Abstract
We found that certain mid-range consumer level digital SLR cameras using full-frame CMOS sensors outperform x-ray film in acquiring signals from immunoblots that use enhanced chemiluminescence for detection. These cameras exhibit a sensitivity comparable to x-ray film, yet provide a 3-fold increase in linear dynamic range and substantial cost saving over time, are more convenient to use, and eliminate the chemical waste associated with processing film.
Keywords: camera, chemiluminescence, CMOS, dot blot, ECL, imaging, immunoblot, SLR, Western blot, x-ray film
Due to its high sensitivity, ECL (enhanced chemiluminescence) is currently the dominant signal-generating method for immuno-detection of proteins via Western blots and dot blots [1]. Signal acquisition is typically accomplished either with x-ray film or specialized cameras that employ cooled CCD (charge-coupled device) sensors. X-ray film is more popular because of its greater sensitivity and/or resolution for a given exposure time, and because it requires only a modest expenditure for equipment. The main drawbacks of film are its limited and non-linear dynamic range, and the fact that automated film processors are expensive to maintain and generate chemical waste. Dedicated CCD-based imagers exhibit a significantly better dynamic range and linearity than film and do not generate chemical waste, but their combination of sensitivity and pixel resolution are generally inferior to film. Moreover, the cost of these imagers ($30-60k) is out of the range of many academic research labs.
Several new single lens reflex (SLR) digital cameras in the mid-range consumer market have been shown to achieve remarkable low-light sensitivity using high resolution full-frame 35 mm sensors based on CMOS (complementary metal oxide semiconductor) technology in combination with advanced noise-reduction firmware. Here we report a comparison of one such camera vs x-ray film for acquiring ECL signals from dot blots and Western blots.
In our first test we spotted serial dilutions (in triplicate) of horseradish peroxidase-coupled antibody (HRP-Ab) directly onto two separate, but identically treated, nitrocellulose membranes. The membranes were developed with a commercial ECL reagent kit. One was imaged by a 4.0 min exposure to a Nikon D700 SLR camera fitted with a 50 mm f/1.4 lens and 12 mm extension tube, and the other by a 4.0 min exposure to x-ray film. To process the SLR image, the resulting 14-bit RAW file (produced in parallel with an 8-bit JPEG file) was opened in the Camera RAW application that is included with Adobe Photoshop CS3. Exposure and Black levels were optimized and the image was then ported into the main Photoshop application where the blue light channel only was used to create a grayscale white-on-black TIFF. The TIFF was opened in the NIH ImageJ application for quantitative analysis of dot densities. A reversed (black-on-white) version of the TIFF (Fig. 1a) was also generated in Photoshop for visual comparison to the x-ray film. To digitize the film image, it was photographed with trans-illumination using the same D700 SLR camera. The resulting 14-bit RAW image was opened in Camera RAW and the default Exposure and Black level settings were accepted prior to importing the image into the main Photoshop program. The film image was then converted to a grayscale TIFF (with no color filtering) (Fig. 1b), and also to a reversed (white-on-black) version of the same TIFF for quantitative analysis in ImageJ. The image processing procedures outlined above were designed to achieve maximum dynamic range and linearity of the integrated density data and are described in more detail in the on-line supplement to this paper.
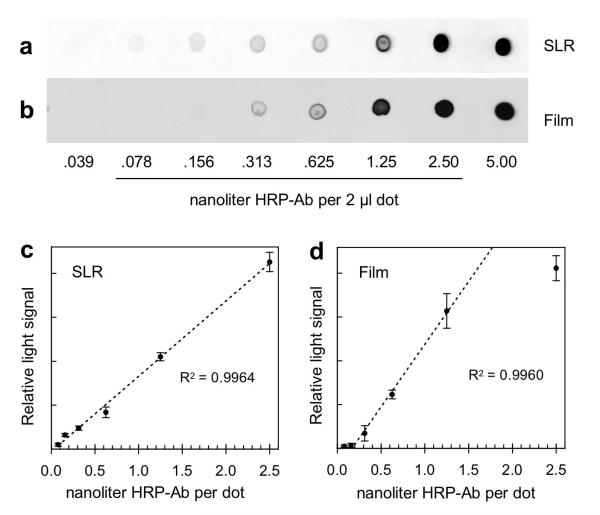
Fig. 1.
Comparison of dot blot data obtained with the D700 SLR camera vs x-ray film. Serial dilutions of HRP-Ab were spotted onto nitrocellulose and processed for ECL as described in Methods. The ECL signals were recorded in 4.0 min exposures using either the D700 SLR camera (a,c) or x-ray film (b,d). (a,b) Representative dot blot images obtained with the camera and x-ray film, respectively. These are black-on-white versions of the same TIFFs used for the ImageJ analyses that generated the plots in panels c and d. The underlined dilution numbers indicate the range of data that were plotted. The highest dilution (0.039 nanoliter of HRP-Ab) was not plotted because it was below the limit of detection in both sets of dot blots. The lowest dilution (5.0 nanoliter of HRP-Ab) was not plotted because the images were oversaturated in both sets of dot blots. (c) Plot of ECL signal vs amount of HRP-Ab recorded with the camera. Each point is the average signal of three blank-corrected triplicate dots, with standard deviations indicated by error bars. The digital RAW image from the camera was processed for maximum dynamic range (as described in Methods), and the resulting TIFF was analyzed by ImageJ. (d) Plot of ECL signal vs amount of HRP-Ab recorded on x-ray film. Each point is the average signal of three blank-corrected triplicate dots, with standard deviations indicated by error bars. The film was digitized using the SLR camera with trans-illumination and the resulting RAW image was converted to a white-on-black TIFF for analysis by ImageJ. The regression coefficient (R2) shown in panel c is based on a fit to all 6 data points relative to the dashed line; this was generated by the linear trend line function in Microsoft Excel. The same applies to panel d, except that the lowest and highest points in that plot were excluded from the calculation.
The greater dynamic range of the SLR camera compared to x-ray film is apparent by comparing dots containing 0.156 and 1.25 nanoliter of HRP-Ab in Figs. 1a and 1b. At 1.25 nanoliters, the film image appears darker than does the SLR image. In contrast, the 0.156 nanoliter dot from the SLR appears darker than the corresponding dot on the x-ray film. These subjective impressions were confirmed by ImageJ density analysis. The SLR camera produced a plot of integrated density signal vs amount of HRP-Ab spotted that was linear over a 32-fold range (.078-2.50 nanoliters) of HRP-Ab (Fig. 1c). In contrast, the x-ray film produced a plot that was markedly sigmoidal, with a linear range of only 10-fold (.156~1.50 nanoliters) (Fig. 1d).
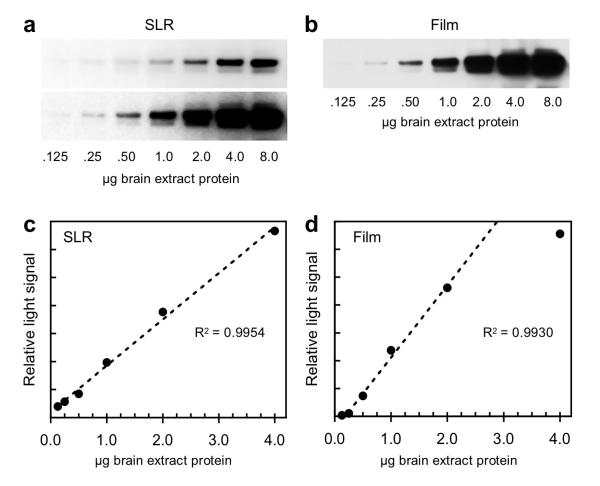
A second test of ECL image acquisition with the D700 SLR vs x-ray film was carried out on a Western blot that employed a series of dilutions of mouse brain extract immunostained for CRMP2 (collapsin response mediator protein 2). In this experiment, the same blot was imaged on film for 20 sec (Fig. 2b), and a few minutes later, with the D700 SLR for 60 sec (Fig. 2a). A RAW to TIFF conversion of the SLR image file (Fig. 2a, upper panel) was carried out as described above and then analyzed with ImageJ to generate a plot of signal intensity vs protein load (Fig. 2c). Similarly, a TIFF file of the corresponding x-ray film image was generated as above and used to create the plot shown in Fig. 2d. The SLR data were linear over a 32-fold range of protein load (.125-4.0 μg), while the film data were linear over a 10-fold range (0.25~2.5 μg).
Fig. 2.
Comparison of Western blot data obtained with the SLR camera vs x-ray film. Serial dilutions of mouse brain extract were subjected to SDS-PAGE and Western blotting for CRMP2 by ECL as described in Methods. The same blot was used in all four panels. It was first exposed to x-ray film for 20 sec, and approximately 5 min later was imaged with the camera in a 60 sec exposure. (a) Upper panel shows an image file recorded by the camera and processed for maximum dynamic range for the plot shown in panel c, while the lower panel shows a contrast-enhanced JPEG version of the same exposure. (b) Digitized x-ray film image used to generate the plot in panel d. (c) Plot of ECL signal vs amount of brain extract recorded with the camera. (d) Plot of ECL signal vs amount of brain extract recorded on x-ray film. The regression coefficient (R2) shown in panel c is based on a fit to all 6 data points relative to the dashed line; this was generated by the linear trend line function in Microsoft Excel. The same applies to panel d, except that the lowest and highest points in that plot were excluded from the calculation.
For higher protein loads (0.50-8.0 μg), the SLR TIFF image showed less intense bands than the corresponding film image. This is due to desaturation applied to the SLRs RAW file using the exposure control available in the Camera RAW application. A contrast-enhanced JPEG image of the same SLR exposure is shown in the bottom panel of Fig. 2a. This image closely resembles that of the x-ray film (Fig. 2b), but like the film, it has a reduced range of linearity. For qualitative comparison or detection of antigen bands, enhanced JPEGs from the SLR are advantageous.
The camera used in our study has adjustable ISO (light sensitivity) settings that range from 100-25,600. To see how light sensitivity and overall image quality are interrelated, we took a series of 1.0 minute exposures of a single Western blot (similar to that shown in Fig. 2) at four different ISO settings (800, 1600, 6400, and 25,600) from the high end of the available range. The results are shown in the on-line supplement (Fig. S2-1). ISO 25,600 was most sensitive by a small margin, but 6400 gave the best overall ratio of signal to background. We were surprised to find that a qualitatively useable image could be obtained even at an ISO setting as low as 800.
We have demonstrated that a moderately priced digital SLR camera designed for the mid-range consumer/professional photography market is comparable to x-ray film in terms of sensitivity, and is clearly superior in dynamic range. Dedicated CCD-based imagers with similar performance have been available for several years but their cost is typically 10-15 times that of the type of camera used here. The key factors in successful application of this technology are (1) a camera with minimal sensor noise at high ISO settings, (2) a wide aperture lens and short subject to camera distance to maximize light capture, (3) effective noise reduction firmware in the camera, and (4) the ability to capture and process RAW images. We presume there are several digital SLRs on the market that can be used to record ECL signals with good sensitivity and integrity, but cameras with equivalent high ISO settings alone will not all produce equally good images. Sensor design and noise reduction firmware vary between brands and models, and these factors play an important role in image quality, especially at the higher ISO settings.
A sometimes forgotten disadvantage of imaging blots on x-ray film is the cost of maintaining and servicing processors that are often used to develop the film. In our recent experience, this has averaged $4,700 per year per machine. Over a five-year period, replacing one film processor with a $3,500 digital camera/lens setup is expected to generate a net savings of about $20,000, not including the extra savings in reduced x-ray film purchase. As our university campus shifts from film to SLR cameras for ECL, we also expect to eliminate 70-80% of the 1,000 gal per year of silver-laden hazardous waste generated in the form of spent fixer and save significant amounts of water that continuously pass though these machines when they are on. The elimination of x-ray film will also decrease the need for darkrooms. Although we currently use a darkroom to capture ECL signals with the SLR camera, it would be easy to make a portable light-tight enclosure (similar to those that house dedicated CCD imagers) so that image acquisition can be carried out on the bench of a normally lit laboratory. Moreover, some digital SLR cameras in this performance class can be connected directly to a computer, either by USB cable or wireless adapter, allowing remote control of the camera settings as well as rapid image download.
Supplementary Material
Acknowledgements
This work was supported by NIH grant NS17269 and funds from the Faculty Research and Travel Committee, School of Biological Sciences, UC Irvine. D.W.A. would like to thank Mr. Chris Benes for initially suggesting that recent advances in digital camera technology might provide the sensitivity needed to image the ECL reaction, and we all thank Ms. Kelsey Goodman and Mr. Kyle Horvath for preparing Western blots used in an initial “proof of concept” experiment.
Abbreviations
- CCD
charge coupled device
- CMOS
complementary metal oxide semiconductor
- CRMP2
collapsin response mediator protein 2
- ECL
enhanced chemiluminescence
- HRP
horse radish peroxidase
- SLR
single lens reflex
Footnotes
None of the authors have any financial interests or business relationships with any of the companies whose products are mentioned in this report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Dickinson J, Fowler SJ. Quantification of proteins on western blots using ECL. In: Walker JM, editor. The Protein Protocols Handbook. 2nd Edition Humana Press; New Jersey: 2002. pp. 429–437. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.