Disability glare with keratoprosthesis can be reduced by preserving the natural iris as much as practical, or it can be significantly reduced with the use of a soft bandage contact lens with a dark iris tint. Implanting the keratoprosthesis in a patient in whom the fellow eye has normal or near normal vision does not seem to improve visual function.
Abstract
Purpose.
To evaluate the optical characteristics of the Boston Keratoprosthesis (KPro), identify glare sources, evaluate possible glare control, and examine the benefit of implantation when the fellow eye has normal vision.
Methods.
Computed and optical-bench-measured point spread function (PSF) and glare sources were compared. A translucent plastic cornea was used to determine the impact of glare caused by scatter in the cornea and its control with a dark-iris tinted contact lens. The effect of glare in implanted eyes was measured with a brightness acuity test (BAT), with and without the dark-iris contact lens. Computed and measured visual fields were compared. Stereopsis was measured in patients with an intact fellow eye.
Results.
Computed and measured modulation transfer functions for the KPro were found to be very close to the diffraction limit. Both the model-eye measurements and patients' BAT glare responses identified that the hazy corneal graft surrounding the KPro is the main source of glare and can be controlled with a dark-iris contact lens. The lid effectively blocks the light that would be scattered in the hazy cornea of patients in whom the type II KPro was implanted. An intact fellow eye remains the dominant eye, with better acuity, and the KPro eye supports only minimal stereo ability and does not expand the binocular visual field.
Conclusions.
Glare can be reduced significantly with the use of a contact lens with a dark iris. Implanting the KPro in a patient whose fellow eye has normal or near normal vision does not seem to improve visual function.
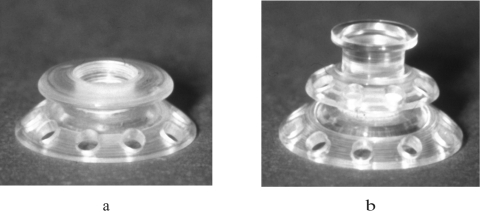
Keratoprosthesis (KPro) has been recognized as a viable alternative to penetrating keratoplasty in the treatment of selective patients with corneal blindness, particularly after repeated graft failures.1 The Boston KPros are illustrated in Figure 1.
Figure 1.
(a) Type 1 KPro. (b) Type 2 KPro includes a protruding cylinder that passes through an opening in the eyelid. A donor cornea is placed between the front and back plates, and the combination is sutured into the patient's corneal opening. The holes in the back plate serve to allow the aqueous humor to diffuse into the donor cornea.
The KPro assembly and surgery were previously described.1,2 Briefly, a donor corneal graft as carrier is used with a 3-mm central hole, through which the optical cylinder of the KPro is inserted. The back plate of the KPro is either screwed onto the stem to firm apposition with the donor tissue, or, since 2007, snapped onto the stem with no rotating movement.3 A titanium locking ring is snapped in place behind the back plate to prevent loosening of the back plate. The graft-prosthesis combination is then transferred to the patient's trephined corneal opening and sutured in place. Finally, for the type 1 KPro, a soft contact lens (usually a Kontur lens; Kontur Kontact Lens Co., Hercules, CA), 16-mm diameter and 9.8-mm base curve, plano power, is placed as a bandage lens.
The type 2 KPro (Fig. 1b) has an anterior cylinder, enabling it to protrude through an opening in the closed lid (Fig. 2c). Type 2 is rarely used, and then only in end-stage dry eye.
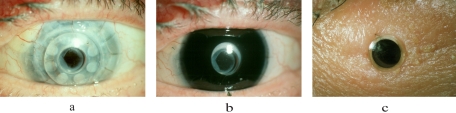
Figure 2.
(a) An eye implanted with a type 1 KPro. Note the light-scattering character of the hazy donor cornea. Also note that an RPM has been only incompletely opened with a YAG laser. (b) The same eye with the dark-iris painted soft contact lens in place. This contact lens blocks much (but not all) of the light path through the light-scattering cornea. (c) An eye implanted with a type 2 KPro protruding through the lid. The patient shown in (a) and (b) was excluded from the study because of poor visual acuity. This image is shown to illustrate a residual RPM.
Multiple modifications have been introduced to the original designs of the Boston KPros, with significant improvement in retention rates, longer conservation of good visual acuity, and reduction in complications. However, little has been published about visual performance and optical properties,4,5 with discussions usually limited to visual acuity changes over time. Indeed, excellent visual acuity results have been demonstrated for the Boston KPro,6 as in the present study where, with selected patients, average acuity with the KPro was 20/30.
Despite the excellent visual acuity results and the patients' general pleasure with the improvement in their visual function, better understanding of the visual function with the KPro may lead to design and practice modifications that will further improve outcomes. Here we addressed two such issues: the effect, sources, and control of glare and the possible binocular vision benefits of implanting a KPro in a patient with a healthy fellow eye. The implantation of the KPro was expected to improve the binocular status of those patients, either by expansion of the visual field or by recovering stereo vision.
Frequently, our patients KPro implants report difficulty in glare situations. Disability glare is defined as a reduction of visual capacity (i.e., visual acuity or contrast sensitivity) caused by a bright light source elsewhere in the field of view.7 It manifests to the observer as a veil of light cast over the scene.8 It results from intraocular light scatter (or stray light) that is cast over the retinal image, thus reducing the contrast of the retinal image.9
Four major causes of glare have been described in the normal eye: light scattered while passing through the cornea, lens, and sclera10 and light scattered after hitting the retina.11 Diseased or postsurgical eyes may have other causes of light scatter in the ocular media, leading to glare. In eyes implanted with the Boston KPros, one would expect the relative impact of these factors to be modified. The KPro itself might be a source of glare because of scattered light within the prosthesis, but the magnitude of this effect is likely to be small. Retroprosthesis membrane (RPM) formation (Fig. 2) may have a glare effect similar to that of posterior lens capsular membrane opacification. Both membranes can be treated with a YAG laser after the KPro implantation. On the other hand, the hazy (translucent) donor cornea surrounding the implant would be expected to be a major source of light scatter and disability glare (Fig. 2a).
Miller and Dohlman12 studied the optical properties of a simulated prosthesis implanted in an excised beef cornea placed on an investigator's cornea. The resolution through the simulated implant was improved by placing a black collar under the front skirt, in front of the beef cornea, thus preventing the entrance of light into the eye through the hazy beef cornea. This addition reduced glare significantly.12
As a partial solution to the scatter through the donor cornea, we propose and evaluate here the benefit of using a dark-iris tinted contact lens in place of the transparent-bandage contact lens used with the type 1 KPro (Fig. 2b). We also evaluated the impact of the lid in type 2 on blocking the light scatter caused by hazy donor cornea in type 2 KPro (Fig. 2c).
We used computerized ray-tracing to derive the theoretical optical properties of the KPro. An optical bench was constructed to create an eye model implanted with a KPro and was used to analyze the optical quality and the effects on glare of a hazy cornea and a dark-iris contact lens. Finally, we evaluated the quality of visual function in 10 patients who received implants by measuring visual acuity and glare sensitivity. We also measured the visual fields and stereoacuity in patients with intact fellow eyes to determine the visual benefit of the implant.
Methods
Optical Analysis
Using the design described by Dohlman,3 an optical design program (Zemax; Focus Software Inc., San Diego, CA) was used to derive the expected optical parameters of the KPro. These included point spread functions (PSFs), spot diagrams, visual fields, and sources of glare by reflection.
Artificial Eye
An optical bench (Fig. 3) allowed measurement of optical quality and the effect of glare on the retinal image. A point-light-source of adjustable intensity and a collimator lens were placed away from an eye-model construct. The resultant “retinal” images were acquired by the sensor of a 4 megapixel static camera and were analyzed using commercial software (MATLAB; The MathWorks, Inc., Natick, MA).
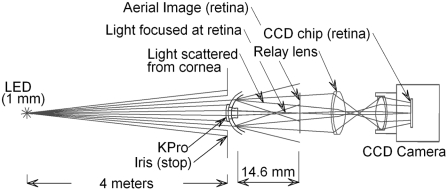
Figure 3.
Schematic drawing of the experimental eye model. A point light source (LED) of adjustable intensity and a collimator lens (not shown) were placed so that the source appeared approximately 4 m from the KPro. A Boston scleral lens that had been sand-blasted until the visual acuity of a patient looking through it was reduced to less than 20/400 was drilled to allow insertion of a type 1 KPro front plate. An adjustable iris was placed in front of the KPro. A 50-mm relay lens was placed 50 mm from the aerial image of the point source of the KPro so that the CCD camera, set for infinite focus, captured the image of the point source and the surrounding glare. The source is a 1-mm white LED, minified to approximately 3 μm at the aerial image. The type 1 KPro used was designed for an aphakic eye (focal length, 14.6 mm in air).
To analyze the effect of glare, the retinal images were taken through the KPro lens mounted in a sand-blasted translucent plastic “cornea,” with and without a dark-iris contact lens in place. To evaluate glare caused by the KPro itself, the KPro was alternately mounted directly into the iris of the system.
Patients
The study, which was conducted in accordance with the tenets of the Declaration of Helsinki, was approved by the Institutional Review Board of the Massachusetts Eye and Ear Infirmary, and informed consent was obtained from all participants. Ten patients, eight who underwent implantation with a type 1 KPro and two who underwent it with a type 2 KPro, were recruited as a convenient sample. All patients had previously undergone previous by the same surgeon (CHD). Patient ages ranged from 19 to 84 years (mean, 53 ± 20 years), and the mean duration since surgery was 2.6 ± 2.4 years (range, 10 months to 7 years). All eyes had a stable visual acuity for 10 months or more after surgery. Two KPros (both type 1) were placed in pseudophakic eyes, and the rest were aphakic. The power of the KPro was adjusted individually for the patients. Five patients each had an intact contralateral eye with good vision (all with type 1); no patients had bilateral KPros. Three of the 10 eyes had glaucoma. Patients with glaucoma were not excluded because glaucoma is a relatively common complication after KPro placement, and 60% of all eyes implanted with KPros had preoperative glaucoma.6 Patients with uncorrected visual acuity worse than 20/60 in the implanted eye were excluded. Routine ophthalmic examination, including slit lamp evaluation, was performed on all patients. The Activities of Daily Vision Scale (ADVS)13 was administered in person.
Glare Sensitivity
Glare sensitivity was measured with a brightness acuity tester (BAT; Mentor O&O, Norwood, MA). Visual acuity measured without BAT was compared with acuity measured under the three available glare intensity settings. After visual acuity was measured using a standard Snellen acuity chart from a distance of 10 feet, glare sensitivity was measured under two conditions. The first was with a dark-iris tinted soft contact lens (Kontur Kontact Lens Co.) with a 5-mm central clear pupil surrounded by a tinted area of 12-mm outer diameter (measured to have 2 log units optical density, Fig. 2b). In the second, visual acuity was measured without glare and under the three BAT glare settings, with a standard transparent contact lens in place. This order of measurement permitted cleaning or replacement (if necessary) of the transparent habitual lens, whereas glare sensitivity was measured with the dark-iris contact lens. Visual acuity measurements for the glare testing were all conducted with no refractive correction (spectacles), to permit uniform apposition of the BAT to the eye (all contact lenses were of plano power).
Additional Tests
Standard screening kinetic Goldmann (Haag-Streit AG, Bern, Switzerland) dynamic perimetry was carried out for each KPro eye. Stereoacuity was measured in those patients who had an intact fellow eye (Wirt Stereo Fly Test; Stereo Optical Co., Inc., Chicago, IL). In patients without measurable stereoacuity, suppression was evaluated using the suppression test provided in the Stereo Fly test.
Statistical Analysis
Visual acuity was converted to the absolute value of the logarithm of the minimum angle of resolution (logMAR).14 For the purpose of statistical analysis, a value of 2.0 logMAR (20/2000) was assigned if acuity was worse than 20/400 during glare testing.15 Only three such readings were used, for 2 patients. All values are expressed as mean ± SD. A 2 × 3 (contact lenses × glare levels) ANOVA was used to determine the impact of use of the dark-iris contact lens on glare sensitivity. All analyses were conducted using commercial software (SPSS; SPSS, Inc., Chicago, IL), with the level of significance taken as P < 0.05.
Results
Optical Quality
Computed modulation transfer function and PSF were derived using Zemax (Fig. 4). The system was found to be close to diffraction limited, as might be expected for an aperture of 3 mm and a focal length of approximately 25 mm.
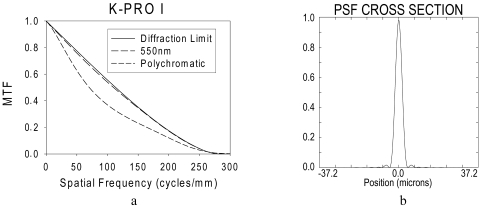
Figure 4.
(a) The computed monochromatic (550 nm) and polychromatic modulation transfer functions (assuming photopic efficiency) are compared with the diffraction limit for the same 550 nm of a PMMA type 1 KPro at the image plane. The retina was assumed to be 22.2 mm from the last surface of the KPro. The performance is close to the diffraction limit. (b) A cross-section of the monochromatic point spread function also illustrates the high quality of the KPro optics.
In the model eye optical bench setup, the type 1 KPro front plate was tightly installed first in a metal iris (3-mm diameter). As such, light reaching the KPro focal plane would only project onto the charge-coupled device (CCD) chip by going through the KPro front plate. A crisp image of the distant point source of light was obtained that was converted into a light intensity plot, representing the Kpro PSF (Fig. 5). This measured, sharp PSF also represented a high-quality system. Both the computational and the measured PSF and modulation transfer function are consistent with the excellent visual acuity results typical of the KPro.
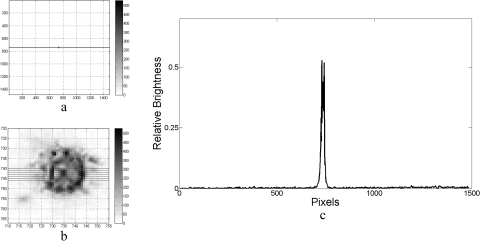
Figure 5.
Measured PSF of the KPro alone, obtained in the artificial-eye optical bench setup. (a) An image of the far point source obtained with the CCD camera. (b) The PSF image spot is magnified and underexposed to display the pixilation and the noise. (c) Profile through the PSF spot imaged at the camera “retina” by the KPro. The profile shown is an average of the readings taken along the horizontal lines shown in (b). The full-width-at-half-maximum (c) is approximately 25 pixels, corresponding to 1/4000 radians (50 seconds of arc).
Glare Effects
We tested for two possible sources of disability glare in KPro-implanted eyes: the optical cylinder (ghosts) and the surrounding hazy cornea. Zemax computation of glare arising from reflections of light from the various surfaces of the KPro was conducted by assuming a worst case of all surfaces to be 100% reflecting (mirrors). The low ray density obtained (not shown) confirmed that little stray light could be generated by the KPro itself. Polymethylmethacrylate (PMMA)/tissue and PMMA/aqueous humor interfaces are actually less than 10% reflective and would, therefore, be expected to result in little stray light.
We then tested for glare by increasing the brightness of the light source in our optical bench setup to the maximal intensity. This led to the appearance of some ghost images surrounding the image of the point source (not shown). These very faint ghost images seem to be the result of scatter in the optical cylinder, as predicted by the Zemax analysis.
To test for the role of the hazy cornea, the same KPro front plate was inserted into a tight 3-mm opening made in a finely sand-blasted Boston scleral lens.16 The scleral lens-KPro combination was mounted behind a metal iris with a 10-mm aperture in the optical bench testing system. A halo of scattered light surrounding the central projection of the bright point source was observed in the camera-captured image (Fig. 6a). The scattered light ring is separated from the light-emitting diode (LED) image because the diffusing cornea is not complete, and the KPro casts a “shadow” of clear imaging centrally. As shown previously, decreasing the aperture of the metal iris down to 3 mm resulted in the elimination of the scatter that was thus shown to be caused by the sand-blasted Boston lens.
Figure 6.
PSF of the KPro measured without the blocking contact lens (dark iris), showing a ring of glare resulting from the translucent cornea. (a) Camera image is overexposed enough to display the glare surrounding it. (b) Magnified overexposed PSF image showing the saturation in this case. (c) Profile measured along the horizontal lines shown in the other panels. Note that in the profile outside the glare, the background noise is not dependent on the light. This noise is camera (electronic) noise because it is the same as that in Figure 5, which is underexposed. The horizontal scale is the same as in Figure 5.
With the adjustable iris again opened to 10-mm aperture, a soft contact lens (Kontur) with the opaque (1% transmissive) iris was then placed on top of the scleral lens-KPro combination. This resulted in a significant decrease in the size of the surrounding scatter halo. However, it was not completely reduced to the level provided by the 3-mm metal aperture, possibly because the clear pupil of the contact lens, which measured 5 mm in diameter, left a 1-mm annular region around the KPro that was not closed off. Light passing through this opening was scattered by the sand-blasted scleral lens, causing the residual glare effect.
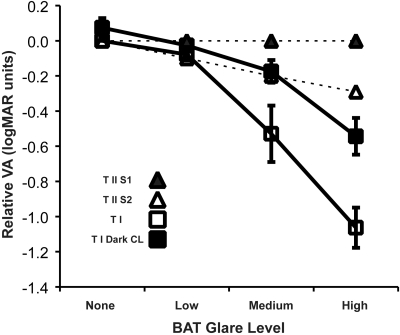
To verify these glare and contact lens effects in our patients, we measured visual acuity under increasing glare settings with the BAT. A substantial reduction in visual acuity was observed with the increase of BAT glare level in the eight patients who underwent implantation with the type 1 KPro (Fig. 7 open squares). With the dark-iris contact lens, some decline in acuity with increased glare level was still apparent, but the effect was reduced compared with the transparent contact lens condition (Fig. 7, filled squares). ANOVA revealed a significant effect of glare (F = 55.73; P < 0.001), a significant effect of the contact lens used (F = 6.053; P = 0.043), and a significant interaction of the glare and contact lens (F = 6.132; P < 0.033). Post hoc parametric analysis found the effect of the dark-iris contact lens to be significant only for the high-level BAT setting (t = 3.76; P = 0.013), whereas the nonparametric Wilcoxon signed rank test found the effect to be significant for both the medium and the high glare levels (z = −2.1 [P = 0.036] and z = −2.37 [P = 0.018], respectively). Three of these subjects had a residual myopic refractive error of >3.00 D, but they did not use spectacle correction habitually, and their acuities were surprisingly good given such high refractive errors (perhaps because of the small pupil imposed by the KPro). We recomputed the ANOVA without these three subjects, and the effects of glare and interactions remained significant, and the effect of contact lens use approached significance (P = 0.064).
Figure 7.
Effect of glare measured using BAT on visual acuity. Relative acuity is normalized to the average acuity measured with no glare and no glare treatment (leftmost open square). Open squares: average relative acuity of eight eyes with the type 1 KPro. Acuity declines significantly with increased glare level. Filled squares: relative acuity of the same eight eyes measured while the patients wore dark-iris soft contact lenses. Acuity was improved at all glare levels with the dark iris. (Error bars are SEM.) Open triangles: relative acuity for the two patients who underwent implantation with a type 2 KPro mounted through the lid. Filled triangles: the patient experienced no loss of acuity from glare because of darkly pigmented skin (African American).
The effect on acuity of increasing glare was substantially reduced in the two eyes with a type 2 KPro (Fig. 7, triangles). With the lid largely blocking light from reaching the scattering cornea, the drop in acuity attributed to increased glare was minimal at all levels for one patient. The drop in acuity for the other patient was less than that noted for all patients with a type 1 KPro and a dark contact lens. These findings further support the hypothesis that the source of glare was not scatter in the KPro itself. The patient with no loss of acuity from glare was African American, whose darker pigmented lid skin served as better protection from glare than did the lighter pigmented lid skin of the other patient, who was of Asian descent (Fig. 2c).
Visual Fields
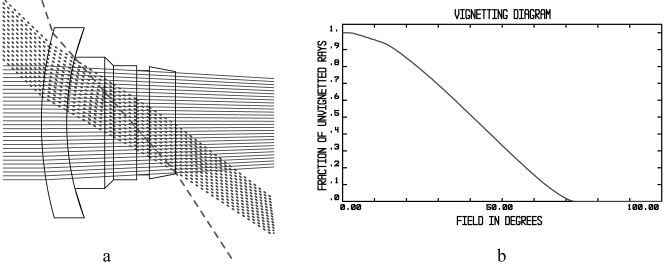
The Zemax model computation of the visual field for the type 1 KPro is shown in Figure 8. The extreme rays that pass through the system without refraction or reflection at the walls cover 71.6° of half-field (Fig. 8a, dashed ray), indicating a maximal possible field of 142.2°. The computation of the half-luminance field (the field in which the nonvignetting rays cover half the diameter of the exit pupil; Fig. 8a, dotted rays) was found to have a half-field extent of 43.7° (Fig. 8b), corresponding to a full-field diameter of 87.4°). The half-luminance field is usually used to estimate the visual field through a device.
Figure 8.
(a) Zemax computation of the theoretical field-extent possible with the KPro. (b) Computed vignetting factor as a function of eccentricity in the object visual field. On-axis, the retaining ring acts as an aperture stop, but at a certain eccentricity the last surface becomes an aperture stop. Because both are very close to each other, vignetting always occurs; hence, 100% efficiency is never achieved.
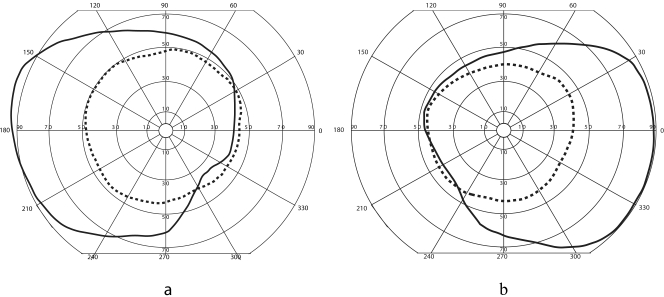
Consistent results were obtained using Goldmann perimetry for those patients with no glaucoma. The V-4e isopters measured for patients with a type 1 KPro and a type 2 KPro are shown in Figures 9a and 9b, respectively. For all the patients without glaucoma, the field was on average approximately 95° wide in eyes with a type 1 KPro and approximately 90° wide in eyes with a type 2 KPro. These values correspond closely to a theoretical half-luminance field and provide patients with fairly wide functional peripheral vision.
Figure 9.
Monocular visual fields (Goldmann V-4e), when one eye has undergone successful implantation with a KPro (dashed line) and the other eye (superimposed) has normal vision (solid line). (a) The right field of a patient with a type 1 KPro superimposed on the normal field of a left eye. (b) The field of a patient with a type 2 KPro in the left eye superimposed with the normal field of a right eye. Note that the contribution from the KPro eye does not enlarge the binocular field.
Stereopsis
Of the five patients with a healthy fellow eye, three were evaluated for stereoacuity and ocular dominance. In all three patients, vision was better in the non-KPro–implanted eye (by one to four lines). The fellow eye was found to be dominant for all three patients. Stereopsis at 16 inches ranged from 400 to 800 seconds of arc, reflecting minimal stereo vision.
Activities of Daily Vision Scale
The ADVS questionnaire13 was administered to six patients who underwent implantation with a type 1 KPro for subjective evaluation of their visual function. Each subscale of the ADVS is scored between 100 (no visual difficulty) and 0 (inability to perform the activity because of visual difficulty). The average overall score (compared with 100 patients scheduled for cataract surgery whose mean visual acuity was 20/150, evaluated by Mangione et al.13) was 74 ± 16 (61 ± 31). Scores for the respective subtests were as follows: night driving, 55 ± 38 (69 ± 30); day driving, 51 ± 29 (42 ± 32); far vision, 76 ± 7 (66 ± 25); near vision, 85 ± 17 (69 ± 25); glare disability, 81 ± 17 (61 ± 31). Thus our patients tended to score higher than did patients with poorer visual acuity before cataract surgery on all except possibly the night driving score. In particular, glare seems to affect patients with KPro implants to a lesser extent than it does patients with moderate cataracts.
Discussion
Despite excellent visual acuity, patients with KPro implants frequently report glare. Light scattered through the donor cornea was thought to be the major cause of the glare, and our optical bench experiments confirmed this. Indeed, veiling glare was virtually eliminated when the KPro was implanted into an opaque metal iris. Using a translucent sand-blasted scleral lens covered with opaque dark-tinted iris soft lens did not eliminate glare but substantially reduced it. The residual light scatter could be attributed to the larger 5-mm diameter clear pupil of the contact lens in comparison with the 3-mm diameter of the KPro stem. This difference translates to an area 177% larger than the stem, allowing passage of a great deal of light through the translucent light-scattering donor cornea.
In patients, we similarly attempted to block light passing through the hazy cornea surrounding the PMMA optical cylinder of the KPro by using an opaque-iris contact lens. This resulted in an improvement in visual acuity of approximately 38%. In concordance with our optical bench experiments, patients with the type 2 KPro were even less sensitive to increased glare settings, reflecting the better blockage of light by the lid tissues around the central post of the KPro.
A large-diameter-bandage contact lens has routinely been used in patients with type 1 KPro implants because it is highly protective against evaporative damage to the corneal tissue around the device.17 Changing to an opaque-iris contact lens should improve vision under glare conditions without much change in postoperative care. It can also be of cosmetic value in many patients because the color and pattern of the contralateral iris can be approximated. However, a lens of that sort must be carefully fitted because significant movement of the lens off axis may result in its opaque part blocking part of the KPro pupil.
The beneficial effect of an opaque contact lens could potentially be mimicked or supplemented by keeping the natural iris as intact as possible during the surgery. Furthermore, a large pupil size (i.e., the absence of iris) would result in significant reduction in visual acuity secondary to glare.
Light internally reflected off the walls of the optical cylinder has previously been described as a possible source of glare.18 The reflected light would result in diffusion circles or circular stripes that can cause a decrease in visual acuity and contraction of the visual field.18 Sokol et al.4 painted the side of the cylinders black, which resulted in improved image sharpness and contrast, particularly in the periphery. We were able to illustrate a similar phenomenon in the KPro using computer simulation, assuming that the inner surface of the optical cylinder behaves as a perfect polished mirror. This is the worst-case assumption for the contribution of the internal reelection within the KPro. However, the optical bench experiments revealed that its contribution to total glare is minimal, possibly because the walls of the cylinder are not polished. The rough surface would actually favor diffusion rather than specular reflection of rays on the surface, minimizing both the amount of light that actually hits the retina by this route and the focused ghost images.
Anterior surface irregularities may be another possible KPro-related glare-causing factor. Scratches on the anterior surface of KPro implants are occasionally identified during slit lamp examination. The contact lens (Kontur) would be expected to smooth these defects, even though its refractive index of approximately 1.37 differs from that of PMMA (1.49). However, no significant change in baseline glare was seen when defects in the front surface were eliminated,12 suggesting that the magnitude of this effect is small.
The RPM that may form on the back surface of the KPro, as seen in Figure 2, may be a source of significant glare. This membrane can be treated with a YAG laser. Our glare measurement did not reveal the effect of the RPM because comparisons were carried out within subjects and the effect of the RPM would be the same with and without the opaque contact lens. Given that only two of our subjects had that membrane (in one it was very mild), it was not possible to determine its effect on glare by comparisons between subjects in our small group.
Visual fields in KPros are dependent on the diameter and length of the optical cylinder, and we found good agreement between the theoretical visual fields derived from the dimensions of the optical cylinder and those obtained in patients who received the implanted devices. In eyes with type 1 KPros, we found approximately 50° in the temporal direction compared with 44° computed; in eyes with type 2 KPros, we found approximately 40° in the temporal direction. These results are also consistent with the 70° diameter measured by Sokol4 for a prosthetic of similar dimensions (3.1-mm diameter, 4.5-mm length) and with the approximately 100° computed by Rol et al.5 (3-mm diameter, 3-mm length). Although these are very wide fields, it is obvious that if a patient has one normal eye and one with severe corneal damage, expanding the visual field is not a good reason to implant KPro in the damaged eye. The normal eye has a nasal field of approximately 60°, thus providing more than the expected temporal field of the potential KPro eye. Limited stereoacuity seemed to result from implanting a KPro in a patient with a healthy fellow eye. This is expected because of the acuity difference between the eyes and the possibility of magnification differences in the images of both eyes. As pointed by Sokol et al.,4 a prosthetic of similar general design results in an approximately 20% increase in retinal image size. Although that increase in size supports better visual acuity, it does impede stereoacuity. Thus, field expansion and return of stereoacuity are rejected as reasons for implanting the KPro device in a patient with one normal eye. The often-expressed “improvement” after successful KPro surgery in one eye in a patient with a normal fellow eye may be inconclusive.
In summary, the optics of the KPro can provide patients excellent vision and wide visual fields. It can allow visual access to the fundus, if the vitreous is clear. Glare from light passing through the hazy cornea surrounding the KPro is the main cause of glare, but this can be controlled if the iris is kept intact in surgery (which is often not possible). Otherwise, wearing a soft lens with a clear pupil and a tinted dark iris can be effective in reducing glare. In the presence of a healthy, well-functioning, contralateral eye, we found no functional benefit for replacing the damaged eye with an implant.
Acknowledgments
The authors thank Jill Beyer for fitting the dark-iris contact lenses.
Footnotes
Supported in part by National Institutes of Health Grant EY12890 (EP) and by the Keratoprosthesis Fund, Massachusetts Eye and Ear Infirmary.
Disclosure: R.R. Sayegh, None; L.A. Diaz, None; F. Vargas-Martín, None; R.H. Webb, None; C.H. Dohlman, None; E. Peli, None
References
- 1.Ma JJ, Graney JM, Dohlman CH. Repeat penetrating keratoplasty versus the Boston keratoprosthesis in graft failure. Int Ophthalmol Clin 2005; 45: 49–59 [DOI] [PubMed] [Google Scholar]
- 2.Doane MG, Dohlman CH, Bearse G. Fabrication of a keratoprosthesis. Cornea 1996; 15: 179–184 [DOI] [PubMed] [Google Scholar]
- 3.Dohlman CH, Harissi-Dagher M, Graney J. The Boston keratoprosthesis: a new threadless design. Digital J Ophthalmol 2007: 5 [Google Scholar]
- 4.Sokol A, Bertelsen TI, Teigland N. The optical function of keratoprostheses. Acta Ophthalmol (Copenh) 1977; 55: 317–332 [DOI] [PubMed] [Google Scholar]
- 5.Rol P, Parel J-M, Lacombe E, Legeais J-M, Villain F. Optics of keratoprostheses. Refract Corneal Surg 1993; 9: 212–213 [Google Scholar]
- 6.Zerbe BL, Belin MW, Ciolino JB. Results from the multicenter Boston type 1 Keratoprosthesis Study. Ophthalmology 2006; 113: 1771–1777 [DOI] [PubMed] [Google Scholar]
- 7.Koch DD. Glare and contrast sensitivity testing in cataract patients. J Cataract Refract Surg 1989; 15: 158–164 [DOI] [PubMed] [Google Scholar]
- 8.Vos JJ. On the cause of disability glare and its dependence on glare angle, age and ocular pigmentation. Clin Exp Ophthalmol 2003; 86: 363–370 [DOI] [PubMed] [Google Scholar]
- 9.Aslam TM, Haider D, Murray IJ. Principles of disability glare measurement: an ophthalmological perspective. Acta Ophthalmol Scand 2007; 85: 354–360 [DOI] [PubMed] [Google Scholar]
- 10.van den Berg TJ, Ijspeert JK, de Waard PW. Dependence of intraocular straylight on pigmentation and light transmission through the ocular wall. Vision Res 1991; 31: 1361–1367 [DOI] [PubMed] [Google Scholar]
- 11.Erie JC, Bandhauer MH. Intraocular lens surfaces and their relationship to postoperative glare. J Cataract Refract Surg 2003; 29: 336–341 [DOI] [PubMed] [Google Scholar]
- 12.Miller D, Dohlman CH. Optical properties of buried corneal silicone prostheses. Am J Ophthalmol 1968; 66: 633–640 [DOI] [PubMed] [Google Scholar]
- 13.Mangione CM, Phillips RS, Seddon JM, et al. Development of the “Activities of Daily Vision Scale”: a measure of visual functional status. Med Care 1992; 30: 1111–1126 [DOI] [PubMed] [Google Scholar]
- 14.Holladay JT. Visual acuity measurements. J Cataract Refract Surg 2004; 30: 287–290 [DOI] [PubMed] [Google Scholar]
- 15.Colenbrander A. Visual acuity measurement standard. Ital J Ophthalmol 1988; 2: 1–15 [Google Scholar]
- 16.Schein OD, Rosenthal P, Ducharme C. A gas-permeable scleral contact lens for visual rehabilitation. Am J Ophthalmol 1990; 109: 318–322 [DOI] [PubMed] [Google Scholar]
- 17.Dohlman CH, Dudenhoefer EJ, Khan BF, Morneault S. Protection of the ocular surface after keratoprosthesis surgery: the role of soft contact lenses. CLAO J 2002; 28: 72–74 [PubMed] [Google Scholar]
- 18.Cardona H. Keratoprostheses: elimination of light reflection from the walls of the optical cylinder. Int Ophthalmol Clin 1966; 6: 111–118 [PubMed] [Google Scholar]