Abstract
Medulloblastomas are the most frequent malignant brain tumors in children. Sunitinib is an oral multitargeted tyrosine kinase inhibitor used in clinical trials as an antiangiogenic agent for cancer therapy. In this report, we demonstrate that sunitinib induced apoptosis and inhibited cell proliferation of both a short-term primary culture (VC312) and an established cell line (Daoy) of human medulloblastomas. Sunitinib treatment resulted in the activation of caspase-3 and cleavage of PARP, and upregulation of pro-apoptotic genes, Bak and Bim, as well as inhibiting expression of survivin, an anti-apoptotic protein. Sunitinib treatment also down-regulated cyclin E, D2 and D3, and up-regulated p21Cip1, all of which are involved in regulating cell cycle. In addition, it inhibited phosphorylation of Signal Transducer and Activator of Transcription 3 (STAT3) and AKT (protein kinase B) in the tumor cells. De-phosphorylation of STAT3 (Tyr705) induced by sunitinib was contributed by a reduction in activities of JAK2 and Src. Additionally, sodium vanadate, an inhibitor of protein tyrosine phosphatases, partially blocked the inhibition of phosphorylated STAT3 by sunitinib. Loss of phosphorylated AKT after sunitinib treatment was accompanied by decreased phosphorylation of downstream proteins, GSK-3β and mTOR. Expression of a constitutively activated STAT3 mutant or myristoylated AKT partially blocked the effects of sunitinib in these tumor cells. Sunitinib also inhibited the migration of medulloblastoma tumor cells in vitro. These findings suggest that potential use of sunitinib for treatment of pediatric medulloblastomas.
Keywords: sunitinib, medulloblastoma, STAT3, AKT, apoptosis
Introduction
Medulloblastomas are neuroepithelial tumors arising from neural stem cell precursors in the granular cell layer of the cerebellum and account for about 20% of all intracranial tumors in children (1). The etiology of medulloblastomas is still unclear, although several signaling pathways that control cell proliferation are thought to be involved in disease progression. Deregulation of the Sonic hedgehog and Wingless pathways have been linked to the development of medulloblastomas (2, 3). The phosphoinositide 3-kinase (PI3K)/AKT cell survival pathway is activated in many cancers, and elevated phosphorylation of AKT at Ser473, which is linked to proliferation of medulloblastoma cells, is observed in human medulloblastoma samples (4). The activity of STAT (signal transducer and activator of transcription) proteins, particularly STAT3, is frequently elevated in a variety of solid tumors and hematological malignancies (5). STAT3 is also constitutively activated in medulloblastomas and the level of STAT3 activation in medulloblastomas exceeds that of several other brain tumors examined, including glioblastomas, ependymomas and astrocytomas (6, 7). Thus, the formation and maintenance of medulloblastomas is likely regulated in part by activated STAT3.
Sunitinib (SU11248, Sutent) is an orally bioavailable molecule that inhibits several receptor tyrosine kinases relevant to tumor angiogenesis, including vascular endothelial growth factor receptors (VEGFRs) and platelet-derived growth factor receptors (PDGFRs) (8). Sunitinib also inhibits receptors such as c-KIT, FLT3 and RET, which are important for the growth of solid tumors and hematologic malignancies (9). Definitive efficacy of sunitinib from clinical trials has been demonstrated in advanced renal carcinoma and in imatinib-refractory GISTs (gastrointestinal stromal tumors), leading to US Food and Drug Administration approval of sunitinib for treatment of these two diseases (9, 10). Clinically sunitinib antitumor activity was also demonstrated in neuroendocrine and breast cancers (11, 12). Both STAT3 and AKT are known to be downstream of various tyrosine kinase receptor signaling pathways, including the ones mentioned above. However, the antitumor effects of sunitinib have been attributed to its ability to inhibit tumor angiogenesis (9, 10). Recently, it was reported that inhibition of STAT3 by sunitinib induced renal cell carcinoma tumor cell apoptosis and growth arrest (13). In vivo studies with sunitinib in the mouse syngeneic RENCA tumor model further showed that sunitinib induced tumor cell apoptosis prior to tumor vasculature collapse (13). STAT3 activity, in both tumor cells and tumor-associated immune cells, is known to play a critical role in tumor immune evasion. The ability of sunitinib to inhibit STAT3 activity in RENCA tumors was associated with reduction of immunosuppressive cells (13).
In the present study, we show that sunitinib induces tumor cell apoptosis and growth arrest in a primary culture (VC312) and an established cell line (Daoy) of medulloblastomas. The biological effects of sunitinib on medulloblastoma tumor cells were associated with inhibition of STAT3 and AKT signaling pathways. Our findings suggest the potential use of sunitinib for the treatment of medulloblastoma.
Results
Effects of sunitinib on the expression of pro-apoptotic and anti-apoptotic genes in a primary culture of human medulloblastoma
To determine whether sunitinib has direct killing effects on medulloblastoma tumor cells, dose-response and time-course studies were performed in VC312 cells, a primary culture derived from human medulloblastoma specimens (14). Cells were treated with either vehicle (DMSO) or increasing concentrations of sunitinib for 24 h and 48 h. Apoptotic cells were Annexin V-positive and defined by flow cytometry (Fig. 1A). Sunitinib markedly induced apoptosis of medulloblastoma cells in a dose- and time-dependent manner. Activation of caspase-3, a critical mediator of apoptosis (15), resulted in cleavage of poly(ADP-ribose) polymerase (PARP), which is known to help cells to maintain their viability (16). To further confirm that the cell death induced by sunitinib is apoptosis, immunoblotting analyses were employed to detect the activation of caspase-3 and cleavage of PARP in total cell lysate after 24 h sunitinib treatment. Sunitinib increased the cleaved caspase-3 (active form of caspase-3) and cleaved PARP (inactive form of PARP) levels in a dose-dependent manner (Fig. 1B).
Figure 1.
Sunitinib induced tumor cell apoptosis and changes in expression of pro-apoptotic and anti-apoptotic genes in the primary culture (VC312) of medulloblastoma. (A) Tumor cells were treated with sunitinib at indicated concentrations for 24h and 48 h, and Annexin V-APC positive apoptotic cells were determined by flow cytometry. (B) Expression of full-length or cleaved caspase-3 and PARP were analyzed by immunoblottings with total cell lysates after 24 h sunitinib treatment. Anti-β-actin monoclonal antibody was used as a loading control. (C) Effects of sunitinib on the expression of pro-apoptotic proteins, Bim, Bak, Bax, Puma and Noxa or anti-apoptotic proteins, survivin, Mcl-1, Bcl-xL and Bcl-2 were also evaluated by immunoblottings. (D) Sunitinib (2.5 μM) inhibited expression of survivin mRNA after 24 h treatment, which was determined by real-time PCR (left panel). Transient transfection of human survivin expression vector partially decreased the effect of sunitinib on the cell viability (right panel). (E) Sunitinib increased the levels of Bak and Bim mRNA and pre-treatment of AcD, a transcriptional inhibitor, blocked the effects of sunitinib in VC312 cells. Data shown are the mRNA ratio of Bak or Bim to GAPDH and determined by real-time quantitive PCR.
The anti-apoptotic protein survivin is a known negative prognostic marker in childhood medulloblastoma (17). We therefore investigated whether its expression was inhibited by sunitinib. Sunitinib reduced the expression of survivin at both protein (Fig. 1C) and mRNA levels (Fig. 1D, left pannel) in VC312 cells, as determined by immunoblotting assays and real-time PCR, respectively. Comparing to DMSO control, sunitinib decreased about 60% of survivin mRNA after 24 h treatment. To further demonstrate the role of survivin in the effects of sunitinib, a human survivin expressing vector was transiently transfected into VC312 cells. After 24 h transfection, cells were treated with 5 μM sunitinib, and viability of cells was determined after 24 h. Result in Figure 1D showed that forced expression of survivin modestly increased the viability of VC312 cells after sunitinib treatment.
Bcl-2 family proteins also have a critical role in survival of normal and tumor cells (18). The expression of three anti-apoptotic proteins, Mcl-1, Bcl-2 and Bcl-xL, and five pro-apoptotic proteins, Bak, Bax, Bim, Puma and Noxa from the Bcl-2 family was further investigated in VC312 tumor cells after sunitinib treatment. Though Mcl-1, Bcl-2, Bcl-xL, Bax, Puma and Noxa were not affected by sunitinib, increased Bak and Bim protein levels were observed after sunitinib treatment in VC312 tumor cells (Fig. 1C). mRNA levels of Bak and Bim were also increased by sunitinib (2.5 μM) after 24 h treatment (Fig. 1E). If cells were pre-treated with AcD, a RNA polymerase II inhibitor to inhibit the transcription, the increased mRNA of Bak and Bim by sunitinib was blocked (Fig. 1E). Therefore, sunitinib increased mRNA levels of Bak and Bim via increasing their transcriptional rates.
Effects of sunitinib in medulloblastoma tumor cells on proliferation and expression of regulatory proteins important for cell growth
To evaluate the effects of sunitinib on cell growth of VC312 tumor cells, cells were treated with either vehicle (DMSO) or sunitinib for 24 h and 48 h. Cell proliferation was determined by MTS assay as described in Materials and Methods. Sunitinib inhibited VC312 cell proliferation in a dose- and time-dependent manner (Fig. 2A). To investigate whether sunitinib affected the expression of regulatory proteins involved in cell cycle, immunoblotting analyses were performed for cyclin E and cyclin D1/D2/D3, the positive regulators for cell cycle, and p21Cip1 and p27Kip1, the negative regulators for cell cycle. Figure 2B showed that cyclin E, cyclin D2 and cyclin D3 were downregulated by sunitinib, while p21Cip1 was upregulated. These results correlated with the inhibition of cell proliferation induced by sunitinib in these cells.
Figure 2.
Sunitinib inhibited tumor cell proliferation and activities of STAT3 and AKT in VC312 cells. (A) Cells were treated with 0, 1.25, 2.5, or 5 μM sunitinib for 24 h and 48 h and cell proliferation was evaluated by MTS assay. (B) Expression of cyclin E, D1, D2, D3, p21Cip1 and p27Kip1 which are important for cell cycle was evaluated by immunoblotings after 24 h sunitinib treatment. (C) Immunoblotting analyses showed effects of sunitinib on total or phosphorylated STAT3, AKT and MAPK(44/42) after 4 h treatment. (D) Inhibition of STAT3 (Tyr705) and AKT (Ser473) phosphorylation were quickly induced by sunitinib after 5 μM sunitinib treatment. (E) Pre-incubation of sunitinib (5 μM) for 20 min inhibited the phosphorylation of STAT3 (Tyr705) induced by IL-6 (10 ng/ml). Anti-β-actin monoclonal antibody was used as a loading control.
Sunitinib rapidly inhibits phosphorylation of STAT3 (Tyr705) and AKT (Ser473) in VC312 cells
Since sunitinib is a known tyrosine inhibitor, we examined major downstream signalings of tyrosine kinases including STAT3, AKT and MAPK. Phosphorylated STAT3, AKT and MAPK represent activated form of these proteins. We investigated the levels of total and phosphorylated STAT3, AKT and MAPK (p44/42) proteins in VC312 cells following 4 h sunitinib treatment. Total protein levels of STAT3, AKT, and MAPK were not significantly changed after sunitinib treatment (Fig. 2C). By contrast, phosphorylation of STAT3 at Tyr705 and AKT at Ser473 was substantially reduced following sunitinib treatment (Fig. 2C). Phosphorylated STAT3 at Ser727 and MAPK(44/42) was not affected by sunitinib. To determine whether inhibition of STAT3 and AKT phosphorylation was an early event, primary cultures from medulloblastoma were treated with 5 μM sunitinib for 0, 1, 5, 15, 30 and 45 min. Immunoblotting analyses (Fig. 2D) showed that the inhibition of phosphorylated STAT3 (Tyr705) and AKT (Ser473) by sunitinib was detected at 5 min to 15 min in the tumor cells.
The ability of IL-6 to directly activate the STAT3 via the JAK family kinases is critical for progression of neoplasia (19). IL-6 receptor (IL-6R) was expressed in medulloblastoma tumors and IL-6 worked as a paracrinal growth factor in the proliferation of medulloblastoma tumor cells (20, 21). To assess whether sunitinib also inhibits the phosphorylation of STAT3 at Tyr705 induced by IL-6, tumor cells were treated with sunitinib for 20 min, followed by the addition of IL-6 (10 ng/ml) for 10 min. Immunoblotting assays showed that sunitinib greatly inhibited the phosphorylation of STAT3 (Tyr705) induced by IL-6 in primary cultures from medulloblastoma (Fig. 2E). In contrast, phosphorylated STAT3 at Ser727 was not affected by IL-6 treatment (Fig. 2E).
Expression of constitutively activated STAT3 partially blocks the effects of sunitinib
To further confirm that inhibition of STAT3 activity is critical for the biological effects of sunitinib on medulloblastoma tumor cells, a constitutively-activated STAT3 mutant (pSTAT3-C) (22) was transfected into VC312 cells. Stable cell lines were established by antibiotic selection (G418) and confirmed by immunoblotting analysis (Fig. 3A, top pannel). pRC was used as a control vector in the transfection and subsequent analyses. Cells containing either pSTAT3-C or control vector were treated with sunitinib, and proliferation assays were performed. Expression of constitutively-activated STAT3 increased the resistance of VC312 cells to sunitinib compared to untransfected cells or control transfected cells (Fig. 3A).
Figure 3.
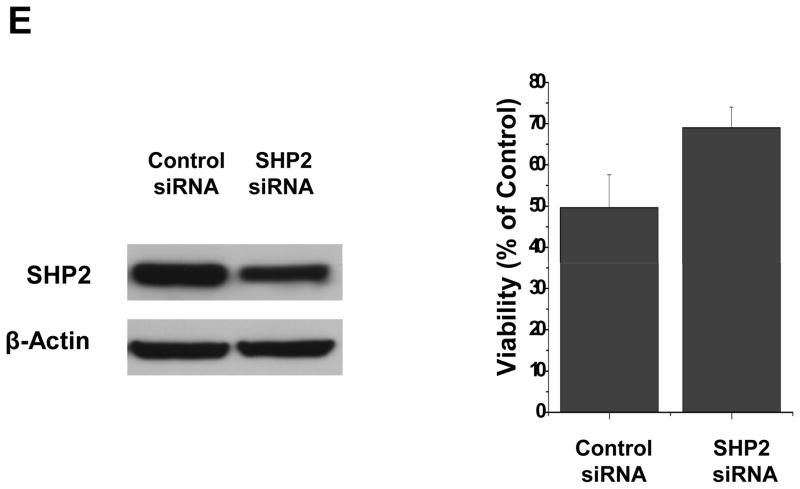
Inhibition of phosphorylated STAT3 induced by sunitinib may involve in multiple mechanisms in VC312 cells. (A) A key role of STAT3 in sunitinib induced anti-tumor effects. VC312 cells were stably transfected with a constitutively activated STAT3 mutant, pSTAT3-C, and the success of transfection was confirmed by immunobloting assay with flag-antibody (top panel). VC312 cells transfected with pSTAT3-C or vector only were treated with 2.5 μM sunitinib for 48 h and cell proliferation was evaluated by MTS assay. Untransfected cells (Untran) treated in the same way as transfected cells were used as positive control. (B) Sunitinib inhibited phosphorylated JAK2 and Src, upstream activators of STAT3. Total and phosphorylated JAK2 and Src were determined by immunoblotting analyses after 5 μM sunitinib treatment at indicated times. (C) Protein tyrosine phosphatases may be involved in de-phosphorylation of STAT3 induced by sunitinib. Sodium vanadate (Na3VO4) reverses the de-phosphorylation of STAT3 (Tyr705) induced by sunitinib. Pre-treatment with 0.5 mM or 1 mM sodium vanadate, a general inhibitor of protein tyrosine phosphatases, inhibited the de-phosphorylation induced by sunitinib (5 μM). (D) Expression of total or phosphorylated SHP2 after sunitinib treatment. Anti-β-actin monoclonal antibody was used as a loading control. (E) Transfection of SHP2 siRNA partially decreased SHP2 expression (left panel) and the effect of sunitinib (2.5 μM) on cell viability after 24 h treatment.
Possible mechanism(s) for inhibition of phosphorylated STAT3 by sunitinib in VC312 cells
Phosphorylation of STAT3 at Tyr705 is usually mediated by receptor-associated tyrosine kinases (JAKs) or non-receptor tyrosine kinases (Src) (5). Many of these tyrosine kinases have been reported to be targets of sunitinib (23) and are deregulated in medulloblastoma (24). Inhibiting the phosphorylation of STAT3 (Tyr705) by sunitinib was detected at 5 and 15 min after treatment (Fig. 2D) in VC312 cells. To elucidate how sunitinib rapidly inhibited phosphorylated STAT3 at Tyr705, total and phosphorylated proteins of JAK2, and Src were examined after sunitinib treatment in VC312 cells. Inhibiting phosphorylated JAK2 and Src by sunitinib was detected after 5 min treatment (Fig. 3B), which was associated with the inhibition of phosphorylated STAT3 at Tyr705 (Fig. 2D).
We also tested the possibility that de-phosphorylation of STAT3 is contributed by direct effects of protein tyrosine phosphatases. Tumor cells were treated with 0.5 or 1 mM sodium vanadate, a general inhibitor for tyrosine phosphatases, for 25 min prior to 5 μM sunitinib treatment for an additional 30 min. Immunoblotting assays showed that sodium vanadate partially reversed the effects of sunitinib in primary cultures from medulloblastoma (Fig. 3C). These results suggest a contributive role for protein tyrosine phosphatases in STAT3 de-phosphorylation induced by sunitinib. SHP2 is a ubiquitously expressed, nonreceptor protein tyrosine phosphatase (PTP). It plays an essential role in the control of cell growth, differentiation, migration and death (25). Since there is evidence that SHP2 is a negative regulator for STAT3 activity (25), total and phosphorylated SHP2 proteins was investigated after sunitinib treatment in VC312 cells. Sunitinib increased the phosphorylation of SHP2 at Tyr580 after 4 h or 24 h sunitinib treatment (Fig. 3D), and total protein levels of SHP2 were not affected by sunitinib. These data suggested that de-phosphorylation of STAT3 at Tyr705 induced by sunitinib may be in part mediated by SHP2. To further confirm a role of SHP2 in sunitinib mediated cytotoxicity, SHP2 siRNA was transfected into VC312 cells. After 24 h SHP2 siRNA transfection, sunitinib was added to the cells, and cell viability was determined after 24 h. Partial knockdown of SHP2 modestly increased cell viability after treatment of sunitinib (Fig. 3E).
Effects of sunitinib on activities of GSK-3β and mTOR, downstream genes of AKT in medulloblastoma cells
Dephosphorylation of AKT (Ser473) induced by sunitinib was an early response in VC312 cells. To confirm whether de-phosphorylation of AKT (Ser473) influenced the downstream targets of AKT signaling, the activities of Glucogen Synthase Kinase-3β (GSK-3β) and Mammalian Target of Rapamycin (mTOR) were investigated after 24 h treatment with sunitinib. GSK-3β is an important component of diverse signaling pathways involved in protein synthesis, proliferation, survival and glycogen metabolism (26). GSK-3β is a negative regulator of cell cycle and viability, and its activity is inhibited by AKT through phosphorylation of GSK-3β at Ser9. Data shown in Figure 4A indicated that GSK-3β phosphorylation was decreased and its activity was increased. mTOR is a positive regulator for cell cycle progression and cellular proliferation, and is activated by AKT via phosphorylation of mTOR at Ser2448 (27). We found that mTOR phosphorylation at Ser2448 was inhibited by sunitinib in a dose-dependent manner (Fig. 4A). In contrast, phosphorylation of mTOR at Ser2481, a site for autophosphorylation, is not affected by sunitinib (Fig. 4A). These results indicated that sunitinib inhibited activities of AKT and downstream targets of AKT.
Figure 4.
Sunitinib inhibits phosphorylation of GSK-3β and mTOR and expression of activated AKT partially reversed effect of sunitinib on VC312 cells. (A) Effects of sunitinib on the expression of total or phosphorylated GSK-3β and mTOR were detected by immunoblotting analyses with specific antibodies. (B) VC312 cells were transiently transfected with a constitutively activated AKT expressing plasmid for 24h, and then cells were treated with 1.25 μM sunitinib for 48 h. Cell proliferation was evaluated by MTS assay. (C) Effects of expressing activated STAT3 and AKT on regulatory proteins for cell cycle and apoptosis. VC312 cells contained stable transfeted pSTAT3-C were transiently transfected with pAKT-C for 24 h, followed by treating with 5 μM sunitinib for another 24 h. Immunoblotting analyses were performed with specific antibodies.
Expression of constitutively activated AKT partially reverses the effects of sunitinib on medulloblastoma cells
To further confirm that the inhibition of AKT signaling was involved in the effects of sunitinib on medulloblastoma cells, we transfected pAKT-C, an expressing vector contained the myristoylated (constitutively activated) form of AKT, into VC312 cells. After 24 h transient transfection with either pAKT-C construct or an empty control vector, cells were treated with 1.25 μM sunitinib for 48 h and cell proliferation was determined by MTS assay. Untransfected VC312 cells served as positive controls for sunitinib treatment. Our data suggest that over-expression of pAKT-C in VC312 cells partially reversed the inhibition of sunitinib on cell proliferation (Fig. 4B).
Since expressing either STAT3 or AKT expressing vector can partially rescue medulloblastoma cells from cytotoxicity induced by sunitinib (Fig. 3A and 4B), we further investigate effects of expressing both pSTAT3-C and pAKT-C on regulatory proteins for cell cycle and apoptosis in VC312 cells. VC312 cells stably transfected with pSTAT3-C were transiently transfected with pAKT-C for 24 h, followed by sunitinib treatment. Expression of cyclin E, p21Cip1, survivin, Bak and Bim was examined by immunoblotting analyses (Fig. 4C). Expression of activated STAT3 and AKT increased expression of cyclin E and survivin and interfered with the inhibitory effects of sunitinib on these genes. Induction of p21Cip1, Bak and Bim by sunitinib was also reduced by expressing activated STAT3 and AKT.
Effects of sunitinib on Daoy cells, an established cell line of medulloblastoma
Sunitinib induced apoptosis and inhibited cell growth in primary cultures (VC312) of medulloblastoma (Fig. 1 and Fig. 2). We also investigated whether sunitinib had similar effects on Daoy cells, an established cell line of medulloblastoma. Daoy cells were treated with sunitinib in the same manner as VC312 cells. Sunitinib induced apoptosis (Fig. 5A) and inhibited cell proliferation (Fig. 5B) of Daoy cells in a dose- and time-dependent manner. Expression of cleaved caspase-3 and PARP was increased by sunitinib (Fig. 5C). This inhibitory effect on the proliferation and survival of Daoy cells was similar to that observed in VC312 cells.
Figure 5.
Sunitinib inhibits cell proliferation and induces apoptosis in an established cell line (Daoy) of medulloblastoma. (A) and (B) Induction of apoptosis and inhibition of cell growth after sunitinib treatment were determined by Annexin V-APC staining and MTS assay, respectively. (C) Expression of full-length or cleaved caspase-3 and PARP was analyzed by immunoblottings. (D) Effects of sunitinib on total or phosphorylated STAT3, AKT, MAK(44/42) after 4 h sunitinib treatment.
We further tested the effects of sunitinib on Daoy cells. Following 4 h sunitinib treatment, expression of total and phosphorylated STAT3 and AKT was analyzed by immunoblotting assays. Phosphorylation of STAT3 at Tyr705 and AKT at Ser473 was inhibited by sunitinib (Fig. 5D). By contrast, phosphorylation of MAPK(44/42) was slightly increased (Fig. 5D). Phosphorylated STAT3 at Ser727 was not affected by sunitinib. Total protein levels of STAT3, AKT and MAPK were not affected by sunitinib. Effects of sunitinib on signaling pathways in Daoy cells were similar to those in VC312 cells.
Sunitinib blocks migration of medulloblastoma tumor cells
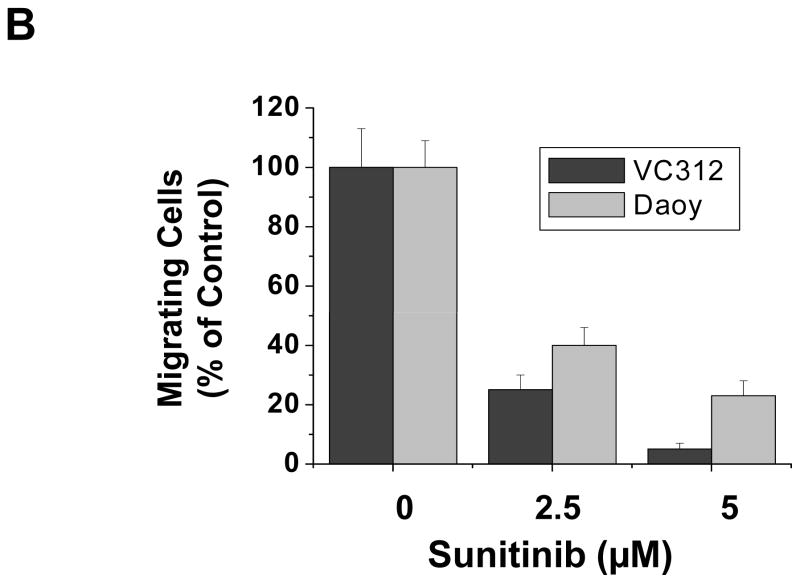
Metastasis, which involves migration of tumor cells, is an important process for medulloblastoma disease progression. To test whether sunitinib might have an effect on medulloblastoma tumor cell migration, we performed migration assays. Approximately 5 X 105 cells of VC312 or Daoy were seeded in each well of six-well culture plates. When cells were fully confluent next day, a “scratch” was made by a pipette tip across the wells. After 24 h treatment with sunitinib (2.5 μm and 5μM) or DMSO, photomicrographs of the scratch were taken and migration was quantified by counting the cells that migrated into the scratch area. Each number represents the average count of cells in two independent experiments in duplicate. Figure 6 showed that more cells migrated to the scratch areas in control treatment (DMSO) compared to those exposed to sunitinib treatments. These results indicated that sunitinib inhibits medullablastoma tumor cell migration in a dose-dependent manner.
Figure 6.
Sunitinib inhibits the migration of human medulloblastoma cells. VC312 and Daoy cells were plated in 6-well plates and next day a single scratch was made in the confluent monolayer, followed by sunitinib or vehicle (DMSO) treatment for 24 h. (A) Each scratch was photographed after 24 h treatment. (B) The data represent the average and SD from two independent experiments in duplicate.
Discussion
Medulloblastoma is the most common brain tumor in children. Standard treatment, which includes surgery, chemotherapy and radiation of the craniospinal axis, is often associated with major long-term side effects (1). Thus, there is a critical need to find less toxic therapies, especially new drugs that target cell signaling pathways implicated as critical mediators in the formation of medulloblastomas. In this study, we report that sunitinib inhibits proliferation and induces apoptosis of medulloblastoma tumor cells, which is associated with inhibition of STAT3 and AKT signaling pathways, as well as their downstream genes involved in tumor cell proliferation and survival.
STAT3 regulates basic biologic processes important in tumorigenesis including cell-cycle progression, apoptosis, tumor angiogenesis, and tumor-cell evasion of the immune system (5, 28). Phosphotyrosine (pTyr705) in STAT3 mediates dimer formation, which is required for the binding of STAT3 to DNA (5). Our results showed that sunitinib inhibited the tyrosine phosphorylation of STAT3 in both primary cultures and an established cell line of medulloblastoma. Consequently, expression of downstream target genes of STAT3, including survivin and p21Cip1 was inhibited by sunitinib treatment, which may in part mediate the observed effects on inhibition of cell growth and survival. Sunitinib also inhibited STAT3 phosphorylation induced by IL-6, which plays a critical role in the progression of neoplasia in general (19).
Like STAT3, AKT is a key cell survival protein involved in antiapoptosis in various cancers (29), including medulloblastoma (4). Sunitinib inhibited phosphorylation of AKT at Ser 473 in both primary cultures and cell line of medulloblastomas. The downstream targets of AKT signaling pathway, such as GSK-3β and mTOR, were also regulated by sunitinib in these cells. mTOR is an important regulator in several transduction pathways that are necessary for cell proliferation. Since mTOR also contributes to cancer cell drug resistance (30), it has emerged for a new target of cancer drug development. Importantly, active AKT has been shown to have a permissive effect on β-catenin mediated transcriptional upregulation, via direct phosphorylation-dependent inactivation of GSK-3β (31). Medulloblastomas also bear sporadic mutations in Axin, a regulator of Wnt signaling-mediated β-catenin activity, leading to transcriptional upregulation of target genes involved in cell cycle progression and metastasis (32).
Sunitinib exhibits strong antiangiogenic activity via inhibition of vascular endothelial growth factor receptors (VEGFRs) and platelet-derived growth factor receptor (PDGFR) (9), which are key regulators of tumor neoangiogenesis in medulloblastoma (33). So, it is possible that sunitinib could inhibit angiogenesis in medulloblastoma. An insidious feature of medullobastomas is their ability to metastasize and disseminate through the cerebrospinal fluid (34). Our results also showed that sunitinb inhibited the migration of medulloblastoma cells. Treatment of medulloblastoma is complicated by the blood-brain barrier, which acts as a physiologic barrier for delivery of drugs to the central nervous system. Various approaches have been developed for local delivery of drugs to brain tumors, including convection-enhanced delivery (35). Therefore, local delivery of sunitinib to the cerebrospinal fluid by convection-enhanced delivery may result in more effective antitumor activity with reduced systemic toxicity. In summary, sunitinib is a potentially promising drug for the treatment of pediatric medulloblastomas.
Materials and Methods
Reagents and antibodies
Sunitinib (SU11248; Sutent) was purchased from City of Hope Cancer Center Pharmacy. Anti-cyclin D1, and anti-cyclin D3 were obtained from Calbiochem. Anti-cyclin E was obtained from BD Biosciences. Anti-cyclin D2, survivin, Noxa and Mcl-1 were obtained from Santa Cruz. Horseradish peroxidase-labeled anti-mouse and anti-rabbit secondary antibodies were from GE Healthcare. All other antibodies were purchased from Cell Signaling.
Cell culture
Human meduloblastoma cell line, Daoy, was from American Type Culture Collection (ATCC). Daoy cells were maintained in MEM (Eagle) with L-glutamine supplemented with 10% fetal bovine serum (FBS), and 1% Antibiotic-Antimycotic (AA). The primary culture (VC312) of medulloblastoma was derived from a tumor of a 4-year old male patient treated at the Virginia Commonwealth University Health System’s Medical College of Virginia Hospital under an IRB approved protocol. Briefly, samples of the tumor were first obtained to allow full neuropathologic evaluation and diagnosis, as required for the clinical management of the patient’s disease. The site of origin of all the tumor samples was cerebellum. The sterile dissection of tumor biopsy was dissociated and plated in 6-well tissue culture plates and expanded in DMEM/F12 medium supplemented with 1% N-2 supplement (Invitrogen), 5% FBS, 20 ng/ml recombinant human EGF and 10 ng/ml recombinant human bFGF (Beckton Dickenson). VC312 cells were subsequently maintained in DMEM (with L-glutamine) supplemented with 10% FBS and utilized at low passage number (below passage 20 for all studies).
Proliferation assay
Cell proliferation assays were performed with CellTiter 96 Aqueous One Solution Cell proliferation Assay (Promega) which contains 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS). Each well of 96-well plates was seeded with 5000 cells in culture medium with 1% FBS. After overnight culture the cells were treated with different concentrations of sorafenib and controls were treated with vehicle (DMSO). After 24 h or 48 h treatments, MTS was added to the cells according to the supplier’s protocol and absorbance was measured at 490 nm using an automated ELISA plate reader.
Apoptosis assay
VC312 or Daoy cells (2 × 105) were seeded in 60 mm culture dishes in culture medium with 1% FBS. The following day the cells were treated with indicated concentrations of sorafenib for a 24 h or 48 h period. After treatment, all cells including both floating and attached cells were collected, and the apoptotic cells were detected by Annexin V-APC Apoptosis Detection Kit (BD Biosciences). The cells were stained with Annexin V-APC according to the supplier’s instructions. Viable and dead cells were detected by flow cytometry in the Analytical Cytometry Core at City of Hope Canter Center.
Immunoblotting analysis
Twenty μg total proteins were resolved in 4–15% gradient Tris-HCl gels (BIO-RAD). After gel electrophoresis, the proteins were transferred to Hybond-C membranes (Amersham). The membranes were blocked for 1 h at room temperature (RT) in 10% non-fat dry milk in PBST (1 X PBS with 0.1% Tween-20), followed by an overnight incubation at 4 ° C with primary antibodies in PBST with 2% non-fat dry milk. The membranes were then incubated with horseradish peroxidase labeled anti-mouse or anti-rabbit secondary antibodies for 1 h at RT. Immunoreactivity was detected with SuperSignal West Pico substrate (Pierce).
Plasmids transfections
The constitutively-activated STAT3 mutant plasmid (pSTAT3-C) was murine STAT3 which was cloned into pRc/CMV vector with a FLAG epitope (22). pSTAT3-C was transfected into VC312 cells by Lipofectamine™ 2000 (Invitrogen). Stable cell line was selected by G418 and confirmed by immunoblotting analysis. Active (myristoylated) AKT plasmid (pAKT-C) from Upstate and a survivin plasmid in pEF/Bsd vector (a generous gift from Dr. Giovanna Tosato, NIH) (36) were transiently transfected to VC312 cells by Lipofectamine™ 2000.
SHP2 siRNA transfection and viability assay
Human SHP2 siRNA was purchased from QIAGEN and transfected to VC312 cells by using RNAiFect™ Transfection Reagent (QIAGEN). After 24 h transfection, cells were treated with sunitinib or DMSO for 24 h, and then cell viability was determined. Viability assay was performed by CellTiter 96 Aqueous One Solution Cell proliferation Assay (Promega).
Actinomycin D treatment
To inhibit transcription, 10 μg/ml actinomycin D (AcD) was added to the culture medium for 30 min before adding 5 μM sunitinib. Cells were collected and total RNA was isolated at 24 h treatment.
Real-time quantitive PCR
Total RNA was extracted by RNeasy kit (Qiagen) and cDNA was synthesized with iScript cDNA Synthesis (Bio-Rad). Primers for human survivin and Bim were purchased from SuperArray Bioscience Corp. Specific human Bak primers are as following: forward, AGACCTGAAAAATGGCTTCGGG; reverse, TAAGGTGACCATCTCTG-GGTCG. Human glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as an endogenous control. Sequence-specific amplification was detected with increased fluorescent signal of SYBR Green (Bio-Rad)
Migration assays
“Wounds” were made by scratching a pipette tip on confluent cells cultured in six-well plates. Cells were then incubated with sunitinib or DMSO vehicle as indicated. Migration of cells into the wound was photographed at × 10 magnification under a microscope.
Acknowledgments
We thank Dr. Wei Wen for the help in the study of AKT signaling pathway, Dr. Sivia Da Costa for editing. This project was partially supported by NIH grants to Dr. Yu.
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Rossi A, Caracciolo V, Russo G, Reiss K, Giordano A. Medulloblastoma: from molecular pathology to therapy. Clin Cancer Res. 2008;14:971–976. doi: 10.1158/1078-0432.CCR-07-2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McNeil DE, Cote TR, Clegg L, Rorke LB. Incidence and trends in pediatric malignancies medulloblastoma/primitive neuroectodermal tumor: a SEER update. Surveillance Epidemiology and End Results. Med Pediatr Oncol. 2002;39:190–194. doi: 10.1002/mpo.10121. [DOI] [PubMed] [Google Scholar]
- 3.Zurawel RH, Chiappa SA, Allen C, Raffel C. Sporadic medulloblastomas contain oncogenic beta-catenin mutations. Cancer Res. 1998;58:896–899. [PubMed] [Google Scholar]
- 4.Hartmann W, Digon-Sontgerath B, Koch A, Waha A, Endl E, Dani I, et al. Phosphatidylinositol 3′-kinase/AKT signaling is activated in medulloblastoma cell proliferation and is associated with reduced expression of PTEN. Clin Cancer Res. 2006;12:3019–3027. doi: 10.1158/1078-0432.CCR-05-2187. [DOI] [PubMed] [Google Scholar]
- 5.Yu H, Jove R. The STATs of cancer--new molecular targets come of age. Nat Rev Cancer. 2004;4:97–105. doi: 10.1038/nrc1275. [DOI] [PubMed] [Google Scholar]
- 6.Schaefer LK, Ren Z, Fuller GN, Schaefer TS. Constitutive activation of Stat3alpha in brain tumors: localization to tumor endothelial cells and activation by the endothelial tyrosine kinase receptor (VEGFR-2) Oncogene. 2002;21:2058–2065. doi: 10.1038/sj.onc.1205263. [DOI] [PubMed] [Google Scholar]
- 7.Cattaneo E, Magrassi L, De-Fraja C, Conti L, Di Gennaro I, Butti G, et al. Variations in the levels of the JAK/STAT and ShcA proteins in human brain tumors. Anticancer Res. 1998;18:2381–2387. [PubMed] [Google Scholar]
- 8.Mendel DB, Laird AD, Xin X, Louie SG, Christensen JG, Li G, et al. In vivo antitumor activity of SU11248, a novel tyrosine kinase inhibitor targeting vascular endothelial growth factor and platelet-derived growth factor receptors: determination of a pharmacokinetic/pharmacodynamic relationship. Clin Cancer Res. 2003;9:327–337. [PubMed] [Google Scholar]
- 9.Chow LQ, Eckhardt SG. Sunitinib: from rational design to clinical efficacy. J Clin Oncol. 2007;25:884–896. doi: 10.1200/JCO.2006.06.3602. [DOI] [PubMed] [Google Scholar]
- 10.Faivre S, Demetri G, Sargent W, Raymond E. Molecular basis for sunitinib efficacy and future clinical development. Nat Rev Drug Discov. 2007;6:734–745. doi: 10.1038/nrd2380. [DOI] [PubMed] [Google Scholar]
- 11.Kulke MH, Lenz HJ, Meropol NJ, Posey J, Ryan DP, Picus J, et al. Activity of sunitinib in patients with advanced neuroendocrine tumors. J Clin Oncol. 2008;26:3403–3410. doi: 10.1200/JCO.2007.15.9020. [DOI] [PubMed] [Google Scholar]
- 12.Burstein HJ, Elias AD, Rugo HS, Cobleigh MA, Wolff AC, Eisenberg PD, et al. Phase II study of sunitinib malate, an oral multitargeted tyrosine kinase inhibitor, in patients with metastatic breast cancer previously treated with an anthracycline and a taxane. J Clin Oncol. 2008;26:1810–1816. doi: 10.1200/JCO.2007.14.5375. [DOI] [PubMed] [Google Scholar]
- 13.Xin H, Zhang C, Herrmann A, Du Y, Figlin R, Yu H. Sunitinib inhibition of Stat3 induces renal cell carcinoma tumor cell apoptosis and reduces immunosuppressive cells. Cancer Res. 2009;69:2506–2513. doi: 10.1158/0008-5472.CAN-08-4323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang F, Van Meter TE, Buettner R, Hedvat M, Liang W, Kowolik CM, et al. Sorafenib inhibits signal transducer and activator of transcription 3 signaling associated with growth arrest and apoptosis of medulloblastomas. Mol Cancer Ther. 2008;7:3519–3526. doi: 10.1158/1535-7163.MCT-08-0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fernandes-Alnemri T, Litwack G, Alnemri ES. CPP32, a novel human apoptotic protein with homology to Caenorhabditis elegans cell death protein Ced-3 and mammalian interleukin-1 beta-converting enzyme. J Biol Chem. 1994;269:30761–30764. [PubMed] [Google Scholar]
- 16.Oliver FJ, de la Rubia G, Rolli V, Ruiz-Ruiz MC, de Murcia G, Murcia JM. Importance of poly(ADP-ribose) polymerase and its cleavage in apoptosis. Lesson from an uncleavable mutant. J Biol Chem. 1998;273:33533–33539. doi: 10.1074/jbc.273.50.33533. [DOI] [PubMed] [Google Scholar]
- 17.Pizem J, Cort A, Zadravec-Zaletel L, Popovic M. Survivin is a negative prognostic marker in medulloblastoma. Neuropathol Appl Neurobiol. 2005;31:422–428. doi: 10.1111/j.1365-2990.2005.00664.x. [DOI] [PubMed] [Google Scholar]
- 18.Maddika S, Ande SR, Panigrahi S, Paranjothy T, Weglarczyk K, Zuse A, et al. Cell survival, cell death and cell cycle pathways are interconnected: implications for cancer therapy. Drug Resist Updat. 2007;10:13–29. doi: 10.1016/j.drup.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 19.Hodge DR, Hurt EM, Farrar WL. The role of IL-6 and STAT3 in inflammation and cancer. Eur J Cancer. 2005;41:2502–2512. doi: 10.1016/j.ejca.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 20.Liu J, Li JW, Gang Y, Guo L, Li H. Expression of leukemia-inhibitory factor as an autocrinal growth factor in human medulloblastomas. J Cancer Res Clin Oncol. 1999;125:475–480. doi: 10.1007/s004320050304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Knupfer H, Knupfer MM, Hotfilder M, Preis R. P450-expression in brain tumors. Oncol Res. 1999;11:523–528. [PubMed] [Google Scholar]
- 22.Bromberg JF, Wrzeszczynska MH, Devgan G, Zhao Y, Pestell RG, Albanese C, et al. Stat3 as an oncogene. Cell. 1999;98:295–303. doi: 10.1016/s0092-8674(00)81959-5. [DOI] [PubMed] [Google Scholar]
- 23.Christensen JG. A preclinical review of sunitinib, a multitargeted receptor tyrosine kinase inhibitor with anti-angiogenic and antitumour activities. Ann Oncol. 2007;18 (Suppl 10):x3–10. doi: 10.1093/annonc/mdm408. [DOI] [PubMed] [Google Scholar]
- 24.Guessous F, Li Y, Abounader R. Signaling pathways in medulloblastoma. J Cell Physiol. 2008;217:577–583. doi: 10.1002/jcp.21542. [DOI] [PubMed] [Google Scholar]
- 25.Xu D, Qu CK. Protein tyrosine phosphatases in the JAK/STAT pathway. Front Biosci. 2008;13:4925–4932. doi: 10.2741/3051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grimes CA, Jope RS. The multifaceted roles of glycogen synthase kinase 3beta in cellular signaling. Prog Neurobiol. 2001;65:391–426. doi: 10.1016/s0301-0082(01)00011-9. [DOI] [PubMed] [Google Scholar]
- 27.Yonezawa K, Yoshino KI, Tokunaga C, Hara K. Kinase activities associated with mTOR. Curr Top Microbiol Immunol. 2004;279:271–282. doi: 10.1007/978-3-642-18930-2_16. [DOI] [PubMed] [Google Scholar]
- 28.Haura EB, Turkson J, Jove R. Mechanisms of disease: Insights into the emerging role of signal transducers and activators of transcription in cancer. Nat Clin Pract Oncol. 2005;2:315–324. doi: 10.1038/ncponc0195. [DOI] [PubMed] [Google Scholar]
- 29.Goswami A, Ranganathan P, Rangnekar VM. The phosphoinositide 3-kinase/Akt1/Par-4 axis: a cancer-selective therapeutic target. Cancer Res. 2006;66:2889–2892. doi: 10.1158/0008-5472.CAN-05-4458. [DOI] [PubMed] [Google Scholar]
- 30.Jiang BH, Liu LZ. Role of mTOR in anticancer drug resistance: perspectives for improved drug treatment. Drug Resist Updat. 2008;11:63–76. doi: 10.1016/j.drup.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McManus EJ, Sakamoto K, Armit LJ, Ronaldson L, Shpiro N, Marquez R, et al. Role that phosphorylation of GSK3 plays in insulin and Wnt signalling defined by knockin analysis. Embo J. 2005;24:1571–1583. doi: 10.1038/sj.emboj.7600633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yokota N, Nishizawa S, Ohta S, Date H, Sugimura H, Namba H, et al. Role of Wnt pathway in medulloblastoma oncogenesis. Int J Cancer. 2002;101:198–201. doi: 10.1002/ijc.10559. [DOI] [PubMed] [Google Scholar]
- 33.Slongo ML, Molena B, Brunati AM, Frasson M, Gardiman M, Carli M, et al. Functional VEGF and VEGF receptors are expressed in human medulloblastomas. Neuro Oncol. 2007;9:384–392. doi: 10.1215/15228517-2007-032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fouladi M, Gajjar A, Boyett JM, Walter AW, Thompson SJ, Merchant TE, et al. Comparison of CSF cytology and spinal magnetic resonance imaging in the detection of leptomeningeal disease in pediatric medulloblastoma or primitive neuroectodermal tumor. J Clin Oncol. 1999;17:3234–3237. doi: 10.1200/JCO.1999.17.10.3234. [DOI] [PubMed] [Google Scholar]
- 35.Sawyer AJ, Piepmeier JM, Saltzman WM. New methods for directdelivery of chemotherapy for treating brain tumors. Yale J Biol Med. 2006;79:141–152. [PMC free article] [PubMed] [Google Scholar]
- 36.Aoki Y, Feldman GM, Tosato G. Inhibition of STAT3 signaling induces apoptosis and decreases survivin expression in primary effusion lymphoma. Blood. 2003;101:1535–1542. doi: 10.1182/blood-2002-07-2130. [DOI] [PubMed] [Google Scholar]