Abstract
Pyruvate carboxylase (PC) activity is enhanced in the islets of obese rats but reduced in the islets of type 2 diabetic rats, suggesting the importance of PC in beta cell adaptation to insulin resistance as well as the possibility that PC reduction might lead to hyperglycemia. However, the causality is currently unknown. We used obese AyL mice as a model to show enhanced beta cell adaptation, and type 2 diabetic db/db mice as a model to show severe beta cell failure. After comparison of the two models, a less severe type 2 diabetic Agouti-K (AyK) mouse model was used to show the changes in islet PC activity during the development of type 2 diabetes (T2DM). AyK mice were separated into two groups, mildly (AyK-M, blood glucose <250 mg/dl) and severely (AyK-S, blood glucose >250mg/dl) hyperglycemic groups. Islet PC activity, but not protein level, was increased 1.7 folds in AyK-M mice; in AyK-S mice, islet PC activity and protein were reduced. All other changes including insulin secretion and islet morphology in AyK-M mice were similar to those observed in AyL mice but worse in AyK-S mice where these parameters closely matched those in db/db mice. In 2-day treated islets, PC activity was inhibited by high glucose but not by palmitate. Our findings suggest that islet PC might play a role in the development of T2DM where reduction of PC activity might be a consequence of mild hyperglycemia and a cause for severe hyperglycemia.
Keywords: Pyruvate carboxylase, Beta cell adaptation, Islets of Langerhans, Insulin secretion, Type 2 diabetes mellitus
Introduction
Pyruvate carboxylase (PC) is located in the mitochondria and converts carbons from pyruvate derived from glucose into the Krebs cycle intermediate oxaloacetate (Macdonald et al. 1996b; Macdonald et al. 1996a). The major roles of PC are related to beta cell adaptation and insulin secretion. Inhibition of PC with its inhibitor phenylacetic acid (PAA) prevents glucose stimulated insulin secretion (GSIS) (Liu et al. 2002; Liu et al. 2005; Farfari et al. 2000). We found that the PAA also reduces the beta cell proliferation response, a portion of adaptation of beta cell, in 60% pancreatectomized rats (Liu et al. 2005) and Zucker fatty rats (Liu et al. 2002). We reduced and increased PC activity in primary beta cells or beta cell line INS-1 cells and found that PC down- and up-regulated GSIS in both cell types and cell proliferation in INS-1 cells (Xu et al. 2008). Hasan et al. (Hasan et al. 2008) used PC siRNA to inhibit PC activity and subsequently inhibit GSIS. 13C-NMR isotopomer analysis (Lu et al. 2002; Cline et al. 2004) has shown a close correlation between flux through PC and the capacity of glucose to stimulate insulin secretion. In addition, Palmer et al (Palmer et al. 2006) found that a single nucleotide polymorphism in the PC gene of African Americans is significantly associated with the magnitude of the acute insulin response.
Oxaloacetate, a product of PC, enters pyruvate–malate shuttle (Macdonald 1995a; Lu et al. 2002) and pyruvate citrate shuttle (Khan et al. 1996). These two shuttles play important roles in NADPH production and insulin secretion in the beta cells (Macdonald 1995a; Lu et al. 2002; Khan et al. 1996). In addition, oxaloacetate can be used for aspartate synthesis (Menendez et al. 1998), and NADPH for lipids and fatty acids synthesis (Infante & Huszagh 1998; Dmitriev 2001) and anti-apoptosis (Sheline & Choi 1998; Brune et al. 1992), and importantly for insulin secretion (Macdonald 1995a; Macdonald 2003).
Pyruvate is also catalyzed into acetyl-CoA (Wallace 1985) in the mitochondria by pyruvate dehydrogenase (PDH) (Zhou et al. 1996; Zhou et al. 1995). In most cell types, the PDH pathway predominates. However, pancreatic beta cells express unusually high levels of PC (Macdonald 1995b). As a result, the beta cell is unique in that approximately equal amounts of pyruvate enter into Krebs cycle via PC and PDH (Khan et al. 1996). A primary role of PC in tissues such as liver and kidney is to provide substrate for gluconeogenesis (Bahl et al. 1997; Baverel et al. 2003). However, as pointed out by MacDonald (Macdonald 1995a), beta cells lack the essential gluconeogenic enzyme phosphoenolpyruvate carboxykinase, therefore the high levels of PC must be required for a different function in the beta cells such as insulin secretion. On the other hand, entry of pyruvate into the Krebs cycle via PDH does not appear to be important since activation of PDH had only a minor affect on insulin release (Nicholls et al. 2002). We have demonstrated that inhibition of PDH activity by overexpressing pyruvate dehydrogenase kinase 4 in INS-1 cells could not significantly reduce insulin secretion (Xu et al. 2008).
Type 2 diabetes mellitus (T2DM) is characterized by insulin resistance and beta cell failure (DeFronzo & Prato 1996). Once insulin resistance occurs, pancreatic beta cells must secrete more insulin to maintain normal glucose levels. Increased insulin secretion requires beta cell adaptation, a process that includes both enhanced insulin secretion and beta cell proliferation. During compensated obesity, islet PDH activity is reduced while PC activity is unaffected (Liu et al. 2002; Liu et al. 2005), indicating that normal PDH activity is not important for beta cell adaptation. In T2DM, the failure of beta cell adaptation coincides with reduced mRNA levels of PC (Jonas et al. 1999; Kjorholt et al. 2005) and activities of PC (Macdonald et al. 1996b; Macdonald et al. 1996a; Macdonald et al. 2009) and PDH (Zhou et al. 1996; Zhou et al. 1995). These reports suggest that PC, not PDH, plays a key role in the development of T2DM.
However, the cause and effect of reduction of PC activity in the islets of type 2 diabetic mice on hyperglycemia is still unknown. In the current study we used an obese mouse model (AyL) and two type 2 diabetic mouse models (AyK and db/db) to observe the correlation between islet PC activity and hyperglycemic levels during the pathogenesis of T2DM, and tested whether high glucose or fatty acid inhibits PC activity in mouse primary islets.
Materials and Methods
Animals and blood glucose and plasma insulin assay
Male type 2 diabetic Agouti-KK (AyK, KK background) and control KK mice, obese Agouti mice (AyL, C57BL/6 background) and control C57BL/6 mice as well as type 2 diabetic db/db and control db/+ mice (all were from Jackson Laboratory, Bar Harbor, ME) with age of 10 to 20 weeks were used for this research. The principles of animal laboratory care under the guidelines of both NIH and the University of Louisville and Research Institute for Children’s Institutional Animal Care and Use Committees were followed strictly. The mice were maintained at 25 °C with a 12-h light/dark cycle. Body weight was determined and blood was collected after tail snipping. Blood glucose was measured with a Glucose Analyzer (Analox Instruments). For measuring blood insulin, blood samples were collected in heparinized capillary tubes and plasma insulin levels were determined with the Ultrasensitive Mouse Insulin-ELISA Test Kit (Mercodia).
Glucose tolerance test
After three hours of fasting, mice were administered intraperitoneally 1.0 g glucose per kg body weight. Blood glucose levels were determined at 0, 15, 30, 60, and 120 minutes and blood samples were collected (at 0, 15, and 30 min only) to measure plasma insulin levels
Islet isolation, culture and high glucose and palmitate treatment
Islets were isolated from the mice by an adaptation of the Gotoh method (Gotoh et al. 1987). Islets were cultured using previous method (Han et al. 2005). For high glucose or palmitate treatment, the islets isolated from 10 weeks old KK mice were divided into seven groups the next morning, and different concentrations of glucose or palmitate (see Figure 6 legend) were added to each group. After 48-hours of cultivation, the islets were collected for PC activity assay.
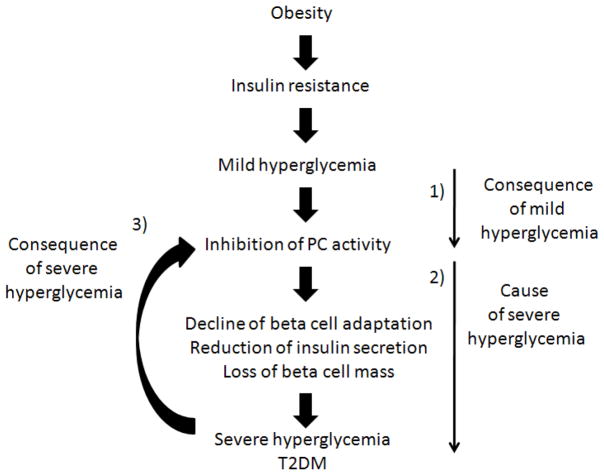
Figure 6.
Hypothetic scheme for islet PC activity as a consequence and cause of hyperglycemia during the pathogenesis of T2DM. 1) indicates that mild hyperglycemia leads to an inhibition in PC activity in the islets; 2) indicates that the inhibition of islet PC activity works as a cause of severe hyperglycemia; 3) indicates that severe hyperglycemia in turn inhibits islet PC activity.
Islet DNA, protein and insulin content
DNA was measured by the Labarca method (Labarca & Paigen 1980) and protein by a commercial kit that used BSA as standard (Bio-Rad, Hercules, CA). Islets were homogenized in acid ethanol and stored at −20 °C pending assay for insulin contents.
Insulin secretion
Ten islets were cultured in each 5-ml vial with 1 ml KRB (Krebs-Ringer bicarbonate buffer supplemented with 10 mmol/l Hepes, pH 7.4 and 0.1% BSA, bubbled with 5% CO2, 95% O2) containing 2.8, 5.5, and 16.7 mmol/l glucose in a 37°C shaking water bath as we have previously described (Liu et al. 1998). After incubation for 60 min, vials were moved from water bath to ice to stop the reaction followed by a brief centrifugation at 4°C, 0.5 ml KRB was moved into a glass tube and stored at −20 °C pending assay for insulin as described above.
PC activity assay and PC protein detection
PC activity was measured according to the method of MacDonald et al. (Macdonald et al. 1996b). PC protein was detected using method of MacDonald et al. (Macdonald 1995b; Xu et al. 2008).
Active PDH activity assay
Active PDH assay was measured as described previously (Liu et al. 1999; Zhou et al. 1995).
Islet morphology, immunohistochemistry and beta cell mass
Immunohistochemistry was carried out as previously described (Jetton et al. 2001; Liu et al. 2002).
Data presentation and statistical methods
All data are expressed as mean ± S.E.M. The listed n values represent the number of individual experiments performed and every experiment was duplicated. Comparisons between two groups were performed by Student’s t test. Comparisons between multiple groups were performed by one-way ANOVA (Tukey post hoc test). A value of p<0.05 was considered significant.
Results
Mildly and severely hyperglycemic AyK mice
The hyperglycemia in AyK mice was less severe than those in db/db mice; we simply designed a blood glucose value of 250 mg/dl as a standard point to separate the AyK mice into mildly (<250 mg/dl, AyK-M) and severely (>250 mg/dl, AyK-S) hyperglycemic groups. We used this value (250 mg/dl) to separate the mice because we found that most parameters including insulin secretion and PC activity in isolated islets were significantly changed if blood glucose levels were over 250 mg/dl and consistently maintained for over two weeks. About 17% of AyK mice at age 10 weeks and 39% at age 20 weeks became severely hyperglycemic, respectively. Because of the slowly developmental process of severe diabetes, AyK mice we kept provided an excellent model for investigating the pathogenesis of T2DM. db/db mice were not ideal for this purpose because they became severely diabetic in a very short period of time: 57% of db/db mice at age 6 weeks and 100% of db/db mice at age 10 weeks became severely hyperglycemic, respectively. However, db/db mice would be a good control model for showing severe diabetes.
General characteristics of 10 and 20 weeks old AyK, AyL, and db/db mice
Body weights were measured periodically and 3-hour fasting blood glucose and insulin levels in 10 and 20 weeks old AyL, AyK, and db/db mice compared with their parallel normal controls. As shown in Table 1, AyL, AyK-M and AyK-S, and db/db mice were obese and had similar body weight, but db/db mice were the heaviest. Compared with control KK mice, glucose and insulin levels of 10 and 20 weeks old AyK mice significantly increased especially in AyK-S mice. Blood glucose levels in db/db mice were higher and blood insulin levels were lower than those in AyK-S mice. Islet protein and DNA contents were significantly increased in 20 weeks old AyL, AyK-M, AyK-S and db/db mice (Table 2), however, islet insulin contents in AyK-S and db/db mice were markedly reduced, especially in db/db mice. In contrast, islet insulin content in AyK-M mice was significantly increased, similar to those in AyL mice. The following results were obtained from 20 weeks old mice except the data in figure 5.
Table 1.
General characteristics of 10 and 20 weeks old KK, AyK, C57BL/6, AyL, db/+ and db/db mice. KK means KK mice for control of AyK mice, AyK-M indicates mildly hyperglycemic AyK mice; AyK-S indicates severely hyperglycemic AyK mice, AyL indicates obese mice (Ay gene mutation on C57BL/6 background), db/+ means littermates without leptin gene mutation and used for control of db/db mice, db/db indicates db/db mice with leptin gene mutation. Week indicates age.
| Strains (n) | Body weight, g | 3h fasting blood glucose levels, mg/dl | 3h fasting plasma insulin levels, ng/ml | |||
|---|---|---|---|---|---|---|
| 10-week | 20-week | 10-week | 20-week | 10-week | 20-week | |
| KK (30) | 22.3 ± 1.2 | 32.4 ± 2.3 | 163 ± 17 | 171 ± 15 | 2.8 ± 0.6 | 3.4 ± 0.5 |
| AyK-M (32–40) (<250 mg/dl) | 24.2 ± 1.1* | 37.8 ± 2.8* | 214 ± 22* | 232 ± 15* | 6.5 ± 1.9* | 9.9 ± 1.2* |
| AyK-S (10–15) (>250 mg/dl) | 25.4 ± 1.6* | 39.4 ± 2.5* | 315 ± 32**§§ | 357 ± 56**§§ | 22.3 ± 2.3**§§ | 26.0 ± 2.6**§§ |
| C57BL/6 (30) | 22.4 ± 1.3 | 33.6 ± 2.5 | 165 ± 15 | 174 ± 18 | 2.3 ± 0.7 | 2.8 ± 0.8 |
| AyL (30) | 24.3 ± 1.4 | 38.6 ± 2.1* | 152 ± 12 | 165 ± 13 | 3.2 ± 1.2 | 3.5 ± 1.4* |
| db/+ (30) | 23.5 ± 1.6 | 32.4 ± 3.3 | 174 ± 18 | 176 ± 16 | 2.1 ± 0.8 | 3.4 ± 0.7 |
| db/db (30) | 26.6 ± 1.7* | 44.6 ± 2.8** | 365 ± 42** | 436 ± 67** | 4.5± 1.3** | 5.3 ± 1.5** |
Data are mean ± SE.
p<0.05,
p<0.01 vs. parallel controls;
p<0.05,
p<0.01 vs. AyK-M.
Table 2.
General characteristics of the islets isolated from 20 weeks old KK, AyK, C57BL/6, AyL, db/+ and db/db mice.
| Group (n=6) | Protein content (μg/islet) | DNA content (ng/islet) | Insulin content (ng/islet) | Insulin content (μg/mg) |
|---|---|---|---|---|
| KK | 0.72 ± 0.15 | 20.6 ± 5.3 | 56 ± 12 | 78 ± 17 |
| AyK-M | 1.35 ± 0.24** | 48.7 ± 9.6** | 115 ± 38** | 85 ± 28 |
| AyK-S | 1.87 ± 0.32** | 52.4 ± 12.4** | 73 ± 18*§ | 39 ± 8*§§ |
| C57BL/6 | 0.67 ± 0.18 | 18.9 ± 4.5 | 47 ± 15 | 70 ± 19 |
| AyL | 1.28 ± 0.25** | 43.6 ± 9.7** | 152 ± 32** | 117 ± 26* |
| db/+ | 0.75 ± 0.17 | 22.4 ± 5.7 | 64 ± 17 | 85 ± 16 |
| db/db | 1.66 ± 0.38** | 46.8 ± 12.3** | 35 ± 9** | 21 ± 5** |
Data are mean ± SE.
p<0.05,
p<0.01 vs. parallel controls;
p<0.05,
p<0.01 vs. AyK-M.
Figure 5.
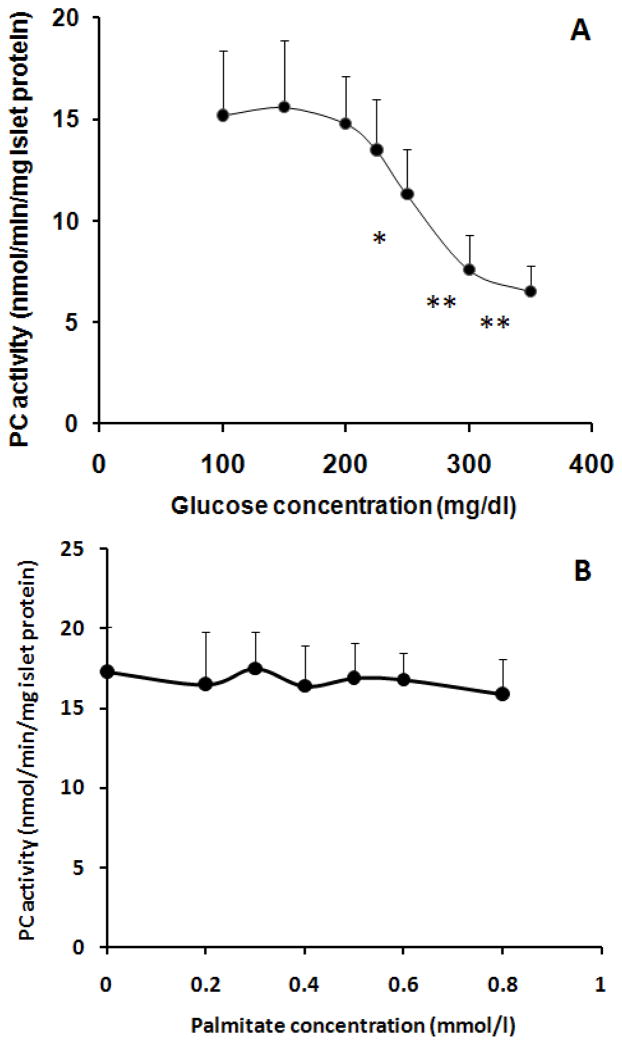
PC activity in high glucose (A) and palmitate (B) 2-day treated islets isolated from 10 weeks old KK mice. In A, glucose was added to each group of the islets to yield 5.5, 8.3, 11.1, 12.5, 13.9, 16.7, and 19.4 mmol/l (final concentration), respectively, and 5.5 mmol/l glucose treated group was used as control. In B, palmitate was added to each group of the islets to yield 0.2, 0.3, 0.4, 0.5, 0.6, and 0.8 mmol/l (final concentration); a control group of islets was not treated with palmitate (0 mmol/l). All groups of islets in B were supplied with 5.5 mmol/l glucose. Palmitate was dissolved in 10% fatty acid free-BSA as a stock solution (8 mmol/l); the latter was diluted with 10% fatty acid free-BSA to yield different concentrations (10x of final concentration) just before use, and final concentration of fatty acid free-BSA in the culture medium in all groups including control was 1%. Data are mean ± SE, n=4. In A, * p<0.05, **p<0.01 vs. control (5.5 mmol/l glucose).
Glucose tolerance tests and plasma insulin levels
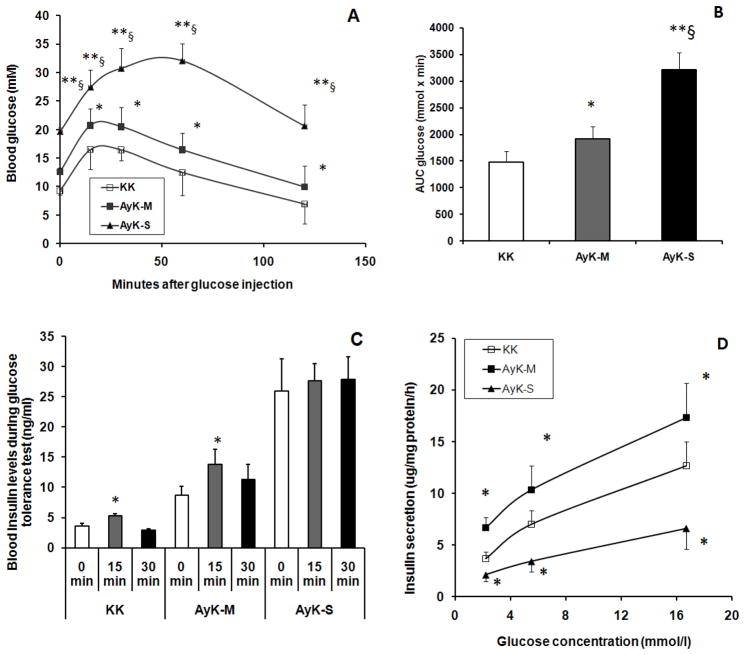
As shown in Figure 1A, during the course of each time point, the blood glucose levels of AyK-M mice elevated slightly but significantly compared to that of the control KK mice, suggesting the predominance of insulin resistance, but not beta cell failure. In AyK-S mice, however, blood glucose levels were strikingly elevated at every time point compared with those of either AyK-M or KK mice; indicating severe insulin resistance and beta cell failure developed. Figure 1B indicates AUCglucose for Figure 1A; the values are consistent with curve changes in Figure 1A. Fasting blood glucose levels in AyK-S mice were lower than those in db/db mice (Table 1); suggesting that diabetes in AyK-S was less severe than those in db/db mice. We measured plasma insulin levels at 0, 15 and 30 min time points during glucose tolerance tests. As shown in Figure 1C, the highest levels of fasting plasma insulin (0 min) were observed in AyK-S mice; fasting plasma insulin levels in AyK-M mice were also higher than those in control KK mice. During glucose tolerance tests, similar to KK mice, plasma insulin levels in AyK-M mice at 15 min time point were significantly increased; indicating partial beta cell function in response to high glucose stimulation was preserved. In contrast, AyK-S mice showed no significant change in insulin plasma level at the 15 min time point; suggesting that AyK-S mouse beta cells lost the responsive capability to glucose stimulation.
Figure 1.
Glucose tolerance tests (A), AUCglucose (B), plasma insulin levels during glucose tolerance tests (C), and in vitro GSIS in isolated islet (D) in control KK and AyK mice. KK: KK mice for control of AyK mice, AyK-M: mildly hyperglycemic AyK mice; AyK-S: severely hyperglycemic AyK mice. Data are mean ± S.E. In A and B, n=8–10, * p<0.05, ** p<0.01 vs. control KK mice; § p<0.01 vs. AyK-M. In C, n=8–10, * p<0.05 vs. 0 min. In D, n=5, * p<0.05 vs. control KK mice.
Insulin secretion from islets isolated from AyK mice
To confirm the results of in vivo insulin secretion, we isolated the islets from AyK-M and AyK-S mice and perform in vitro GSIS in the presence of 2.8, 5.5 and 16.7 mmol/l glucose. As shown in Figure 1D, compared to control group, islet GSIS was significantly increased in AyK-M mice but reduced in AyK-S mice at each glucose concentration; indicating that beta cell insulin secretory function was significantly enhanced in AyK-M mice but definitely impaired in AyK-S mice. These data are consistent with the in vivo observation (Figure 1C).
PC and PDH activities in the islets of AyK-M and AyK-S mice
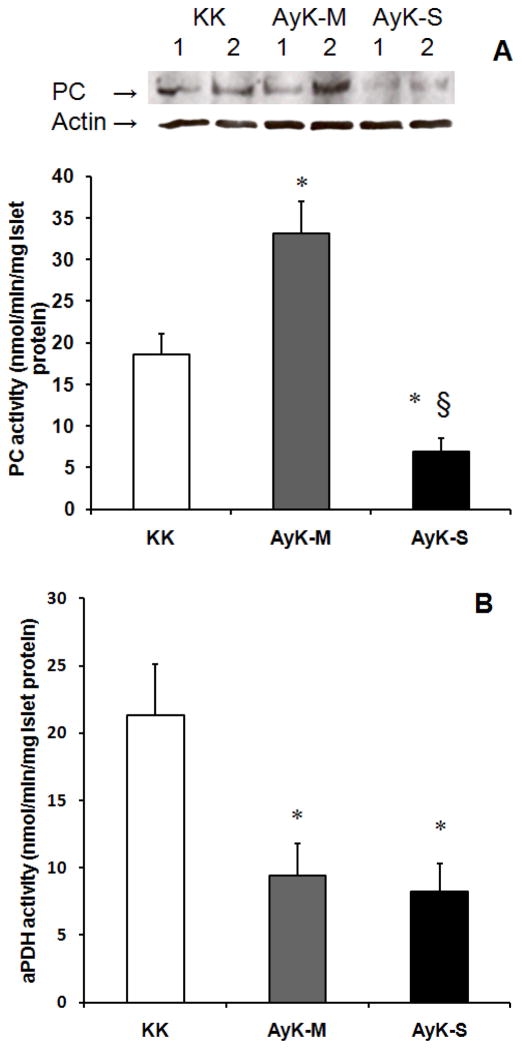
Because of the importance of PC activity described in the Introduction section, we measured PC activity in AyK islets. As shown in Figure 2A, islet PC activity but not protein expression level significantly increased (1.7 fold) in AyK-M mice; quantified PC protein contents were 1.3±0.2-fold increase after normalizing to actin (p<0.05 vs. KK islets; n=6); not consistent with increased PC activity (1.7-fold increase) but this change may account for the increase in PC activity. In contrast, the reduction of PC activity and protein level observed in the AyK-S mice. Quantified PC protein contents in AyK-S islets were 45±8% of control KK after normalizing to actin (p<0.01 vs. KK islets; n=6); this change accounts for the reduction in PC activity. The changes of PC in AyK-M islets are consistent with those in an adaptative 60% pancreatectomized model, where islet PC activity is increased while protein concentration does not (Liu et al. 2005). However, the changes of PC in AyK-S islets are similar to those in human severe type 2 diabetic islets, both Pc mRNA (significantly) and PC activity (slightly) levels are reduced (Macdonald et al. 2009). We measured active PDH (aPDH) activity in AyK islets to know whether the changes in PDH activity were consistent with those in obese rat islets (Liu et al. 2002). Our data shown in Figure 2B demonstrate that islet aPDH activity was significantly reduced in both AyK-M and AyK-S mice. These data suggest that islet PC might play a compensatory role in beta cell adaptation to insulin resistance when PDH is reduced in AyK-M islets, and reduced islet PC activity in AyK-S mice might associate with beta cell failure.
Figure 2.
Islet PC activity and protein expression (A) and aPDH (B) activity in 20 weeks old KK and AyK mice. KK: KK mice for control of AyK mice, AyK-M: mildly hyperglycemic AyK mice; AyK-S: severely hyperglycemic AyK mice. Western blot on the top of figure A shows PC protein (~130 kDa) contents and actin; each lane was loaded with 20 μg of protein extract obtained from islets isolated from 1 animal; similar results were obtained in other 2 independent experiments (total 6 mice). Quantified PC protein contents are given in the Results section. Data are mean ± S.E. * p<0.01 vs. KK control mice, § p<0.01 vs. AyK-M mice. n=6.
PC and PDH activities and insulin secretion in the islets of AyL and db/db mice
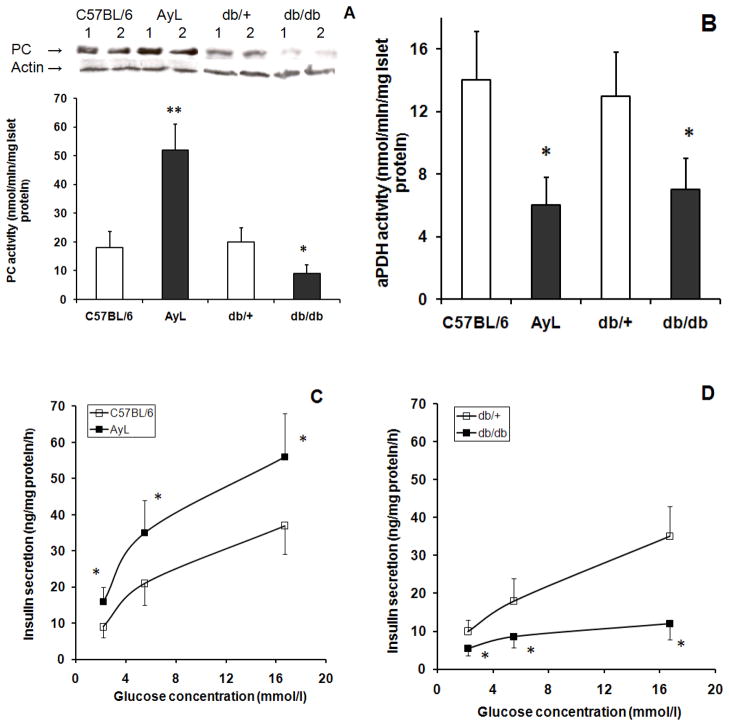
AyL and db/db mice were used as control models as described above, thus we measured islet PC and aPDH activities and insulin secretion in these mice. As shown in Figure 3A, a 2.8 fold increased was observed in PC activity of AyL mice compared with that of C57BL/6 mice. This elevation was higher than those observed in AyK mice (1.7 fold increase, Figure 2A). Like AyK-M islets, PC protein levels in AyL islets were slightly increased; quantified PC protein contents were 1.4±0.2-fold increase after normalizing to actin (p<0.05 vs. C57BL/6 islets; n=6); not consistent with increased PC activity (2.8±0.4-fold increase). Similar to AyK-S mice, both PC activity and protein levels in db/db mouse islets were significantly reduced (Figure 3A). Quantified PC protein contents in db/db islets were 35±6% of control db/+ after normalizing to actin (p<0.01 vs. db/+; n=6); this change accounts for the reduction in PC activity. Islet aPDH activity (Figure 3B) was reduced in both AyL and db/db mice, which was similar to those in AyK-M and AyK-S mice (Figure 2B). Islet GSIS was significantly increased in AyL mice (Figure 3C) but it was reduced in db/db mice (Figure 3D), and these results were also similar to those observed in AyK-M and AyK-S mice, respectively (Figure 1D). These data demonstrate that the changes in AyK-M islets were similar to those in AyL mice, and AyK-S mice similar to db/db mice. In other words, beta cell adaptation was enhanced in both AyL and AyK-M mice; beta cell failure occurred in both AyK-S and db/db mice.
Figure 3.
PC activity and protein expression (A), aPDH activity (B) and GSIS (C and D) in isolated islets of 20 weeks old C57BL/6, AyL, db/+ and db/db mice. C57BL/6 mice were for control of AyL mice; AyL indicates obese (not diabetic) mice on C57BL/6 background; db/+ means littermates without leptin gene mutation and used for control of db/db mice; db/db indicates db/db mice. Western blot on the top of figure A shows PC protein (~130 kDa) contents and actin; each lane was loaded with 20 μg of protein extract obtained from islets isolated from 1 animal; similar results were obtained in other 2 independent experiments (total 6 mice). Quantified PC protein contents are given in the Results section. Data are mean ± S.E. * p<0.05, **p<0.01 vs. parallel controls. In A and B, n=6; in C and D, n=4.
Comparation of physiological and biochemical parameters and islet characteristics in six strains of 20 weeks old mice
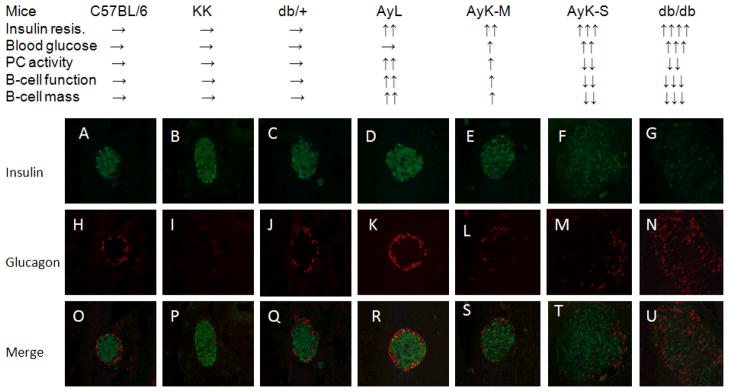
To clearly highlight the importance of PC during the development of T2DM, we summarize the dynamic changes of insulin resistance, blood glucose levels, PC activity, beta cell secretory function and beta cell mass in Figure 4, and that data is compared with islet morphological alterations. We also used C57BL/6 (control of AyL mice) and db/+ (control of db/db mice) mice as normal controls, followed by obese AyL, diabetic AyK-M and AyK-S as well as db/db mice. The progressive process of T2DM is demonstrated clearly in all groups from normal, obese, mild diabetic to severe diabetic. Insulin resistance was gradually enhanced starting at obese AyL mice; blood glucose levels were slightly increased in AyK-M mice and further increased in AyK-S mice and db/db mice; the latter had the highest blood glucose levels. Interestingly, PC activity was paralleled with beta cell function and mass, and these three parameters were increased in AyL mice; indicating that increased PC activity was associated with enhanced beta cell adaptation. In AyK-M mice, beta cell function and mass were also enhanced, but these parameters were partially suppressed because of slight reduction in PC activity compared to that in AyL mice. Beta cell function and mass were significantly reduced in AyK-S and db/db mice while PC activity was significantly reduced. Importantly, significant reduction in PC activity was also paralleled with the aberrations in islet morphology. All diabetic mice had large islet size. However, islet insulin staining was gradually reduced starting at AyK-M mice, and this change was paralleled with the reduction in PC activity. Islet insulin staining in these mice was consistent with the changes in insulin contents in isolated islets shown in Table 2. In db/db mice, they are supposed to be at an ending stage of T2DM, most islet insulin staining was lost; in contrast, glucagon staining in the islets was greatly enhanced and aberrant glucagon distribution occurred. Figure 4 demonstrates a strong association between PC activity and beta cell function/mass and islet morphological aberrations during the pathogenesis of T2DM.
Figure 4.
Summary of physiological parameters and islet morphology in seven strains of mice. Physiological and beta cell mass parameters are summarized on the top. → means normal, ↑ means increase, and ↓ means decrease, double or more ↑ or ↓ mean more or much more significant changes. Micrographs (A to U, magnification × 40) show insulin (A to G) and glucagon (H to N) staining of typical of appearance in the islets of 20 weeks old C57BL/6 (A, H and O), KK (B, I and P), db/+ (C, J and Q), AyL (D, K and R), AyK-M (E, L and S), AyK-S (F, M and T) and db/db (G, N and U) mice. Micrographs from O to U are merged of insulin plus glucagon. Each micrograph (O to U) shows a representative islet taken from a section of separate mouse pancreas. Green color indicates insulin staining, red color indicates glucagon staining.
PC activity in high glucose and palmitate 2-day treated islets
Although Figure 4 has demonstrated an association between PC activity level and blood glucose level, the causality remains unknown. We hypothesized that slightly increased blood glucose level might suppress PC activity, because in mildly hyperglycemic AyK-M mice islet PC activity seems to be reduced to 1.7-fold if comparing it with AyL mice (2.8-fold increased). To test this hypothesis and test what level of hyperglycemia can initially inhibit PC activity, we isolated the islets from 10 weeks old KK mice, and treated these islets with different concentrations of glucose for two days, and then measured PC activity. We used these mice because these mice are controls of AyK mice. As shown in Figure 5A, 11.1 mmol/l (equals to 200 mg/dl) glucose had no effect on islet PC activity; PC activity was initially suppressed by 12.5 mmol/l (225 mg/dl) glucose, and significantly reduced by 13.9 mmol/l (250 mg/dl) glucose. Interestingly, fasting blood glucose levels (Table 1) in AyK-M mice were consistent with these glucose concentrations. This result clearly demonstrated that mild hyperglycemia inhibits PC activity in KK mouse islets. To test if high fatty acids contribute to islet PC reduction, we treated the same islets with palmitate (up to 0.8 mmol/l, bound with BSA) for 2 days. Our date shown in Figure 5B indicate that 2-day palimtate treatment did not significantly inhibit islet PC activity.
Discussion
Most data regarding the importance of PC were obtained from rat pancreatic islets (Macdonald et al. 1996b; Macdonald et al. 1996a; Liu et al. 2002) or rat beta cell lines (Lu et al. 2002; Cline et al. 2004), few studies have tested islet PC activity in type 2 diabetic mice. In current study, we tried to observe whether islet PC activity is reduced in type 2 diabetic mice. As expected, islet PC activity and protein level were significantly reduced in severely diabetic AyK-S and db/db mice, and these results are consistent with those in type 2 diabetic rats (Macdonald et al. 1996b; Macdonald et al. 1996a) and human (Macdonald et al. 2009). Surprisingly, islet PC activity and protein concentration in mildly diabetic AyK-M mice were significantly increased (1.7-fold and 1.3-fold, respectively). As a distinct compensatory model of obesity, AyL mice have shown a higher level of islet PC activity (2.8-fold, Figure 3A) compared with C57BL/6 mouse islets. Thus the elevation of islet PC activity in AyK-M mice may be a portion of enhanced beta cell adaptation to insulin resistance; reduced islet PC activity and protein level may relate to beta cell failure in AyK-S and db/db mice (Figures 2 and 3), and this observation is consistent with previous reports (Macdonald et al. 1996b; Macdonald et al. 1996a; Macdonald et al. 2009). Our recent results (Xu et al. 2008) in PC up- and down-regulated beta cells support this hypothesis. Although a strong correlation between islet PC activity and hyperglycemia or beta cell failure was observed, this correlation, however, did not uncover the causality. In addition, whether PC regulates GSIS is still controversial; Jensen et al. have observed that Pc siRNA did not inhibit GSIS in beta cell line INS-1 cells, and they have suggested an acetyl carnitine mechanism that compensates for PC inhibition in these cells (Jensen et al. 2006).
We have known that obesity enhances insulin resistance (Shoelson et al. 2006; White 2003), the latter can slightly elevate blood glucose levels after a meal challenge comparing to non-insulin resistant individuals (Ahren et al. 1999; Bajaj et al. 2002). Once significant insulin resistance develops, elevated blood glucose is expected to be higher and last for a longer time (Bruning et al. 1997; Gabriely et al. 2002). Comparing to frank diabetes, however, this elevation in insulin resistant individuals is mild (Zhou et al. 1999; Kido et al. 2000). Based on this recognition, we tested whether mild hyperglycemia inhibits islet PC activity. We isolated the islets from KK mice and treated these islets with different concentrations of glucose, from physiological to diabetic levels. This in vitro study confirmed that mild high glucose (12.5 mmol/l, equals to 225 mg/dl) initially reduced islet PC activity (Figure 5A). This result helps us to interpret islet PC activity in AyK-M, AyK-S and db/db mice. First, in AyK-M mice, islet PC activity was preserved because blood glucose levels were lower than 250 mg/dl (Table 1; mean values were 214 mg/dl at 10 weeks of age and 232 mg/dl at 20 weeks of age). Second, assuming 2.8-fold increase of islet PC activity in AyL mice is a “normal level” for beta cell adaptation, 1.7-fold increase in AyK-M islets would be a “reduced level”. Because 12.5 mmol/l (equal to 225 mg/dl) glucose initially inhibited islet PC activity, a portion of elevated islet PC activity (the values from 1.7-fold to 2.8-fold) in AyK-M mice may be suppressed by mild hyperglycemia (232 ± 15 mg/dl, Table 1), thus only 1.7-fold rather than 2.8-fold increase in PC activity was observed in AyK-M islets. Third, based on the results shown in Figure 5A, if the levels of glycemia are elevated to or over 13.9 mmol/l (equal to 250 mg/dl), islet PC activity would be significantly inhibited. Because the average value of blood glucose in AyK-S mice was 315 mg/dl at 10 weeks of age or 357 mg/dl at 20 weeks of age (Table 1), islet PC activity may be significantly suppressed (Figure 2A) by higher levels of glycemia. Low islet PC activity in db/db mice (Figure 3A) may also be explained by the same mechanism described above. Fourth, because PC has been demonstrated to be important in beta cell adaptation to insulin resistance and in insulin secretion (Liu et al. 2002; Liu et al. 2005; Xu et al. 2008), inhibited islet PC activity might contribute to beta cell failure; thus severe hyperglycemia caused by beta cell failure would in turn suppress islet PC activity. This hypothesis is supported by the result shown in Figure 5A, where high glucose (16.7 or 19.4 mmol/l, equal to 300 or 350 mg/dl, respectively) significantly reduced islet PC activity; this may explain PC reduction in AyK-S and db/db mice (Table 1, Figure 2A, and Figure 3A). Although our data can partially explain the inhibition of PC activity by hyperglycemia in diabetic mouse islets, and this is consistent with previous report in rat islet research (Liu et al. 2004), however, the “real” mechanisms by which hyperglycemia inhibits PC activity are entirely unknown, thus further efforts are needed to reveal the “real” mechanisms.
Because high levels of free fatty acids (FFA) are also present in obese individuals (Liu et al. 2002; Unger 1995), which may be a factor to inhibit islet PC activity. Actually, Busch et al. (Busch et al. 2002) have found that treating mouse cancer cell line MIN6 cells with palmitate and oleate for 48h resulted in a 2.2- and 1.8-fold reduction in Pc mRNA, respectively. Our data, however, demonstrated that palmitate (up to 0.8 mmol/l) did not inhibit PC activity in 2-day treated KK mouse primary islets (Figure 5B), and these results were consistent with our previous observation in rat islets (Liu et al. 1999; Xu et al. 2006). Comparing to high glucose, FFA may not be a key factor to inhibit PC activity in 2-day treated mouse primary islets.
If AyL and db/db mice are considered as pre-diabetic and severely diabetic, respectively, diabetic states in AyK-M and AyK-S mice would be in the early middle and late middle stages of the pathogenesis of T2DM. The islet micrographs shown in Figure 4 clearly demonstrate that AyK-M and AyK-S mice stay on the way between pre-diabetes and severe diabetes: islet structure and staining density and distributions of insulin and glucagon in AyK-M mice are similar to those in AyL mice but slightly worse. In contrast, all these changes in AyK-S mice are similar to those in db/db mice but less severe. Importantly, PC activity levels were paralleled with beta cell function and mass during the pathogenesis of T2DM; strongly suggesting that PC is associated with insulin secretion and beta cell proliferation.
In summary, we found that islet PC activity and insulin secretion were increased in AyL and AyK-M mice and reduced in AyK-S and db/db mice; additionally islet morphological aberrations, a clear association between PC activity and hyperglycemia/beta cell function/mass has been demonstrated. An in vitro study has shown that islet PC activity was inhibited by high glucose but not by fatty acid palmitate. Our results suggest that increased islet PC activity might play an important role in beta cell adaptation to insulin resistance, and the reduction of islet PC activity in type 2 diabetic mice might be a consequence of mild hyperglycemia and a cause of severe hyperglycemia. We summarized our hypothesis in Figure 6. Having said that, the results in our current study would not be strong enough to draw a precise conclusion; beta cell Pc transgenic or knockout mouse models would be excellent tools for future studies. Because PC activity is sensitive to mild hyperglycemia indicated in this study, strictly control of blood glucose levels would be very important to prevent the onset of frank diabetes in the patients with pre-diabetes or mild T2DM.
Acknowledgments
This work was supported by grants from the National Institutes of Health (P20 RR/DE17702 from the COBRE Program of the National Center for Research Resources, and 1R01DK077624-01 from NIDDK), and the American Diabetes Association (Junior Faculty Award). This project was also supported by a grant (6931) from The Research Institute for Children, Children’s Hospital at New Orleans. We wish to thank Mr. Naeem Uddin for critical reading of the manuscript.
Abbreviations
- PC
Pyruvate carboxylase
- PDH
pyruvate dehydrogenase
- GSIS
glucose stimulated insulin secretion
- T2DM
Type 2 diabetes mellitus
- AyK
Agouti KK mice (KK background)
- AyL
Agouti L mice (C57BL/6 background)
Reference List
- Ahren B, Gudbjartsson T, Al Amin AN, Martensson H, Myrsen-Axcrona U, Karlsson S, Mulder H, Sundler F. Islet perturbations in rats fed a high-fat diet. Pancreas. 1999;18:75–83. doi: 10.1097/00006676-199901000-00010. [DOI] [PubMed] [Google Scholar]
- Bahl JJ, Matsuda M, DeFronzo RA, Bressler R. In vitro and in vivo suppression of gluconeogenesis by inhibition of pyruvate carboxylase. Biochem Pharmacol. 1997;53:67–74. doi: 10.1016/s0006-2952(96)00660-0. [DOI] [PubMed] [Google Scholar]
- Bajaj M, Berria R, Pratipanawatr T, Kashyap S, Pratipanawatr W, Belfort R, Cusi K, Mandarino L, DeFronzo RA. Free fatty acid-induced peripheral insulin resistance augments splanchnic glucose uptake in healthy humans. Am J Physiol Endocrinol Metab. 2002;283:E346–E352. doi: 10.1152/ajpendo.00329.2001. [DOI] [PubMed] [Google Scholar]
- Baverel G, Conjard A, Chauvin MF, Vercoutere B, Vittorelli A, Dubourg L, Gauthier C, Michoudet C, Durozard D, Martin G. Carbon 13 NMR spectroscopy: a powerful tool for studying renal metabolism. Biochimie. 2003;85:863–871. doi: 10.1016/j.biochi.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Brune B, Dimmeler S, Lapetina EG. NADPH: a stimulatory cofactor for nitric oxide-induced ADP-ribosylation reaction. Biochem Biophys Res Commun. 1992;182:1166–1171. doi: 10.1016/0006-291x(92)91854-j. [DOI] [PubMed] [Google Scholar]
- Bruning JC, Winnay J, Bonner-Weir S, Taylor SI, Accili D, Kahn CR. Development of a novel polygenic model of NIDDM in mice heterozygous for IR and IRS-1 null alleles. Cell. 1997;88:561–572. doi: 10.1016/s0092-8674(00)81896-6. [DOI] [PubMed] [Google Scholar]
- Busch AK, Cordery D, Denyer GS, Biden TJ. Expression profiling of palmitate- and oleate-regulated genes provides novel insights into the effects of chronic lipid exposure on pancreatic beta-cell function. Diabetes. 2002;51:977–987. doi: 10.2337/diabetes.51.4.977. [DOI] [PubMed] [Google Scholar]
- Cline GW, LePine RL, Papas KK, Kibbey RG, Shulman GI. 13C NMR isotopomer analysis of anaplerotic pathways in INS-1 cells. J Biol Chem. 2004;279:44370–44375. doi: 10.1074/jbc.M311842200. [DOI] [PubMed] [Google Scholar]
- DeFronzo RA, Prato SD. Insulin resistance and diabetes mellitus. J Diabetes Complications. 1996;10:243–245. doi: 10.1016/1056-8727(96)00046-3. [DOI] [PubMed] [Google Scholar]
- Dmitriev LF. Activity of key enzymes in microsomal and mitochondrial membranes depends on the redox reactions involving lipid radicals. Membr Cell Biol. 2001;14:649–662. [PubMed] [Google Scholar]
- Farfari S, Schulz V, Corkey B, Prentki M. Glucose-regulated anaplerosis and cataplerosis in pancreatic beta- cells: possible implication of a pyruvate/citrate shuttle in insulin secretion. Diabetes. 2000;49:718–726. doi: 10.2337/diabetes.49.5.718. [DOI] [PubMed] [Google Scholar]
- Gabriely I, Ma XH, Yang XM, Rossetti L, Barzilai N. Leptin resistance during aging is independent of fat mass. Diabetes. 2002;51:1016–1021. doi: 10.2337/diabetes.51.4.1016. [DOI] [PubMed] [Google Scholar]
- Gotoh M, Maki T, Satomi S, Porter J, Bonner-Weir S, O’Hara CJ, Monaco AP. Reproducible high yield of rat islets by stationary in vitro digestion following pancreatic ductal or portal venous collagenase injection. Transplantation. 1987;43:725–730. doi: 10.1097/00007890-198705000-00024. [DOI] [PubMed] [Google Scholar]
- Han J, Xu J, Epstein PN, Liu YQ. Long-term effect of maternal obesity on pancreatic beta cells of offspring: reduced beta cell adaptation to high glucose and high-fat diet challenges in adult female mouse offspring. Diabetologia. 2005;48:1810–1818. doi: 10.1007/s00125-005-1854-8. [DOI] [PubMed] [Google Scholar]
- Hasan NM, Longacre MJ, Stoker SW, Boonsaen T, Jitrapakdee S, Kendrick MA, Wallace JC, Macdonald MJ. Impaired anaplerosis and insulin secretion in insulinoma cells caused by small interfering RNA-mediated suppression of pyruvate carboxylase. J Biol Chem. 2008;283:28048–28059. doi: 10.1074/jbc.M804170200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Infante JP, Huszagh VA. Analysis of the putative role of 24-carbon polyunsaturated fatty acids in the biosynthesis of docosapentaenoic (22:5n-6) and docosahexaenoic (22:6n-3) acids. FEBS Lett. 1998;431:1–6. doi: 10.1016/s0014-5793(98)00720-0. [DOI] [PubMed] [Google Scholar]
- Jensen MV, Joseph JW, Ilkayeva O, Burgess S, Lu D, Ronnebaum SM, Odegaard M, Becker TC, Sherry AD, Newgard CB. Compensatory responses to pyruvate carboxylase suppression in islet beta-cells. Preservation of glucose-stimulated insulin secretion. J Biol Chem. 2006;281:22342–22351. doi: 10.1074/jbc.M604350200. [DOI] [PubMed] [Google Scholar]
- Jetton TL, Liu YQ, Trotman WE, Nevin PW, Sun XJ, Leahy JL. Enhanced expression of insulin receptor substrate-2 and activation of protein kinase B/Akt in regenerating pancreatic duct epithelium of 60 %- partial pancreatectomy rats. Diabetologia. 2001;44:2056–2065. doi: 10.1007/s001250100011. [DOI] [PubMed] [Google Scholar]
- Jonas JC, Sharma A, Hasenkamp W, Ilkova H, Patane G, Laybutt R, Bonner-Weir S, Weir GC. Chronic hyperglycemia triggers loss of pancreatic beta cell differentiation in an animal model of diabetes. J Biol Chem. 1999;274:14112–14121. doi: 10.1074/jbc.274.20.14112. [DOI] [PubMed] [Google Scholar]
- Khan A, Ling ZC, Landau BR. Quantifying the carboxylation of pyruvate in pancreatic islets. J Biol Chem. 1996;271:2539–2542. doi: 10.1074/jbc.271.5.2539. [DOI] [PubMed] [Google Scholar]
- Kido Y, Burks DJ, Withers D, Bruning JC, Kahn CR, White MF, Accili D. Tissue-specific insulin resistance in mice with mutations in the insulin receptor, IRS-1, and IRS-2. J Clin Invest. 2000;105:199–205. doi: 10.1172/JCI7917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjorholt C, Akerfeldt MC, Biden TJ, Laybutt DR. Chronic hyperglycemia, independent of plasma lipid levels, is sufficient for the loss of beta-cell differentiation and secretory function in the db/db mouse model of diabetes. Diabetes. 2005;54:2755–2763. doi: 10.2337/diabetes.54.9.2755. [DOI] [PubMed] [Google Scholar]
- Labarca C, Paigen K. A simple, rapid, and sensitive DNA assay procedure. Anal Biochem. 1980;102:344–352. doi: 10.1016/0003-2697(80)90165-7. [DOI] [PubMed] [Google Scholar]
- Liu YQ, Han J, Epstein PN, Long YS. Enhanced rat beta-cell proliferation in 60% pancreatectomized islets by increased glucose metabolic flux through pyruvate carboxylase pathway. Am J Physiol Endocrinol Metab. 2005;288:E471–E478. doi: 10.1152/ajpendo.00427.2004. [DOI] [PubMed] [Google Scholar]
- Liu YQ, Jetton TL, Leahy JL. beta-Cell adaptation to insulin resistance. Increased pyruvate carboxylase and malate-pyruvate shuttle activity in islets of nondiabetic Zucker fatty rats. J Biol Chem. 2002;277:39163–39168. doi: 10.1074/jbc.M207157200. [DOI] [PubMed] [Google Scholar]
- Liu YQ, Moibi JA, Leahy JL. Chronic high glucose lowers pyruvate dehydrogenase activity in islets through enhanced production of long chain acyl-CoA: prevention of impaired glucose oxidation by enhanced pyruvate recycling through the malate-pyruvate shuttle. J Biol Chem. 2004;279:7470–7475. doi: 10.1074/jbc.M307921200. [DOI] [PubMed] [Google Scholar]
- Liu YQ, Tornheim K, Leahy JL. Fatty acid-induced beta cell hypersensitivity to glucose. Increased phosphofructokinase activity and lowered glucose-6-phosphate content. J Clin Invest. 1998;101:1870–1875. doi: 10.1172/JCI1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YQ, Tornheim K, Leahy JL. Glucose-fatty acid cycle to inhibit glucose utilization and oxidation is not operative in fatty acid-cultured islets. Diabetes. 1999;48:1747–1753. doi: 10.2337/diabetes.48.9.1747. [DOI] [PubMed] [Google Scholar]
- Lu D, Mulder H, Zhao P, Burgess SC, Jensen MV, Kamzolova S, Newgard CB, Sherry AD. 13C NMR isotopomer analysis reveals a connection between pyruvate cycling and glucose-stimulated insulin secretion (GSIS) Proc Natl Acad Sci USA. 2002;99:2708–2713. doi: 10.1073/pnas.052005699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald MJ. Feasibility of a mitochondrial pyruvate malate shuttle in pancreatic islets. Further implication of cytosolic NADPH in insulin secretion. J Biol Chem. 1995a;270:20051–20058. [PubMed] [Google Scholar]
- Macdonald MJ. Influence of glucose on pyruvate carboxylase expression in pancreatic islets. Arch Biochem Biophys. 1995b;319:128–132. doi: 10.1006/abbi.1995.1274. [DOI] [PubMed] [Google Scholar]
- Macdonald MJ. Export of metabolites from pancreatic islet mitochondria as a means to study anaplerosis in insulin secretion. Metabolism. 2003;52:993–998. doi: 10.1016/s0026-0495(03)00149-5. [DOI] [PubMed] [Google Scholar]
- Macdonald MJ, Efendic S, Ostenson CG. Normalization by insulin treatment of low mitochondrial glycerol phosphate dehydrogenase and pyruvate carboxylase in pancreatic islets of the GK rat. Diabetes. 1996a;45:886–890. doi: 10.2337/diab.45.7.886. [DOI] [PubMed] [Google Scholar]
- Macdonald MJ, Longacre MJ, Langberg EC, Tibell A, Kendrick MA, Fukao T, Ostenson CG. Decreased levels of metabolic enzymes in pancreatic islets of patients with type 2 diabetes. Diabetologia. 2009;52:1087–1091. doi: 10.1007/s00125-009-1319-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald MJ, Tang J, Polonsky KS. Low mitochondrial glycerol phosphate dehydrogenase and pyruvate carboxylase in pancreatic islets of Zucker diabetic fatty rats. Diabetes. 1996b;45:1626–1630. doi: 10.2337/diab.45.11.1626. [DOI] [PubMed] [Google Scholar]
- Menendez J, Delgado J, Gancedo C. Isolation of the Pichia pastoris PYC1 gene encoding pyruvate carboxylase and identification of a suppressor of the pyc phenotype. Yeast. 1998;14:647–654. doi: 10.1002/(SICI)1097-0061(199805)14:7<647::AID-YEA269>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Nicholls LI, Ainscow EK, Rutter GA. Glucose-stimulated insulin secretion does not require activation of pyruvate dehydrogenase: impact of adenovirus-mediated overexpression of PDH kinase and PDH phosphate phosphatase in pancreatic islets. Biochem Biophys Res Commun. 2002;291:1081–1088. doi: 10.1006/bbrc.2002.6567. [DOI] [PubMed] [Google Scholar]
- Palmer ND, Langefeld CD, Campbell JK, Williams AH, Saad M, Norris JM, Haffner SM, Rotter JI, Wagenknecht LE, Bergman RN, Rich SS, Bowden DW. Genetic Mapping of Disposition Index and Acute Insulin Response Loci on Chromosome 11q: The Insulin Resistance Atherosclerosis Study (IRAS) Family Study. Diabetes. 2006;55:911–918. doi: 10.2337/diabetes.55.04.06.db05-0813. [DOI] [PubMed] [Google Scholar]
- Sheline CT, Choi DW. Neuronal death in cultured murine cortical cells is induced by inhibition of GAPDH and triosephosphate isomerase. Neurobiol Dis. 1998;5:47–54. doi: 10.1006/nbdi.1998.0177. [DOI] [PubMed] [Google Scholar]
- Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest. 2006;116:1793–1801. doi: 10.1172/JCI29069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unger RH. Lipotoxicity in the pathogenesis of obesity-dependent NIDDM. Genetic and clinical implications. Diabetes. 1995;44:863–870. doi: 10.2337/diab.44.8.863. [DOI] [PubMed] [Google Scholar]
- Wallace JC. Pyruvate carboxylase. In: Keech DB, Wallace JC, editors. CRC Series in Enzyme Biology. Boca Raton: CRC Press; 1985. pp. 5–63. [Google Scholar]
- White MF. Insulin Signaling in Health and Disease. Science. 2003;302:1710–1711. doi: 10.1126/science.1092952. [DOI] [PubMed] [Google Scholar]
- Xu J, Han J, Epstein PN, Liu YQ. Regulation of PDK mRNA by high fatty acid and glucose in pancreatic islets. Biochem Biophys Res Commun. 2006;344:827–833. doi: 10.1016/j.bbrc.2006.03.211. [DOI] [PubMed] [Google Scholar]
- Xu J, Han J, Long YS, Epstein PN, Liu YQ. The role of pyruvate carboxylase in insulin secretion and proliferation in rat pancreatic beta cells. Diabetologia. 2008;51:2022–2030. doi: 10.1007/s00125-008-1130-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou YP, Berggren PO, Grill V. A fatty acid-induced decrease in pyruvate dehydrogenase activity is an important determinant of beta-cell dysfunction in the obese diabetic db/db mouse. Diabetes. 1996;45:580–586. doi: 10.2337/diab.45.5.580. [DOI] [PubMed] [Google Scholar]
- Zhou YP, Cockburn BN, Pugh W, Polonsky KS. Basal insulin hypersecretion in insulin-resistant Zucker diabetic and Zucker fatty rats: role of enhanced fuel metabolism. Metabolism. 1999;48:857–864. doi: 10.1016/s0026-0495(99)90219-6. [DOI] [PubMed] [Google Scholar]
- Zhou YP, Ostenson CG, Ling ZC, Grill V. Deficiency of pyruvate dehydrogenase activity in pancreatic islets of diabetic GK rats. Endocrinology. 1995;136:3546–3551. doi: 10.1210/endo.136.8.7628391. [DOI] [PubMed] [Google Scholar]