Abstract
Hemolytic Uremic Syndrom after kidney transplantation affects an increasing number of patients. It is characterized as recurrent and de novo. Older age at onset of HUS, shorter mean interval between HUS and transplantation or ESRD, living related donor and treatment with CNI have been associated with an increased risk of recurrence. Patients who lost the first transplant because of HUS recurrence should not receive a second transplant. The outcome of recurring HUS after transplantation is worse in familial forms leading invariably to graft loss and for this reason doctors should discourage the use of living related donors in this setting.
De novo HUS is not a rare complication after kidney transplantation and may be associated with infection, CNI or mTOR inhibitor toxicity, antibody use (OKT3), or acute vascular rejection. The clinical picture is obscure and treatment rests on removal of inciting factor with or without plasma exchange / FFP infusion.
Keywords: hemolytic uremic syndrome, kidney transplantation
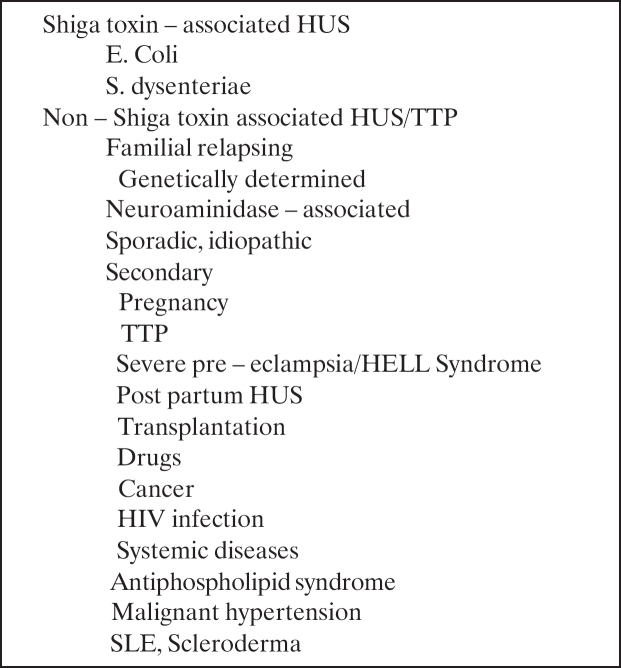
Thrombotic microngiopahty (TMA) is a well recognized serious complication of renal transplantation. The term has been applied to a diverse group of conditions that share the common pathomechanism of endothelial damage (Table 1). Histopathologically, TMA in the kidney is characterized by subendothelial accumulation of amorphous material in glomeruli, with narrowing or occlusion of the capillaries, fibrinoid or mucoid change in the intimal of small arteries, glomerular and/or arterial fibrin thrombi and fragmented red blood cells in the vascular wall, glomeruli or interstitium. The majority of cases represent de novo TMA, which occurs in 2.8%-3.5% of renal transplant recipients and is associated with 22% rate of graft loss1. The clinical presentation of TMA is variable. TMA may present systematically as hemolytic uremic syndrome (HUS) with the classic findings of renal failure, hemolytic anemia, schistocytes and thrombocytopenia or may be localized in the allograft only with worsening of renal function or delayed graft function (DGF) but few or no systemic manifestations of HUS. It is not known whether patients with localized or systemic TMA have different characteristics and clinical courses. The optimal management of post-transplantation TMA also remains controversial.
Table 1. Causes of thrombotic microangiopathy.
HUS and thrombotic thrombocytopenic purpura (TTP) are in fact a single entity2. They are two different expressions of the same disease and this is consistent with the reports of familial cases that, despite the same genetic abnormality were classified as HUS or TTP in different members of the same family3. More convincing are the reports of different episodes of recurrent disease in the same patient that were in some occasions defined as HUS and in others as TTP in spite the fact that they were associated with the same genetic defect4. The term HUS tends to be preferred in children with renal insufficiency and the term TTP in adults with predominantly neurologic involvement. However, some evidence suggests that the two entities may be distinguished based upon the presence and/or activity of the von Willebrand factor cleaving protease (ADAMST13).
HUS is characterized by microangiopathic hemolytic anemia, thrombocytopenia and renal failure. It affects 1 in 100,000 adults and leads to end stage renal failure (ESRF) in 50% of them. In children the average frequency is 2 every 100,000, with peak incidence in Argentina (20 in 100,000). The prognosis in children is much better with only 2% to 4% of them progressing in ESRF in Western Countries5–7. The prognosis difference of HUS between adult and children is mainly due to the largely benign Shiga – toxin (STX) – associated HUS that affects children in almost 80% of cases while in adults the incidence is only 5%.
Pathogenetically, the activation of microvascular endothelium leads to endothelium–blood cell interaction and platelet thrombosis and furthermore to occlusion of capillaries and small vessels of target organ.
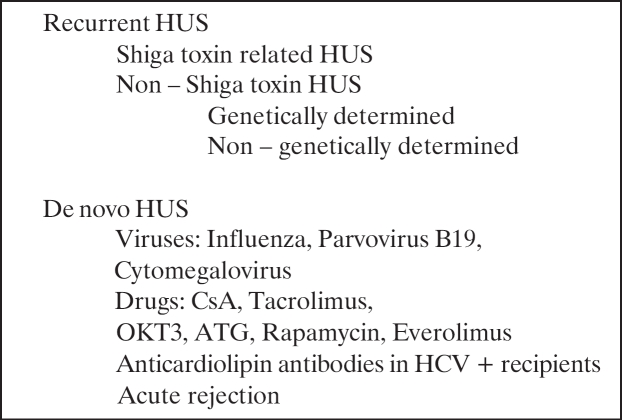
Classification of post-transplant HUS
HUS after kidney transplantation appears to affect an increasing number of patients. The frequency of HUS is higher in transplant patients compared to general population. After transplantation, HUS may be characterized recurrent or de novo HUS (Table 2).
Table 2. Causes of HUS after kidney transplantation.
Recurrent HUS
The first case of recurrent HUS was reported in 1976. Since then an extremely variable rate of recurrence ranging from 9% to 54% has been reported in different series8. Differentiation of recurrent HUS from other conditions largely accounts for these findings. A recent meta. analysis showed that the recurrence rate is 27%8. Older age at onset of HUS, shorter mean interval between HUS and transplantation or ESRD, living related transplant and treatment with calcinurin inhibitors have been associated with an increased risk of recurrence. Conceivably, older age at onset and faster progression to ESRD both reflect non-STX . associated HUS, whereas the increased risk associated with living related transplantation most likely disclosed a genetic (familial) predisposition to the disease. Later on, it was suggested that the progression to ESRD was associated with the type of HUS and not the patient age.
Recurrent disease occurs in most patients with familial HUS which is usually due to mutations in the gene for complement factor H9 and the gene for complement factor I10,11. Recurrence is independent of the source of the transplant (CD or LD) or the immunosuppressive regimen12. Reports of children with end stage renal disease who underwent continued kidney and liver transplantation, the latter to normalize factor H concentration and function are not encouraging 13,14. Patients with mutations in the gene for membrane cofactor protein (MCP), a membrane protein highly expressed in the kidney have successful transplantations with no disease recurrence15,16.
Today we know that STX-associated HUS does not recur after transplantation (0.8% recurrence in children)17. There is evidence that anti-STX-neutralizing antibodies persist over the long term in the circulation of these patients and render extremely unlikely the possibility of HUS recurrence18. Even adult patients with STX.related HUS are virtually without risk of post-transplant recurrence.
Non-STX HUS presents a substantial risk of recurrence and graft loss after renal transplantation both in children and in adults. Children present a recurrence rate ranging from 50% to 90%19–21. In all series the most recurrences occurred within the first two months after transplantation. Graft outcome was poor with graft loss occurring two or three weeks after HUS recurrence and ranging from 80% to 90%. In adults, recurrence of non-STX HUS is frequent and happens early after transplantation. The risk of recurrence is lower in patients with pre-transplant bilateral nephrectomy compared to non-nephrectomized patients21. Overall graft success may be diminished in patients with recurrent HUS, the one and five year graft survival estimated to be 33% and 19% respectively in one series compared to 57% for patients without recurrent HUS. The outcome of recurring HUS after transplantation is worse in familial forms of HUS leading invariably to graft loss and for this reason doctors should discourage the use of living related donors in this setting.
Screening for complement factor H, factor I and membrane cofactor protein genotype could be useful in patients with ESRD due to no-STX HUS who wish to have a kidney transplant.
Management
Prophylaxis: The use of low dose aspirin and dipyridamole has been reported after transplantation with inconsistent results22,23. CsA and antilymphocyte globulin should be used with caution. Heavy inadequacy of the specific protease cleaving the vWillebrand factor must be treated with FFP infusion every 15 days after kidney transplantation17,24. Landau has proposed high dose FFP infusion in patients with Factor H deficiency to prevent post-transplant HUS in children25 but it is uncertain if this approach has any success.
Treatment: It consists of plasma exchange with FFP or FFP infusion with reduction or discontinuation of cyclosporine at the first sign of recurrence with limited success22,26–28. Fulminant recurrence of haemolytic uremic syndrome has been noticed in children during a CNI free immunosuppression 29 . Nephrectomy of native kidneys has been performed with limited success. Combined kidney . liver transplantation has been performed in cases with factor H with discouraging results.
De novo postransplant HUS
De novo HUS is not a rare complication after kidney transplantation and frequently is associated with CNI / mTOR inhibitor toxicity or acute vascular rejection.
Viral infections
Influenza A viruses30, parvoviruses and cytomegalovirus have been implicated in the pathogenesis of de novo HUS. Parvoviruses can infect the endothelial cell through a specific binding to the P-antigen on the cell surface. The consequent endothelial injury can then sustain the microangiopathic process. Similar mechanisms have been involved in the setting of infection with cytomegalovirus, which, in addition to directly damaging the endothelial cells, can sustain the platelet adhesion to the microvascular wall by inducing the expression of adhesion molecules and the release of vWF31–33.
Anticardiolipinic antibodies and de novo HUS
Patients with hepatitis C and anticardiolipinic antibodies may present HUS after kidney transplantation34
Use of calcinurin inhibitors
Association of CsA with HUS was reported initially in bone marrow transplantation (BMT), later in liver transplantation and finally in kidney and heart transplantation35. Today treatment with calcinurin inhibitors is a well-established risk factor for the development of de novo TMA36.
The frequency after kidney transplantation was higher in patients treated with Neoral and its increased bioavailability. The use of tacrolimus (FK506) in kidney transplantation was accompanied by reports of its successful use in transplant recipient with CsA-associated HUS. Later on it was proved that tacrolimus could induce HUS after renal transplantation37.
Both CsA and tacrolimus – associated HUS can present with a spectrum of clinical signs ranging from variable degrees of haematologic or renal abnormalities to the full blown pentad of microangiopathic haemolytic anaemia, thrombocytopenia, neurological signs, fever and renal failure. The haematological changes can precede or follow the signs of target organ dysfunction that occasionally can be the only sign of the disease.
The diagnosis of HUS due to CsA or tacrolimus can be done on the basis of graft biopsies performed to determine the cause of delayed graft function or to rule out acute allograft rejection. Every organ may present vascular lesions due to CsA or tacrolimus HUS. Pulmonary, dermatologic, musculoskeletal, hepatic and gastrointestinal involvement has been described in CsA – associated HUS34.
The pathogenesis of calcinurin inhibitors associated HUS is not completely understood. They cause endothelial injury and reduction of both prostacyclin synthesis and the prostacyclin to thromboxane A2 ratio. These changes lead to vasoconstriction, platelet aggregation and thrombus formation38,39. Calcinurin inhibitors have direct and indirect preglomerular constricting properties, the latter through a stimulatory effect on endothelin secretion40, which cause increased vascular sear stress, abnormal vWF fragmentation and platelet activation and further amplification of the microangiopathic process.
A recent analysis of the United States Renal Data System (USRDS) and Medicare claims identified multiple additional risk factors including younger recipient age, female gender of the recipient, longer duration of dialysis before transplantation, previous renal transplant, delayed graft function, allograft rejection, increased peak panel – reactive antibody and treatment with sirolimus41.
Use of mTOR inhibitors
Initially it was thought that sirolimus (rapamycin, SRL) did not induce HUS and would be a solution to the problem of patients with HUS due to CsA or FK506. Recently it has been reported de novo HUS after rapamycin use42. Recent evidence suggests that treatment with SRL may be followed by the development of TMA. First, TMA has been noticed in patients on protocols containing SRL in conjunction with CNIs43,44. Secondly, a recent analysis of the USRDS identified SRL use post-transplantation as a risk factor for TMA41 at least at the same level as CsA. Thirdly, there are recent reports describing patients who developed TMA on a regimen containing SRL in the absence of CNIs41,45. Recently, it was estimated that the relative risk of developing TMA after kidney transplantation is 16.1 (incidence 20.7%) for the combination of CsA+SRL and 4.7 (incidence 6.1%) for FK+SRL46.
The pathomechanism by which sirolimus causes de novo HUS is obscure. A working hypothesis is that SRL acts to inhibit endothelial cell proliferation47. In the transplant setting endothelial cell may be injured by a variety o mechanisms including direct drug toxicity, infection, immune processes or OKT3 use. In response to injury endothelial cell may be repopulated by recipient derived endothelial cells48. The antiproliferative effect of SRL may prevent repopulation of the allograft vasculature by reparative endothelial proliferation, thereby promoting local activation of the clotting cascade, consumption of platelets and red blood cell destruction. This hypothesis is supported by the observation that the majority of de novo TMA is renal-limited. SRL has also been shown to cause platelet aggregation49 thus providing another potential mechanism by which SRL could promote TMA. Another scenario is the clotting of vasculature in cases of reperfusion injury or acute rejection because of high expression of tissue factor in patients taking SRL50.
Antibodies
OKT3 antibodies administration has been shown to cause HUS rarely. This complication is most likely to occur with high-dose OKT3 (10 mg/d than the current dose of 5 mg/d). The pathogenesis of OKT3-HUS is due to a four-fold to six-fold increase of prothrombin activity, complement activation and increased TNF-a re lease51,52.
Antibodies against the von Willebrant factor-cleaving metalloprotease DAMTS13 are responsible for most cases of idiopathic TTP53.
Acute rejection and HUS
The procedure of rejection with its detrimental effect on endothelium could be a triggering factor to TMA in patients predisposed to develop HUS. Some investigators have suggested an association of acute vascular rejection with HUS54. Patients with HUS as primary renal disease present more and heavier rejection episodes compared to patients without HUS and higher frequency of CAN has been reported in patients with HUS as primary renal disease who did not develop HUS after kidney transplantation. In fact this relationship, if there is any, has to be clarified in the future.
HUS is the result of a primary non-inflammatory toxic lesion of the endothelium followed by a secondary local intravascular coagulation, fibrin formation and platelet activation. On the other hand acute vascular rejection is the result of an inflammatory reaction of the immune system due to complement classical pathway activation and antibody action. Recent evidence suggests that the only clue for differential diagnosis is C4d deposits in the peritubular capillaries in cases of acute vascular rejection55. Follow up biopsies would be informative in patients who have been treated successfully for HUS.
Clinical Picture
The clinical picture of post – transplant HUS is obscure. Anemia is usually mild and thrombocytopenia is reported in no more than 50% of patients. Renal dysfunction can be the only non – specific sign of post . transplant HUS. However, since concomitant complications such as acute rejection, CsA or tacrolimus nephrotoxicity or cytomegalovirus infection can confound the clinical picture, the definite diagnosis almost invariably rests on biopsy findings56. Typical changes include glomerular and arterial thrombosis, endothelial cell swelling and detachment from the basement membrane, glomerular ischemia and in the healing phase, onion skin hypertrophy of the arteriolar walls. These changes, however, can reflect an acute vascular rejection that should always be considered in the differential diagnosis with HUS, in particular when tubulitis and interstitial infiltration are accompanied by severe endovasculitic lessons affecting the entire vascular tree of the graft36.
Patients with parvovirus infection may present fever, fatigue, athralgia, aplastic anemia and thrombocytopenia followed by deterioration of renal function
Management
Treatment of post-transplant HUS rests on removal of the inciting factor, relief of symptoms and plasma infusion or exchange. Drug withdrawal is first line therapy for de-novo CsA or FK506 associated HUS but is effective in less than 50% of cases. A significantly higher success rate has been reported with drug withdrawal plus plasma infusion or exchange26,58. In adults the suggested dose of FFP is 20 mg/kg/d initially every day and later every second day according to the evolution of haemolysis, thrombocytopenia and decrease in serum creatinine level58. In cases with anuria, cardiac overload or hypertension, plasma exchange was given every 48 hours between hemodialysis sessions and 2 lt of plasma volume was exchanged with 1200-600 ml FFP + 500 ml albumin or macromolecular solutions58.
Plasma exchange has been suggested as a first line therapy in heavy cases and as secondary in cases with no response to treatment with FFP infusion. It has been reported the use of steroids (1 mg/kg/d) as well as the use of anti-platelet agents concomitantly with the plasma exchange. A similar response has been noticed with intravenous infusion of IgG (0.4 g/kg) alone or in combination with plasma exchange. The rational of this therapy is the neutralization of circulating cytotoxic or platelet agglutinating factors33.
After remission is achieved it is better to switch from one drug to another or treat with mycophenolate mofetil59,60. Monoclonal anti IL-2 receptor antibodies also can be a valid option for maintaining adequate immunosuppression and avoiding the toxic effects of calcinurin inhibitors. The outcome of de novo forms occurring in the setting of viral infection parallels the response to treatment of the underlying disease30,31,33.
In spite of the above, we must have in mind that the complication rate of plasma exchange may be as high as 30% some of which may be fatal61. Patients with localized TMA respond to decreasing, changing or temporarily discontinuing NCIs and do not routinely require plasma exchange for graft salvage1.
Having in mind these data, renal transplantation should be considered an effective and safe treatment of ESRD for patients with STX-associated HUS17,55,62 and patients who lost the first kidney graft for recurrence should not receive another transplant. Living related renal transplant in familial forms should be avoided because it has the risk, among the others, to precipitate the disease onset on the donor63.
References
- 1.Schwimmer J, Nadasdy TA, Spitalnik PF, Kaplan KL, Xand MS. De novo thrombotic microangiopathy in renal transplant recipients: a comparison of hemolytic uremic syndrome with localized renal thrombotic microangiopathy. Am J Kidney Dis. 2003;41:471–479. doi: 10.1053/ajkd.2003.50058. [DOI] [PubMed] [Google Scholar]
- 2.Remuzzi G. HUS and TTP: Variable expression of a single entity. Kidney Int. 1987;32:292–308. doi: 10.1038/ki.1987.206. [DOI] [PubMed] [Google Scholar]
- 3.Kaplan BS, Kaplan B. Hemolytic uremic syndrome in families. In: Kaplan BS, Trompeter RS, Moake JL, editors. Hemolytic Uremic Syndrome and Thrombotic Thrombocytopenic Purpura. NY: Marcel Dekker; 1992. pp. 23–225. [Google Scholar]
- 4.Meroni M, Volpi A, Battini G, et al. Reccurent hemolytic syndrome: Case report. Nephron. 1986;44:263–264. doi: 10.1159/000184001. [DOI] [PubMed] [Google Scholar]
- 5.Pettit RM. Thrombotic thrombocytopenic purpura; a thirty year review. Semin Thromb Hemost. 1980;6:350–355. doi: 10.1055/s-2007-1005108. [DOI] [PubMed] [Google Scholar]
- 6.Schieppati A, Ruggeneti P, Plata Cornejo R, et al. For the Italian Regitry of hemolytic uremic syndrome. Renal function at hospital admission as a prognostic factor in adult hemolytic uremic syndrome. J Am Soc Nephrol. 1992;2:1640–1644. doi: 10.1681/ASN.V2111640. [DOI] [PubMed] [Google Scholar]
- 7.Ruggeneti P, Noris M, Remmuzi G. Thrombotic microangiopathy, hemolytic uremic syndrome and thrombotic thrombocytopenic purpura. Kidney Int. 2001;60:831–846. doi: 10.1046/j.1523-1755.2001.060003831.x. [DOI] [PubMed] [Google Scholar]
- 8.Ducloux D, Rebibou JM, Semhoun-Ducloux S, et al. Recurrent hemolytic-uremic syndrome in renal transplant recipients. Transplantation. 1998;65:1405–1407. doi: 10.1097/00007890-199805270-00023. [DOI] [PubMed] [Google Scholar]
- 9.Noris M, Remmuzi G. Genetic abnormalities of complement regulators in hemolytic uremic syndrome: how do they affect patient management? Nature Clinical Practice. Nephrology. 2005;1:2–3. doi: 10.1038/ncpneph0018. [DOI] [PubMed] [Google Scholar]
- 10.Fremeaux-Bacchi V. Complement factor I: a succeptibility gene for atypical haemolytic uraemic syndrome. J Med Gen. 2004;41:e84. doi: 10.1136/jmg.2004.019083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kavanagh D. Mutations in complement factor I predispose to development of typical hemolytic uremic syndrome. J Am Soc Nephrol. 2005;16:150–2155. doi: 10.1681/ASN.2005010103. [DOI] [PubMed] [Google Scholar]
- 12.Caprioli J, Bettinagliou P, Zipfer PF, et al. The molecular basis of familial hemolytic uremic syndrome: mutation analysis of factor H gene reveals a hot spot consensus repeat. Am Soc Nephrol. 2001;12:297. doi: 10.1681/ASN.V122297. [DOI] [PubMed] [Google Scholar]
- 13.Remuzzi G, Ruggeneti P, Codazzi D, et al. Combined kidney and liver transplantation for familial hemolytic uremic syndrome. Lancet. 2002;359:1671–1672. doi: 10.1016/S0140-6736(02)08560-4. [DOI] [PubMed] [Google Scholar]
- 14.Remuzzi G, Ruggeneti P, Colledan M, et al. Hemolytic uremic syndrome: A fatal outcome after kidney and liver transplantation performed to correct factor H gene mutation. Am J Transplant. 2005;5:1146–1150. doi: 10.1111/j.1600-6143.2005.00783.x. [DOI] [PubMed] [Google Scholar]
- 15.Noris M, Remuzzi G. Hemolytic uremic syndrome. J Am Soc Nephrol. 2005;16:1035–1050. doi: 10.1681/ASN.2004100861. [DOI] [PubMed] [Google Scholar]
- 16.Goodship Th. Mutations in CD46, a complement regulatory protein, predispose to atypical HUS. Trends Mol Med. 2004;10:226–231. doi: 10.1016/j.molmed.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 17.Loirat Ch, Niaudet P. The risk of recurrence of hemolytic uremic syndrome after renal transplantation in children. Pediatr Nephrol. 2003;18:1095–1101. doi: 10.1007/s00467-003-1289-8. [DOI] [PubMed] [Google Scholar]
- 18.Ashkenazi S, Cleary TG, Lopez E, Pickering LK. Anticytotoxin-neutralizing antibodies inimmune globulin preparations: Potential use in hemolytic uremic syndrome. J Pediatr. 1988;113:1008–1014. doi: 10.1016/s0022-3476(88)80572-9. [DOI] [PubMed] [Google Scholar]
- 19.Hebert D, Kim EM, Sibley RK, Mauer SM. Post-transplantation outcome of patients with hemolytic uremic syndrome: Update. Pediatr Nephrol. 1991;5:162–167. doi: 10.1007/BF00852876. [DOI] [PubMed] [Google Scholar]
- 20.Miller RB, Burke BA, Achmidt WJ, et al. Recurrence of hemolytic uremic syndrome in renal transplants: a single center report. Nephrol Dial Transplant. 1997;12:1425–1430. doi: 10.1093/ndt/12.7.1425. [DOI] [PubMed] [Google Scholar]
- 21.Lahlou A, Lang P, Charpentier B, Group Cooperatife De L'Ile-De France hemolytic uremic syndrome: Recurrence after renal transplantation. Medicine (Baltimore) 2000;79:90–102. doi: 10.1097/00005792-200003000-00003. [DOI] [PubMed] [Google Scholar]
- 22.Agarwal A, Mauer SM, Matas AJ, Nath KA. Recurrent haemolytic uremic syndrome in an adult renal allograft recipient: Current concepts and management. J Am Soc Nephrol. 1995;6:160. doi: 10.1681/ASN.V641160. [DOI] [PubMed] [Google Scholar]
- 23.Hebert D, Sibley RK, Muer SM. Recurrence of haemolytic uremic syndrome in renal transplant recipients. Kidney Int. 1986;(Suppl 19):S51. [PubMed] [Google Scholar]
- 24.Veyradier A, Obert B, Haddad E, et al. Severe difficiency of the specific von Willebrant factor-cleaving protease (ADAMTS 13) activity in a subgroup of children with atypical haemolytic uremic syndrome. J Pediatr. 2003;142:310–317. doi: 10.1067/mpd.2003.79. [DOI] [PubMed] [Google Scholar]
- 25.Landau D, Shalev H, Levy-Finer G, Polonsky A, Segev Y, Katchko L. Familial haemolytic uremic syndrome associated with complement factor H deficiency. J Pediatr. 2001;138:412–417. doi: 10.1067/mpd.2001.112649. [DOI] [PubMed] [Google Scholar]
- 26.Zarifian A, Meleg-Smith S, O'Donovan R, et al. Cyclosporine associated thrombotic microangiopathy in renal allografts. Kidney Int. 1999;55:2457–2466. doi: 10.1046/j.1523-1755.1999.00492.x. [DOI] [PubMed] [Google Scholar]
- 27.Springate J, Fildes R, Anthone S, et al. Recurrent haemolytic uremic syndrome after renal transplantation. Transplanr Proc. 1988;20:559. [PubMed] [Google Scholar]
- 28.Stevenson JA, Dumke A, Glassock RJ, Cohen AH. Thrombotic microangiopathy: Recurrence following renal transplant and response to plasma infusion. Am J Nephrol. 1982;2:227. doi: 10.1159/000166651. [DOI] [PubMed] [Google Scholar]
- 29.Florman S, Banchimol F, Lieberman K, Burrows L, Bromberg JS. Fulminant recurrence of atypical haemolytic uremic syndrome during a calcineurin inhibitor free immunosuppression regimen. Paediatr Transplantation. 2000;6:352–355. doi: 10.1034/j.1399-3046.2002.t01-1-00002.x. [DOI] [PubMed] [Google Scholar]
- 30.Asaka M, Ishikawa I, Nakazawa T, et al. Hemolytic uremic syndrome associated with influenza A virus infection in an adult renal allograft recipient: Case report and review of the literature. Nephron. 2000;84:258–266. doi: 10.1159/000045586. [DOI] [PubMed] [Google Scholar]
- 31.Murer L, Zacchello G, Bianchi T, et al. Thrombotic microangiopathy associated with Parvovirus B19 infection after renal transplantation. J Am Soc Nephrol. 2000;11:1132–1137. doi: 10.1681/ASN.V1161132. [DOI] [PubMed] [Google Scholar]
- 32.Waiser J, Budde K, Rudolph B, et al. De novo hemolytic uremic syndrome post renal transplant after cytomegalovirus infection. Am J Kidney Dis. 1999;34:556–560. doi: 10.1016/s0272-6386(99)70085-5. [DOI] [PubMed] [Google Scholar]
- 33.Hochstetler LA, Flanigan MJ, Lager DJ. Transplant - associated thrombotic microangiopathy: the role of IgG administration a initial therapy. Am J Kidney Dis. 1994;23:444–450. doi: 10.1016/s0272-6386(12)81010-9. [DOI] [PubMed] [Google Scholar]
- 34.Van Buren D, Van Buren CT, Flechner M, et al. De novo haemolytic uremic syndrome in renal transplant recipients immunosuppressed with cyclosporine. Surgery. 1985;98:54–62. [PubMed] [Google Scholar]
- 35.Holman MJ, Gonwa T, Cooper B, et al. FK506 - associated thrombotic thrombocytopenic purpura. Transplantation. 1992;55:205–206. [PubMed] [Google Scholar]
- 36.Liapis H. Thrombotic microangiopathy involving the kidney: A histopathologic Perspective. Hippokratia. 2003;7:152–158. [Google Scholar]
- 37.Baid S, Pasqual M, Williams WW, Jr, et al. Renal thrombotic microangiopathy associated with anti - cardiolipine antibodies in hepatitis C-positive renal allograft recipients. J Am Soc Nephrol. 1999;10:146–153. doi: 10.1681/ASN.V101146. [DOI] [PubMed] [Google Scholar]
- 38.Remmuzi G, Bertani T. Renal vacular and thrombotic effect of cycloporin. Am J Kidney Dis. 1989;13:261–272. doi: 10.1016/s0272-6386(89)80032-0. [DOI] [PubMed] [Google Scholar]
- 39.Collins P, Wilkie M, Razak K, et al. Cyclosporine and cremaphor modulate von Willebrand factor release from cultured human endothelial cells. Transplantation. 1993;56:1218–1223. doi: 10.1097/00007890-199311000-00032. [DOI] [PubMed] [Google Scholar]
- 40.Moutabaric A, Ishibashi M, Fukunaga M, et al. FK506 mechanism of nephrotoxicity: Stimulatory effect on endothelin secretion by cultured kidney cells and tubular cell toxicity in vitro. Transplant Proc. 1991;23:3133–3136. [PubMed] [Google Scholar]
- 41.Reynolds JC, Agodoa LY, Yuan CM, Abbot KC. Thrombotic microangiopathy after renal transplantation in the United States. Am J Kidny Dis. 2003;42:1058–1068. doi: 10.1016/j.ajkd.2003.07.008. [DOI] [PubMed] [Google Scholar]
- 42.Crew RJ, Radhaktishnam J, Coen DJ, et al. De novo thrombotic microangiopathy following treatment with sirolimus: report of two cases. Nephrol Dial Transplant. 2005;20:203–209. doi: 10.1093/ndt/gfh334. [DOI] [PubMed] [Google Scholar]
- 43.Saikali JA, Truong LD, Suki WN. Sirolimus may promote thrombotic microangiopathy. Am J Transplant. 2003;3:229–230. doi: 10.1034/j.1600-6143.2003.00018.x. [DOI] [PubMed] [Google Scholar]
- 44.Barone GW, Gurley BG, Abul-Ezz SR, Gogden N. Sirolimus induced thrombotic microangiopathy in a renal transplant recipient. Am J Kidney Dis. 2003;42:202–206. doi: 10.1016/s0272-6386(03)00424-4. [DOI] [PubMed] [Google Scholar]
- 45.Langer RM, Van Buren CT, Katz SM, Kahan BD. De novo hemolytic uremic syndrome after kidney transplantation in patients treated with cyclosporine -sirolimus combination. Transplantation. 2002;73:756–760. doi: 10.1097/00007890-200203150-00017. [DOI] [PubMed] [Google Scholar]
- 46.Fortin M, Raymond M, Madore F, et al. Increased of thrombotic microangiopathy in patients receiving a cyclosporine - sirolimus combination. Am J Transplant. 2004;4:946–952. doi: 10.1111/j.1600-6143.2004.00428.x. [DOI] [PubMed] [Google Scholar]
- 47.Lieberthal W, Fuhro R, Andry CC, et al. Rapamycin impairs recovery from acute renal failure: role of cell ? cycle arrest and apoptosis of tubular cells. Am J Physiol. 2001;281:F693–F706. doi: 10.1152/ajprenal.2001.281.4.F693. [DOI] [PubMed] [Google Scholar]
- 48.Lagaaij EL, Cramer-Knijnenburg GF, van Kemenade FJ, van Es LA, Bruijn JA, van Krieken JHJM. Endothelial cell chymerism after renal transplantation and vascular rejection. Lancet. 2001;357:33–37. doi: 10.1016/S0140-6736(00)03569-8. [DOI] [PubMed] [Google Scholar]
- 49.Babinska A, Markell MS, Salifu MO, Akoad M, Ehrlich YH, Kornecki E. Enhancement of human platelet aggregation and secretion induced by rapamycin. Nephrol Dial Transplant. 1998;13:3153–3159. doi: 10.1093/ndt/13.12.3153. 50. [DOI] [PubMed] [Google Scholar]
- 50.Guba M, Yezhelyev M, Eichhorm ME, et al. Rapamycin induces tumor - specific thrombosis via tissue factor in the presence of VEGF. Blood. 2005;105:4463–4469. doi: 10.1182/blood-2004-09-3540. [DOI] [PubMed] [Google Scholar]
- 51.Abramowicz D, Pradier O, Marchant A, et al. Induction of thrombosis within renal grafts by high-dose prophylactic OKT3. Lancet. 1992;339:777–778. doi: 10.1016/0140-6736(92)91897-h. [DOI] [PubMed] [Google Scholar]
- 52.Chatenaud L, Ferran C, Reuter A, et al. Sytemic reaction to anti-T cell monoclonal antibody OKT3 in relation to serum levels of tumor necrosis factor and interferon - gamma. N Engl J Med. 1989;329:1420–1421. doi: 10.1056/NEJM198905253202117. [DOI] [PubMed] [Google Scholar]
- 53.Pham PT, Danovitch GM, Wilkinson AH, et al. Inhibitors of ADAMTS13: A potential factor in the course of thrombotic microangiopathy in a renal allograft recipient. Transplantation. 2002;74:1077. doi: 10.1097/00007890-200210270-00003. [DOI] [PubMed] [Google Scholar]
- 54.Schlumpf R, Candinas D, Weber W, et al. Acute vascular rejection with hemolytic uremic syndrome in kidneys from non-heart-beating donors: Associated with secondary grafts and early cyclosporine treatment? Transplant Proc. 1993;25:1518–1521. [PubMed] [Google Scholar]
- 55.Artz MA, Steenbrgen EJ, Hoitsma AJ, Monnons LA, Wetzels JFM. Renal transplantation in patients with hemolytic uremic syndrome: high rate of recurrence and increased incidence of acute rejections. Transplantation. 2003;76:821–826. doi: 10.1097/01.TP.0000085083.74065.1B. [DOI] [PubMed] [Google Scholar]
- 56.Hebert D, Mauer SM. Hemolytic uremic syndrome and transplantation. In: Kaplan BS, Trompeter RS, Moake JL, editors. Hemolytic Uremic Syndrome and Thrombotic Thrombocytopenic Purpura. NY: Marcel Dekker; 1992. pp. 179–186. [Google Scholar]
- 57.Pham PTT, Peng A, Wilkinson AH, et al. Cyclosporine and tacrolimus ? associated thrombotic microangiopathy. Am J Kidney Dis. 1999;36:556–560. doi: 10.1053/ajkd.2000.17690. [DOI] [PubMed] [Google Scholar]
- 58.Tostivint I, Mougenot B, Flahault A, et al. Adult haemolytic and uraemic syndrome: causes and prognostic factors in the last decade. Nephrol Dial Transplant. 2002;17:1228–1234. doi: 10.1093/ndt/17.7.1228. [DOI] [PubMed] [Google Scholar]
- 59.Grup C, Schmidt F, Braun F, et al. Hemolytic uremic syndrome during treatment with cyclosporine A after renal transplantation- Is tacrolimus the answer? Nephrol Dial Transplant. 1998;13:1629–1631. doi: 10.1093/ndt/13.7.1629. [DOI] [PubMed] [Google Scholar]
- 60.McGregor DO, Robson RA, Lynn KL. Hemolytic uremic syndrome in a renal transplant recipient treated with conversion to mycophenolate mofetil. Nephron. 1998;80:365–366. doi: 10.1159/000045205. [DOI] [PubMed] [Google Scholar]
- 61.Rizvi MA, Vesely SK, George JN, et al. Complications of plasma exchange in 71 consecutive patients treated for clinically suspected thrombotic thrombocytopenic purpura - hemolytic - uremic syndrome. Transfusion. 2000;40:896–901. doi: 10.1046/j.1537-2995.2000.40080896.x. [DOI] [PubMed] [Google Scholar]
- 62.Ferraris JR, Ramirez JA, Ruiz S, et al. Shiga toxin - associated hemolytic uremic syndrome. Absence of recurrence after renal transplantation. Pediatr Nephrol. 2002;17:809–814. doi: 10.1007/s00467-002-0936-9. [DOI] [PubMed] [Google Scholar]
- 63.Donne RL, Abbs I, Barany P, et al. Recurrence of hemolytic uremic syndrome after live related renal transplantation associated with subsequent de novo disease in the donor. Am J Kidney Dis. 2002;40:E22. doi: 10.1053/ajkd.2002.36938. [DOI] [PubMed] [Google Scholar]