Abstract
Background
The United States Food and Drug Administration requires clinical trial noninferiority margins to preserve a fraction (eg, 50%) of the established comparator drug's efficacy versus placebo. Lack of placebo-controlled trials for many infections complicates noninferiority margin justification for and, hence, regulatory review of new antimicrobial agents. Noninferiority margin clarification is critical to enable new antimicrobial development. In the absence of placebo-controlled trials, we sought to define the magnitude of efficacy of antimicrobial agents and resulting noninferiority margins for studies of complicated skin and skin-structure infection (SSSI).
Methods
We systematically reviewed literature on complicated SSSI published during 1900–1950 (before widespread penicillin resistance) to define treatment outcomes and confidence intervals (CIs). Antimicrobial efficacy was calculated as the lower limit CI of the cure rate with antimicrobials minus the upper limit CI of the cure rate without antimicrobials.
Results
We identified 90 articles describing >28,000 patients with complicated SSSI. For cellulitis/erysipelas, cure rates were 66% (95% CI, 64%–68%) without antibiotics and 98% (95% CI, 96%–99%) for penicillin-treated patients, and penicillin reduced mortality by 10%. Cure rates for wound/ulcer infections were 36% (95% CI, 32%–39%) without antibiotics and 83% (95% CI, 81%–85%) for penicillin-treated patients. For major abscesses, cure rates were 76% (95% CI, 71%–80%) without antibiotics and 96% (95% CI, 94%–98%) for penicillin-treated patients; penicillin reduced mortality by 6%.
Conclusion
Systematic review of historical literature enables rational noninferiority margin justification in the absence of placebo-controlled trials and may facilitate regulatory review of noninferiority trials. Noninferiority margins of 14% for cellulitis/erysipelas, 21% for wound/ulcer infections, and 7% for major abscesses would preserve ≥50% of antibiotic efficacy versus placebo for these complicated SSSI subsets.
Over the past several decades, increasing antimicrobial resistance has driven a critical need to develop new antimicrobial agents [1]. However, recent controversy over acceptable margins of noninferiority for registrational clinical trials has served as a major impediment to successful development of new antibiotics [2, 3]. As for many infectious diseases, selection of an appropriate noninferiority margin is problematic for clinical trials of complicated skin and skin-structure infection (SSSI), because antimicrobials became available in an era prior to randomized, placebo-controlled trials. Complicated SSSIs are among the most common medical conditions in the United States (US) and throughout the world and impart substantial patient morbidity and cost to the US and global health care systems [4–11]. Furthermore, the spread of community-acquired methicillin-resistant Staphylococcus aureus has provided a major impetus to develop new antimicrobial agents for these infections [9, 11–20].
As an example of rational noninferiority margin justification in a manner compliant with US Food and Drug Administration (FDA) [21] and International Congress on Harmonization (ICH) E9 and E10 guidances [22, 23], we sought to define appropriate noninferiority margins for clinical trials of antimicrobial agents in the treatment of complicated SSSI. As a basis for margin justification, we conducted a systematic review of historical literature to determine the effect size of a “gold standard” antimicrobial agent relative to no active therapy against these infections.
Methods
Systematic review
We conducted a systematic review of the peer-reviewed literature on skin infection in the preantibiotic and immediate postantibiotic era (1900–1950). To increase the relevance of our search to modern clinical studies (which exclude enrollment of patients infected with bacteria resistant to the comparator drug), we focused the literature search on a period prior to the dissemination of β-lactamase–mediated resistance to penicillin in both hospital inpatient and community settings in the 1950s [24–29].
PubMed, ScienceDirect, and Google were searched for English language articles published during 1900–1950 using the search combinations “cellulitis OR erysipelas,” “wound AND infection,” or “abscess OR carbuncle.” In addition, references to other SSSI studies found in identified articles were reviewed.
Definitions and statistics
Definitions of clinical cure or failure were adapted from each manuscript on the basis of the criteria available. Objective criteria used to indicate failure included death, septic complications, worsening of infection after initiation of therapy, persistence of lesions after completion of therapy or for ≥28 days while receiving therapy, relapse or recurrence of infection after termination of therapy, failure to heal wounds or wound dehiscence, failure of skin grafts, or amputation.
Skin infections were divided into 1 of 3 major complicated SSSI categories: (1) cellulitis/erysipelas; (2) infection of trauma, surgical, or combat wounds or ulcers; and (3) major abscesses. Patients with a furuncle (ie, an uncomplicated abscess [30]) were excluded from the analysis whenever they were described separately from patients with a major abscess or carbuncle.
Treatments were divided into 3 categories: (1) no active antimicrobial therapy, (2) sulfonamide therapy, and (3) penicillin therapy. Weighted averages of successful treatment were calculated across studies. Confidence intervals (CIs) were calculated using standard linear combination variance formulas [31]. This method allowed inclusion of 1-armed studies and non-randomized 2-armed studies, which is not possible with meta-analytic techniques [32]. Antimicrobial efficacy was conservatively defined as the lower limit of the 95% CI of the cure rate with antimicrobial therapy minus the upper limit of the 95% CI of the cure rate with no antimicrobial therapy.
Egger's test for publication bias and the χ2 Q test for heterogeneity were calculated using Comprehensive Meta-Analysis Software (Biostat). A χ2 or Fisher's Exact test was used to compare proportions of cure or mortality. A 2-tailed P value ≤.05 was considered to be significant.
Results
Literature summary
Ninety peer-reviewed publications from 1900 through 1950 were included in the analysis, describing cure or mortality rates for >28,000 patients with a complicated SSSI. Additional studies were excluded because specific cure rates could not be determined [33–36]. Studies of chlortetracycline or streptomycin treatment of complicated SSSIs were also excluded [37–39], as were studies of erysipeloid (ie, cellulitis caused by Erysipelothrix rhusiopathiae) [40, 41].
Two additional studies reported population-based mortality rates from erysipelas spanning the pre- and postantibiotic eras (figure 1). In the first study, Hoyne et al. [42] described the mortality among patients with erysipelas treated at Cook County Hospital during 1929–1938. In another study, Madsen [43] reported the population-based mortality rates for erysipelas over a 90-year period with use of a national database in Norway. The magnitude of mortality reduction reported immediately after the availability of first sulfonamides and then penicillin was dramatic (figure 1).
Figure 1.
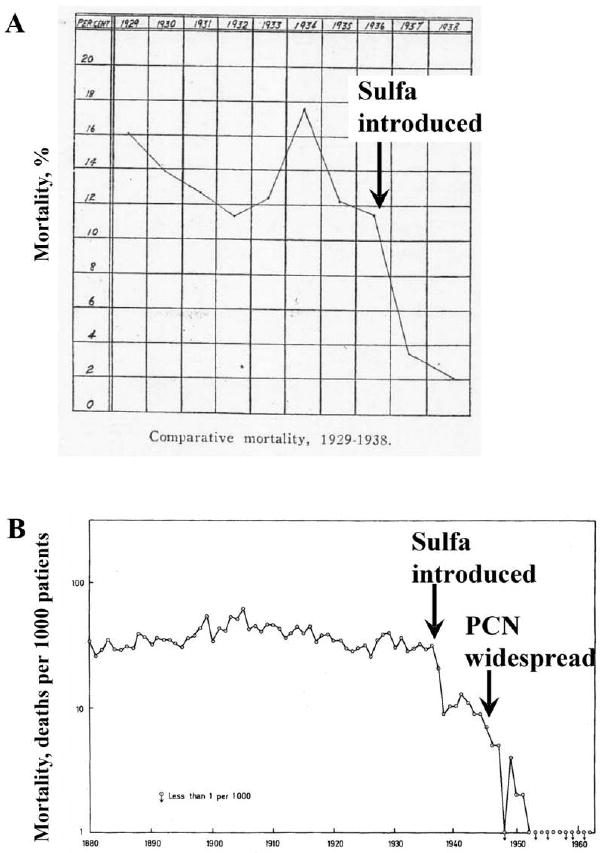
Mortality rates associated with erysipelas before and after the introduction of antimicrobial agents. A, Mortality rates of erysipelas at Cook County Hospital in Chicago, Illinois, from 1929–1938. Sulfonamides (sulfa) became generally available in 1936. Adapted from Hoyne et al. [42] with permission. B, Mortality of erysipelas from a national registry in Norway. Sulfonamides (sulfa) became generally available between 1936 and 1937. Penicillin (PCN) was first used in patients in 1941 but did not become generally available for civilian use until after World War II, between 1946 and 1947. Adapted from Madsen [43] with permission.
Cure rates for cellulitis/erysipelas
Thirty-seven studies reported cure rates for cellulitis/erysipelas for children and adults (table 1). Concordant with modern experience, β-hemolytic streptococci were the predominant organisms identified, with S. aureus being the second most common (table 1) [44].
Table 1. Clinical cure rates for cellulitis/erysipelas.
| Treatment | |||
|---|---|---|---|
| Outcome | Othera | Sulfonamide | Penicillin |
| Overall, proportion of patients cured | 1520/2294 | 1423/1573 | 196/200 |
| Percentage of patients cured (95% CI) | 66 (64–68) | 91 (89–92) | 98 (96–99) |
| Effect size, % (95% CI) | … | 24 (21–28) | 32 (28–36) |
| Lower limit of effect size,b % | … | … | 28 |
Note. CI, confidence interval. This table is available in its entirety in the online version of the journal.
“Other” refers to nonantimicrobial therapies, including topical creams (eg, magnesium sulfate, glycerin, etc.), blood transfusion, injection of anti-streptococcal serum into lesions, X-ray or ultraviolet therapy, or bacteriophage therapy.
Lower limit of effect size is calculated by subtracting the upper bound of the 95% CI of cure with no antibiotic therapy (68%) from the lower bound of the 95% CI of cure with penicillin (96%).
No difference in cure rates was found for topical creams or ointments, ultraviolet radiation therapy, X-ray therapy, active vaccination, antitoxin serum, bacteriophage therapy, or injection of autologous blood (data not shown). Therefore, all non-antimicrobial treatments were grouped as “other” treatments for analysis (table 1). The average cure rate for cellulitis/erysipelas was 66% for non–antimicrobial-treated patients, 91% for systemic sulfonamide-treated patients, and 98% for systemic penicillin-treated patients. The lower limit of efficacy for systemic penicillin was 28% (table 1).
Two additional studies reported the efficacy of topical or local penicillin treatment for a combined total of 37 patients with cellulitis/erysipelas [45, 46]. Topical or local penicillin treatment was less effective than was systemic penicillin, resulting in a cure rate of 89% (95% CI, 80%–98%).
Significant heterogeneity was detected in studies reporting cure rates for no antimicrobial therapy or a sulfonamide treatment (P < .001 for both, by Q test). However, no heterogeneity was detected in studies reporting penicillin cure rates (P = .9). Heterogeneity was largely accounted for by differences in the factors used by the studies to define treatment failure. Specifically, studies tended to report higher cure rates when they considered fewer factors in the definition of treatment failure. In contrast, studies reported lower cure rates when they considered more factors in the definition of treatment failure. Publication bias was not detected by Egger's test for any of the groups (P = .6, P = .1, and P = .4 for no antimicrobial, sulfonamide, and penicillin treatment, respectively).
Cure rates for wound/ulcer infections
Twenty-three studies reported outcomes of traumatic, surgical, or combat wound or ulcer cutaneous infections (table 2). Again, consistent with modern experience, S. aureus was the most common pathogen isolated, with streptococci (predominantly β-hemolytic streptococci) being the second most common (table 2). Several studies also reported culturing Enterococcus species, gram-negative rods, and/or anaerobes from polymicrobial wound infections.
Table 2. Clinical cure rates for trauma, surgery, or combat wounds or ulcer infections.
| Treatment | |||
|---|---|---|---|
| Outcome | Othera | Sulfonamide | Penicillin |
| Overall, proportion of patients cured | 160/449 | 385/531 | 1476/1775 |
| Percentage of patients cured (95% CI) | 36 (32–39) | 73 (70–76) | 83 (81–85) |
| Effect size, % (95% CI) | … | 37 (30–43) | 48 (42–53) |
| Lower limit of effect size,b % | … | … | 42 |
Note. CI, confidence interval. This table is available in its entirety in the online version of the journal.
“Other” refers to nonantimicrobial therapies, including topical creams (eg, magnesium sulfate, glycerin, etc.), blood transfusion, injection of anti-streptococcal serum into lesions, X-ray or ultraviolet therapy, or bacteriophage therapy.
Lower limit of effect size is calculated by subtracting the upper bound of the 95% CI of cure with no antibiotic therapy (68%) from the lower bound of the 95% CI of cure with penicillin (96%).
The cure rate was 36%, 73%, or 83% for patients treated with no antimicrobial, a sulfonamide, or penicillin, respectively. The lower CI limit of penicillin efficacy compared with no antimicrobial therapy was 42% (table 2). Significant heterogeneity in cure rates was detected for all 3 treatment groups (P < .001, by Q test) because of the diverse types of wounds and the mixture of studies using topical or local versus systemic routes of antimicrobial administration. Publication bias was not detected by Egger's test for any of the groups (P = .1, P = .9, and P = .1 for no antimicrobial, sulfonamide, and penicillin treatment, respectively).
Seven studies were identified in which 109 patients were clearly described to have received only systemic, and not topical or local, penicillin treatment of wound or ulcer infections [47–53]. The cure rate in these studies of systemic penicillin therapy was 89% (95% CI, 83%–95%). There was no significant heterogeneity in these studies (P = .5) and no evidence of publication bias (P = .3).
Cure rates for major abscesses
Thirty-four studies reported cure rates for children and adults with a major abscess (table 3). S. aureus was by far the most common etiologic organism identified, with streptococci identified much less often (table 3). Abscesses (mostly carbuncles) in the historical literature were typically complicated and accompanied by fever and other systemic signs of illness. Furuncles constituted <10% of the analyzed cases.
Table 3. Clinical cure rates for major abscesses.
| Treatment | |||
|---|---|---|---|
| Outcome | Othera | Sulfonamide | Penicillin |
| Overall, proportion of patients cured | 254/336 | 60/69 | 282/293 |
| Percentage of patients cured (95% CI) | 76 (71–80) | 87 (80–94) | 96% (94–98) |
| Effect size, % (95% CI) | … | 11 (0–23) | 21 (14–27) |
| Lower limit of effect size,b % | … | … | 14 |
Note. CI, confidence interval. This table is available in its entirety in the online version of the journal.
“Other” refers to nonantimicrobial therapies, including topical creams (eg, magnesium sulfate, glycerin, etc.), blood transfusion, injection of anti-streptococcal serum into lesions, X-ray or ultraviolet therapy, or bacteriophage therapy.
Lower limit of effect size is calculated by subtracting the upper bound of the 95% CI of cure with no antibiotic therapy (68%) from the lower bound of the 95% CI of cure with penicillin (96%).
The cure rate for major abscesses was 76% with no antimicrobial treatment, 87% with sulfonamide treatment, and 96% with systemic penicillin treatment. The lower limit of systemic penicillin efficacy compared with no active antimicrobial was 14% (table 3). When studies that included any furuncles were excluded from the analysis, the cure rates were changed marginally (77%; [95% CI, 72%–81%] for no antimicrobial treatment vs. 97% [95% CI, 95%–100%] for penicillin treatment). In contrast, in 3 studies including 69 patients primarily treated with local or topical penicillin rather than systemic therapy [45–47], the cure rate (84% [95% CI, 76%–93%]) was not significantly different from placebo.
Excluding studies in which surgical management was not clearly described [47, 52, 54–58], surgical intervention was significantly more common among patients treated without antimicrobial therapy than among those treated with penicillin (98 [37%] of 268 patients vs. 18 [16%] of 112 patients; P < .001). Furthermore, cure rates were not significantly different with or without surgery among patients treated without an antimicrobial agent (75 [77%] of 98 patients vs. 131 [77%] of 180 patients; P > .99) or among those treated with penicillin (18 [100%] of 18 patients vs. 93 [99%] of 94 patients; P = .8).
Significant heterogeneity in cure rates was detected in studies of no antimicrobial therapy (P < .001, by Q test) but not in studies of penicillin treatment (P = .8). Publication bias was not detected (P = .3 and P = .4 for no antimicrobial or penicillin treatment, respectively).
Mortality rates for skin infections
Ultraviolet therapy, sulfonamides, and penicillin each significantly reduced the mortality rate of cellulitis/erysipelas compared with other nonantimicrobial treatments (table 4, P < .001 for all 3 comparisons). Sulfonamide and penicillin treatment were also more effective than ultraviolet therapy (P < .001 for both comparisons), and penicillin was more effective than sulfonamide treatment (P = .02, by Fisher's exact test). Penicillin mediated a 10% absolute reduction in the mortality rate attributable to cellulitis/erysipelas compared with no antimicrobial therapy.
Table 4. Mortality rates for cellulitis/erysipelas.
| Treatment | ||||
|---|---|---|---|---|
| Outcome | Othera | UV | Sulfonamide | Penicillin |
| Overall, proportion of patients cured | 2528/23657 | 409/4936 | 35/1593 | 1/325 |
| Percentage of patients cured (95% CI) | 10.7 (10.3–11.1) | 8.3 (7.5–9.1) | 2.2 (1.5–2.9) | 0.3 (0–0.8) |
| Effect size, % (95% CI) | … | −2.4 (−1.3 to −3.6) | −8.5 (−7.4 to −9.6) | −10.4 (−9.5 to −11.1) |
Note. CI, confidence interval. This table is available in its entirety in the online version of the journal.
“Other” refers to nonantimicrobial therapies, including topical creams (eg, magnesium sulfate, glycerin, etc.), blood transfusion, injection of anti-streptococcal serum into lesions, X-ray therapy (but not ultraviolet therapy), or bacteriophage therapy.
Mortality data were available from 33 studies of major abscesses (table 5). In 4 studies, sulfonamides did not significantly reduce mortality versus no antimicrobial therapy. In contrast, penicillin mediated a 6% absolute reduction in the mortality rate attributable to major abscesses relative to no antimicrobial therapy (P < .001).
Table 5. Mortality rates for carbuncles and furuncles.
| Treatment | ||
|---|---|---|
| Outcome | Other | Penicillin |
| Overall, proportion of patients cured | 64/1016 | 0/337 |
| Percentage of patients cured (95% CI) | 6 (5–8) | 0 (0–0) |
| Effect size, % (95% CI) | … | −6 (−5 to −8) |
Note. CI, confidence interval. This table is available in its entirety in the online version of the journal.
“Other” refers to nonantimicrobial therapies, including topical creams (eg, magnesium sulfate, glycerin, etc.), blood transfusion, injection of anti-streptococcal serum into lesions, X-ray therapy (but not ultraviolet therapy), or bacteriophage therapy.
Modern dose-escalation data
A recent, phase II dose-escalation study of dalbavancin for complicated SSSI provided evidence of the minimal efficacy of active antimicrobial therapy for this disease. Seltzer et al. [59] randomized patients with complicated SSSI to receive a single infusion of 1100 mg of dalbavancin or a single 1000 mg infusion followed by a 500 mg infusion 1 week later. The clinical cure rate at the test-of-cure visit for the clinically evaluable population was 94% for patients treated with 2 doses of dalbavancin and 62% for patients treated with 1 dose of dalbavancin. For the intention-to-treat population, the cure rate was 91% for patients who received 2 doses of dalbavancin versus 60% for patients who received a single dose of dalbavancin. Compared with the single dose dalbavancin regimen, the 2-dose regimen was 32% more effective in the clinically evaluable population and 31% more effective in the intention-to-treat population.
Discussion
A comprehensive review of complicated SSSI from the preantibotic era and the immediately postantibiotic era revealed a substantial treatment effect of antimicrobial therapy. Penicillin was more effective than sulfonamides, consistent with both extensive historical experience and the fact that sulfonamide monotherapy has not been used since the advent of combination sulfonamide-dihydrofolate reductase inhibitors 40 years ago. Therefore, systemic penicillin was the clear historical gold standard antimicrobial therapy on which the basis for noninferiority margins should be justified.
ICH E9 and E10 guidances indicate that noninferiority margins should preserve a clinically meaningful fraction of the lower limit of the established efficacy of the comparator drug [22, 23]. Preservation of 50% of the comparator drug's efficacy relative to placebo or no therapy has been suggested as reasonable when setting noninferiority margins [60–64], but others have argued that this method is highly conservative [65], and this issue has not been clarified [64]. To preserve one-half of the lower limit of the treatment effects we found, the non-inferiority margin should be 14% for cellulitis/erysipelas, 21% for wound or ulcer infection, and 7% for major abscess. In practice, the noninferiority margin for a specific complicated SSSI trial should be weighted for the proportion of enrolled patients with cellulitis/erysipelas, wound or ulcer infections, and major abscesses. For example, if a 1:1:1 ratio of patients with cellulitis, wound or ulcer infection, or major abscess were enrolled, the noninferiority margin should be 14% (average of 14%, 21%, and 7%). We emphasize that, because of the low mortality of complicated SSSI treated with antibiotics, it may be reasonable to preserve <50% of the gold standard comparator's efficacy, resulting in wider noninferiority margins, especially if the new agent offered other clinical benefits [64], such as enhanced activity against antimicrobial-resistant bacteria and an enhanced safety profile, compared with currently available agents.
Our calculations for antibiotic efficacy were also conservative in that they were generated by subtracting the upper limit of the 95% CI of no antimicrobial therapy efficacy from the lower limit of the 95% CI of penicillin efficacy. Therefore, our calculations likely resulted in underestimates of the actual efficacy of penicillin relative to no antimicrobial therapy.
FDA [21] and ICH guidances [22, 23] also emphasize that the data used to justify a noninferiority margin must be relevant to modern studies (the “Constancy Assumption” standard). Several elements in our analysis support fulfillment of the Constancy Assumption standard for the efficacy of antimicrobial agents for complicated SSSI. First, the efficacy of 2 doses versus 1 dose of dalbavancin in a modern study of complicated SSSI provided an estimate of antibiotic efficacy that was strikingly similar to the historical datasets, and this conclusion was conservative, because 1 dose of dalbavancin was likely more effective than placebo. Second, the microbiology of the historical studies of cellulitis/erysipelas, wound or ulcer infection, and major abscess was similar to the microbiology of such infections in the modern era. Finally, the mortality rates associated with complicated SSSI treated with penicillin were similar to modern mortality rates of complicated SSSI when effective antimicrobial therapy is used.
We emphasize that previous studies evaluating antibiotic efficacy compared with placebo or in the setting of discordant therapy have focused on uncomplicated SSSI rather than complicated SSSI [11, 15, 17–19, 66–68]. In contrast, we specifically excluded analysis of furuncles (ie, uncomplicated abscesses) to focus on complicated SSSI. The 6% mortality rate among patients with a major abscess receiving no antimicrobial therapy reinforces the fact that these infections represented complicated SSSI rather than uncomplicated SSSI.
Because of the low mortality associated with antimicrobial-treated complicated SSSI, mortality is not viable as the only outcome measure for modern complicated SSSI clinical trials. Nevertheless, the historical mortality data underscore the efficacy of antimicrobial agents for treatment of complicated SSSI. Indeed, the fact that cellulitis/erysipelas caused an 11% mortality rate in the preantibiotic era has been largely forgotten in the antibiotic era. The magnitude of that mortality rate is emphasized by its similarity to the 12% mortality rate of myocardial infarction in the placebo arm of a modern, randomized, double-blinded study [69].
It is highly unlikely that changes in background medical care affected the mortality rates during the period of our survey, or indeed subsequent to it. For example, during >50 years leading up to 1936, there was no change in the mortality rate of erysipelas (figure 1) [43]. Immediately after the availability of sulfonamides, the mortality rate decreased by >3 fold [42, 43], at a time when no other new medical technology was being introduced. When penicillin became available there was another immediate 10-fold decrease in mortality, to rates comparable to those in the modern era, with no further change during the subsequent >20 years. Published testimonials from physicians caring for patients in the 1930s and 1940s affirm that it was the introduction of antibiotics, and not another change in medical practice, that led to the immediate decrease in mortality attributable to infection [70–73].
The primary limitations of our analysis include heterogeneity, the potential for publication bias, and the large proportion of single-armed, observational studies in the analysis. The heterogeneity of outcomes for patients receiving no antimicrobial therapy was largely accounted for by higher cure rates reported when fewer criteria were used to define treatment failure, and thus, results were likely biased toward a lower efficacy of antimicrobial agents rather than a higher efficacy. For wound or ulcer infections, heterogeneity was driven by inclusion of patients who received topical or local antimicrobial therapy in the analysis. Again, this resulted in a more conservative estimate of antibiotic efficacy, because the efficacy of penicillin relative to no antimicrobial therapy was higher (53%; 95% CI, 44%–62%) in the 7 studies that exclusively evaluated systemic therapy.
We found no statistical evidence of publication bias. However, given the limitations of such analyses [74], we cannot exclude the possibility that publication bias existed. If publication bias did exist, it likely affected the publication of results for both antimicrobial and nonantimicrobial therapeutic efficacy. For example, published cases in which topical ointments, dye solutions, ultraviolet therapy, or X-ray therapy were used to treat skin infection are likely selected for those cases in which more favorable results were observed.
Ideally, rigorous establishment of the magnitude of efficacy of antimicrobial versus no antimicrobial therapy for complicated SSSI would rely upon contemporary, double-blinded, placebo-controlled trials. However, the clear establishment of efficacy of sulfonamides and penicillin [60, 70, 72, 75–78] predated, by several decades, the widespread use of randomized, placebo-controlled trials. Nor can placebo-controlled trials of antimicrobial agents be conducted today for most types of infection [60, 79]. The limitations of the current analysis must be considered in the context of the public health imperative to develop new antimicrobial agents for resistant infection [1]. Antimicrobial agents are unique, not just among all drugs, but among all technologies, in that they continually lose efficacy over time in a transmissible manner. Therefore, unlike any other class of drugs, there is a perpetual need to develop new antimicrobial agents to enable treatment of bacteria that are continually becoming resistant to currently available drugs.
The ICH E10 guidance emphasizes that “the determination of the margin in a non-inferiority trial is based on both statistical reasoning and clinical judgment” [23, p. 9]. Therefore, in light of (1) the critical need for new antimicrobial agents, (2) the robustness of the datasets reviewed, (3) the conservative nature of the calculations, (4) the evidence of a large magnitude of antimicrobial efficacy for treatment of complicated SSSI, and (5) our compliance with critical features of the ICH E9 and E10 and FDA guidances, we believe that the current findings are sufficient to enable a rational justification for noninferiority margins for complicated SSSI clinical trials.
Acknowledgments
The authors thank Dr. Peter Christensen, from the Los Angeles Biomedical Research Institute and the General Clinical Research Center at Harbor-UCLA Medical Center, for statistical support. The authors also thank reference librarians Pat Taylor, Jenna Oh, and Kelley Talevich at the Parlow Library at Harbor-UCLA Medical Center for their tremendous assistance with obtaining much of the historical literature. Finally, the authors thank Drs. Henry Masur, Eric Brass, and Roger Lewis for helpful comments.
Footnotes
Potential conflicts of interest. B.S. has received research grant support from Astellas, Gilead, Novartis, Merck, and Pfizer; serves on the scientific advisory board of Merck; has consulted for Arpida, Basilea, Advanced Life Sciences, Novo Nordisk, and Theravance; owns equity in NovaDigm Therapeutics; and has previously received speaker's honoraria from Merck, Astellas, and Pfizer. G.T. serves, or has recently served, as a consultant to the Actelion, Avera, Bausch and Lomb, Calixa, Cempra, Cerexa, Cubist, Ipsat, Middlebrook, Nabriva, PTC, Rib-X, Shire, Targanta, Tetraphase, Theravance, ViroPharma, and Wyeth; and owns equity in Calixa and Mpex. J.B.'s employer has received research grants from AstraZeneca, Cubist, Johnson & Johnson, and Wyeth, and has received reimbursement for J.B.'s role in consulting for AstraZeneca, Cubist, Johnson & Johnson, Wyeth, Forest/ Cerexa, Pfizer, Schering Plough, and Trius. H.B. serves as an advisor/consultant to Astellas, Basilea, Cubist, Johnson & Johnson, Merck, Pfizer, Rib-X, Targanta, and Theravance and has owned shares of Pfizer and Cubist. D.G. serves as an advisor/consultant to Pfizer, Advanced Life Sciences, Pacific Bioscience, Schering-Plough, Roche, Wyeth, and Pfizer and is on the speakers' bureau for Merck. W.M.S. serves on advisory boards of Pfizer, Cubist, and GlaxoSmithKline. J.E.E. serves on the scientific advisory boards of Pfizer, Merck, and Gilead; has participated in educational programs regarding fungal infections funded by Pfizer, Merck, and Astellas; has received research laboratory support from Pfizer, Merck, and Gilead; and has participated in the Bristol-Myers Squibb Freedom to Discovery research program. J.G.B. serves on the HIV advisory boards for Bristol-Myers Squibb, Abbott Laboratories, and GlaxoSmithKline.
References
- 1.Spellberg B, Guidos R, Gilbert D, et al. The epidemic of antibiotic-resistant infections: a call to action for the medical community from the Infectious Diseases Society of America. Clin Infect Dis. 2008;46:155–64. doi: 10.1086/524891. [DOI] [PubMed] [Google Scholar]
- 2.Shlaes DM, Moellering RC., Jr The United States Food and Drug Administration and the end of antibiotics. Clin Infect Dis. 2002;34:420–2. doi: 10.1086/338976. [DOI] [PubMed] [Google Scholar]
- 3.Gilbert DN, Edwards JE., Jr Is there hope for the prevention of future antimicrobial shortages. Clin Infect Dis. 2002;35:215–6. doi: 10.1086/341958. author reply 216–7. [DOI] [PubMed] [Google Scholar]
- 4.Lipsky BA, Weigelt JA, Gupta V, Killian A, Peng MM. Skin, soft tissue, bone, and joint infections in hospitalized patients: epidemiology and microbiological, clinical, and economic outcomes. Infect Control Hosp Epidemiol. 2007;28:1290–8. doi: 10.1086/520743. [DOI] [PubMed] [Google Scholar]
- 5.Ellis Simonsen SM, van Orman ER, Hatch BE, et al. Cellulitis incidence in a defined population. Epidemiol Infect. 2006;134:293–9. doi: 10.1017/S095026880500484X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McNamara DR, Tleyjeh IM, Berbari EF, et al. Incidence of lower-extremity cellulitis: a population-based study in Olmsted county, Minnesota. Mayo Clin Proc. 2007;82:817–21. doi: 10.4065/82.7.817. [DOI] [PubMed] [Google Scholar]
- 7.Fry DE. The economic costs of surgical site infection. Surg Infect (Larchmt) 2002;3 1:S37–43. doi: 10.1089/sur.2002.3.s1-37. [DOI] [PubMed] [Google Scholar]
- 8.Leaper DJ, van Goor H, Reilly J, et al. Surgical site infection—a European perspective of incidence and economic burden. Int Wound J. 2004;1:247–73. doi: 10.1111/j.1742-4801.2004.00067.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee SY, Kuti JL, Nicolau DP. Antimicrobial management of complicated skin and skin structure infections in the era of emerging resistance. Surg Infect (Larchmt) 2005;6:283–95. doi: 10.1089/sur.2005.6.283. [DOI] [PubMed] [Google Scholar]
- 10.Hersh AL, Chambers HF, Maselli JH, Gonzales R. National trends in ambulatory visits and antibiotic prescribing for skin and soft-tissue infections. Arch Intern Med. 2008;168:1585–91. doi: 10.1001/archinte.168.14.1585. [DOI] [PubMed] [Google Scholar]
- 11.Daum RS. Clinical practice. Skin and soft-tissue infections caused by methicillin-resistant Staphylococcus aureus. N Engl J Med. 2007;357:380–90. doi: 10.1056/NEJMcp070747. [DOI] [PubMed] [Google Scholar]
- 12.Chambers HF. Community-associated MRSA—resistance and virulence converge. N Engl J Med. 2005;352:1485–7. doi: 10.1056/NEJMe058023. [DOI] [PubMed] [Google Scholar]
- 13.Talbot GH, Bradley J, Edwards JE, Jr, et al. Bad bugs need drugs: an update on the development pipeline from the Antimicrobial Availability Task Force of the Infectious Diseases Society of America. Clin Infect Dis. 2006;42:657–68. doi: 10.1086/499819. [DOI] [PubMed] [Google Scholar]
- 14.Klevens RM, Morrison MA, Nadle J, et al. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA. 2007;298:1763–71. doi: 10.1001/jama.298.15.1763. [DOI] [PubMed] [Google Scholar]
- 15.Moran GJ, Krishnadasan A, Gorwitz RJ, et al. Methicillin-resistant S. aureus infections among patients in the emergency department. N Engl J Med. 2006;355:666–74. doi: 10.1056/NEJMoa055356. [DOI] [PubMed] [Google Scholar]
- 16.Moellering RC., Jr The growing menace of community-acquired methicillin-resistant Staphylococcus aureus. Ann Intern Med. 2006;144:368–70. doi: 10.7326/0003-4819-144-5-200603070-00014. [DOI] [PubMed] [Google Scholar]
- 17.Moellering RC., Jr Current treatment options for community-acquired methicillin-resistant Staphylococcus aureus infection. Clin Infect Dis. 2008;46:1032–7. doi: 10.1086/529445. [DOI] [PubMed] [Google Scholar]
- 18.Abrahamian FM, Talan DA, Moran GJ. Management of skin and soft-tissue infections in the emergency department. Infect Dis Clin North Am. 2008;22:89–116. vi. doi: 10.1016/j.idc.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 19.Stevens DL, Bisno AL, Chambers HF, et al. Practice guidelines for the diagnosis and management of skin and soft-tissue infections. Clin Infect Dis. 2005;41:1373–406. doi: 10.1086/497143. [DOI] [PubMed] [Google Scholar]
- 20.MMWR Morb Mortal Wkly Rep. Vol. 51. 2002. Vancomycin-resistant Staphylococcus aureus—Pennsylvania, 2002; p. 902. [PubMed] [Google Scholar]
- 21.US Department of Health and Human Services Food and Drug Administration. Center for Drug Evaluation and Research (CDER); 2007. [15 June 2009]. Guidance for Industry Antibacterial drug products: use of noninferiority studies to support approval. Available at: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm070951.pdf. [Google Scholar]
- 22.Guidance for Industry. E9, Statistical Principles for Clinical Trials. International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use; 1998. [15 June 2009]. Available at: http://www.emea.europa.eu/pdfs/human/ich/036396en.pdf. [Google Scholar]
- 23.Guidance for Industry. E10, Choice of Control Group and Related Issues in Clinical Trials. International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use; 1998. [15 June 2009]. Available at: http://www.fda.gov/downloads/RegulatoryInformation/Guidances/ucm129460.pdf. [Google Scholar]
- 24.Chambers HF. The changing epidemiology of Staphylococcus aureus? Emerg Infect Dis. 2001;7:178–82. doi: 10.3201/eid0702.010204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beigelman PM, Rantz LA. The clinical importance of coagulase-positive, penicillin-resistant Staphylococcus aureus. N Engl J Med. 1950;242:353–8. doi: 10.1056/NEJM195003092421002. [DOI] [PubMed] [Google Scholar]
- 26.Dowling HF, Lepper MH, Jackson GG. Observations on the epidemiological spread of antibiotic-resistant staphylococci, with measurements of the changes in sensitivity to penicillin and aureomycin. Am J Public Health Nations Health. 1953;43:860–8. doi: 10.2105/ajph.43.7.860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lepper MH, Dowling HF, Jackson GG, Hirsch MM. Epidemiology of penicillin- and aureomycin-resistant staphylococci in a hospital population. AMA Arch Intern Med. 1953;92:40–50. doi: 10.1001/archinte.1953.00240190052003. [DOI] [PubMed] [Google Scholar]
- 28.Rountree PM, Barbour RG. Incidence of penicillin-resistant and streptomycin-resistant staphylococci in a hospital. Lancet. 1951;1:435–6. doi: 10.1016/s0140-6736(51)92031-4. [DOI] [PubMed] [Google Scholar]
- 29.Summers GA. Penicillin-resistant staphylococci; distribution among out patients. Lancet. 1952;1:135–7. doi: 10.1016/s0140-6736(52)92432-x. [DOI] [PubMed] [Google Scholar]
- 30.US Department of Health and Human Services. Food and Drug Administration. Center for Drug Evaluation and Research (CDER); 1998. [15 June 2009]. Guidance for Industry Uncomplicated and complicated skin and skin structure infections—developing antimicrobial drugs for treatment. Available at: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm071185.pdf. [Google Scholar]
- 31.Dixon WJ, Massey FJ. Introduction to statistical analysis. 4th. New York: McGraw Hill; 1983. p. 463. [Google Scholar]
- 32.Deeks JJ, Altman DG, Bradburn MJ. Statistical methods for examining heterogeneity and combining trials. In: Egger M, Davey Smith G, Altman DG, editors. Systematic reviews in healthcare: meta-analysis in context. London: BMJ Books; 2001. [Google Scholar]
- 33.Herrell WE. The clinical use of penicillin: an antibacterial agent of biologic origin. JAMA. 1944;124:622–6. [Google Scholar]
- 34.Bedford PD. Penicillin in general practice. Lancet. 1946;1:977. [Google Scholar]
- 35.Griffiths E, Jones PF, Shooter RA, Heady JA. The comparative merits of sodium and procaine penicillin given frequently. Br Med J. 1949;2:958–61. doi: 10.1136/bmj.2.4634.958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.King P. Erysipeloid: survey of 115 cases. Lancet. 1946;2:196–8. [PubMed] [Google Scholar]
- 37.Logan MA, Metzger WI, Wright LT, Prigot A, Robinson EA. Aureomycin in soft tissue infections. Am J Surg. 1950;79:229–43. doi: 10.1016/0002-9610(50)90161-9. [DOI] [PubMed] [Google Scholar]
- 38.Howes EL. Topical use of streptomycin in wounds. Am J Med. 1947;2:449–56. doi: 10.1016/0002-9343(47)90090-9. [DOI] [PubMed] [Google Scholar]
- 39.Bloom J. Streptomycin therapy in established wound infections. Oral Surg Oral Med Oral Pathol. 1949;3:534. [PubMed] [Google Scholar]
- 40.Bush RA. Case of erysipeloid of Rosenbach treated with penicillin. Br Med J. 1949;2:964–5. doi: 10.1136/bmj.2.4634.964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barber M, Nellen M, Zoob M. Erysipeloid of Rosenbach: response to penicillin. Lancet. 1946;1:125–7. doi: 10.1016/s0140-6736(46)91267-6. [DOI] [PubMed] [Google Scholar]
- 42.Hoyne AL, Wolf AA, Prim L. Mortality rates in the treatment of 998 erysipelas patients. JAMA. 1939;113:2279–81. [Google Scholar]
- 43.Madsen ST. Scarlet fever and erysipelas in Norway during the last hundred years. Infection. 1973;1:76–81. doi: 10.1007/BF01638479. [DOI] [PubMed] [Google Scholar]
- 44.Panton PN, Adams JE. An investigation into the etiology of erysipelas and allied infections. Lancet. 1909;2:1065–71. [Google Scholar]
- 45.Taylor PH, Hughes KEA. Infective dermatoses treated with penicilin. Lancet. 1944;2:780–4. [Google Scholar]
- 46.Meleney FL. Penicillin in the treatment of established surgical infections. Ann Surg. 1946;124:962–78. [PMC free article] [PubMed] [Google Scholar]
- 47.Keefer CS, Blake FG, Marshall EK, Jr, Lockwood JS, Wood BW. Penicillin in the treament of infections. JAMA. 1943;122:1217–24. [Google Scholar]
- 48.Herrell WE. Further observations on the clinical use of penicillin. Proceedings of the Staff Meetings of the Mayo Clinic; 1943. pp. 65–76. [Google Scholar]
- 49.Bentley FH. The treatment of flesh wounds by early secondary suture and penicillin. Br J Surg. 1944;32:132–9. [Google Scholar]
- 50.Dawson MH, Hobby GL. The clinical use of penicillin: observations in one hundred cases. JAMA. 1944;124:611–21. [Google Scholar]
- 51.Lyons C. An investigation of the role of chemotherapy in wound management in the Mediterranean theater. Ann Surg. 1946;123:902–23. [PMC free article] [PubMed] [Google Scholar]
- 52.Altemeier WA. Penicillin therapy with prolonged interval dosage schedules. Ann Surg. 1948;128:708–13. doi: 10.1097/00000658-194810000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Southworth J, Dabbs C. Prolonged interval dosing of aqueous penicillin in surgical infections. South Med J. 1949;42:981–3. doi: 10.1097/00007611-194911000-00012. [DOI] [PubMed] [Google Scholar]
- 54.Jern HZ, Howes EL, Meleney FL. Studies in bacteriophage. J Lab Clin Med. 1934;19:1257–71. [Google Scholar]
- 55.King CO. Radiation therapy of carbuncles. South Med J. 1937;30:903–6. [Google Scholar]
- 56.Robinson JA, Hirsh HL, Dowling HF. Oral penicillin in the treatment of various bacterial infections. Am J Med. 1948;4:716–23. doi: 10.1016/s0002-9343(48)90395-7. [DOI] [PubMed] [Google Scholar]
- 57.Wellman WE, Herrell WE. Procaine penicillin G in sesame oil: a study of reactions and results in 400 cases. Proceedings of the Staff Meetings of the Mayo Clinic; 1948. pp. 595–600. [PubMed] [Google Scholar]
- 58.Davies AM. Penicillin therapy in scarlet fever. Lancet. 1948;1:810. doi: 10.1016/s0140-6736(48)90879-4. [DOI] [PubMed] [Google Scholar]
- 59.Seltzer E, Dorr MB, Goldstein BP, Perry M, Dowell JA, Henkel T. Once-weekly dalbavancin versus standard-of-care antimicrobial regimens for treatment of skin and soft-tissue infections. Clin Infect Dis. 2003;37:1298–303. doi: 10.1086/379015. [DOI] [PubMed] [Google Scholar]
- 60.Spellberg B, Talbot GH, Brass EP, Bradley JS, Boucher HW, Gilbert D. Position paper: recommended design features of future clinical trials of antibacterial agents for community-acquired pneumonia. Clin Infect Dis. 2008;47 3:S249–65. [PMC free article] [PubMed] [Google Scholar]
- 61.Kaul S, Diamond GA, Weintraub WS. Trials and tribulations of non-inferiority: the ximelagatran experience. J Am Coll Cardiol. 2005;46:1986–95. doi: 10.1016/j.jacc.2005.07.062. [DOI] [PubMed] [Google Scholar]
- 62.Hung HMJ, Wang SJ, Tsong Y, Lawrence J, O'Neil RT. Some fundamental issues with non-inferiority testing in active controlled trials. Stat Med. 2003;22:213–25. doi: 10.1002/sim.1315. [DOI] [PubMed] [Google Scholar]
- 63.D'Agostino RB, Sr, Massaro JM, Sullivan LM. Non-inferiority trials: design concepts and issues—the encounters of academic consultants in statistics. Stat Med. 2003;22:169–86. doi: 10.1002/sim.1425. [DOI] [PubMed] [Google Scholar]
- 64.Active control non-inferiority studies: theory, assay sensitivity, choice of margin. [5 November 2008];US Food and Drug Administration. 2002 Available at: http://www.fda.gov/ohrms/dockets/ac/02/slides/3837s1_02_Temple.ppt.
- 65.Carroll KJ. Active-controlled, non-inferiority trials in oncology: arbitrary limits, infeasible sample sizes and uninformative data analysis is there another way? Pharm Stat. 2006;5:283–93. doi: 10.1002/pst.218. [DOI] [PubMed] [Google Scholar]
- 66.Stryjewski ME, Chambers HF. Skin and soft-tissue infections caused by community-acquired methicillin-resistant Staphylococcus aureus. Clin Infect Dis. 2008;46 5:S368–77. doi: 10.1086/533593. [DOI] [PubMed] [Google Scholar]
- 67.Chambers HF, Moellering RC, Jr, Kamitsuka P. Clinical decisions: management of skin and soft-tissue infection. N Engl J Med. 2008;359:1063–7. doi: 10.1056/NEJMclde0708359. [DOI] [PubMed] [Google Scholar]
- 68.Rajendran PM, Young D, Maurer T, et al. Randomized, double-blind, placebo-controlled trial of cephalexin for treatment of uncomplicated skin abscesses in a population at risk for community-acquired methicillin-resistant Staphylococcus aureus infection. Antimicrob Agents Chemother. 2007;51:4044–8. doi: 10.1128/AAC.00377-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Randomised trial of intravenous streptokinase, oral aspirin, both or neither among 17, 187 cases of suspected acute myocardial infarction: ISIS-2. ISIS-2 (Second International Study of Infarct Survival) Collaborative Group. Lancet. 1988;2:349–60. [PubMed] [Google Scholar]
- 70.Thomas L. The youngest science notes of a medicine-watcher. New York: The Viking Press; 1983. [Google Scholar]
- 71.Grossman CM. The first use of penicillin in the United States. Ann Intern Med. 2008;149:135–6. doi: 10.7326/0003-4819-149-2-200807150-00009. [DOI] [PubMed] [Google Scholar]
- 72.Goldner M. Three generations of experience and thought in microbiology and infection. Can J Infect Dis. 2003;14:329–35. doi: 10.1155/2003/925927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lesch JE. The first miracle drugs: how the sulfa drugs transformed medicine. New York: Oxford University Press; 2007. [Google Scholar]
- 74.Lau J, Ioannidis JP, Terrin N, Schmid CH, Olkin I. The case of the misleading funnel plot. BMJ. 2006;333:597–600. doi: 10.1136/bmj.333.7568.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Northey EH. Monograph #106. New York: Reinhold Publishing; 1948. The sulfonamides and allied compounds. 1948 American Chemical Society Monograph Series. [Google Scholar]
- 76.Dixon B. Sulfa's true significance. Microbe. 2006;1:500–1. [Google Scholar]
- 77.Chain E, Florey HW, Gardner AD, et al. Penicillin as a chemotherapeutic agent. Lancet. 1940;2:226–8. [Google Scholar]
- 78.Abraham EP, Chain E, Fletcher CM, et al. Further observations on penicillin. Lancet. 1941;2:177–188. [Google Scholar]
- 79.Ellenberg SS, Temple R. Placebo-controlled trials and active-control trials in the evaluation of new treatments. Part 2: practical issues and specific cases. Ann Intern Med. 2000;133:464–70. doi: 10.7326/0003-4819-133-6-200009190-00015. [DOI] [PubMed] [Google Scholar]


