Abstract
Apolipoproteins are the protein components of lipoproteins that have the innate ability to inter convert between a lipid-free and a lipid-bound form in a facile manner, a remarkable property conferred by the helix bundle motif. Composed of a series of four or five amphipathic α-helices that fold to form a helix bundle, this motif allows the en face orientation of the hydrophobic faces of the α-helices in the protein interior in the lipid-free state. A conformational switch then permits helix-helix interactions to be substituted by helix-lipid interactions upon lipid binding interaction. This review compares the apolipoprotein high resolution structures and the factors that trigger this switch in insect apolipophorin III and the mammalian apolipoproteins, apolipoprotein E and apolipoprotein A-I, pointing out the commonalities and key differences in the mode of lipid interaction. Further insights into the lipid bound conformation of apolipoproteins are required to fully understand their functional role under physiological conditions.
Keywords: apolipophorin, apolipoprotein, lipophorin, lipoprotein, protein structure, cholesterol, diacylglycerol, triacylglycerol
1. Introduction
Lipoproteins are the primary vehicles responsible for the transport of hydrophobic material through the aqueous circulatory system between different sites in animals. They are large macromolecular complexes of lipids and proteins that exist in a wide dynamic range of particle size, density and composition. Lipoproteins are typically composed of a hydrophobic core of neutral lipids surrounded by a monolayer of amphipathic proteins and lipids on the surface. Proteins are embedded in the lipid environment for optimal packing and solubility. Apolipoproteins, the protein component of lipoproteins, serve a structural role to maintain the integrity and stability of the lipoprotein as they go through a dynamic remodeling process. Larger (lower density) lipoproteins require the presence of large integral proteins (75–500 kDa) without which the particle loses its integrity. However, the transport of hydrophobic material between different sites is often facilitated by the smaller (5–40 kDa) exchangeable apolipoproteins. The physiological, structural and biochemical aspects of different types of exchangeable apolipoproteins from several animal species have been studied in detail for several years, for example, apolipophorin III (apoLp-III), apolipoprotein A-I (apoA-I), apoA-II, apoA-IV, apoA-V, apoC-I, apoC-II, apoC-III, apoD, and apoE (see Jonas and Phillips (2008) for a recent review of lipoprotein structure and composition). Yet, the molecular mechanisms involved in their lipid binding ability and exchangeability are emerging only now, in part due to the availability of high-resolution structural information and biophysical data for these proteins. In this review, we compare the structural basis for lipid interaction of the insect apolipoprotein (known as apolipophorin) apoLp-III, with vertebrate apoE and apoA-I.
2. Lipid transport and lipoprotein metabolism
Remarkably, regardless of their source, an underlying theme that is critical for the exchangeable nature of apolipoproteins is their ability to superficially and reversibly associate with the lipoprotein particles depending on the structural status and physiological need of lipids. Insect hemolymph (blood) contains large numbers of lipoprotein particles, which are termed lipophorins. They are composed of two (non-exchangeable) structural apolipoproteins, apolipophorin (apoLp) -I and -II, which are responsible for the packing of diacylglycerol (DG), hydrocarbons and phospholipids, and maintaining lipoprotein integrity and solubility (Smolenaars et al., 2005). A third apolipophorin, apoLp-III, is found in certain insects that rely heavily on lipids to fuel flight activity (Van der Horst, 1990; Ryan 1990; Soulages and Wells, 1994a).
The role of apoLp-III in lipid transport processes has been well documented in the locust Locusta migratoria (Orthoptera) and tobacco hornworm Manduca sexta (Lepidoptera) (Ryan and Van der Horst, 2000). ApoLp-III is a crucial factor in the transport of large amounts of DG to the energy-demanding flight muscles. Present abundantly in the hemolymph of resting insects, the majority of apoLp-III occurs in the lipid-free state. However, the protein has the ability to rapidly respond to changes in lipophorin lipid composition, during which time these insect lipoproteins aid in the transport of DG. Lipid reserves are stored as triacylglycerol (TG) in the fat body, a tissue carrying out metabolic processes similar to liver and adipose tissue in vertebrates. During insect flight, adipokinetic hormones (AKH) are released by a small endocrine gland (corpus cardeacum), shifting the energy balance from carbohydrates, predominantly trehalose (a disaccharide), to free fatty acids to satisfy the highly increased energy demands during flight. AKH trigger an intracellular response in fat body cells, resulting in the hydrolysis of TG by a specific lipase and the appearance of DG (Van der Horst et al., 2001). At cell surface binding sites in the fat body, DG is transferred to a pre-existing lipophorin, known as high-density lipophorin (HDLp) named for its density characteristics in flotation ultracentrifugation protocol (Beenakkers et al., 1988). The transfer of lipids to HDLp is facilitated by lipid transfer particle, a multiunit high molecular weight protein (Ryan et al., 1988, 1990; Van Heusden and Law, 1989). Increase of the lipophorin DG content triggers apoLp-III binding to ensure the structural integrity of the expanding particle, resulting in the formation of low density lipophorin (LDLp). Up to 16 molecules of apoLp-III can be found on the LDLp surface (Wells et al., 1987; Van der Horst et al., 1991), the binding of which increases the lipoprotein diameter from 17 nm to ~ 30 nm (Van Antwerpen et al., 1988). DG hydrolysis at the flight muscles by lipoprotein lipase releases apoLp-III from the lipophorin surface and leads to reformation of HDLp. Both apoLp-III and HDLp can then be used for subsequent cycles of DG transport, thereby acting as a reusable lipoprotein shuttle. The reversible association of apoLp-III to the lipophorin surface is the key feature of the function of this apolipoprotein. Unlike insects, mammals have a more complex lipoprotein pattern and metabolism. Of direct relevance to this review, is the issue of the variety of exchangeable apolipoproteins that are present in mammals. Thus, while insects have apoLp-III as the only identified exchangeable apolipoprotein, mammals have a variety of these proteins, of which we will discuss only apoE and apoA-I. In the plasma of mammals, specifically in humans, apoE is primarily located on TG-rich chylomicron remnants, very low density lipoproteins (VLDL), intermediate density lipoproteins and a sub-class of high density lipoproteins (HDL), while in the brain and cerebrospinal fluid it is localized on HDL-sized particles, the major lipoprotein class identified in the central nervous system (CNS) (Fagan et al., 1999). Thus, in contrast to apoLp-III, which is found predominantly in LDLp, apoE is distributed on a wide variety of lipoprotein classes that vary in density, particle diameter, protein and lipid composition. This presents a scenario in which the dynamics of inter-conversion of the lipoproteins in the mammalian system involves complex apoE association-dissociation characteristics. It can therefore be envisaged that the inter-conversion is regulated by a variety of factors, with changes in lipid composition being a significant factor. ApoE-null mice display massive accumulation of TG-rich lipoproteins and extraordinarily high plasma levels of cholesterol (Zhang et al., 1992; Plump et al., 1992), while transgenic mice overexpressing apoE display a marked resistance to developing high plasma cholesterol levels when maintained on a high-cholesterol diet (Shimano et al., 1992). These observations are directly attributed to the critical role of apoE in maintaining plasma cholesterol and TG homeostasis, and in extracellular cholesterol and TG transport. In this manner, apoE is analogous to apoLp-III that plays a central role in DG transport and metabolism in insects (Van der Horst et al., 2002). As the lipoprotein particles are being remodeled, it is believed that apoE transitions between the different lipoprotein classes, illustrating its exchangeable character.
In humans apoE exists as three isoforms that are encoded by a polymorphic gene on chromosome 19 (Das et al., 1985). The three alleles APOE ε2, ε3 and ε4 occur with frequencies of ~8, 77 and 15%, respectively in the human population. The occurrence of apoE isoforms is unique to humans among mammals. The three isoforms, apoE2, apoE3 and apoE4 vary in the amino acids at positions 112 and 158, with apoE2 bearing Cys and apoE4 bearing Arg at these locations. ApoE3 has a Cys and an Arg, respectively, at these locations. It is believed that APOE ε4 is the ancestral gene in primates, an inference based on its sequence similarity with primate APOE (Fullerton et al., 2000). APOE ε3 is believed to have evolved from APOE ε4 recently (Finch and Stanford, 2004), plausibly as a selection for ‘meat-adaptive genes’ as a result of changes in the diet from a predominantly plant-based (i.e., low cholesterol) to a meat-based (i.e. lipid-rich, high cholesterol, high TG) diet. In concurrence, apoE3 is generally considered as an anti-atherogenic protein, while apoE4 is associated with risk for cardiovascular disease. In addition, apoE4 is considered a risk factor for Alzheimer’s disease. APOE ε2 appears to have arisen from APOE ε3. Subjects homozygous for the APOE ε2 allele are at a high risk for developing type III hyperlipoproteinemia and premature atherosclerosis.
ApoA-I is the main protein component of HDL and mediates many essential functions: 1) it acts as the major structural component of HDL; 2) it interacts with the ATP-binding cassette transporter A1 (ABCA1) to mediate efflux of phospholipid and cholesterol from cells; 3) it interacts with the plasma enzyme lecithin:cholesterol acyltransferase (LCAT) to convert free cholesterol to cholesteryl ester; and 4) it binds to the scavenger receptor class B type I (SR-BI) to mediate transport of HDL-derived cholesteryl ester into the cell. Although these functions seem diverse, they are all working together to generate and stabilize HDL in the plasma as part of the reverse cholesterol transport hypothesis (Yokoyama, 1998). In this hypothesis, lipid-free apoA-I interacts with the ABCA1 transporter on the cell surface and the ABCA1 donates phospholipid and free cholesterol to apoA-I generating a discoidal immature HDL particle. Although highly controversial, it is believed that ABCA1 acts as a phospholipid translocase, flopping phospholipid (predominantly phosphatidylcholine) to the outer leaflet, creating an excess of phospholipid that may partially detach or bulge. ApoA-I can then interact with the loosely associated phospholipid, removing it from the plasma membrane and generating a phospholipid:apoA-I complex. This partially lipidated apoA-I (also termed pre-β HDL) represents the model discoidal complexes that we use in functional assays. The pre-β HDL can now act as a hydrophobic lipid acceptor and acquire more cholesterol from the cell surface through non-specific binding (Wang and Rader, 2007). LCAT can interact with pre-β HDL converting free cholesterol to cholesteryl ester, generating a more hydrophobic lipid that will reside in the core of the HDL particle. Through these continued and concerted actions, a larger spherical HDL will be generated and this particle is extremely stable and represents the bulk of HDL found in the plasma. HDL generation through this mechanism can occur at the liver or in the periphery (cells from all other parts of the body excepting the liver and intestine). It is then supposed that HDL thus generated would bring cholesterol from the peripheral cells to the liver (therefore termed reverse cholesterol transport) as a means of regulating total body cholesterol. The cholesteryl ester can be delivered directly to the liver by the receptor SR-BI or indirectly after being transferred to other lipoproteins. Phospholipid transfer protein and cholesteryl ester transfer protein help dynamically modify the coat (phospholipid) and core (cholesteryl ester) components of HDL in an effort to promote turnover and transport of cholesterol. As well, HDL becomes an acceptor and carrier of hydrophobic lipids (including bioactive, oxidized lipids) and bioactive proteins (protease inhibitors, lipases, inflammatory markers, complement factors involved in innate immunity). For this reason, HDL has been ascribed numerous functions beyond cholesterol delivery (Feig et al., 2008). ApoA-I, by generating and stabilizing HDL, becomes an essential regulator of total body cholesterol metabolism and therefore also heart disease (Marcel and Kiss, 2003).
Lipoprotein particles are in a state of dynamic interconversion, constantly changing their size and lipid composition. This requires a class of proteins that responds rapidly to changes in lipid surface packing in order to maintain lipoprotein integrity. The exchangeable apolipoproteins are ideal candidates for this role. However, there are subtle variations in the specific role played by each of the apolipoproteins in lipid transport processes. The flexible structure of the exchangeable apolipoproteins such as apoLp-III, apoE, and apoA-I, provide the framework to fulfill this role.
3. Helix bundle architecture: A signature motif of lipid free exchangeable apolipoproteins
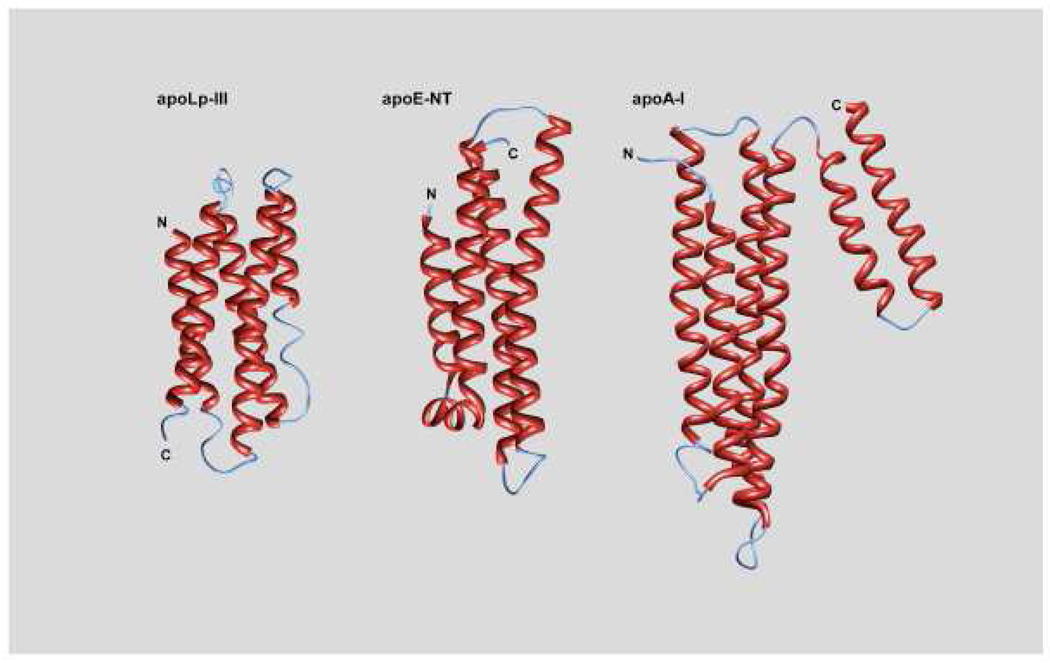
Analyzing the lipid binding property from a structural perspective of a limited number of crystallized exchangeable apolipoproteins, it can be seen that there is a remarkable similarity amongst them with a helix bundle molecular fold being a signature feature in the lipid-free state. High-resolution structural information is available for L. migratoria apoLp-III (PDB ID 1AEP and 1LS4), M. sexta apoLp-III (PDB ID 1EQ1) (Breiter et al., 1991; Fan et al., 2003a; Wang et al., 2002), the N-terminal domain (residues 1–191) of all three major human apoE isoforms (1LE2, Wilson et al., 1994; 1LPE, Wilson et al., 1991; 1LE4, Dong et al., 1996), mouse apoE (1AY9, Hatters et al., 2005a) and human apoA-I (2A01, Ajees et al., 2006). As predicted from their sequence, these proteins (and other exchangeable apolipoproteins for which high resolution structural information is not available), are characterized by an abundance of amphipathic α-helices interrupted by short loops or turns. The helices are arranged in a simple up-and-down topology commonly found in other helix bundle proteins not related to lipid transport function (Kohn et al., 1997). For the most part, the hydrophobic side chains are buried in the protein interior, while most polar and charged residues are exposed on the protein surface. This bundle motif is a stable arrangement that allows the protein to exist in an aqueous environment such as the blood and the hemolymph. Despite the stability associated with this state, the lipid-free apolipoproteins are relatively more prone to proteolytic degradation than their corresponding lipid-bound state. While the overall helix bundle fold is the theme in all the apolipoprotein structures identified so far, there are small but significant variations amongst them, most noticeably the number of helices in the helix bundle, i.e. four or five, and the presence and location of a short helix. Looking down the helix bundle from one end, it can be seen that the helices in a 5-helix bundle have a narrower hydrophobic phase (each subtends an angle of 72°), compared to the 4-helix bundles, these helices of which subtend an angle of 90°. Thus, the helices in a 5-helix bundle have a narrower hydrophobic phase, which may have implications for protein folding and lipid binding. In this section, we discuss the structural details of these proteins in some detail.
L. migratoria and M. sexta are evolutionarily divergent insects and apoLp-III from these species (~165 amino acid residues, protein mass ~ 18 kDa) shares very little amino acid sequence similarity. However, their three-dimensional structures are strikingly similar and are composed of a bundle of five amphipathic α-helices (numbered 1 to 5) connected by short loops. In addition, they bear significant functional resemblance and mode of lipid interaction, as demonstrated by studies using hybrid systems, in which L. migratoria apoLp-III was able to associate with M. sexta lipophorin (Van der Horst, 1988; Chetty et al., 2003a). The dimensions of L. migratoria apoLp-III are 53 × 22 Å (length × width), with a helix length of 23–31 residues (Figure 1, left). M. sexta apoLp-III has similar dimensions and bears an additional short helix situated between helix 3 and helix 4, roughly perpendicular to the helix bundle axis (not shown). NMR solution studies of L. migratoria apoLp-III suggest the presence of a putative 4-residue helix situated between helix 4 and helix 5 not originally identified in the X-ray structure. The 5-helix bundle protein is monomeric in solution (Ryan et al., 1993; Weers et al., 1994), but dimers were observed after deletion of two helices in apoLp-III from the greater wax moth Galleria mellonella, which is closely related to M. sexta; the dimerization was possibly due to the compensation for the exposure of hydrophobic surfaces in the 3-helix mutant proteins (Dettloff et al., 2001). M. sexta apoLp-III is relatively rich in positively and negatively charged residues (25 Lys/Arg and 28 Glu/Asp), while the content of these residues is much less in L. migratoria apoLp-III (10 Lys/Arg and 18 Glu/Asp). ApoLp-III from most Orthoptera species contains N-linked carbohydrate chains. In L. migratoria apoLp-III the two glycosylation sites were occupied by complex structures with carbohydrate linked 2-aminoethylphosphonate (Hård et al., 1993). NMR studies of bacterially-expressed recombinant L. migratoria apoLp-III (i.e. devoid of N-linked carbohydrates) indicate that the protein backbone is structurally almost indistinguishable from insect derived glycosylated protein (Fan et al., 2003; Weers et al., 2000a). Most Lepidopteran apoLp-III does not appear to be glycosylated, and the function of carbohydrates in apoLp-III remains unknown.
Figure 1. Helix bundle structure of lipid-free apolipoproteins.
From left to right: apoLp-III from L. migratoria, N-terminal domain of human apoE3 (residues 1–191) and full length human apoA-I.
In contrast to insect apoLp-III, mammalian apoE is 299 residues long, with the structure organized as two domains: an N-terminal (NT) and a C-terminal (CT) domain, comprising residues 1–191 and 201–299, respectively (Weisgraber, 1994; Hatters et al., 2006). The two domains are linked by a protease-sensitive linker loop. The NT domain is made up of a series of amphipathic α-helices that adopt a helix bundle fold (Figure 1, middle). The helices (numbered 1 to 4) are longer than those of the insect counterpart, giving the apoE NT domain a dimension of 65 × 24 Å (length × width). A 5th short helix (termed helix 1′, containing 9 residues, position 44–53) links helices 1 and 2 in apoE, similar to a short helix connecting helix 3 and helix 4 in M. sexta. The elongated helix bundle structure is stabilized by a predominance of hydrophobic interactions contributed by leucine and valine side chains. In addition, there are abundant salt bridges that occur within helices and between neighboring helices. These interactions in the helix bundle interior confer a higher degree of stability compared to the insect apolipoproteins, as confirmed by the high concentration of chaotropic agent required to unfold this domain (discussed in more detail in Section 5).
A defining functional feature of apoE not observed in apoLp-III or apoA-I is its ability to serve as a high-affinity ligand for the cell surface localized lipoprotein super family of receptors including the low density lipoprotein (LDL) receptor (LDLr) and related members such as LDLr related protein (LRP). This is a major factor in its role as an anti-atherogenic protein as it facilitates cellular binding and uptake of the entire lipoprotein molecule (with the bound apoE), thereby lowering plasma cholesterol levels. Located on the 4th helix and its vicinity in the NT domain, the LDLr binding site is characterized by an abundance of positively charged residues that interact with acidic residues on the LDLr. Helix 4 also bears sites for interaction with other members of the lipoprotein receptor family (Herz and Bock, 2002) and to cell surface localized heparan sulfate proteoglycans, which are rich in negatively charged moieties (Saito et al., 2003; Libeu et al., 2001). A similar apolipophorin ligand that can mediate the uptake and internalization of lipophorin particles in insect species is yet to be identified. Nevertheless, “swapping” residues 131 to 151 from helix 4 of apoE NT domain for residues 146 to 166 of helix 5 from M. sexta apoLp-III, conferred LDLr binding competency to the chimeric apolipoprotein “apoLp-IIIE” (Kiss et al., 2003). This observation suggests that the minimal required residues for receptor binding are contained within this receptor-binding domain, and that the receptor active conformation can be maintained within the context of a model apolipoprotein. A lipophorin receptor (HDLp receptor) has been identified in the fat body of L. migratoria (Dantuma et al., 1999; Van Hoof et al., 2002). It appears to belong to the lipoprotein receptor super family of proteins, and the corresponding ligand is HDLp, which resembles human LDL (for recent review see Van der Horst et al., 2009).
Despite knowledge of the structural aspects of the NT domain for over 15 years, the organization of the CT domain of apoE remains largely unknown. Our current understanding of this domain comes from in silico secondary structure predictions, circular dichroism and fluorescence spectroscopic analyses (Segrest et al., 1992, 1994; Choy et al, 2003). The terminal helix, encompassing residue 266–289, appears to form a G* helix, with a characteristic random arrangement of acidic and basic residues on the polar face of the amphipathic helix. C-terminal truncation analysis indicates that the terminal helix likely forms the subunit interface via inter-molecular interactions between the hydrophobic surfaces of the amphipathic helices (Westerlund and Weisgraber, 1993). In concurrence, mutation of five large hydrophobic residues between positions 253 and 289 to smaller or polar residues results in a monomeric form of apoE in the lipid-free state (Fan et al., 2004; Zhang et al., 2007). It is possible that residues 210–265, which also have the propensity to form an amphipathic helix (Choy et al., 2003), are involved in intermolecular helix-helix interaction; further dimerization of two dimers likely leads to apoE tetramerization. Thus, apoE intermolecular interaction via the CT domain appears to be an important feature in protecting exposure of the hydrophobic sites in the absence of a lipid surface, an aspect also relevant in apoA-I. More studies are required to get a better understanding of the CT domain of apoE and its conformation in the context of the entire protein.
Human apoA-I is 243 amino acids in length, 28 kDa in size, and is composed predominantly of α-helices. Secondary structure algorithms predict the presence of up to 10 proline-punctuated α-helices separated by short hinge regions (Segrest et al., 1994). Circular dichroism experiments estimate the overall helical content at ~50–57% (Leroy and Jonas, 1994). Human apoA-I is known to self associate; early sedimentation equilibrium ultracentrifugation studies on chicken apoA-I, which does not self-associate but is functionally analogous to human apoA-I (Kiss et al., 1993, 1999, 2001), revealed an axial ratio of 4.0 for the monomer. The molecular dimensions were 25.2 × 100.8 Å, strongly indicative of a helix-bundle arrangement (Kiss et al., 1993). Fluorescence spectroscopic analysis supported a model in which the hydrophobic surfaces of the α-helices were sequestered from the aqueous environment, consistent with a helix bundle (Kiss et al., 1999; Brouillette et al., 2005). Recent X-ray analysis of human apoA-I reveals that it is composed of a 4-helix bundle N-terminal domain Figure 1, right (residues 1–186) (Ajees et al., 2006). Interestingly, the helices of the bundle did not correspond to the predicted proline-punctuated helices and were much longer (~40 residues), with an overall dimension of full-length protein of 88 × 50 × 27 Å including the 2-helix CT domain, or 88 × 27 Å (length×width) for the 4-helix bundle alone. There are no short helices in the hinge regions as seen in apoLp-III or apoE. A hydrophobic patch consisting of Leu42, Leu44, Leu46, Leu47 at one end of the helix bundle, evident in the crystal structure, may serve as the lipid binding initiation site, very similar to the proposed model in L. migratoria apoLp-III (discussed in Section 6). Although far less stable than the apoE NT domain, apoA-I helix bundle is stabilized by salt bridges and H-bonding interactions (Kiss et al.,, 1993; Ajees et al., 2006) and a negatively charged patch that is proposed to be involved in ABCA1 binding (Ajees et al., 2006). The CT domain (residues 187–243) is composed of two short antiparallel helices. This is in agreement with studies that demonstrate that cleavage of the two terminal helices left an intact, folded structure that was stable in solution (Burgess et al., 1999; Fang et al., 2003). The CT domain of apoA-I is known to have the highest lipid binding affinity compared with other segments of the protein and may also be responsible for most of the self-association properties of human apoA-I in the absence of lipids (Zhu et al., 2007), as observed for apoE.
Despite the overall resemblance in helix bundle structure, the actual molecular organization of lipid-free apoA-I under physiological conditions is proposed to be at variance with the only reported high resolution structure available (reviewed in depth by Davidson & Thompson, 2007), given the dynamic and flexible nature of this protein. As pointed out by these authors, the 4-helix bundle organization obtained by X-ray analysis did not fit with a number of data obtained from biophysical studies. This is perhaps best exemplified by: (i) the lower helical content of the molecule reported in general by biophysical means, and (ii) the less helical organization of N-terminal third of the molecule as suggested by fluorescence and electron paramagnetic spectroscopy studies (Davidson et al., 1999; Lagerstedt et al. 2007). Interestingly, a novel mutant with the first 43 and last 57 residues deleted (residues 44–186) was also folded and stable in solution (Beckstead et al., 2005). Based on the crystal structure, this mutant lacks the first helix of the four helix bundle, and the C-terminal domain. Either apoA-I can be stable as a three-helix bundle or there is a massive reorganization of the helical boundaries to create an altered stable structure in the deletion mutant.
Taken together, it can be noted that while the helix bundle fold is the most favorable motif across the species to sequester the functional hydrophobic sites involved in lipid binding, additional structural features appear to have evolved in higher animals, with the proteins organized into discrete domains. However, a key question remains unanswered about the specificity of the reversible lipid binding process associated with the helix bundle. Further, whereas in insects the helix bundle plays a role in simple shuttling of lipids from and to sites of utilization, in mammals it appears to play additional roles such as a ligand for the lipoprotein receptor, co-activator for the LCAT enzyme or in cholesterol efflux, and possibly in a regulatory capacity. It is envisaged that the tendency of the CT domain of apoE or apoAI to self-associate may be driven by formation of an inter-molecular helix bundle in the lipid-free state. Further studies are in progress by several laboratories to evaluate the structural and functional features of these proteins.
4. Belt conformation: A recurrent structural theme in lipid-associated apolipoproteins
While the compact helix bundle organization offers insights into the structural basis of this class of proteins in the lipid-free state, it is inadequate to explain the conformation that they adopt in the lipid-associated state (interchangeably referred to as lipoprotein-bound apolipoproteins throughout the review). However, there is sufficient circumstantial evidence to support the concept that the helix bundle organization undergoes a dramatic conformational change upon encountering a lipid surface. Our current understanding of the lipid-bound conformation of apolipoproteins was greatly facilitated by the ability to use vesicles of synthetic phospholipids as a surrogate of the naturally occurring lipoproteins. To this end, vesicles of dimyristoylphosphatidylcholine (DMPC) have been employed routinely. Lipid packing defects in the phosphatidylcholine (PC) bilayer at the gel to liquid crystalline transition temperature generate binding sites for apolipoprotein interaction. This binding interaction results in a spontaneous remodeling of the DMPC vesicles, creating small discoidal particles (10–20 nm diameter) that resemble the nascent HDL found in mammals in vivo (O’Connor et al., 1998). As these particles mature, they develop a core containing neutral lipids and transform into a spherical HDL particle. The discoidal particles are composed of a bilayer of PC that is surrounded by the apolipoproteins in a conformation distinct from that of the lipid-free helix bundle organization. The exact conformation adopted by apolipoproteins in this state is unclear and is currently under debate.
Structural biologists in the lipoprotein research field have entertained several descriptors to portray the conformation and orientation of the apolipoproteins helices with respect to the fatty acyl chains of the lipid bilayer of HDL based on biophysical and spectroscopic evidence. Earlier studies reported that the protein helical axes are oriented parallel to the axes of the phospholipid fatty acyl chains (i.e. perpendicular to the plane of the bilayer). Referred to as the “picket fence” model, this concept was rapidly disregarded when information from several newer studies suggested an extended helix circumscribing the discoidal bilayer, in a manner resembling a belt, called the “belt” model. There is a general consensus regarding the belt model that suggests that the helical axis is oriented perpendicular to the axis of the fatty acyl chains (i.e. parallel to the plane of the bilayer) in a reconstituted discoidal HDL particle. Remarkably, all the apolipoproteins, whether of mammalian or insect origin, appear to adopt this lipid-bound conformation in general. The specifics of each case will be briefly discussed below.
Initial evidence for the conformational opening of the helix bundle came from studies involving M. sexta apoLp-III interaction with DMPC vesicles, with important clues provided by biophysical and biochemical methods (Wientzek et al., 1994; Narayanaswami et al, 1996). Helix bundle opening was inferred from observations that the fluorescence emission spectrum of apoLp-III displayed dramatically altered characteristics upon lipid association (Wientzek et al., 1994; Weers et al., 2000b, Soulages and Arrese, 2001). In addition, tethering the helix bundle, which was achieved by substituting cysteine residues at strategic locations that held selected helices together in close spatial proximity due to the covalent disulfide bond formation, prevented apoLp-III from binding to spherical lipoprotein surfaces (Narayanaswami et al, 1996; Sahoo et al., 2002; Chetty et al., 2003b). However, the ability of the variant to interact with PC vesicles was not completely impaired suggesting that the helix bundle may open by alternate modes.
A critical step in the binding process of apoLp-III is the repositioning of the first and last helices. When helix 1 and helix 5 were tethered by a disulfide bond, the protein was unable to bind lipid surfaces as demonstrated for M. sexta, L. migratoria, and G. mellonella apoLp-III (Garda et al., 2002; Soulages et al., 2001; Sahoo et al., 2002; Leon et al., 2006). In addition, biophysical studies showed that helix 1 and helix 5 appear to have the highest conformational flexibility (Soulages and Arrese, 2000). This initial separation of the two helices may facilitate further opening of the hydrophobic helix interior, allowing the protein to completely spread out on the lipid surface. Regardless of the mode of opening, lipid-bound apoLp-III appears to orient its helices perpendicular to the fatty acyl chains as inferred by Fourier transform infrared spectroscopic analyses (Raussens et al., 1995, 1996). The α-helices are in a fully extended conformation, with two apoLp-III molecules juxtaposed in an antiparallel orientation with the possibility of a one-helix overhang, and probably four protein molecules per discoidal particle (Garda et al., 2002; Sahoo et al., 2002). Thus, helices of neighboring apoLp-III molecules bridge the gap between the PC head groups of the bilayer leaflets, shielding the acyl chains from exposure to the aqueous environment. The open conformation allows the protein’s hydrophobic interior to interact with the hydrophobic lipid surface; consequently the non-polar face of the amphipathic α-helices are now in direct contact with the PC acyl chains (Soulages and Arrese, 2001). Efforts are under way to obtain further insights into the lipid-associated conformation of apoLp-III.
In the case of apoE, lipid association is a crucial factor for the NT domain to elicit optimal interaction with the LDLr (Weisgraber, 1994). It appears to involve opening of the helix bundle and movement of the helices away from each other (Lu et al., 2000) as deduced from a helix tethering approach. This observation was independently confirmed by fluorescence resonance energy transfer studies, which suggested that helix 1 moves away from helix 3 (Fisher et al., 2000), and that eventually the entire domain opens to form an extended helical conformation (Raussens et al., 1998; Narayanaswami et al., 2004). More studies are needed to determine which pair of hinges is involved in the bundle opening: those located between helix 1/helix 2, and between helix 3/helix 4 or those between helix 2/helix 3, and between helix 3/helix 4. In addition, fluorescence studies also postulate that the NT and the CT domains “unfurl” away from each other in apoE3, with the unstructured linker loop also adopting an α-helical structure (Narayanaswami et al., 2001; Gupta et al., 2006). Lastly, studies with the isolated CT domain demonstrate that the helices orient perpendicular to the plane of the bilayer in the lipid-associated state (Raussens et al., 2005). Thus, considering that there is a loss in sensitivity to proteolysis upon lipid interaction (Narayanaswami et al., 2004), spectroscopic and biochemical evidence suggest that the entire apoE3 forms an extended helical structure surrounding the lipid bilayer. Whether this is applicable to the apoE4 isoform as well is unclear at the moment. Fluorescence analysis of apoE4 using spatially sensitive probes that monitor the NT and CT domains indicates that selected sites in these domains remain in close spatial proximity in discoidal complexes with DMPC, suggesting alternate possibilities exist to the extended “belt” model, such as the “looped-back belt” model (Drury and Narayanaswami, 2005). Such a possibility was independently confirmed using fluorescence resonance energy transfer and electron paramagnetic resonance spectroscopy (Hatters et al., 2005b). However, small angle X-ray scatter studies at 10 Å -resolution suggests an alternative model of apoE4 in complex with dipalmitoylphosphatidylcholine (Peters-Libeu et al., 2006, 2007). Lacking a bilayer, this spheroidal-shaped lipoprotein bears two apoE molecules that make extensive contact with the polar head groups of the phospholipid, and a core composed of the phospholipid fatty acyl chains; in this model, the hydrophobic faces of the helices of apoE4 face each other and the molecule adopts a hairpin conformation. More studies are needed to fully understand the molecular organization of lipoprotein-associated apoE in complex with lipids, and in spherical lipoproteins containing a neutral lipid core. In addition, we are faced with the challenge of deciphering the structural basis of the isoform-specific and particle lipid composition-specific differences amongst different apoE isoforms, and their role in diseases.
Opening of the apoE helix bundle may be a regulatory step in lipoprotein metabolism (Narayanaswami and Ryan, 2000; Saito et al., 2001, 2004). In the lipid-bound state, it is envisaged that apoE adopts two different conformations: whereas the CT domain is lipid-associated in both conformations, the NT domain can be lipid-associated or lipid-free. This model ensures that the CT domain serves to anchor the entire protein to the lipoprotein. From a functional perspective, this model suggests that the conformation of the NT domain (closed helix bundle, LDLr-inactive or the open LDLr-active state), determines the lipoprotein particle’s “readiness” to be recognized by the LDLr and internalized by the cell. Studies are in progress to obtain evidence for this model from a structural perspective.
Lipid-associated apoA-I has received researchers’ intense scrutiny for the longest period of time. Despite this, we have a poor consensus on its conformation, in part due to the dynamic nature of the particle and the heterogeneity associated with apoA-I conformation per se. The exact conformation and the registry of the apoA-I molecules depends on the study (Thomas et al., 2006). Three models were originally tested: the “picket-fence” (in which the helices are oriented parallel to the fatty acyl chains in an up-and-down fashion), the “hairpin” (in which the apoA-I helices protein folds back on itself perpendicular to the acyl chains) and the “double-belt” (in which two apoA-I proteins orient side by side around the periphery of the disc). The picket-fence model initially gained some experimental support from Fourier Transform Infrared spectroscopy (FTIR) (Wald et al., 1990), but was later discarded. Jonas and colleagues provided support for a hairpin model based on fluorescence resonance energy transfer analysis (Tricerri et al., 2001). However, other studies using similar approaches provided evidence for the belt model (Koppaka et al., 1999; Panagotopulos et al., 2001; Li et al., 2000), such that now the consensus is that apoA-I orients itself perpendicular to the acyl chains. To distinguish between the remaining two models, chemical crosslinking, followed by in gel trypsin digestion and mass spectrometry were used. This method was effectively able to separate intra- and inter molecular interactions and identify the model of binding for a defined discoidal particle (Davidson and Hilliard, 2003; Davidson and Silva, 2005). The authors’ conclusion was that, in general, apoA-I forms a belt around the disc periphery similar to that noted for apoLp-III and apoE. In fact, apoA-I adopted multiple conformations with sliding registries. One additional complication is that the disc particle can contain 2, 3, or 4 molecules of apoA-I (Thomas et al., 2006). In this case, disc particles containing 3 apoA-I proteins may have both double-belt and hairpin structures. Most recently, a “belt-buckle” model was proposed. This model was obtained by crosslinking and mass spectrometry and unique products were identified indicating a variation to the belt model; two apoA-I molecules form an anti-parallel belt around the discoidal structure, with the N-terminus of one apoA-I molecule forming a hairpin which makes contact with the C-terminus of the second apoA-I molecule (Bhat et al., 2005). It is also possible that a central part of the anti-parallel belt may loop out (dissociate from the lipid surface) to accommodate varying sizes of disc complexes (Martin et al., 2006). Although it was not appreciated at the time, the structure of apoA-I on disc particles and the size of disc particles are flexible and likely reflect the varied observations to date.
A crystal structure of an N-terminal deletion mutant of apoA-I (Δ1–43) revealed a drawn-out helical horseshoe, which the authors proposed represented a lipid-bound conformation, although the structure was obtained in the absence of lipids (Borhani et al., 1997). Modeling studies and fluorescence techniques are in general agreement with the conformation of apoA-I on spherical HDL particles as pairs of elongated α-helices (reviewed in Thomas et al., 2008). How the discoidal apoA-I relates to apoA-I on spherical HDL is less clear. Some researchers have proposed that apoA-I on HDL continues to function as an antiparallel dimer which wraps around the circumference of the spherical HDL (Silva et al., 2008). In discoidal HDL, the hydrophobic surface of apoA-I interacts with acyl chains of the phospholipids around the periphery of the disc. However, in spherical HDL, the hydrophobic face of apoA-I must penetrate the phospholipid surface and gain access to the hydrophobic acyl chains, although only peripherally (Kiss et al., 1999). Silva et al. (2008) showed, using cross-linking and mass spectrometry, that the overall organization of apoA-I on discoidal and spherical HDL particles was similar regardless of the diameter of the complex. However, HDL that contain three apoA-I molecules would not fit the model of Silva et al. with antiparallel dimers, and thus other models would have to be invoked. Furthermore, because of the lack of restriction on movement that is present on the surface of a lipoprotein, unlike a discoidal particle, most studies have been unable to pin down one structure and it is likely that there are many conformations that apoA-I can adopt on the HDL surface. Opening of the apoA-I helix bundle may be an important regulatory step in lipoprotein metabolism. Activation of the plasma enzyme LCAT is a necessary step for the maturation and stability of HDL. Like apoE in the lipid-bound state, it is envisaged that apoA-I also adopts two different conformations. In both conformations the CT domain anchors the entire protein to the lipoprotein surface; the NT domain can be either lipid-free or lipid-bound. It was more recently shown that the extreme N-terminal helix also possesses a strong lipid binding domain that must also bind to the lipoprotein to allow the central domain flexibility to interact with LCAT, facilitating cholesteryl ester formation and HDL maturation (Rogers, et al., 1997, 1998; Palgunachari et al., 1996). Mutants of apoA-I that lack the N-terminal helix or have decreased lipid binding affinity have impaired LCAT activation ability in vitro and in vivo (Scott et al., 2001). Therefore, facilitated opening of the helix bundle in apoA-I serves a structural and functional role.
5. Trigger for lipid binding: lipid packing defects and helix bundle stability
While we have a fair understanding of apolipoprotein structure prior to and post-lipid binding, details regarding what initiates the conformational switch and lipid binding (not necessarily in this order) are unclear. As the lipoprotein particle is constantly undergoing remodeling and the lipids are metabolized, it is expected that the changes in the particle lipid composition or physical state induce complementary changes in the protein structure that trigger protein interaction with lipid. Intuitively, it is believed that these factors are mutually complementary.
Changes in the lipid composition of the lipoprotein particle may arise from: enzyme mediated changes including cholesterol esterification (mediated by LCAT), cholesteryl ester hydrolysis (cholesterol esterase activity), changes in the cholesterol content (following cellular efflux to lipid-poor lipoprotein particles mediated by the ATP-binding cassette family of proteins), cholesteryl ester transfer protein mediated exchange of cholesteryl ester and TG, or in the case of insects, DG loading or hydrolysis. Experimentally, the latter is best illustrated by in vivo studies that demonstrate that following loading of the lipophorin particle at the fat body site, DG partitions between the hydrophobic core and the amphipathic monolayer surrounding the core of the particle (Shapiro et al., 1988). The appearance of DG in the phospholipid monolayer causes severe packing defects and impairment in monolayer integrity (Soulages and Wells, 1994b), similar to that noted for cholesterol (Pownall et al., 1979). The appearance of packing defects in the lipid layer is a cue for apoLp-III binding to the particle surface (Kawooya et al., 1991). Subsequent lipoprotein lipase-mediated DG hydrolysis at flight muscle binding site results in dissociation of apoLp-III from the lipoprotein surface. Lipid packing defects have been simulated by sustaining a lipid vesicle preparation at the gel to liquid crystalline transition temperature of the phospholipid (Surewicz et al., 1986). Alternatively, the packing defects have been generated in vitro by treating a spherical lipoprotein surface such as LDL with phospholipase C, which cleaves the phospholipid head group leaving DG in the phospholipid monolayer (Liu et al., 1993). At this stage, the lipid-free apolipoproteins present in the surrounding solution bind to the exposed DG to prevent the latter’s exposure to aqueous environment. There is ample evidence that other apolipoproteins such as apoE and apoA-I can also be induced to bind to DG-exposed particles. In the absence of phospholipase C, no binding occurs. In addition, proteins that are known for their non-specific interaction, such as albumin, do not bind to the DG-exposed lipoprotein either. These observations support the notion that lipid-packing defects are an important factor to trigger binding of apolipoproteins.
Whereas the lipid composition forces lipid binding interaction of the apolipoprotein, the binding per se and the accompanying conformational opening may be attributed to the low stability of the helix bundle structure. There are several independent biophysical studies involving chemical denaturant (guanidine-HCl and urea)-induced protein unfolding that have contributed tremendously to our understanding of apolipoprotein-lipid interaction. The relative concentration of guanidine-HCl required to cause a 50% loss in protein secondary structure, [Gdn-HCl]½, is used to infer the stability of a protein. A low stability is interpreted to depict ease of helix bundle opening. ApoLp-III displays a relatively low helix bundle stability, with a [Gdn-HCl]½ of 0.3–0.6 M and an apparent ΔG° of unfolding of 1–2 kcal mol−1 (Ryan et al., 1993; Weers et al., 1994). Similarly, apoA-I (either the monomeric chicken apoA-I or the multimeric human apoA-I) elicits a low [Gdn-HCl]½ of 0.6–1.0 M (Kiss et al, 1993; Edelstein and Scanu, 1980). Both apoLp-III and apoA-I interact with lipids rapidly. In addition, isolated apoE CT domain (whose helices make extensive inter-molecular helical contacts) displays [Gdn-HCl]½ of ≤ 1.0 M (Wetterau et al, 1988; Aggerbeck et al., 1988; Morrow et al., 2000) and a higher lipid binding affinity compared to the NT domain (Morrow et al., 2000, Choy et al., 2003). By contrast, the isolated NT domain helix bundle of all the three apoE isoforms (apoE2, apoE3 and apoE4) display a relative resistance to unfolding ([Gdn-HCl]½ of ~ 2.5 M) (Morrow et al., 2000). As expected, the NT domain is slow to respond to stimulation to bind lipids or are required in larger amounts to display binding rates comparable to that of apoA-I or apoLp-III at neutral pH.
Probing the molecular basis of the lower helix bundle stability of apoLp-III, it was postulated that the presence of polar residues in the hydrophobic interior tends to destabilize the protein structure (Fan et al., 2003). A typical example is the presence of Thr31 in the protein interior in L. migratoria apoLp-III. Thr31 substitution by Leu increased the protein stability but resulted in a decreased ability to bind to lipid. On the other hand, introducing lysine residues in the helix core to destabilize the protein promoted lipid binding to a large extent (Weers et al., 2005). Other helix bundle destabilization simulations also indicate the correlation between protein stability and lipid binding. For example, lowering the pH of the reaction to 3.0 –4.0 induces a molten globule form, with retention of secondary structure but with decreased tertiary contacts (Soulages and Bendavid, 1998; Weers at al., 2001a, 2001b, 2003; Morrow et al., 2002; Gursky and Atkinson, 1996). In this state, apolipoproteins display an order of magnitude higher DMPC vesicle solubilization rates (Soulages and Bendavid, 1998, Weers et al., 2001a, 2001b; Morrow et al., 2002). Taken together, these studies indicate a direct correlation between protein instability and lipid binding ability. A flexible α-helical bundle structure therefore appears to be a structural feature making it possible for apolipoproteins to rapidly associate with lipid surfaces following appearance of lipid packing defects.
6. Helix bundle end zones involved in initiation of lipid binding
It is generally believed that, whereas lipid binding involves the hydrophobic face of the helix bundle as such, the initiation of binding itself is initiated at one end of the helix bundle. Several models have been proposed to describe the initial binding steps, which are discussed below.
Short helix at one end of helix bundle
The ability to sense lipid packing defects on the lipoprotein surface has been attributed to a 6-residue short helix in M. sexta apoLp-III (Narayanaswami et al., 1999) or a 4-residue helix in L. migratoria apoLp-III (Fan et al., 2003) (schematically represented in Figure 2). Replacement of the short helix (PDVEKE) by a β-turn (NPNG) in M. sexta apoLp-III resulted in defective binding to spherical lipoproteins without significant alterations to the helix bundle stability (Narayanaswami et al., 1999). Val 97 was identified as a critical residue for initiating lipoprotein binding. In L. migratoria apoLp-III, a putative short helix exists (AWAP) on the end opposite to that noted in M. sexta apoLp-III. No experimental evidence is available to support a function for this short helix. In the case of apoE NT domain, there is evidence to support initiation of lipid binding at either end of the helix bundle (Redmond et al., 2006, Segelke et al., 2000). The end bearing helix 1′ (EQVQEELLS) was suggested as being responsible for initiation of lipid interaction (Redmond et al., 2006). In contrast to M. sexta apoLp-III, replacement of the short helix by a β-turn enhanced the lipid binding ability (to spherical lipoproteins) of apoE3 NT compared to the wild type protein. In the DMPC vesicle solubilization assay, the β-turn-bearing mutant was more effective than the wild type apoE3 NT. The β-turn-bearing mutant revealed a lower stability compared to the wild type counterpart. While they do not significantly increase our understanding of initiation of lipid binding interaction, these observations suggest a possible ‘loosening’ of the helix bundle in the β-turn-bearing mutant, which allows the helix bundle to open with ease.
Figure 2. Initiation of lipid binding by apolipoproteins.
The helix bundle is represented as a blue oval, and the C-terminal tail as a green oval. The pink edges at one end of the helix bundle represent the hydrophobic patch or the short helix as putative sites of initiation. Two representative models of initiation of lipid binding to a hydrophobic surface (orange oval) on the lipoprotein particle are shown: (i): Binding of a single domain helix bundle protein, exemplified by insect apolipoproteins. Initiation of binding at one end of the bundle (step 1) is followed by subsequent helix bundle opening (step 2); (ii) Binding of a two-domain protein comprising a helix bundle and a C-terminal tail as seen for apoE and apoA-I. The CT tail initiates lipid binding due to its high affinity for lipids (step 1); there are two possible conformations of lipid-bound protein: one where the NT domain is lipid free (step 2) and a second where it is lipid-bound (step 3), similar to that seen for insect apolipoproteins.
Studies carried out at the other end of the helix bundle opposite of helix-1′ suggest that the flexible loop linking helices 2 and 3 is mobile, though it was traced unambiguously in the electron density map (Segelke et al., 2000), and that it plays a role in lipid interaction of apoE. Termed the 80’s loop, this segment has an abundance of negatively charged residues (5 Glu in a sequence of 12 residues) (Segelke et al., 2000). It is postulated that the negative charges complement the positive charge of the phosphate head groups of phospholipids. A recent NMR study of the NT domain of apoE3 indicates the presence of short helices towards the terminal ends (one towards the N- and a second towards the C-terminal end of the helix bundle) (Sivashanmugam and Wang, 2009). Although lacking experimental evidence, the authors suggest that the latter has the potential to be an initiator of lipid binding interaction. More studies are needed to define the role of these residues in initiating lipid binding.
Hydrophobic patch at one end of helix bundle
Other studies suggest an alternative to the short helix as initiator of lipid binding: the presence of conserved Leu residues (Leu 32, Leu 34, and Leu 95) at or around loops on one end of the protein, opposite of the short helix in L. migratoria apoLp-III (Weers et al., 1999). Mutations that changed the leucines to arginine residues, thereby creating a net positive charge, decreased its ability to compete with WT protein for lipoprotein surface, with Leu 95 being the most critical. More subtle mutations are needed to pinpoint this end of the protein as the initiation region, in addition to studies to clarify the role of the short helix. Similar to L. migratoria apoLp-III, a hydrophobic patch of Leu 42, Leu 44, Leu 46, and Leu 47 in apoA-I may serve a role in lipid binding initiation (Ajees et al., 2006). However, there is insufficient experimental evidence to provide support for the role of this hydrophobic patch in lipid initiation.
Terminal helices initiate lipid binding in full-length mammalian apolipoproteins
The presence of a CT domain in addition to the helix bundle domain in apoE and apoA-I requires further consideration in terms of initiation of lipid binding. It is smaller in size, composed of ~ 2 α-helices, and for apoE, bears a significantly lower protein stability and is therefore believed to display a stronger association with lipids. Indeed, it may serve as an anchor on the lipid surface for the entire protein, thereby facilitating the existence of two different lipid-bound conformations: one wherein the NT domain in a helix bundle (LDLr binding-incompetent) state and a second where the helix bundle is an open (LDLr binding-competent) state (Narayanaswami & Ryan, 2000; Segall, et al., 2002; Saito et al., 2004). As mentioned earlier, this conformational regulation is unique to apoE as it may serve to sense the competency of the lipoprotein particle to interact with lipoprotein receptors and be internalized by the cell.
Similarly, the initiation of lipid binding for apoA-I likely occurs via one the helices in its CT domain, with which the protein is anchored to HDL. Under appropriate conditions, such as exposure of hydrophobic surface area due to cholesterol loading, the N-terminal domain can also bind, opening up the helix bundle for interaction with the lipid surface (or with ABCA1 or LCAT) (Kono et al., 2008). However, other data suggest that, in addition to the C-terminal helix, the first helix (including residues 8–33) may also act as a lipid-binding anchor, allowing the central domain of apoA-I to dissociate intermittently (Tanaka et al., 2006). This notion is supported by other studies that indicate that residues 121–187 were highly dynamic in their interaction with lipid, suggesting that these residues may occasionally dissociate from the disc particle, putatively to interact with LCAT or ABCA1 (Maiorano et al., 2004). Thus, apoA-I is a dynamic and flexible molecule that can adopt many conformations to accommodate varying lipid-binding conditions (Davidson and Thompson, 2007).
5. Concluding remarks and future perspective
The three apolipoproteins discussed in the present review are remarkably similar in their structural properties and lipid binding function. All are helix bundle proteins, with a core of amphipathic α-helical segments. Insect apoLp-III is a single-domain protein composed of a 5-helix bundle. In case of apoE and apoA-I, one or two additional helices at the C-terminal end complement the 4-helix bundle and serve to anchor the protein to the lipoprotein particle. The C-terminal helices may have evolved to fulfill the more complex role in vertebrate lipid transport processes. It is plausible that the 5th helix in insect apoLp-III provides further instability, thereby facilitating a rapid switch between a lipid-free to a lipid-bound state and allowing apoLp-III to be recycled efficiently. The lipid binding character is undoubtedly inherited by the presence of the amphipathic helices. Interestingly, the C-terminal portion of TIP47, a protein localized on intracellular neutral lipid droplets and belonging to the same family as perilipin and adipose differentiation-related protein, was shown to have a 4-helix bundle motif (Hickenbottom et al., 2004). Localized in the cytosol, TIP47 is thought to associate with the phospholipid surface that coats the TG core of lipid droplets. TIP47 is also able to transform DMPC vesicles and form nanodiscs (Bulankina et al., 2009). Thus, the simple molecular fold of a helix bundle is a signature, underlying theme that provides an excellent structural arrangement for a variety of proteins to bind to lipid surfaces. Near future research will need to obtain the structure of full length apoE, as well as high resolution structures of the exchangeable apolipoproteins in their lipid-bound conformation, a key requirement to fully understand the mechanistic aspects of apolipoprotein-lipid and lipoprotein-receptor interaction and cellular lipoprotein uptake. These aspects are critical to understand the role of apoE in regulating lipoprotein metabolism. The dynamic nature of lipid-free and lipid-bound apoA-I presents a far more complex problem, which will require a multi-faceted approach to get a complete handle on the structure. Finally, it will be necessary to obtain the structure of not only the “convenient” discoidal particles but also of spherical lipoproteins.
Acknowledgements
This work is a culmination of research funded by NIH R15 HL077135 and SCORE GM 063119 (PMMW), CIHR MOP – 89972 (RSK) and the American Heart Association 07551374, CSULB SCAC Award, Tobacco-Related Disease Research Program (17 RT-0165) and the Drake Family Trust (VN).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aggerbeck LP, Wetterau JR, Weisgraber KH, Wu CS, Lindgren FT. Human apolipoprotein E3 in aqueous solution. II. Properties of the amino- and carboxyl-terminal domains. J. Biol. Chem. 1988;263:6249–6258. [PubMed] [Google Scholar]
- Ajees AA, Anantharamaiah GM, Mishra VK, Hussain MM, Murthy HM. Crystal structure of human apolipoprotein A-I: insights into its protective effect against cardiovascular diseases. Proc. Natl. Acad. Sci. USA. 2006;103:2126–2131. doi: 10.1073/pnas.0506877103. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Beckstead JA, Block BL, Bielicki JK, Kay CM, Oda MN, Ryan RO. Combined N- and C-terminal truncation of human apolipoprotein A-I yields a folded, functional central domain. Biochemistry. 2005;44:4591–4599. doi: 10.1021/bi0477135. [DOI] [PubMed] [Google Scholar]
- Beenakkers AMTh, Chino H, Law JH. Lipophorin nomenclature. Insect Biochem. 1988;18:1–2. [Google Scholar]
- Bhat S, Sorci-Thomas MG, Alexander ET, Samuel MP, Thomas MJ. Intermolecular contact between globular N-terminal fold and C-terminal domain of ApoA-I stabilizes its lipid-bound conformation: studies employing chemical cross-linking and mass spectrometry. J. Biol. Chem. 2005;280:33015–33025. doi: 10.1074/jbc.M505081200. [DOI] [PubMed] [Google Scholar]
- Borhani DW, Rogers DP, Engler JA, Brouillette CG. Crystal structure of truncated human apolipoprotein A-I suggests a lipid-bound conformation. Proc. Natl. Acad. Sci. USA. 1997;94:12291–12296. doi: 10.1073/pnas.94.23.12291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breiter DR, Kanost MR, Benning MM, Wesenberg G, Law JH, Wells MA, Rayment I, Holden HM. Molecular structure of an apolipoprotein determined at 2.5 Å resolution. Biochemistry. 1991;30:603–608. doi: 10.1021/bi00217a002. [DOI] [PubMed] [Google Scholar]
- Brouillette CG, Dong WJ, Yang ZW, Ray MJ, Protasevich II, Cheung HC, Engler JA. Förster resonance energy transfer measurements are consistent with a helical bundle model for lipid-free apolipoprotein A-I. Biochemistry. 2005;44:16413–16425. doi: 10.1021/bi051018v. [DOI] [PubMed] [Google Scholar]
- Bulankina AV, Deggerich A, Wenzel D, Mutenda K, Wittmann JG, Rudolph MG, Burger KNJ, Höning S. TIP47 functions in the biogenesis of lipid droplets. J. Cell Biol. 2009;185:641–655. doi: 10.1083/jcb.200812042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess JW, Frank PG, Franklin V, Liang P, McManus DC, Desforges M, Rassart E, Marcel YL. Deletion of the C-terminal domain of apolipoprotein A-I impairs cell surface binding and lipid efflux in macrophage. Biochemistry. 1999;38:14524–14533. doi: 10.1021/bi990930z. [DOI] [PubMed] [Google Scholar]
- Chetty PS, Arrese EL, Soulages JL. In vivo lipoprotein binding assay of the insect exchangeable apolipoprotein, apolipophorin-III. Protein Pept. Lett. 2003a;10:469–473. doi: 10.2174/0929866033478681. [DOI] [PubMed] [Google Scholar]
- Chetty PS, Arrese EL, Rodriguez V, Soulages JL. Role of helices and loops in the ability of apolipophorin-III to interact with native lipoproteins and form discoidal lipoprotein complexes. Biochemistry. 2003b;42:15061–15067. doi: 10.1021/bi035456i. [DOI] [PubMed] [Google Scholar]
- Choy N, Raussens V, Narayanaswami V. Inter-molecular coiled-coil formation in human apolipoprotein E C-terminal domain. J. Mol. Biol. 2003;334:527–539. doi: 10.1016/j.jmb.2003.09.059. [DOI] [PubMed] [Google Scholar]
- Dantuma NP, Potters M, De Winther MP, Tensen CP, Kooiman FP, Bogerd J, Van der Horst DJ. An insect homolog of the vertebrate very low density lipoprotein receptor mediates endocytosis of lipophorins. J. Lipid Res. 1999;40:973–978. [PubMed] [Google Scholar]
- Das HK, McPherson J, Bruns GA, Karathanasis SK, Breslow JL. Isolation, characterization, and mapping to chromosome 19 of the human apolipoprotein E gene. J. Biol. Chem. 1985;260:6240–6247. [PubMed] [Google Scholar]
- Davidson WS, Arnvig-McGuire K, Kennedy A, Kosman J, Hazlett TL, Jonas A. Structural organization of the N-terminal domain of apolipoprotein A-I: studies of tryptophan mutants. Biochemistry. 1999;38:14387–14395. doi: 10.1021/bi991428h. [DOI] [PubMed] [Google Scholar]
- Davidson WS, Hilliard GM. The spatial organization of apolipoprotein A-I on the edge of discoidal high density lipoprotein particles: a mass specrometry study. J. Biol. Chem. 2003;278:27199–27207. doi: 10.1074/jbc.M302764200. [DOI] [PubMed] [Google Scholar]
- Davidson WS, Silva RA. Apolipoprotein structural organization in high density lipoproteins: belts, bundles, hinges and hairpins. Curr. Opin. Lipidol. 2005;16:295–300. doi: 10.1097/01.mol.0000169349.38321.ad. [DOI] [PubMed] [Google Scholar]
- Davidson WS, Thompson TB. The structure of apolipoprotein A-I in high density lipoproteins. J. Biol. Chem. 2007;282:22249–22253. doi: 10.1074/jbc.R700014200. [DOI] [PubMed] [Google Scholar]
- Dettloff M, Weers PMM, Niere M, Kay CM, Ryan RO, Wiesner A. An N-terminal three-helix fragment of the exchangeable insect apolipoprotein apolipophorin III conserves the lipid binding properties of wild-type protein. Biochemistry. 2001;40:3150–3157. doi: 10.1021/bi0013804. [DOI] [PubMed] [Google Scholar]
- Dong LM, Parkin S, Trakhanov SD, Rupp B, Simmons T, Arnold KS, Newhouse YM, Innerarity TL, Weisgraber KH. Novel mechanism for defective receptor binding of apolipoprotein E2 in type III hyperlipoproteinemia. Nat. Struct. Biol. 1996;3:718–722. doi: 10.1038/nsb0896-718. [DOI] [PubMed] [Google Scholar]
- Drury J, Narayanaswami V. Examination of lipid-bound conformation of apolipoprotein E4 by pyrene excimer fluorescence. J. Biol. Chem. 2005;280:14605–14610. doi: 10.1074/jbc.M414019200. [DOI] [PubMed] [Google Scholar]
- Edelstein C, Scanu AM. Effect of guanidine hydrochloride on the hydrodynamic and thermodynamic properties of human apolipoprotein A-I in solution. J. Biol. Chem. 1980;255:5747–5754. [PubMed] [Google Scholar]
- Fagan AM, Holtzman DM, Munson G, Mathur T, Schneider D, Chang LK, Getz GS, Reardon CA, Lukens J, Shah JA, LaDu MJ. Unique lipoproteins secreted by primary astrocytes from wild type, apoE (−/−), and human apoE transgenic mice. J. Biol. Chem. 1999;274:30001–30007. doi: 10.1074/jbc.274.42.30001. [DOI] [PubMed] [Google Scholar]
- Fan D, Zheng Y, Yang D, Wang J. NMR solution structure and dynamics of an exchangeable apolipoprotein, Locusta migratoria apolipophorin III. J. Biol. Chem. 2003;278:21212–21220. doi: 10.1074/jbc.M208486200. [DOI] [PubMed] [Google Scholar]
- Fan D, Li Q, Korando L, Jerome WG, Wang J. A monomeric human apolipoprotein E carboxyl-terminal domain. Biochemistry. 2004;43:5055–5064. doi: 10.1021/bi035958w. [DOI] [PubMed] [Google Scholar]
- Fang Y, Gursky O, Atkinson D. Structural studies of N- and C-terminally truncated human apolipoprotein A-I. Biochemistry. 2003;42:6881–6890. doi: 10.1021/bi034152t. [DOI] [PubMed] [Google Scholar]
- Feig JE, Shamir R, Fisher EA. Atheroprotective effects of HDL: beyond reverse cholesterol transport. Curr. Drug Targets. 2008;9:196–203. doi: 10.2174/138945008783755557. [DOI] [PubMed] [Google Scholar]
- Finch CE, Stanford CB. Meat-adaptive genes and the evolution of slower aging in humans. Q. Rev. Biol. 2004;79:3–50. doi: 10.1086/381662. [DOI] [PubMed] [Google Scholar]
- Fisher CA, Narayanaswami V, Ryan RO. The lipid-associated conformation of the low density lipoprotein receptor binding domain of human apolipoprotein E. J. Biol. Chem. 2000;275:33601–33606. doi: 10.1074/jbc.M002643200. [DOI] [PubMed] [Google Scholar]
- Fullerton SM, Clark AG, Weiss KM, Nickerson DA, Taylor SL, Stengârd JH, Salomaa V, Vartiainen E, Perola M, Boerwinkle E, Sing CF. Apolipoprotein E variation at the sequence haplotype level: implications for the origin and maintenance of a major human polymorphism. Am. J. Hum. Genet. 2000;67:881–900. doi: 10.1086/303070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garda HA, Arrese EL, Soulages JL. Structure of apolipophorin III in discoidal lipoproteins. Interhelical distances in the lipid-bound state and conformational change upon binding to lipid. J. Biol. Chem. 2002;277:19773–19782. doi: 10.1074/jbc.M110089200. [DOI] [PubMed] [Google Scholar]
- Gupta V, Narayanaswami V, Budamagunta MS, Yamamato T, Voss JC, Ryan RO. Lipid-induced extension of apolipoprotein E helix 4 correlates with low density lipoprotein receptor binding ability. J. Biol. Chem. 2006;281:39294–39299. doi: 10.1074/jbc.M608085200. [DOI] [PubMed] [Google Scholar]
- Gursky O, Atkinson D. Thermal unfolding of human high-density apolipoprotein A-1: implications for a lipid-free molten globular state. Proc. Natl. Acad. Sci. USA. 1996;93:2991–2995. doi: 10.1073/pnas.93.7.2991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hård K, Van Doorn JM, Thomas-Oates JE, Kamerling JP, Van der Horst DJ. Structures of the Asn-linked oligosaccharides of apolipophorin III from the insect Locusta migratoria. Carbohydrate-linked 2-aminoethylphosphonate as a constituent of a glycoprotein. Biochemistry. 1993;32:766–775. doi: 10.1021/bi00054a005. [DOI] [PubMed] [Google Scholar]
- Hatters DM, Peters-Libeu CA, Weisgraber KH. Engineering conformational destabilization into mouse apolipoprotein E. A model for a unique property of human apolipoprotein E4. J. Biol. Chem. 2005a;280:26477–26482. doi: 10.1074/jbc.M503910200. [DOI] [PubMed] [Google Scholar]
- Hatters DM, Budamagunta MS, Voss JC, Weisgraber KH. Modulation of apolipoprotein E structure by domain interaction: differences in lipid-bound and lipid-free forms. J. Biol. Chem. 2005b;280:34288–34295. doi: 10.1074/jbc.M506044200. [DOI] [PubMed] [Google Scholar]
- Hatters DM, Peters-Libeu CA, Weisgraber KH. Apolipoprotein E structure: insights into function. Trends Biochem. Sci. 2006;31:445–454. doi: 10.1016/j.tibs.2006.06.008. [DOI] [PubMed] [Google Scholar]
- Herz J, Bock HH. Lipoprotein receptors in the nervous system. Annu. Rev. Biochem. 2002;71:405–434. doi: 10.1146/annurev.biochem.71.110601.135342. [DOI] [PubMed] [Google Scholar]
- Hickenbottom SJ, Kimmel AR, Londos C, Hurley JH. Structure of a lipid droplet protein: The PAT family member TIP47. Structure. 2004;12 doi: 10.1016/j.str.2004.04.021. 11-99-1207. [DOI] [PubMed] [Google Scholar]
- Jonas A, Phillips MC. Lipoprotein structure. In: Vance DE, Vance JE, editors. Biochemistry of Lipids, Lipoproteins and Membranes. Amsterdam: Elsevier BV; 2008. pp. 485–506. [Google Scholar]
- Jones MK, Catte A, Patterson JC, Gu F, Chen J, Li L, Segrest JP. Thermal stability of apolipoprotein A-I in high-density lipoproteins by molecular dynamics. Biophys. J. 2009;96:354–371. doi: 10.1016/j.bpj.2008.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawooya JK, van der Horst DJ, van Heusden MC, Brigot BL, van Antwerpen R, Law JH. Lipophorin structure analyzed by in vitro treatment with lipases. J. Lipid Res. 1991;32:1781–1788. [PubMed] [Google Scholar]
- Kiss RS, Ryan RO, Hicks LD, Oikawa K, Kay CM. Physical properties of apolipoprotein A-I from the chicken, Gallus domesticus. Biochemistry. 1993;32:7872–7878. doi: 10.1021/bi00082a006. [DOI] [PubMed] [Google Scholar]
- Kiss RS, Kay CM, Ryan RO. Amphipathic alpha-helix bundle organization of lipid-free chicken apolipoprotein A-I. Biochemistry. 1999;38:4327–4334. doi: 10.1021/bi982597p. [DOI] [PubMed] [Google Scholar]
- Kiss RS, Ryan RO, Francis GA. Functional similarities of human and chicken apolipoprotein A-I: dependence on secondary and tertiary rather than primary structure. Biochim. Biophys. Acta. 2001;1531:251–259. doi: 10.1016/s1388-1981(01)00109-3. [DOI] [PubMed] [Google Scholar]
- Kiss RS, Weers PMM, Narayanaswami V, Cohen J, Kay CM, Ryan RO. Structure-guided protein engineering modulates helix bundle exchangeable apolipoprotein properties. J. Biol. Chem. 2003;278:21952–21959. doi: 10.1074/jbc.M302676200. [DOI] [PubMed] [Google Scholar]
- Kohn DW, Man CT, Hodges RS. α-Helical protein assembly motifs. J. Biol. Chem. 1997;272:2583–2586. doi: 10.1074/jbc.272.5.2583. [DOI] [PubMed] [Google Scholar]
- Kono M, Okumura Y, Tanaka M, Nguyen D, Dhanasekaran P, Lund-Katz S, Phillips MC, Saito H. Conformational flexibility of the N-terminal domain of apolipoprotein a-I bound to spherical lipid particles. Biochemistry. 2008;47:11340–11347. doi: 10.1021/bi801503r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koppaka V, Silvestro L, Engler JA, Brouillette CG, Axelsen PH. The structure of human lipoprotein A-I. Evidence for the "belt" model. J. Biol. Chem. 1999;274:14541–14544. doi: 10.1074/jbc.274.21.14541. [DOI] [PubMed] [Google Scholar]
- Koyama M, Tanaka M, Dhanasekaran P, Lund-Katz S, Phillips MC, Saito H. Interaction between the N- and C-terminal domains modulates the stability and lipid binding of apolipoprotein A-I (dagger) Biochemistry. 2009;48:2529–2537. doi: 10.1021/bi802317v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagerstedt JO, Budamagunta MS, Oda MN, Voss JC. Electron paramagnetic resonance spectroscopy of site-directed spin labels reveals the structural heterogeneity in the N-terminal domain of apoA-I in solution. J. Biol. Chem. 2007;282:9143–9149. doi: 10.1074/jbc.M608717200. [DOI] [PubMed] [Google Scholar]
- Leon LJ, Idangodage H, Wan C-PL, Weers PMM. Apolipophorin III: lipopolysaccharide binding requires helix bundle opening. Biochem. Biophys. Res. Commun. 2006;348:1328–1322. doi: 10.1016/j.bbrc.2006.07.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leroy A, Jonas A. Native-like structure and self-association behavior of apolipoprotein A-I in a water/n-propanol solution. Biochim. Biophys. Acta. 1994;1212:285–294. doi: 10.1016/0005-2760(94)90202-x. [DOI] [PubMed] [Google Scholar]
- Li H, Lyles DS, Thomas MJ, Pan W, Sorci-Thomas MG. Structural determination of lipid-bound ApoA-I using fluorescence resonance energy transfer. J. Biol. Chem. 2000;275:37048–37054. doi: 10.1074/jbc.M005336200. [DOI] [PubMed] [Google Scholar]
- Libeu CP, Lund-Katz S, Phillips MC, Wehrli S, Hernáiz MJ, Capila I, Linhardt RJ, Raffaï RL, Newhouse YM, Zhou F, Weisgraber KH. New insights into the heparan sulfate proteoglycan-binding activity of apolipoprotein. J. Biol. Chem. 2001;276:39138–39144. doi: 10.1074/jbc.M104746200. [DOI] [PubMed] [Google Scholar]
- Liu H, Scraba DG, Ryan RO. Prevention of phospholipase-C induced aggregation of low density lipoprotein by amphipathic apolipoproteins. FEBS Lett. 1993;316:27–33. doi: 10.1016/0014-5793(93)81730-n. [DOI] [PubMed] [Google Scholar]
- Lu B, Morrow JA, Weisgraber KH. Conformational reorganization of the four-helix bundle of human apolipoprotein E in binding to phospholipid. J. Biol. Chem. 2000;275:20775–20781. doi: 10.1074/jbc.M003508200. [DOI] [PubMed] [Google Scholar]
- Maiorano JN, Jandacek RJ, Horace EM, Davidson WS. Identification and structural ramifications of a hinge domain in apolipoprotein A-I discoidal high-density lipoproteins of different size. Biochemistry. 2004;43:11717–11726. doi: 10.1021/bi0496642. [DOI] [PubMed] [Google Scholar]
- Marcel YL, Kiss RS. Structure-function relationships of apolipoprotein A-I: a flexible protein with dynamic lipid associations. Curr. Opin. Lipidol. 2003;14:151–157. doi: 10.1097/00041433-200304000-00006. [DOI] [PubMed] [Google Scholar]
- Martin DD, Budamagunta MS, Ryan RO, Voss JC, Oda MN. Apolipoprotein A-I assumes a "looped belt" conformation on reconstituted high density lipoprotein. J. Biol. Chem. 2006;281:20418–20426. doi: 10.1074/jbc.M602077200. [DOI] [PubMed] [Google Scholar]
- Morrow JA, Segall ML, Lund-Katz S, Phillips MC, Knapp M, Rupp B, Weisgraber KH. Differences in stability among the human apolipoprotein E isoforms determined by the amino-terminal domain. Biochemistry. 2000;39:11657–11666. doi: 10.1021/bi000099m. [DOI] [PubMed] [Google Scholar]
- Morrow JA, Hatters DM, Lu B, Hochtl P, Oberg KA, Rupp B, Weisgraber KH. Apolipoprotein E4 forms a molten globule. A potential basis for its association with disease. J. Biol. Chem. 2002;277:50380–50385. doi: 10.1074/jbc.M204898200. [DOI] [PubMed] [Google Scholar]
- Narayanaswami V, Wang J, Kay CM, Scraba DG, Ryan RO. Disulfide bond engineering to monitor conformational opening of apolipophorin III during lipid binding. J. Biol. Chem. 1996;271:26855–26862. doi: 10.1074/jbc.271.43.26855. [DOI] [PubMed] [Google Scholar]
- Narayanaswami V, Wang J, Schieve D, Kay CM, Ryan RO. A molecular trigger of lipid-binding induced opening of a helix bundle exchangeable apolipoprotein. Proc. Natl. Acad. Sci. U.S.A. 1999;96:4366–4371. doi: 10.1073/pnas.96.8.4366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanaswami V, Ryan RO. Molecular basis of exchangeable apolipoprotein function. Biochim. Biophys. Acta. 2000;1483:15–36. doi: 10.1016/s1388-1981(99)00176-6. [DOI] [PubMed] [Google Scholar]
- Narayanaswami V, Szeto SS, Ryan RO. Lipid association-induced N- and C-terminal domain reorganization in human apolipoprotein E3. J. Biol. Chem. 2001;276:37853–37860. doi: 10.1074/jbc.M102953200. [DOI] [PubMed] [Google Scholar]
- Narayanaswami V, Maiorano JN, Dhanasekaran P, Ryan RO, Phillips MC, Lund-Katz S, Davidson WS. Helix orientation of the functional domains in apolipoprotein E in discoidal high density lipoprotein particles. J. Biol. Chem. 2004;279:14273–14279. doi: 10.1074/jbc.M313318200. [DOI] [PubMed] [Google Scholar]
- O’Connor PM, Zysow BR, Schoenhaus SA, Ishida BY, Kunitake ST, Naya-Vigne JM, Duchateau PN, Redberg RF, Spencer SJ, Mark S, Mazur M, Heilbron DC, Jaffe RB, Malloy MJ, Kane JP. J. Lipid Res. 1998;39:670–678. [PubMed] [Google Scholar]
- Palgunachari MN, Mishra VK, Lund-Katz S, Phillips MC, Adeyeye SO, Alluri S, Anantharamaiah GM, Segrest JP. Only the two end helixes of eight tandem amphipathic helical domains of human apo A-I have significant lipid affinity. Implications for HDL assembly. Arterioscler. Thromb. Vasc. Biol. 1996;16:328–338. doi: 10.1161/01.atv.16.2.328. [DOI] [PubMed] [Google Scholar]
- Panagotopulos SE, Horace EM, Maiorano JN, Davidson WS. Apolipoprotein A-I adopts a belt-like orientation in reconstituted high density lipoproteins. J. Biol. Chem. 2001;276:42965–42970. doi: 10.1074/jbc.M106462200. [DOI] [PubMed] [Google Scholar]
- Peters-Libeu CA, Newhouse Y, Hatters DM, Weisgraber KH. Model of biologically active apolipoprotein E bound to dipalmitoylphosphatidylcholine. J. Biol. Chem. 2006;281:1073–1079. doi: 10.1074/jbc.M510851200. [DOI] [PubMed] [Google Scholar]
- Peters-Libeu CA, Newhouse Y, Hall SC, Witkowska HE, Weisgraber KH. Apolipoprotein E*dipalmitoylphosphatidylcholine particles are ellipsoidal in solution. J. Lipid Res. 2007;2007(48):1035–1044. doi: 10.1194/jlr.M600545-JLR200. [DOI] [PubMed] [Google Scholar]
- Plump AS, Smith JD, Hayek T, Aalto-Setälä K, Walsh A, Verstuyft JG, Rubin EM, Breslow JL. Severe hypercholesterolemia and atherosclerosis in apolipoprotein E-deficient mice created by homologous recombination in ES cells. Cell. 1992;71:343–353. doi: 10.1016/0092-8674(92)90362-g. [DOI] [PubMed] [Google Scholar]
- Pownall HJ, Massey JB, Kusserow SK, Gotto AM., Jr Kinetics of lipid--protein interactions:effect of cholesterol on the association of human plasma high-density apolipoprotein A-I with L-alpha-dimyristoylphosphatidylcholine. Biochemistry. 1979;18:574–579. doi: 10.1021/bi00571a004. [DOI] [PubMed] [Google Scholar]
- Raussens V, Goormaghtigh E, Narayanaswami V, Ryan RO, Ruysschaert JM. Alignment of apolipophorin III α-helices in complex with dimyristoylphosphatidylcholine: a unique spatial orientation. J. Biol. Chem. 1995;270:12542–12547. doi: 10.1074/jbc.270.21.12542. [DOI] [PubMed] [Google Scholar]
- Raussens V, Narayanaswami V, Goormaghtigh E, Ryan RO, Ruysschaert JM. Hydrogen/deuterium exchange kinetics of apolipophorin-III in lipid-free and phospholipid-bound states. An analysis by Fourier transform infrared spectroscopy. J. Biol. Chem. 1996;271:23089–23095. doi: 10.1074/jbc.271.38.23089. [DOI] [PubMed] [Google Scholar]
- Raussens V, Fisher CA, Goormaghtigh E, Ryan RO, Ruysschaert JM. The low density lipoprotein receptor active conformation of apolipoprotein E. Helix organization in N-terminal domain-phospholipid disc particles. J. Biol. Chem. 1998;273:25825–25830. doi: 10.1074/jbc.273.40.25825. [DOI] [PubMed] [Google Scholar]
- Raussens V, Drury J, Forte TM, Choy N, Goormaghtigh E, Ruysschaert JM, Narayanaswami V. Orientation and mode of lipid-binding interaction of human apolipoprotein E C-terminal domain. Biochem J. 2005;387:747–754. doi: 10.1042/BJ20041536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redmond KA, Murphy C, Narayanaswami V, Kiss RS, Hauser P, Guigard E, Kay CM, Ryan RO. Replacement of helix 1' enhances the lipid binding activity of apoE3 N-terminal domain. EBS J. 2006;273:558–567. doi: 10.1111/j.1742-4658.2005.05089.x. [DOI] [PubMed] [Google Scholar]
- Rogers DP, Brouillette CG, Engler JA, Tendian SW, Roberts L, Mishra VK, Anantharamaiah GM, Lund-Katz S, Phillips MC, Ray MJ. Truncation of the amino terminus of human apolipoprotein A-I substantially alters only the lipid-free conformation. Biochemistry. 1997;36:288–300. doi: 10.1021/bi961876e. [DOI] [PubMed] [Google Scholar]
- Rogers DP, Roberts LM, Lebowitz J, Datta G, Anantharamaiah GM, Engler JA, Brouillette CG. The lipid-free structure of apolipoprotein A-I: effects of amino-terminal deletions. Biochemistry. 1998;37:11714–11725. doi: 10.1021/bi973112k. [DOI] [PubMed] [Google Scholar]
- Ryan RO, Senthilathipan KR, Wells MA, Law JH. Facilitated diacylglycerol exchange between insect hemolymph lipophorins. Properties of Manduca sexta lipid transfer particle. J. Biol. Chem. 1988;263:14140–14145. [PubMed] [Google Scholar]
- Ryan RO, Howe A, Scraba DG. Studies of the morphology and structure of the plasma lipid transfer particle from the tobacco hornworm, Manduca sexta. J Lipid Res. 1990;31:871–879. [PubMed] [Google Scholar]
- Ryan Dynamics of insect lipophorin metabolism. J. Lipid. Res. 1990;31:1725–1739. [PubMed] [Google Scholar]
- Ryan RO, Oikawa K, Kay CM. Conformational, thermodynamic and stability properties of Manduca sexta apolipophorin III. J. Biol. Chem. 1993;268:1525–1530. [PubMed] [Google Scholar]
- Ryan RO, Van der Horst DJ. Lipid transport biochemistry and role in energy production. Ann. Rev. Entomol. 2000;45:233–260. doi: 10.1146/annurev.ento.45.1.233. [DOI] [PubMed] [Google Scholar]
- Sahoo D, Weers PMM, Ryan RO, Narayanaswami V. Lipid triggered molecular switch of apoLp-III helix bundle to an extended helix conformation. J. Mol. Biol. 2002;321:201–214. doi: 10.1016/s0022-2836(02)00618-6. [DOI] [PubMed] [Google Scholar]
- Saito H, Dhanasekaran P, Baldwin F, Weisgraber KH, Lund-Katz S, Phillips MC. Lipid binding-induced conformational change in human apolipoprotein E. Evidence for two lipid-bound states on spherical particles. J. Biol. Chem. 2001;276:40949–40954. doi: 10.1074/jbc.M106337200. [DOI] [PubMed] [Google Scholar]
- Saito H, Dhanasekaran P, Nguyen D, Baldwin F, Weisgraber KH, Wehrli S, Phillips MC, Lund-Katz S. Characterization of the heparin binding sites in human apolipoprotein E. J. Biol. Chem. 2003;278:14782–14787. doi: 10.1074/jbc.M213207200. [DOI] [PubMed] [Google Scholar]
- Saito H, Lund-Katz S, Phillips MC. Contributions of domain structure and lipid interaction to the functionality of exchangeable human apolipoproteins. Prog. Lipid Res. 2004;43:350–380. doi: 10.1016/j.plipres.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Scott BR, McManus DC, Franklin V, McKenzie AG, Neville T, Sparks DL, Marcel YL. The N-terminal globular domain and the first class A amphipathic helix of apolipoprotein A-I are important for lecithin:cholesterol acyltransferase activation and the maturation of high density lipoprotein in vivo. J. Biol. Chem. 2001;276:48716–48724. doi: 10.1074/jbc.M106265200. [DOI] [PubMed] [Google Scholar]
- Segall ML, Dhanasekaran P, Baldwin F, Anantharamaiah GM, Weisgraber KH, Phillips MC, Lund-Katz S. Influence of apoE domain structure and polymorphism on the kinetics of phospholipid vesicle solubilization. J. Lipid Res. 2002;43:1688–1700. doi: 10.1194/jlr.m200157-jlr200. [DOI] [PubMed] [Google Scholar]
- Segelke BW, Forstner M, Knapp M, Trakhanov SD, Parkin S, Newhouse YM, Bellamy HD, Weisgraber KH, Rupp B. Conformational flexibility in the apolipoprotein E amino-terminal domain structure determined from three new crystal forms: implications for lipid binding. Protein Sci. 2000;9:886–897. doi: 10.1110/ps.9.5.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segrest JP, Jones MK, De Loof H, Brouillette CG, Venkatachalapathi YV, Anantharamaiah GM. The amphipathic helix in the exchangeable apolipoproteins: a review of secondary structure and function. J. Lipid Res. 1992;33:141–166. [PubMed] [Google Scholar]
- Segrest JP, Garber DW, Brouillette CG, Harvey SC, Anantharamaiah GM. The amphipathic alpha helix: a multifunctional structural motif in plasma apolipoproteins. Adv. Protein Chem. 1994;45:303–369. doi: 10.1016/s0065-3233(08)60643-9. [DOI] [PubMed] [Google Scholar]
- Shapiro JP, Law JH, Wells MA. Lipid transport in insects. Annu. Rev. Entomol. 1988;33:297–318. doi: 10.1146/annurev.en.33.010188.001501. [DOI] [PubMed] [Google Scholar]
- Shimano H, Yamada N, Katsuki M, Yamamoto K, Gotoda T, Harada K, Shimada M, Yazaki Y. Plasma lipoprotein metabolism in transgenic mice overexpressing apolipoprotein E. Accelerated clearance of lipoproteins containing apolipoprotein B. J. Clin. Invest. 1992;90:2084–2091. doi: 10.1172/JCI116091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva RA, Huang R, Morris J, Fang J, Gracheva EO, Ren G, Kontush A, Jerome WG, Rye KA, Davidson WS. Structure of apolipoprotein A-I in spherical high density lipoproteins of different sizes. Proc. Natl. Acad. Sci. USA. 2008;105:12176–12181. doi: 10.1073/pnas.0803626105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivashanmugam A, Wang J. A unified scheme for initiation and conformational adaptation of human apolipoprotein E N-terminal domain upon lipoprotein binding and for receptor binding activity. J. Biol. Chem. 2009;284:14657–14666. doi: 10.1074/jbc.M901012200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smolenaars MM, Kasperaitis MA, Richardson PE, Rodenburg KW, Van der Horst DJ. Biosynthesis and secretion of insect lipoprotein: involvement of furin in cleavage of the apoB homolog, apolipophorin-II/I. J. Lipid Res. 2005;46:412–421. doi: 10.1194/jlr.M400374-JLR200. [DOI] [PubMed] [Google Scholar]
- Soulages JL, Wells MA. Lipophorin: the structure of an insect lipoprotein and its role in lipid transport in insects. Adv. Prot. Chem. 1994a;45:371–415. doi: 10.1016/s0065-3233(08)60644-0. [DOI] [PubMed] [Google Scholar]
- Soulages JL, Wells MA. Effect of diacylglycerol content on some physicochemical properties of the insect lipoprotein, lipophorin. Correlation with the binding of apolipophorin-III. Biochemistry. 1994b;33:2356–2362. doi: 10.1021/bi00175a002. [DOI] [PubMed] [Google Scholar]
- Soulages JL, Bendavid OJ. The lipid binding activity of the exchangeable apolipoprotein apolipophorin-III correlates with the formation of a partially folded conformation. Biochemistry. 1998;37:10203–10210. doi: 10.1021/bi980622l. [DOI] [PubMed] [Google Scholar]
- Soulages JL, Arrese EL. Fluorescence spectroscopy of single tryptophan mutants of apolipophorin-III in discoidal lipoproteins of dimyristoylphosphatidylcholine. Biochemistry. 2000;34:10574–10580. doi: 10.1021/bi0007223. [DOI] [PubMed] [Google Scholar]
- Soulages JL, Arrese EL. Interaction of the alpha-helices of apolipophorin III with the phospholipid acyl chains in discoidal lipoprotein particles: a fluorescence quenching study. Biochemistry. 2001;40:14279–14290. doi: 10.1021/bi010949d. [DOI] [PubMed] [Google Scholar]
- Surewicz WK, Epand RM, Pownall HJ, Hui SW. Human apolipoprotein A-I forms thermally stable complexes with anionic but not with zwitterionic phospholipids. J. Biol. Chem. 1986;261:16191–16197. [PubMed] [Google Scholar]
- Tanaka M, Dhanasekaran P, Nguyen D, Ohta S, Lund-Katz S, Phillips MC, Saito H. Contributions of the N- and C-terminal helical segments to the lipid-free structure and lipid interaction of apolipoprotein A-I. Biochemistry. 2006;45:10351–10358. doi: 10.1021/bi060726t. [DOI] [PubMed] [Google Scholar]
- Thomas MJ, Bhat S, Sorci-Thomas MG. The use of chemical cross-linking and mass spectrometry to elucidate the tertiary conformation of lipid-bound apolipoprotein A-I. Curr. Opin. Lipidol. 2006;17:214–220. doi: 10.1097/01.mol.0000226111.05060.f4. [DOI] [PubMed] [Google Scholar]
- Thomas MJ, Bhat S, Sorci-Thomas MG. Three-dimensional models of HDL apoA-I: implications for its assembly and function. J. Lipid Res. 2008;49:1875–1783. doi: 10.1194/jlr.R800010-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tricerri MA, Behling Agree AK, Sanchez SA, Bronski J, Jonas A. Arrangement of apolipoprotein A-I in reconstituted high-density lipoprotein disks: an alternative model based on fluorescence resonance energy transfer experiments. Biochemistry. 2001;40:5065–5074. doi: 10.1021/bi002815q. [DOI] [PubMed] [Google Scholar]
- Van Antwerpen R, Linnemans WAM, Van der Horst DJ, Beenakkers AMTh. Immunocytochemical localization of lipophorins in the flight muscles of the migratory locust (Locusta migratoria) at rest and during flight. Cell Tissue Res. 1998;252:661–668. doi: 10.1007/BF00216654. [DOI] [PubMed] [Google Scholar]
- Van Hoof D, Rodenburg KW, Van der Horst DJ. Insect lipoprotein follows a transferrin-like recycling pathway that is mediated by the insect LDL receptor homologue. J. Cell Sci. 2002;115:4001–4012. doi: 10.1242/jcs.00113. [DOI] [PubMed] [Google Scholar]
- Van der Horst DJ, Ryan RO, Van Heusden MC, Schulz TKF, Van Doorn JM, Law JH, Beenakkers AMTh. An insect lipoprotein hybrid helps to define the role of apolipophorin III. J. Biol. Chem. 1988;263:2027–2033. [PubMed] [Google Scholar]
- Van der Horst DJ. Lipid transport function of lipoproteins in flying insects. Biochim. Biophys. Acta. 1990;1047:195–211. doi: 10.1016/0005-2760(90)90518-3. [DOI] [PubMed] [Google Scholar]
- Van der Horst DJ, Van Doorn JM, Voshol H, Kanost MR, Ziegler R, Beenakkers AMTh. Different isoforms of an apoprotein (apolipophorin III) associate with lipoproteins in Locusta migratoria. Eur. J. Biochem. 1991;196:509–517. doi: 10.1111/j.1432-1033.1991.tb15843.x. [DOI] [PubMed] [Google Scholar]
- Van der Horst DJ, Van Marrewijk WJA, Diederen JHB. Adipokinetic hormones of insect: release, signal transduction, and responses. Int. Rev. Cytol. 2001;211:179–240. doi: 10.1016/s0074-7696(01)11019-3. [DOI] [PubMed] [Google Scholar]
- Van der Horst DJ, Van Hoof D, Van Marrewijk WJA, Rodenburg KW. Alternative lipid mobilization: the insect shuttle system. Mol. Cell. Biochem. 2002;239:113–119. [PubMed] [Google Scholar]
- Van der Horst DJ, Roosendaal SD, Rodenburg KW. Circulatory lipid transport: lipoprotein assembly and function from an evolutionary perspective. Mol Cell Biochem. 2009 doi: 10.1007/s11010-008-0011-3. in press. [DOI] [PubMed] [Google Scholar]
- Van Heusden MC, Law JH. An insect lipid transfer particle promotes lipid loading from fat body to lipoprotein. J. Biol. Chem. 1989;264:17287–17292. [PubMed] [Google Scholar]
- Wang J, Sykes BD, Ryan RO. Structural basis for the conformational adaptability of apolipophorin III, a helix-bundle exchangeable apolipoprotein. Proc. Natl. Acad. Sci. USA. 2002;99:1188–1193. doi: 10.1073/pnas.032565999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Rader DJ. Molecular regulation of macrophage reverse cholesterol transport. Curr. Opin. Cardiol. 2007;22:368–372. doi: 10.1097/HCO.0b013e3281ec5113. [DOI] [PubMed] [Google Scholar]
- Wald JH, Goormaghtigh E, De Meutter J, Ruysschaert JM, Jonas A. Investigation of the lipid domains and apolipoprotein orientation in reconstituted high density lipoproteins by fluorescence and IR methods. J. Biol. Chem. 1990;265:20044–20050. [PubMed] [Google Scholar]
- Weers PMM, Kay CM, Oikawa K, Wientzek M, Van der Horst DJ, Ryan RO. Factors affecting the stability and conformation of Locusta migratoria apolipophorin III. Biochemistry. 1994;33:3617–3624. doi: 10.1021/bi00178a019. [DOI] [PubMed] [Google Scholar]
- Weers PMM, Narayanaswami V, Kay CM, Ryan RO. Interaction of an exchangeable apolipoprotein with phospholipid vesicles and lipoprotein particles. Role of leucines 32, 34, and 95 in Locusta migratoria apolipophorin III. J. Biol. Chem. 1999;274:21804–21810. doi: 10.1074/jbc.274.31.21804. [DOI] [PubMed] [Google Scholar]
- Weers PMM, Van der Horst DJ, Ryan RO. Interaction of locust apolipophorin III with lipoproteins and phospholipid vesicles: effect of glycosylation. J. Lipid Res. 2000a;41:416–423. [PubMed] [Google Scholar]
- Weers PMM, Prenner EJ, Kay CM, Ryan RO. Lipid binding of the exchangeable apolipoprotein, apolipophorin III induces major changes in fluorescence properties of Trp 115 and Trp 130. Biochemistry. 2000b;39:6874–6880. doi: 10.1021/bi992891x. [DOI] [PubMed] [Google Scholar]
- Weers PMM, Narayanaswami V, Ryan RO. Modulation of the lipid binding properties of the N-terminal domain of human apolipoprotein E3. Eur. J. Biochem. 2001a;268:3728–3735. doi: 10.1046/j.1432-1327.2001.02282.x. [DOI] [PubMed] [Google Scholar]
- Weers PMM, Kay CM, Ryan RO. Conformational changes of an exchangeable apolipoprotein, apolipophorin III from Locusta migratoria, at low pH: correlation with lipid binding. Biochemistry. 2001b;40:7754–7760. doi: 10.1021/bi010410f. [DOI] [PubMed] [Google Scholar]
- Weers PMM, Cabrera J, Abdullahi WE, Hsu T-C. Role of buried polar residues in helix bundle stability and lipid binding of apolipophorin III: destabilization by threonine 31. Biochemistry. 2005;44:8810–8816. doi: 10.1021/bi050502v. [DOI] [PubMed] [Google Scholar]
- Weers PMM, Ryan RO. Apolipophorin III: role model apolipoprotein. Insect Biochem. Mol. Biol. 2006;36:231–240. doi: 10.1016/j.ibmb.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Weisgraber KH. Apolipoprotein E: structure-function relationships. Adv. Protein Chem. 1994;45:249–302. doi: 10.1016/s0065-3233(08)60642-7. [DOI] [PubMed] [Google Scholar]
- Wells MA, Ryan RO, Kawooya JK, Law JH. The role of apolipophorin III in vivo lipoprotein interconversions in adult Manduca sexta. J. Biol. Chem. 1987;262:4172–4176. [PubMed] [Google Scholar]
- Westerlund JA, Weisgraber KH. Discrete carboxyl-terminal segments of apolipoprotein E mediate lipoprotein association and protein oligomerization. J. Biol. Chem. 1993;268:15745–15750. [PubMed] [Google Scholar]
- Wetterau JR, Aggerbeck LP, Rall SC, Jr, Weisgraber KH. Human apolipoprotein E3 in aqueous solution. I. Evidence for two structural domains. J. Biol. Chem. 1988;263:6240–6248. [PubMed] [Google Scholar]
- Wientzek M, Kay CM, Oikawa K, Ryan RO. Binding of insect apolipophorin III to dimyristoylphosphatidylcholine vesicles. Evidence for a conformational change. J. Biol. Chem. 1994;269:4605–4612. [PubMed] [Google Scholar]
- Wilson C, Wardell MR, Weisgraber KH, Mahley RW, Agard DA. Three-dimensional structure of the LDL receptor-binding domain of human apolipoprotein E. Science. 1991;252:1817–1822. doi: 10.1126/science.2063194. [DOI] [PubMed] [Google Scholar]
- Wilson C, Mau T, Weisgraber KH, Wardell MR, Mahley RW, Agard DA. Salt bridge relay triggers defective LDL receptor binding by a mutant apolipoprotein. Structure. 1994;2:713–718. doi: 10.1016/s0969-2126(00)00072-1. [DOI] [PubMed] [Google Scholar]
- Yokoyama S. Apolipoprotein-mediated cellular cholesterol efflux. Biochim. Biophys. Acta. 1998;1392:1–15. doi: 10.1016/s0005-2760(98)00032-0. [DOI] [PubMed] [Google Scholar]
- Zhang SH, Reddick RL, Piedrahita JA, Maeda N. Spontaneous hypercholesterolemia and arterial lesions in mice lacking apolipoprotein E. Science. 1992;258:468–471. doi: 10.1126/science.1411543. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Vasudevan S, Sojitrawala R, Zhao W, Cui C, Xu C, Fan D, Newhouse Y, Balestra R, Jerome WG, Weisgraber K, Li Q, Wang J. A monomeric, biologically active, full-length human apolipoprotein E. Biochemistry. 2007;46:10722–10732. doi: 10.1021/bi700672v. [DOI] [PubMed] [Google Scholar]
- Zhong N, Weisgraber KH. Understanding the association of apolipoprotein E4 with Alzheimer disease: Clues from its structure. J. Biol. Chem. 2009;284:6027–6031. doi: 10.1074/jbc.R800009200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu HL, Atkinson D. Conformation and lipid binding of a C-terminal (198–243) peptide of human apolipoprotein A-I. Biochemistry. 2007;46:1624–1634. doi: 10.1021/bi061721z. [DOI] [PMC free article] [PubMed] [Google Scholar]