, the second - 21 women,(average age 25.6) in the third trimester of normal pregnancy and the third group-13 non-pregnant healthy women, (average age 29.5). Including criteria for the first group were: hypertension, blood pressure > 140/90 mmHg, proteinuria > 1.5 g/24 hours with or without edema, normal renal function - serum creatinine (Scr) up to 100 µmol/l. Pregnant with pregnancy -induced hypertension, or hypertension diagnosed before the pregnancy as well as those with chronic kidney diseases were excluded from the study. The pregnant women with preeclampsia were treated with Dopegyt, Ca- channel blockers and/ or B blockers. After this medication their blood pressure was never under 140 /90 mm Hg but it never increased over 150/100 mmHg. After the delivery their blood pressure was normal and in no patient any antihypertensive drug was necessary.
The non-pregnant women and healthy pregnant women were randomly chosen from the outpatients who visit our hospital. In all three groups Scr was measured at the beginning of the study. Using CU longitudinal (L), transversal (T) and front-back (F-B) diameters were measured. In all the groups the renal volume (RV) and renal parenchyma volume (PV) were calculated out of the mentioned parameters. RV=(L X T X F-B) X 0.523-ellipse volume. In all the women examined CU and DU were carried out with 3.5 MHz, CFM 2.8-5 MHz convex transducer. Color and Doppler ultrasound velocimetric examinations (Pulse Doppler) were performed in both kidneys but the presented results are those which we obtained from the right kidney because there were no significant differences between the results measured in the left kidney. The renal sinus was depicted by using color flow mapping in an attempt to identify the interlobar renal artery which runs between the renal pyramids and extends into the renal cortex. IRA were identified by Color Doppler. The sample volume of the Doppler system was 2 mm and a 50 Hz filter was used to reduce the noise from the pulsating arterial wall. Pulsed Doppler ulrasound waveforms were displayed at sweep speed of 40-60 mm/s. The insolation angles were less then 300 during a period of suspended respiration. DWA of the IRA from the upper and lower renal pole as well as in the middle of the kidney was done. The Doppler waveform analysis (DWA) of the interlobar renal artery (IRA) gave the following indices which are automatically calculated by the machine software: resistance index (RI), pulsatility index (PI), peak systolic velocity (Vmax), end diastolic velocity (Vmin) and mean velocity (Vm). We took three waveforms from the IRA in the upper, lower and middle sides in both kidneys. The average value of each parameter was used for this study. RI and PI can be calculated by the formulas:RI= (Vmax.- Vmin.)/Vmax and PI= (Vmax -Vmin )/ Vm.
All the data were expressed as a mean value (MV) ±SD (standard deviation). One way analysis of the variance followed by Levene test also was used to compare the values within each group. Differences with a P value less than 0.05 were considered to be statistically significant.
Results
The blood pressure and the values of the Scr were measured before the DU examination and in the PE group (I group) they proved to be significantly higher than those in the other two groups - of the healthy pregnant (II group) and non-pregnant women (III group), (Table 1).
Table 1. A comparison of the systolic, diastolic pressure and the Scr between the three examined groups.
All the parameters shown are mean values (MV) ±SD (standard deviation)
A statistically significant difference was not found comparing the MV of RI and PI and V max and V min between the first and the second group as well as between the second and the third (Table 2).
Table 2. A comparison of the MV of RI, PI as well as Vmax, Vmin between the three groups examined.
All the parameters shown are MV ±SD
What were interesting were the results of the CU. The renal volumes (RV) and the renal parenchyma volume ((RPV) in the PE group (I group) are statistically significantly higher then those in the second and third group- (t-criteria, P< 0.05). Actually, this is the only significant difference found by ultrasound in the PE group as compared to the groups of the healthy pregnant and non- pregnant women. No significant difference was found in the kidney volumes between the healthy pregnant and non- pregnant women (Table 3).
Table 3. RV and RPV in all groups examined.
All the parameters shown are MV ±SD
Discussion
The increased plasma volume in the course of the normal pregnancy leads to systemic vasodilatation and decreasing of the blood pressure. Blood pressure mea-sured in the normal pregnancy group are insignificantly lower than those of the healthy non-pregnant women. The renal plasma flow and glomerular filtration rate increases are the result of the kidney vasodilatation during the normal pregnancy5. That's why Scr is lower in the pregnant as compared to the one in the non-pregnant women. In the PE group the Scr approaches the values of that in the non-pregnant group, being higher as compared to the Scr of the normal third trimester pregnant group. This means that there is some renal function reduction in the PE group. RI is a parameter which correlates with the renal function and it is logical that RI increases when the renal function decreases. But such correlation is not found in the preeclampsia group. Probably the lack of such a change can be explained by the short duration of the "endotheliosis", the use of antihypertensive drugs and by the renal autoregulation mechanism. An overlap of the levels of S cr between group I and group II is likely to exist and be related to the lack of difference in RI of the two groups.
The hypertension and the vessel changes are leading pathogenic mechanisms in PE. In this pregnancy complication the generalized vasospasm in response to vasoconstrictors leads to an increased peripheral vascular resistance. The physiological results of these changes are a decrease in glomerular filtration rate and renal blood flow which remains within the physiologically normal range but are near to the parameters of the nonpregnant women6. Can the Doppler wave analysis of the renal arteries and the registered indices and velocities characterize the pathological changes of the blood flow in the context of the theory for the increased vascular resistance in PE? The spectral Doppler analysis of the a. uterine and a. umbilicalis in cases of heavy PE shows typical changes with a RI increase with high-resistant waveform curves and that's why it can be used as an early diagnostic criteria interpreted along with others clinical predictive factors2–4.
Is there a correlation between the parameters detected by Doppler wave analysis of the IRA and the basic pathophysiological mechanism? The answers to this question can be multiple Kublickas and all have found lower RI and PI of IRA in pregnancy complicated with hypertension7. Gudmundssson et al have found a lower PI in preeclampsia as compared to that in normal pregnancy. This difference is explained with arteriovenous shunts in preeclampsia8. Dib and coworkers have found out lower PI in normal pregnancies at the 8-12 week interval of gestational age as compared to non -pregnant controls. Their conclusion is that there are no significant alterations in renal artery RI during normal pregnancy9.
But in our study we did not find significant difference between peak systolic and end diastolic velocity in all examined groups. Resistive and pulsatility indices are the results of these velocities and it is logical for their values not to be different in the three groups. The resistive index is considered to be a parameter more sensitive to reflecting the vascular resistance than the pulsatility one.
In the results published by Liberati there is no significant difference in the flow velocity waveforms obtained in the intralobar renal arteries in healthy women, healthy pregnancies and hypertensive disorders of pregnancy examined between 20 and 39 weeks' gestation10. In this respect we have come to almost the same results. Zimmermann has not found difference between the RI in pregnancies with high risk for hypertension and that of preeclampsia pregnancies. Even in patients with severe preeclampsia with abnormal uterine artery Doppler findings the RI is normal and similar to that of the normal pregnancies11. The results of our study are getting close to those of Akihito Nakai and all who have not found a statistically significant difference for RI, PI, ad V max and V min in PE and third trimester normal pregnancy. They have found also a change in the acceleration time which has not been the task of the present study12. Miayke et al have found prolonged acceleration time of the interlobar renal arteries in women with pregnancy induced hypertension. No change in the other Doppler indices has been announced. In our study the acceleration time has not been examined so we cannot interpret this parameter. Miayke's conclusion that the continuous vasospasm explains the acceleration time changes seems logical and has been confirmed by other researchers13.
The lack of a significant difference in the values of Doppler parameters in the three groups (the lack of the difference in the group of the PE pregnancy being particularly illustrative) can be explained by the kidney autoregulation and the possibility of the kidney to constantly and adequately maintain the intrarenal pressure under the conditions of vasopasm within the vast range of the change of the systemic blood pressure and vessels resistance14. The lack of such a mechanism for autoregulation of the a. uterina can explain the changes in DWA in PE-high RI with a reverse or missing diastolic blood flow in these vessels in the state of vasoconstriction in PE11.
Morphological kidney changes in PE are summarized as "endotheliosis"- edema of the endothelial cells without pronounced proliferation, immune deposits and tubular changes. Gartner has proved that the severity of the PE correlate with histological changes. Sever PE with blood pressure >150/90 and proteinuria > 3 g/24 hours occur when proliferation, hyalinosis and segmental sclerosis appear. In that case a certain increase of the Doppler indices is mentioned15.
In PE there are no substantial changes in the parameters of the DWA just as in the chronic glomerulonephritis without heavy proliferative changes and interstitial damage. The Doppler parameters in these patients are within the normal range. Plat and all formulated the theses that the Doppler indices reflect the physiological changes as well as the type of the kidney involvement16.
In the preeclampsia group renal volumes are significantly higher than those in the other two groups. To prove that the increase of the renal volumes is rather the result of an active renal disease than an anthropomorphic parameter we can point out the fact that 6 months after the delivery the renal volumes in the preeclampsia group (190 ±31 and 28,8 ± 7.6) werent very close to those of the non-pregnant women (178±39 and 27.1±10). All the women in the preeclampsia group had normal laboratory parameters and normal blood pressure 6 months after the delivery. Karabulut et al have found an abnormally reduced venous impedance index in pregnant women in the second and third trimester, which can be explained by reduced vascular compliance from the increased interstitial pressure subsequent to "physiological " dilatation17. The renal volume is the sum of many processes one of which is the venous flow. It can be concluded that the increase of the renal volumes will be parallel to the increase of the degree of "physiological" dilatation. The pregnant women (healthy and with preeclampsia) examined after the 20 weeks gestation have only hydrocalicosis without any ultrasound findings for hydronephrosis. Both groups have "physiological" dilatation but in the preeclampsia group the renal volumes are higher than those in the healthy pregnancies. That comes to show that the "physiological" dilatation has a light effect on the increase of the renal volumes and vascular resistance.
The results we have received are in support of the thesis that only the Doppler wave analysis of the IRA is not enough to evaluate the stage of the kidney involvement in preeclampsia. It should be interpreted within the complex of the clinic symptoms: hypertension, proteinuria and the increase of the renal volumes detected by conventional ulatrasound. DWA can be useful to differentiate kidney diseases which occur or get activated during pregnancy and can not be recognized as preeclampsia.
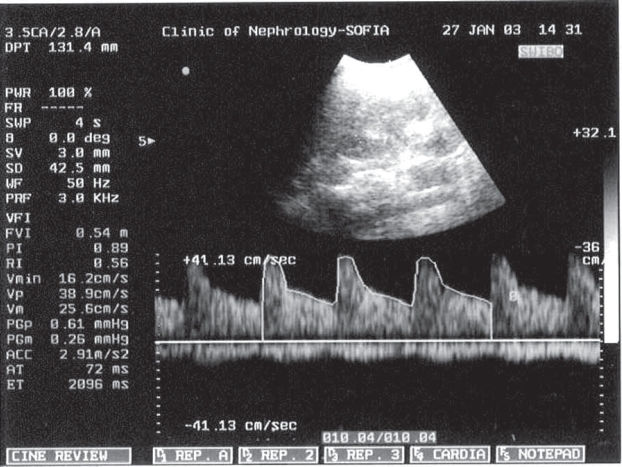
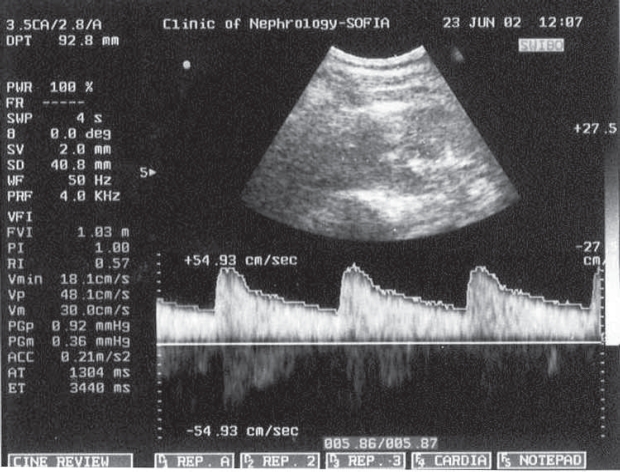
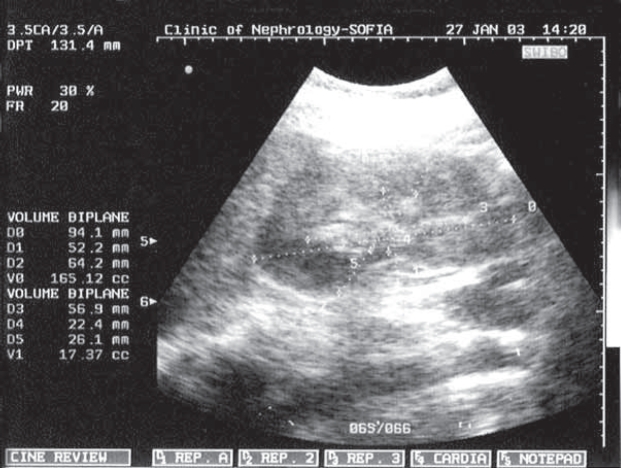
Figure 1. Doppler waveform.
Figure 2. Doppler waveform of the IRA of a healthy pregnant woman the IRA of PE pregnant woman.
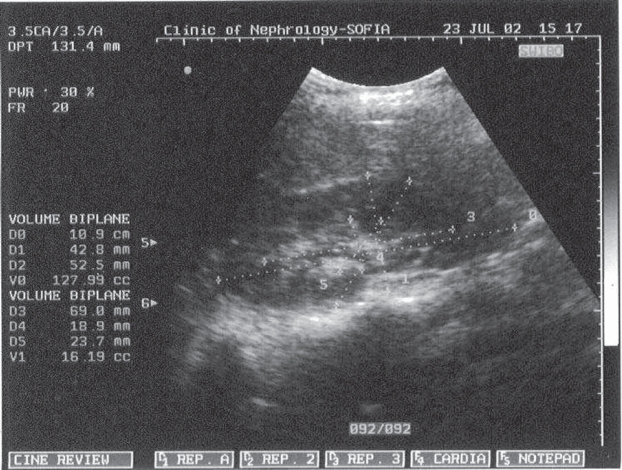
Figure 3. CU - RV and RPV n healthy third trimester preg-nant pregnant woman.
Figure 4. CU - RV and RPV in PE pregnant woman.
References
- 1.Walsh SW. The role of fatty acid peroxidation and antioxidant status in normal pregnancy and in pregnancy complicated by preeclampsia. World Rev Nutr Diet. 1994;76:114–118. doi: 10.1159/000424005. [DOI] [PubMed] [Google Scholar]
- 2.Aquilina J, Thompson O, Thilaganathan B, Harrington K. Improved early prediction of pre-eclampsia by combining second-trimester maternal serum inhibin-A and uterine artery Doppler. Ultrasound Obstet Gynecol. 2001;17:477–484. doi: 10.1046/j.1469-0705.2001.00382.x. [DOI] [PubMed] [Google Scholar]
- 3.Chavarria ME, Lara-Gonzalez L, Gonzalez-Gleason A, Sojo I, Reyes A. Maternal plasma cellular fibronectin concentrations in normal and preeclamptic pregnancies: a longitudinal study for early prediction of preeclampsia. Am J Obstet Gynecol. 2002;187:595–601. doi: 10.1067/mob.2002.123281. [DOI] [PubMed] [Google Scholar]
- 4.Clausen T, Djurovic S, Brosstad FR, Berg K, Henriksen T. Altered circulating levels of adhesion molecules at 18 weeks? gestation among women with eventual preeclampsia: indicators of disturbed placentation in absence of evidence of endothelial dysfunction? Am J Obstet Gynecol. 2000;182:321–325. doi: 10.1016/s0002-9378(00)70218-3. [DOI] [PubMed] [Google Scholar]
- 5.Baylis C, Deng A, Couser WG. Glomerular hemodynamic effects of late pregnancy in rats with experimental membranous glomerulonephropathy. J Am Soc Nephrol. 1995;6:1197–1201. doi: 10.1681/ASN.V641197. [DOI] [PubMed] [Google Scholar]
- 6.Chesley LC. Hypertensive disorders in pregnancy. New York, NY: Appleton Century Crofts; 1987. The kidney; pp. 170–175. [Google Scholar]
- 7.Kublickas M, Lunell NO, Nisell H, Westgren M. Maternal renal artery blood flow velocymetry in normal and hypertensive pregnancies. Acta Obstet Gynecol Scand. 1996;75:715–719. doi: 10.3109/00016349609065733. [DOI] [PubMed] [Google Scholar]
- 8.Gudmundsson S, Marsal K. Doppler ultrasound examination in healthy women, normotensive pregnant women and in preeclampsia. Ultrasound Obstet Gynecol. 1991;1:258–260. doi: 10.1046/j.1469-0705.1991.01040258.x. [DOI] [PubMed] [Google Scholar]
- 9.Dib FR, Sala MM, Ferriani RA, Berezowski AT. Prospective evaluation of renal artery resistance and pulsatility indices in normal pregnant women. Ultrasound Obstet Gynecol. 2003;22:515–519. doi: 10.1002/uog.240. [DOI] [PubMed] [Google Scholar]
- 10.Liberati M, Rotmensch S, Zannolli P, Bellati U. Doppler velocimetry of maternal renal interlobar arteries in pregnancy-induced hypertension. Int J Gynaecol Obstet. 1994;44:129–133. doi: 10.1016/0020-7292(94)90066-3. [DOI] [PubMed] [Google Scholar]
- 11.Zimmrmann P, Ranta T. Doppler assessment of the maternal interlobar renal and uterine arteries in mid-pregnancy in women at low and high risk for pregnancy- induced hypertension. J Clin Ultrasound. 1998;26:239–245. doi: 10.1002/(sici)1097-0096(199806)26:5<239::aid-jcu2>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 12.Akahito N, Akasura H. Pulsed Doppler US findings of renal interlobar arteries in pregnancy -induced hypertension. Radiolology. 1999;213:423–428. doi: 10.1148/radiology.213.2.r99nv18423. [DOI] [PubMed] [Google Scholar]
- 13.Miayke H, Nakai A, Koshino T, Araki T. Doppler velocimetry of maternal renal circulation in pregnancy-induced hypertension. J Clin Ultrasound. 2001;29:449–455. doi: 10.1002/jcu.10007. [DOI] [PubMed] [Google Scholar]
- 14.Easterrling TR, Benedeti TJ. Preeclampsia: A hyperdynamic disease model. Am. J Obstet Gynecol. 1989;160:1447–1453. doi: 10.1016/0002-9378(89)90869-7. [DOI] [PubMed] [Google Scholar]
- 15.Πartner HV. Nephropathy in pregnancy-an endotelial lesion? (Geramn) Zentralbl Gynakol. 1994;116:123–137. [PubMed] [Google Scholar]
- 16.Platt JF, Ellis JH, Rubin JM, DiPietro MA, Sedman AB. Intrarenal arterial Doppler sonography in patients with nononobstructive renal disease: correlation of resistev index with biopsy findings. Am. J Roentgenol. 1990;154:1223–1227. doi: 10.2214/ajr.154.6.2110732. [DOI] [PubMed] [Google Scholar]
- 17.Karabulut N, Baki Yagci A, Karabulut A. Reanl vein Doppler ultrasound of maternal kidneys in normal second nad third trimester pregnancy. Br J Radiol. 2003;76:444–447. doi: 10.1259/bjr/81976752. [DOI] [PubMed] [Google Scholar]