Abstract
CBP and p300 are transcriptional coactivators that physically interact with diverse sequence-specific DNA-binding factors through conserved domains. To further investigate the functional roles of these protein-interaction domains in CBP/p300 regulation, we have identified multiple domains of CBP that interact with FKLF2 and the CH2 domain as a new p53 interacting domain of CBP. Functional studies demonstrate that several domains of CBP are capable of stimulating FKLF2 and p53 DNA binding. In addition, we found that CBP through distinct domain is able to bind DNA directly with no specificity. We identified a 51-residue domain in CBP that is capable of interacting with both transcription factors and DNA. We named this domain PDBD for protein and DNA binding domain. These results unveiled two novel activities of CBP. First, these highly conserved domains of CBP not only function to recruit CBP to the target promoter through interaction with DNA-bound transcription factors, but they also actively regulate the DNA binding activity of their interacting factors. Second, by directly interacting with DNA, CBP may orchestrate the formation of stable and promoter-committed transcriptional complexes through interactions with both proteins and promoter DNA.
Keywords: CBP, DNA binding, Functional domain, Protein interaction, p53, FKLF2
CREB binding protein (CBP) and p300, first isolated as proteins that interact with CREB and adenoviral oncoprotein E1A proteins [1,2], are large multifunctional proteins that regulate many cellular functions including cell growth, development, and transformation [3,4]. Both proteins function as transcriptional coactivators for a large number of transcription activators [5,6]. Currently, it is suggested that CBP/p300 coactivators are recruited to the target gene promoters through protein–protein interactions with sequence-specific DNA binding transcriptional activators. At the target gene promoter, they regulate transcription by several mechanisms. First, as CBP/p300 have been shown to interact with both promoter-bound transcriptional activators and general transcription factors, they may function as bridging molecules to mediate the interaction between upstream DNA-bound transcriptional activators and the basal transcription machinery [7,8]. Second, CBP/p300 are huge molecules that contain multiple protein–protein interaction domains and are capable of interacting with diverse proteins including coactivators, activators, and general transcription factors. They may function as a scaffold for the formation of transcriptional complex [8]. Third, CBP/p300 have intrinsic acetyltransferase activity [9,10] and are able to interact with other acetyltransferases including PCAF [11], SRC-1 [12,13], and ACTR [14]. The acetylation of histones by these acetylases has been associated with transcriptional activity [15–19]. In addition to histones, these acetylases also acetylate a large number of transcription factors. The acetylation of transcription factors has been shown to regulate their transcriptional activity at multiple levels [20].
The multiple functions of CBP/p300 reside in their distinct domains. Except for the acetyltransferase domain, however, the only function identified for the other domains of CBP/p300 is to mediate multiple protein–protein interactions. Whether these domains have other regulatory functions remains unknown. In this study, we carried out detailed protein–protein interaction and functional studies on the CBP domains. We found that multiple domains of CBP interact with FKLF2, a member of the Sp1/KLF family of transcription factors [21]. In addition, we identified CH2 as a new p53 interacting domain in CBP. DNA binding assays using these CBP domains with FKLF2 or p53 revealed two novel functions of the CBP domains. First, we found that in addition to mediating protein interaction with FKLF2 and p53, the TAZ1, CH2, and TAZ2 domains of CBP also stimulated FKLF2 and p53 DNA binding activity. Second, we identified a domain of 51 residues in CBP that has direct DNA binding activity. Since this domain also interacts with FKLF2, we named this domain protein and DNA binding domain (PDBD). These results suggest that these CBP domains not only function to recruit CBP to the target promoters through protein–protein interactions with sequence-specific DNA binding factors, but they also actively regulate the DNA binding activity of DNA binding activators and make direct interactions with the promoter DNA.
Materials and methods
Plasmids
Plasmids for expression of Myc-tagged FKLF2 and Flag-tagged p53 in mammalian cells were constructed by inserting the amplified coding sequences for FKLF2 and p53 into mammalian expression vectors. GST–FKLF2 fusion proteins were constructed by inserting the PCR fragment containing amino acid sequences from 149 to 289 into pGEX-4T-1 (Pharmacia). GST fusion proteins containing different CBP domains were constructed by inserting the respective CBP sequences into pGEX-4T-1. GST p53 containing the full-length p53 was constructed by inserting the full-length p53 sequence into pGEX-4T-1.
Electrophoretic mobility shift assay
EMSA was carried out as described [22]. The sequences of the oligonucleotide probes for EMSA assays are: FKLF2 binding site from the human γ globin promoter: 5′-GCTAAACTCCACCCATGGGTTGG-3′; p53 binding site from GADD45 promoter: 5′-TACAGAACATGTCTAAGCATGCTGG GG-3′ [23]; E2F-1 binding site from DHFR promoter: 5′-ATTTAAG ATTTCCCGCCTTTTCTCAA-3′ [24]; consensus GATA-1 binding site: 5′-CACTTGATAACAGAAAGTGATAACTCT-3′; consensus NFE2 binding site: 5′-TGGGGAACCTGTGCTGAGTCACTGG AG-3′; and consensus Sp1 binding site: 5′-ATTCGATCGGGGCGG GGCGAGC-3′.
Cell extracts and protein purification
Whole cell extracts from COS cells were prepared as described [22]. GST fusion proteins were purified as described [22]. The concentration and purity of the fusion proteins were determined by SDS–PAGE and Coomassie blue staining using bovine serum albumin as a standard.
Protein–protein interaction assays
GST pull-down assays were carried out by incubating whole cell extracts from COS cell expressing Myc-tagged FKLF2 or Flag-tagged p53 with 1 μg of GST fusion proteins containing different domains of CBP immobilized on glutathione–agarose beads in a binding buffer containing 20 mM Tris–HCl (pH 7.9), 10% glycerol, 100mM KCl, 5mM MgCl2, 0.5mM EGTA, 0.5mM EDTA, 2mM DTT, and 0.2% IGEPAL-CA-630 with protease inhibitors. The binding mixture was incubated at room temperature for 2h with gentle mixing. Beads were collected by centrifugation and washed four times with 500 μl of binding buffer each. After being re-suspended in SDS sample buffer and boiled for 5 min, proteins were separated on 10% SDS–PAGE and transferred to nitrocellulose membrane. Myc-tagged FKLF2 and Flag-tagged p53 proteins were detected using anti-Myc 9E10 (Santa Cruz Biotechnology) and anti-Flag M2 (Sigma) monoclonal antibodies, respectively, and Chemiluminescence (ECL, Amersham Pharmacia Biotech).
Results and discussion
FKLF2 and p53 interact with CBP through multiple domains
We have found that the acetyltransferase activity of CBP/p300 is not required for stimulation of the DNA binding and transcription activity of FKLF2 [22]. This result suggests that CBP/p300 may function as coactivators for FKLF2 through protein–protein interaction rather than acetylation. CBP/p300 interacts with diverse transcriptional activators through several highly conserved domains (Fig. 1A) including the KIX domain [25], IBiD domain [26], and three putative zinc finger domains termed CH1 (TAZ1), CH2 (PHD), and CH3 (ZZ and TAZ2) [27,28]. We have found that FKLF2 interacts with CBP sequences from 1680 to 1891, which contains the CH3 (ZZ and TAZ2) domain [22]. Further detailed mapping revealed that FKLF2 interacts with the ZZ and TAZ2 domains individually (Fig. 1B). Since TAZ1 and TAZ2 are very similar in sequence and structure, and CBP/p300 has been shown to interact with a large number of factors through multiple domains, we decided to determine whether FKLF2 also interacts with TAZ1 as well as other domains of CBP. We examined the binding of FKLF2 to a series of CBP sequences fused to glutathione S-transferase (GST) (Fig. 1D). These assays revealed that FKLF2 interacts with multiple conserved domains of CBP including the TAZ1, CH2, ZZ, and TAZ2 domains. In addition to these known protein interaction domains, CBP sequences from amino acid residues 1800 to 1891 are also able to bind FKLF2 (Fig. 1B). This region contains the carboxyl terminal half of the TAZ2 domain (residues 1800–1850), which by itself is insufficient for FKLF2 binding (Fig. 1B, lane 9). Further detailed analyses of this region identified a 51 residue PDBD domain that is sufficient for FKLF2 interaction (shown below). Several domains of CBP have been shown to interact with p53 including the TAZ1 (CH1), CH3, and a region in the C-terminus [29]. The DNA binding and transcriptional activities of p53 have been reported to be regulated by CBP/p300 [23,30,31]. By using a acetylase defective mutant of CBP (L1690K/C1691L), we have found that the acetylase activity of CBP is not required for stimulation of p53 DNA binding (unpublished data). We, therefore, examined the interaction between CBP and p53 in detail with the CBP domains that we have used in the FKLF2 interaction assay (Fig. 1D). Consistent with a previous report [32], p53 interacts with the TAZ1 domain of CBP (Fig. 1C). The CH3 domain has been shown to interact with p53 [33]. Further detailed mapping found that p53 interacts with the ZZ domain and the C-terminal half of TAZ2 within the CH3 domain (Fig. 1C). In addition, the CH2 domain was also found to interact with p53. Although both FKLF2 and p53 interacted with the TAZ1, CH2, ZZ, and TAZ2 domains of CBP, some differences in their interaction with CBP domains are also found. For example, the entire TAZ2 domain is required for FKLF2 interaction, whereas p53 is able to interact with the C-terminal half of TAZ2. The C-terminal portion of CBP (residues 1990–2442) interacted with p53 [23] but not with FKLF2 [22]. The result that the C-terminal half of the TAZ2 domain of CBP (residues 1800–1850) but not that of the N-terminal half (residues 1750–1800) interacts with p53 is in agreement with the results from structural studies on TAZ2 and p53 interaction. The peptide of the N-terminal transcriptional activation domain of p53 interacted with one face of the CBP TAZ2 domain through several residues located in the C-terminal portion of TAZ2 including lys1798, Val1819, Gln1822, Leu1823, and Leu1826, probably having direct hydrogen interaction with the Gln1822 residue [34]. Our result that residues 1800–1850 are sufficient for p53 interaction suggests that lys1798 is not required for CBP interaction with full-length p53. In summary, we found that in addition to previously identified domains including TAZ1, ZZ, and TAZ2, the CH2 domain of CBP also interacts with p53 and CBP interacts with the C-terminal portion of the TAZ2 domain.
Fig. 1.
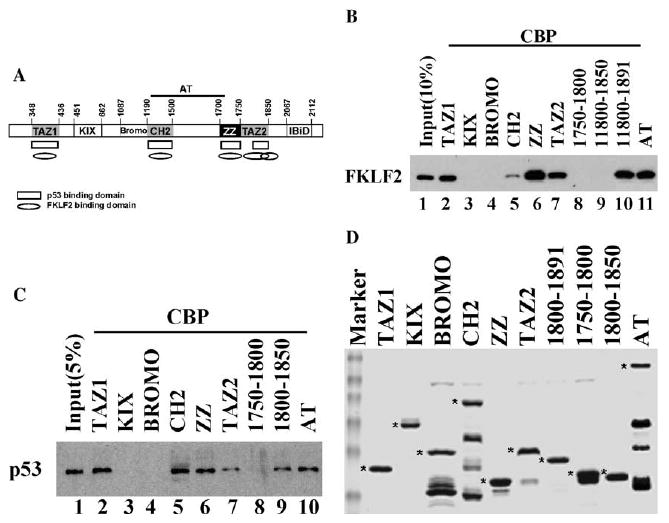
CBP interacts with FKLF2 and p53 through distinct domains. (A) The schematic representation of CBP domain structure. FKLF2 and p53 interacting domains of CBP are indicated as oval and rectangle. (B) GST pull-down assays were carried out by incubating 1 μl of whole cell extracts prepared from COS cells expressing Myc-tagged FKLF2 protein with 1 μg of purified GST–CBP fusion proteins immobilized on glutathione–agarose beads as indicated. The presence of FKLF2 was detected by immunoblotting using anti-Myc 9E10 monoclonal antibody and chemiluminescence. (C) GST pull-down assays were carried out by incubating 1 μl of whole cell extracts prepared from COS cells expressing Flag-tagged FKLF2 protein with 1 μg of purified GST–CBP fusion proteins immobilized on glutathione–agarose beads as indicated. The presence of p53 was detected by immunoblotting using anti-Flag M2 monoclonal antibody and chemiluminescence. (D) Coomassie blue staining of the GST–CBP fusion proteins was used in the assay. The full-length proteins are marked by an asterisk.
Small domains of CBP stimulate FKLF2 and p53 DNA binding activity by direct protein–protein interaction
Current models suggest that the primary function of the conserved domains of CBP/p300, with the exception of the acetyltransferase domain, is to mediate protein–protein interactions with sequence-specific DNA-binding transcription activators, chromatin, cofactors, and the general transcription machinery. It is unknown whether these protein interaction domains have additional regulatory functions. The result that CBP acetylase activity is dispensable for stimulation of FKLF2 DNA binding indicates that the stimulation may be mediated solely by physical interaction between FKLF2 and CBP [22]. To test this possibility and determine whether other conserved domains are able to stimulate FKLF2 DNA binding, we carried out EMSA assays using purified GST fusion proteins containing the CBP domains shown in Figs. 1D and 2C. These assays revealed that the TAZ1, CH2, ZZ, and TAZ2 domains stimulated FKLF2 DNA binding activity, whereas the KIX and Bromo domains showed minimal effects (Fig. 2A). To establish the generality of the observation that the domains of CBP actively regulate the DNA binding activity of their interacting proteins, we wanted to test the effect of these CBP domains on the DNA binding activity of other factors. As purified CBP acetylase domain that contains point mutations (L160K/C161L) and is defective in acetyltransferase activity stimulated p53 DNA binding activity as well as the wild-type acetyltransferase domain (data not shown), we examined the effect of these domains on p53 DNA binding. EMSA assays showed that the TAZ1, CH2, and TAZ2 domains also stimulated p53 DNA binding. The ability of the TAZ1, CH2, and TAZ2 domains to stimulate FKLF2 or p53 DNA binding correlated with their ability to interact with FKLF2 or p53 except for the ZZ domain of CBP, which showed strong binding to both FKLF2 and p53 but weak or no stimulation of FKLF2 and p53 DNA binding, respectively. Since the same amount of purified proteins containing different CBP domains was used in the EMSA assays, the differences in their ability to stimulate FKLF2 and p53 DNA binding is not due to a difference in their amounts used in the assay. The inability of the KIX, Bromo, and ZZ domains to stimulate FKLF2 and p53 DNA binding is also not due to their inactivation during purification. These domains are purified at the same time and different batches of preparations gave the same results. In addition, the ZZ domain is active in FKLF2 and p53 binding but not in stimulation of their DNA binding. The ability of the ZZ domain to interact with both FKLF2 and p53 also indicate that the interaction with FKLF2 and p53 by itself in not sufficient to stimulate their DNA binding.
Fig. 2.
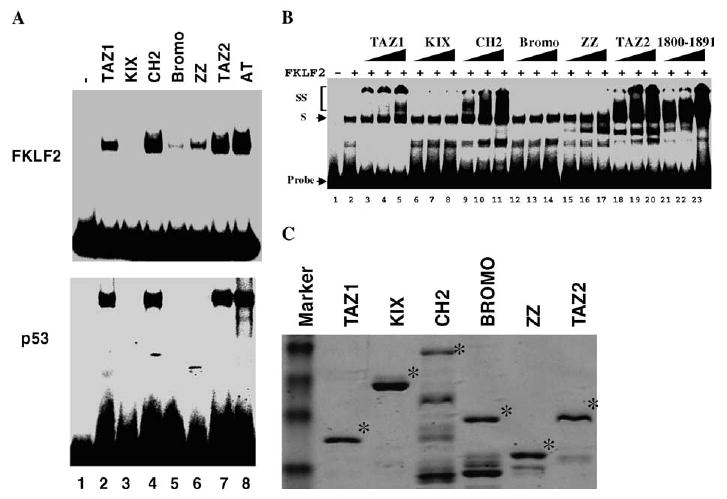
Stimulation of FKLF2 and p53 DNA binding by the conserved domains of CBP. (A) EMSA assays were carried out using purified GST–FKLF2 (149–289) containing the basic and zinc finger DNA binding domains of FKLF2 and GST full-length p53 in the presence or absence of purified GST–CBP domains as indicated. To be sure that each lane received the same amount of recombinant FKLF2 or p53, FKLF2 or p53 was added to a master reaction mixture and aliquots were used in each EMSA reaction. (B) Quantitative EMSA assay was carried out using higher amount of FKLF2 and increasing amount of CBP domains to observe the formation of CBP domains and FKLF2 supershift complex. SS: supershift; S: shift. (C) Coomassie blue staining of the GST–CBP fusion proteins used in the assay. The full-length proteins are marked by an asterisk.
Purified recombinant FKLF2 is not latent in DNA binding activity. Our previous quantitative EMSA assay has shown that CBP acts by stimulating its DNA binding activity but not by converting it from an inactive form to an active form [22]. The EMSA assay shown in Fig. 2A was carried out using a lower amount of FKLF2 to see maximum stimulation by CBP domains. Under this assay condition, the formation of super-shifted complexes containing both FKLF2 and CBP domains was not observed. This may be due to the instability of the complex under this EMSA assay condition. We have shown that the CBPHAT domain stimulated FKLF2 DNA binding independent of its HAT activity [22]. The super-shifted complexes containing CBPHAT and FKLF2 can only be observed under EMSA condition in which higher amounts of both CBPHAT domain and FKLF2 proteins were used (data not shown). We, therefore, carried out quantitative EMSA assays using increasing amounts of CBP domains and a fixed and higher amount of FKLF2 to detect the formation of super-shifted complexes of FKLF2 and CBP domains. Under this condition, FKLF2 showed significant DNA binding by itself (Fig. 2B, lane 2). The addition of CBP domains under this condition resulted in the formation of dose-dependent super-shifts (Fig. 2B). The formation of the super-shift correlates with the ability of these CBP domains to interact with FKLF2 in protein–protein interaction assay (Fig. 1B). The only exception is the ZZ domain that interacts with FKLF2 under protein–protein interaction assay, but stimulated FKLF2 DNA binding weakly (Fig. 2A), and produced no supershift under this EMSA condition (Fig. 2B, lanes 15–17). In conclusion, these results demonstrate that by interacting with sequence-specific DNA binding transcription factors, domains of CBP not only function to bring CBP to the target promoters, but they also actively regulate the DNA binding activity of their interacting factors.
CBP binds DNA directly through a small domain
After finding that small domains of CBP are capable of stimulating FKLF2 and p53 DNA binding activity, we thought that it was necessary to determine whether these CBP domains by themselves can bind to the oligonucleotide probes used in the EMSA assays. EMSA assays using the probes containing FKLF2 or p53 binding site and purified GST–CBP domains showed strong DNA binding by CBP sequences 1800–1891 (Fig. 4B and data not shown). These results are surprising since it is generally believed that CBP/p300 are not DNA binding proteins. To further establish the DNA binding activity of CBP and determine the DNA binding sequence specificity, we examined several EMSA probes containing binding sequences for transcription factors E2F-1, GATA-1, NFE2, and Sp1 that were available in the laboratory. Consistent with the results with probes containing FKLF2 or p53 binding site, EMSA assays using CBP sequences from residues 1800 to 1891 resulted in a distinct shift with all the probes tested. These results demonstrate that CBP residues 1800–1891 have nonspecific DNA binding activity (Fig. 3). No significant DNA binding activity by the KIX, CH2, or ZZ domain was detected under the same condition. Both the TAZ1 and TAZ2 domains also generated complexes with the DNA probes that remain on the top of the gel, indicating they may have DNA binding activity. Both TAZ1 and TAZ2 domains have the HCCC type zinc finger domains and have similar but not same overall structures [34,35]. The DNA binding activity of TAZ1 and TAZ2 is very weak comparing with that of residues 1800–1891. Therefore, we focused our analyses on residues 1800–1891. To further define the DNA binding sequences within 1800–1891, we generated an extensive series of CBP deletion mutants within this region (Fig. 4A). EMSA assays using these mutants of CBP identified that 51 amino acid residues from 1810 to 1860 are sufficient for DNA binding (Fig. 4B). Since sequences from 1800 to 1891 also interact with FKLF2 and stimulate its DNA binding, we examined the FKLF2 binding activity of the same set of CBP deletion mutants. We found that sequences from 1810 to 1860 are required for maximal stimulation of FKLF2 DNA binding activity (Fig. 4A). The TAZ2 domain contains three HCCC type zinc fingers with a structure of four α helices [34]. The sequences from amino acid residues 1810 to 1850 contain the C-terminal one and half of the zinc fingers within α helices 3 and 4. CBP residues 1830–1891 showed a weak but significant FKLF2 binding activity, indicating that the 30 amino acids from 1830 to 1860 may be the minimal sequence for FKLF2 interaction. The results of the deletion analysis of sequences from 1800 to 1891 are summarized in Fig. 4C. Sequence alignment analysis of the PDBD domains from several species showed that human and mouse CBPs have an identical PDBD sequence, and human p300 is 94% identical (Fig. 4D). The corresponding sequence in Drosophila and Caenorhabditis elegans are 78% and 63% identical to human CBP, respectively. Since the C-terminal 10 residues from amino acids 1850 to 1860 of human or mouse CBP are required for DNA binding, the human p300 should be able to bind DNA through this domain. The corresponding sequences within Drosophila and C. elegans CBP are quite divergent from the human and mouse CBP. Further studies will determine whether the Drosophila and C. elegans CBPs can also bind DNA. Studies have shown that p300 binds directly to chromatin through its bromodomain [36], to nucleosome assembly protein-1 (NAP-1), a histone H2A–H2B shuttling protein that promotes histone deposition [37], and modulo, a chromatin associated factor [38]. Together, these results suggest that, in addition to functioning as bridging molecules and acetylases, CBP/p300 coactivators also actively participate in chromatin and DNA interactions to facilitate the formation of transcriptionally competent complexes at the target gene promoter.
Fig. 4.
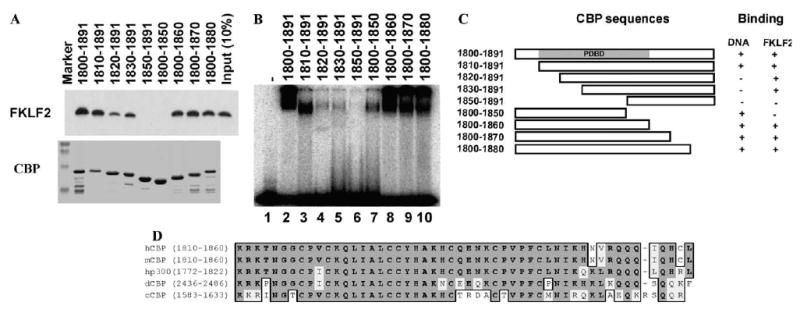
A small and overlapping region of CBP mediates CBP binding to both DNA and FKLF2. (A) GST pull-down assays were carried out by incubating 1 μl of whole cell extracts prepared from COS cells expressing Myc-tagged FKLF2 protein with 1 μg of purified GST–CBP fusion proteins immobilized on glutathione–agarose beads as indicated. The presence of FKLF2 was detected by immunoblotting using anti-Myc 9E10 monoclonal antibody and chemiluminescence. The GST pull-down results were shown at the top. The bottom shows the result of Coomassie blue staining of the GST–CBP fusion proteins used in the assay. (B) EMSA assay using CBP fusion proteins as shown in the bottom of (A). (C) Schematic illustration of CBP domains used in the assay and the summary of their DNA and FKLF2 binding activity. (D) Sequence alignment of the PDBD sequences from human CBP (hCBP), mouse CBP (mCBP), human p300 (hp300), Drosophila CBP (dCBP), and C. elegans CBP (cCBP).
Fig. 3.
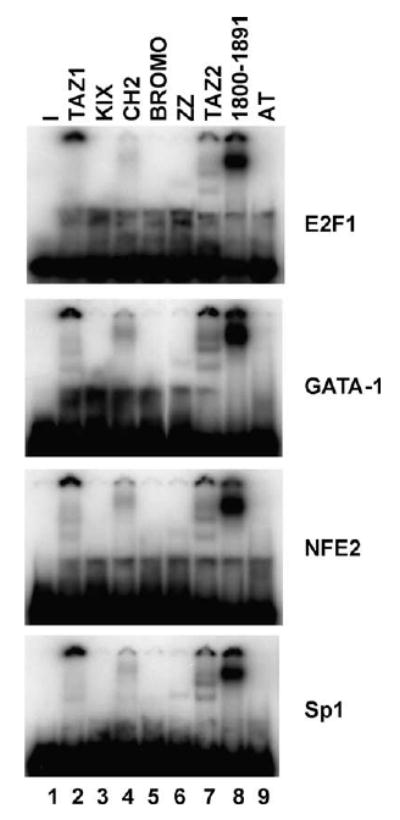
Direct DNA binding activity of CBP domains. EMSA assays were carried out using purified GST–CBP fusion proteins containing different domains of CBP and probes containing binding sites for different factors as indicated. The GST–CBP proteins are as shown in Fig. 2B.
In summary, we have identified multiple FKLF2 interacting domains and a new p53 interacting domain of CBP. Functional studies demonstrate that several of these domains are capable of stimulating FKLF2 and p53 DNA binding through protein–protein interaction. We further show that CBP has direct and nonspecific DNA binding activity. The DNA binding is mediated through a 51 amino acid PDBD domain. These results unveiled two novel functions of the conserved CBP domains and suggest that CBP/p300 coactivators play more dynamic roles in the regulation of transcription.
Acknowledgments
This work was supported by grants from the National Institutes of Health.
References
- 1.Chrivia JC, et al. Phosphorylated CREB binds specifically to the nuclear protein CBP. Nature. 1993;365:855–859. doi: 10.1038/365855a0. [DOI] [PubMed] [Google Scholar]
- 2.Eckner R, et al. Molecular cloning and functional analysis of the adenovirus E1A-associated 300-kDa protein (p300) reveals a protein with properties of a transcriptional adaptor. Genes Dev. 1994;8:869–884. doi: 10.1101/gad.8.8.869. [DOI] [PubMed] [Google Scholar]
- 3.Giordano A, Avantaggiati ML. p300 and CBP: partners for life and death. J Cell Physiol. 1999;181:218–230. doi: 10.1002/(SICI)1097-4652(199911)181:2<218::AID-JCP4>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 4.Goodman RH, Smolik S. CBP/p300 in cell growth, transformation, and development. Genes Dev. 2000;14:1553–1577. [PubMed] [Google Scholar]
- 5.Berger SL. Gene activation by histone and factor acetyltransfe-rases. Curr Opin Cell Biol. 1999;11:336–341. doi: 10.1016/S0955-0674(99)80046-5. [DOI] [PubMed] [Google Scholar]
- 6.Vo N, Goodman RH. CREB-binding protein and p300 in transcriptional regulation. J Biol Chem. 2001;276:13505–13508. doi: 10.1074/jbc.R000025200. [DOI] [PubMed] [Google Scholar]
- 7.Janknecht R, Hunter T. Transcription. A growing coactivator network. Nature. 1996;383:22–23. doi: 10.1038/383022a0. [DOI] [PubMed] [Google Scholar]
- 8.Chan HM, La NB. Thangue, p300/CBP proteins: HATs for transcriptional bridges and scaffolds. J Cell Sci. 2001;114:2363–2373. doi: 10.1242/jcs.114.13.2363. [DOI] [PubMed] [Google Scholar]
- 9.Bannister AJ, Kouzarides T. The CBP co-activator is a histone acetyltransferase. Nature. 1996;384:641–643. doi: 10.1038/384641a0. [DOI] [PubMed] [Google Scholar]
- 10.Ogryzko VV, Schiltz RL, Russanova V, Howard BH, Nakatani Y. The transcriptional coactivators p300 and CBP are histone acetyltransferases. Cell. 1996;87:953–959. doi: 10.1016/s0092-8674(00)82001-2. [DOI] [PubMed] [Google Scholar]
- 11.Yang XJ, Ogryzko VV, Nishikawa J, Howard BH, Nakatani Y. A p300/CBP-associated factor that competes with the adenoviral oncoprotein E1A. Nature. 1996;382:319–324. doi: 10.1038/382319a0. [DOI] [PubMed] [Google Scholar]
- 12.Yao TP, Ku G, Zhou N, Scully R, Livingston DM. The nuclear hormone receptor coactivator SRC-1 is a specific target of p300. Proc Natl Acad Sci USA. 1996;93:10626–10631. doi: 10.1073/pnas.93.20.10626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spencer TE, Jenster G, Burcin MM, Allis CD, Zhou J, Mizzen CA, McKenna NJ, Onate SA, Tsai SY, Tsai MJ, O'Malley BW. Steroid receptor coactivator-1 is a histone acetyltransferase. Nature. 1997;389:194–198. doi: 10.1038/38304. [DOI] [PubMed] [Google Scholar]
- 14.Chen H, et al. Nuclear receptor coactivator ACTR is a novel histone acetyltransferase and forms a multimeric activation complex with P/CAF and CBP/p300. Cell. 1997;90:569–580. doi: 10.1016/s0092-8674(00)80516-4. [DOI] [PubMed] [Google Scholar]
- 15.Grunstein M. Histone acetylation in chromatin structure and transcription. Nature. 1997;389:349–352. doi: 10.1038/38664. [DOI] [PubMed] [Google Scholar]
- 16.Struhl K. Histone acetylation and transcriptional regulatory mechanisms. Genes Dev. 1998;12:599–606. doi: 10.1101/gad.12.5.599. [DOI] [PubMed] [Google Scholar]
- 17.Wade PA, Pruss D, Wolffe AP. Histone acetylation: chromatin in action. Trends Biochem Sci. 1997;22:128–132. doi: 10.1016/s0968-0004(97)01016-5. [DOI] [PubMed] [Google Scholar]
- 18.Workman JL, Kingston RE. Alteration of nucleosome structure as a mechanism of transcriptional regulation. Annu Rev Biochem. 1998;67:545–579. doi: 10.1146/annurev.biochem.67.1.545. [DOI] [PubMed] [Google Scholar]
- 19.Kadonaga JT. Eukaryotic transcription: an interlaced network of transcription factors and chromatin-modifying machines. Cell. 1998;92:307–313. doi: 10.1016/s0092-8674(00)80924-1. [DOI] [PubMed] [Google Scholar]
- 20.Kouzarides T. Acetylation: a regulatory modification to rival phosphorylation? EMBO J. 2000;19:1176–1179. doi: 10.1093/emboj/19.6.1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bieker JJ. Kruppel-like factors: three fingers in many pies. J Biol Chem. 2001;276:34355–34358. doi: 10.1074/jbc.R100043200. [DOI] [PubMed] [Google Scholar]
- 22.Song CZ, Keller K, Murata K, Asano H, Stamatoyannopoulos G. Functional interaction between coactivators CBP/p300, PCAF, and transcription factor FKLF2. J Biol Chem. 2002;227:7029–7036. doi: 10.1074/jbc.M108826200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gu W, Roeder RG. Activation of p53 sequence-specific DNA binding by acetylation of the p53 C-terminal domain. Cell. 1997;90:595–606. doi: 10.1016/s0092-8674(00)80521-8. [DOI] [PubMed] [Google Scholar]
- 24.Martinez-Balbas MA, Bauer UM, Nielsen SJ, Brehm A, Kouzarides T. Regulation of E2F1 activity by acetylation. EMBO J. 2000;19:662–671. doi: 10.1093/emboj/19.4.662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parker D, et al. Phosphorylation of CREB at Ser-133 induces complex formation with CREB-binding protein via a direct mechanism. Mol Cell Biol. 1996;16:694–703. doi: 10.1128/mcb.16.2.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin CH, et al. Small domain of CBP/p300 binds diverse proteins: solution structure and functional studies. Mol Cell Biol. 2001;8:581–590. doi: 10.1016/s1097-2765(01)00333-1. [DOI] [PubMed] [Google Scholar]
- 27.Borrow J, et al. The translocation t(8;16)(p11;p13) of acute myeloid leukaemia fuses a putative acetyltransferase to the CREB-binding protein. Nat Genet. 1996;14:33–41. doi: 10.1038/ng0996-33. [DOI] [PubMed] [Google Scholar]
- 28.Ponting CP, Blake DJ, Davies KE, Kendrick-Jones J, Winder SJ. ZZ and TAZ: new putative zinc fingers in dystrophin and other proteins. Trends Biochem Sci. 1996;21:11–13. [PubMed] [Google Scholar]
- 29.Grossman SR, et al. p300/MDM2 complexes participate in MDM2-mediated p53 degradation. Mol Cell Biol. 1998;2:405–415. doi: 10.1016/s1097-2765(00)80140-9. [DOI] [PubMed] [Google Scholar]
- 30.Espinosa JM, Emerson BM. Transcriptional regulation by p53 through intrinsic DNA/chromatin binding and site-directed cofactor recruitment. Mol Cell Biol. 2001;8:57–69. doi: 10.1016/s1097-2765(01)00283-0. [DOI] [PubMed] [Google Scholar]
- 31.Barlev NA, et al. Acetylation of p53 activates transcription through recruitment of coactivators/histone acetyltransferases. Mol Cell Biol. 2001;8:1243–1254. doi: 10.1016/s1097-2765(01)00414-2. [DOI] [PubMed] [Google Scholar]
- 32.Grossman SR. p300/CBP/p53 interaction and regulation of the p53 response. Eur J Biochem. 2001;268:2772–2778. doi: 10.1046/j.1432-1327.2001.02226.x. [DOI] [PubMed] [Google Scholar]
- 33.Avantaggiati ML, et al. Recruitment of p300/CBP in p53-dependent signal pathways. Cell. 1997;89:1175–1184. doi: 10.1016/s0092-8674(00)80304-9. [DOI] [PubMed] [Google Scholar]
- 34.De Guzman RN, Liu HY, Martinez-Yamout M, Dyson HJ, Wright PE. Solution structure of the TAZ2 (CH3) domain of the transcriptional adaptor protein CBP. J Mol Biol. 2000;303:243–253. doi: 10.1006/jmbi.2000.4141. [DOI] [PubMed] [Google Scholar]
- 35.Dames SA, Martinez-Yamout M, De Guzman RN, Dyson HJ, Wright PE. From the cover: structural basis for Hif-1alpha/CBP recognition in the cellular hypoxic response. Proc Natl Acad Sci USA. 2002;99:5271–5276. doi: 10.1073/pnas.082121399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Manning ET, Ikehara T, Ito T, Kadonaga JT, Kraus WL. p300 forms a stable, template-committed complex with chromatin: role for the bromodomain. Mol Cell Biol. 2001;21:3876–3887. doi: 10.1128/MCB.21.12.3876-3887.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Asahara H, et al. Dual roles of p300 in chromatin assembly and transcriptional activation in cooperation with nucleosome assembly protein 1 in vitro. Mol Cell Biol. 2002;22:2974–2983. doi: 10.1128/MCB.22.9.2974-2983.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bantignies F, Goodman RH, Smolik SM. Functional interaction between the coactivator Drosophila CREB-binding protein and ASH1, a member of the trithorax group of chromatin modifiers. Mol Cell Biol. 2001;20(m 268):2773–2778. doi: 10.1128/mcb.20.24.9317-9330.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]


