Abstract
The pathogenesis of sickle cell disease (HbSS), which has numerous complications including stroke, involves inflammation resulting in alteration of plasma inflammatory protein concentration. We investigated HbSS children with abnormal cerebral blood flow detected by trans-cranial Doppler ultrasound (TCD) who participated in multi-center stroke prevention (STOP) study, to determine if plasma inflammatory protein concentration is associated with the outcome of stroke in the STOP study. Thirty-nine plasma samples from HbSS participants with elevated TCD who had no stroke, HbSS-NS (n=13) or had stroke, HbSS-S (n=13), HbSS steady-state controls (n=7) and controls with normal hemoglobin, HbAA (n=6), were analyzed simultaneously for 27 circulating inflammatory proteins. Logistic regression and receiver operating characteristics curve analysis of stroke on plasma inflammatory mediator concentration, adjusted for age and gender, demonstrated that interleukin-1β (IL-1β) was protective against stroke development (HbSS-NS = 19, 17–23, HbSS-S = 17, 16 – 19 pg/mL, median and 25th–75th percentile; Odds ratio = 0.59, C.I. = 0.36 – 0.96) and was a good predictor of stroke (area under curve = 0.852). This result demonstrates a strong association of systemic inflammation with stroke development in HbSS via moderately increased plasma IL-1β concentration, which is furthermore associated with a decreased likelihood of stroke in HbSS.
Keywords: Sickle cell, Stroke, Interleukin-1β, Cytokine, Chemokine
1. Introduction
Inflammation has a central role in the pathogenesis of sickle cell disease (HbSS)[1] which has numerous complications including cerebro-vascular disease [2] that may culminate in stroke [3]. The risk of development and severity of stroke and other neurodegenerative diseases have been associated with altered plasma concentration of specific inflammatory mediators [4]. The clinical course of HbSS varies widely amongst affected individuals in spite of the shared mutation in the β globin chains of the hemoglobin (Hb) molecule, which accounts for the basic pathology and this has prompted the evaluation of other biochemical pathways besides hemoglobin for answers to the clinical variability. In this study we investigated the association of inflammation with the risk of developing stroke in HbSS children with abnormal cerebral blood flow, by assaying steady state plasma levels for specific inflammatory mediators. The role of plasma inflammatory mediators in sickle cell disease stroke has not been examined as fully as for stroke in the general population and in experimental stroke in animal models. The role of circulating C-reactive protein (CRP) and interleukin (IL)-6 in severity of HbSS was recently demonstrated in sickle mice [5] and children [6], although their predictive value for stroke risk was not ascertained.
Researchers have demonstrated that following brain insult cytokine levels are elevated as a result of increased production from inflammatory cells, glia and neurons [7, 8] with IL-1, IL-6, IL-10, tumor necrosis factor-alpha (TNF-α) and transforming growth factor-beta (TGF-β) being the most studied for stroke [9]. IL-1β and TNF-α have been associated with exacerbation of injury in stroke while IL-6, IL-10 and TGF-β have been found to be neuroprotective [10]. Table 1 summarizes some reported roles of specific cytokine/chemokines in cerebral ischemia and stroke in the general population as well as animal models.
Table 1.
Reported roles of some cytokines/chemokines in cerebral ischemia and stroke
| Name | Sources in Neuroinflammation |
Reported Role in Stroke | Role in Inflammation |
Reference |
|---|---|---|---|---|
| IL-1β | Microglia, astrocytes, neurons, endothelial cell and peripheral immune cells. |
1. Astrocyte activation resulting in increased antioxidant defense. 2. Administration results in increased brain injury in experimental stroke. 3. Reduction of cerebral blood flow. Endothelial cell activation. |
Pro- inflammatory |
[11,20–22,26] |
| IL-1RA | Microglia | Administration rIL-1RA associated with reduced infarct size in experimental stroke. |
Anti- inflammatory |
[23,24] |
| TNF-α | Neurons, microglia, astrocytes and peripheral immune cells |
1. Functions are pleiotropic. 2. Inhibition decrease brain injury. |
Pro- inflammatory |
[7,37–45] |
| 3. Administration of recombinant increases brain injury. |
||||
| 4. Involved in ischemic tolerance which is protective |
||||
| IL-6 | Neurons, atrocytes and peripheral immune cells |
1. Unclear. 2. Lower levels associated |
Pro- inflammatory |
[46] |
| with better outcome in ischemic stroke treated with rIL-1RA. |
||||
| IL-10 | Microglia | 1. Inhibits IL-1 and TNF- alpha expression. Suppresses cytokine receptor expression. |
Anti- inflammatory |
[47–52] |
| 2. Increased levels appear beneficial in cerebral ischemia. |
||||
| 3. Low levels associated with increased stroke risk. |
||||
| TGF-β1 | Astrocytes, microglia and neurons |
Increased expression protective of ischemic neuron damage. |
Anti- inflammatory |
[53–56] |
| Fractalkine | Viable neurons at periphery of cerebral infarct |
Deficiency associated with smaller infract size and lower mortality following transient cerebral ischemia. |
Pro- inflammatory |
[57–59] |
| MCP-1 | Microglia | Inhibition/deficiency associated with reduced cerebral injury following cerebral ischemia. |
Pro- inflammatory |
[60,61] |
| MIP-1α | Microglia | Inhibition/deficiency associated with reduced cerebral injury following cerebral ischemia. |
Pro- inflammatory |
[60,61] |
The goal of this study was to identify plasma inflammatory proteins that are associated with and could predict development of stroke in children with HbSS and abnormal TCD. The hypothesis is that alterations in plasma inflammatory protein concentrations in HbSS individuals with abnormal TCD can predict the likelihood stroke occurrence. We selected cytokines, chemokines and growth factors known to mediate neuro-inflammation and others that have not yet been investigated for this reason.
2. Subjects and Methods
2.1 Subjects
Approximately 11% of HbSS individuals develop overt stroke by age 20 years, the majority of which are ischemic strokes with a few being hemorrhagic [11–13]. Between 1995 and 1997 the Stroke Prevention (STOP) trial in sickle cell anemia was conducted using TCD to detect abnormal cerebral blood flow. One thousand nine hundred and thirty four children 2 to 16 years of age with homozygous HbSS genotype SS or Sβ0-thalassemia and no history of a previous stroke were screened at 14 STOP trial centers via TCD blood flow velocity measurement in the distal internal carotid artery or the proximal middle cerebral artery. Mean velocity measurements below 170 cm/sec were considered normal and those above 200 cm/sec on at least 2 separate occasions were considered abnormal. Two hundred and six individuals qualified for the study, 130 (60 male and 70 female) gave informed consent and were randomly assigned to either standard care (SC), n=67 or the transfusion (TX) arm, n=63, of the study. Annual TCDs were performed during the follow up period with stroke as the primary endpoint, determined by a blinded panel of neurologists who reviewed the clinical and imaging data. The SC arm demonstrated a significantly higher rate of stroke compared with the TX arm during the second interim analysis leading to a premature closure of the study [11, 14]. This present study was ancillary to the STOP study, enabling measurement of the stored anonymized plasma samples that were collected from the study participants for baseline biochemical analysis, upon recruitment into the study. Institutional review boards of Morehouse School of medicine, Medical College of Georgia and the New England Research Institutes, approved this ancillary study.
The stroke group constitutes individuals in the STOP study who developed stroke (HbSS-S) irrespective of the arm of the study to which they belonged (n=13). The no stroke group is composed of participants in the STOP study who did not develop stroke (HbSS-NS) during the follow up period (n=13). HbAA controls are healthy individuals with normal Hb (n=6), and age and race matched for comparison with the HbSS-NS and HbSS-S groups. Steady-State HbSS controls are individuals with no history of stroke or signs indicative of stroke at physical examination and did not have infection or sickle cell crisis at the time of sample collection (n=7). These controls were also age and race matched.
2.2 Samples
The test plasma samples for the STOP study were collected upon recruitment, before any intervention was made, and were stored at −80 degrees Celsius. Plasma samples from HbAA and HbSS individuals in steady state with no history or physical evidence of cerebro-vascular complications provided additional controls.
2.3 Multiplex Microsphere Immunoassay
Duplicate measurements from 50 µl plasma samples (n = 39) were made simultaneously to determine circulating levels of 27 inflammatory proteins [IL-1β, IL-1RA, IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-9, IL-10, IL-12, IL-13, IL-15, IL-17, eotaxin, fibrocyte growth factor (FGF) basic protein, granulocyte-colony stimulating factor (G-CSF), granulocyte monocyte-colony stimulating factor (GM-CSF), interferon-gamma (IFN-γ), 10 kDa interferon-gamma-induced protein (IP-10), MCP-1, MIP-1α, macrophage inflammatory protein-1beta (MIP-1β), platelet derived growth factor −bb (PDGF-bb), regulated upon activation, normal T-cell expressed and secreted (RANTES), TNF-α and vascular endothelial growth factor (VEGF)], using a commercially available multiplex calorimetric bead-based protein array system, the Bio-Rad Bioplex Beadlyte system (Bio-Rad, Hercules, CA) powered by Luminex and using human-specific bead sets. Assays were conducted as instructed by the manufacturer. The results were interpolated from 5-parameter-fit curves generated using the relevant recombinant human protein standards. The samples were tested at a 1:4 dilution.
Intra-assay variability of the duplicate determinations was calculated and expressed as percentage coefficient of variation (CV %) and presented as mean CV±SD% as follows: IL-1β = 8.3 ± 7.8%, IL-1RA = 9.2 ± 6.9%, IL-4 = 9.5 ± 7.1%, IL-5 = 8.7 ± 6.7%, IL-6 = 8.7 ± 11.9%, IL-7 = 8.0 ± 4.9%, IL-8 = 7.7 ± 6.3%, IL-10 = 7.8 ± 4.7%, IL-12 = 7.7 ± 5.0%, IL-13 = 7.8 ± 6.9%, IL-17 = 9.2 ± 10.4%, Eotaxin = 8.9 ± 8.3%, G-CSF = 8.9 ± 7.9%, GM-CSF = 9.9 ± 7.0%, IFN-γ = 7.7 ± 4.5%, IP-10 = 9.6 ± 6.3%, MCP-1 = 9.3 ± 7.0%, MIP-1α = 8.8 ± 6.9%, MIP-1β = 9.0 ± 8.1%, PDGF-bb = 5.3 ± 4.1%, TNF-α = 9.0 ± 6.1%, VEGF = 5.9 ± 4.7%.
2.4 Statistical Analysis
The data were depicted graphically using box plots showing the median, 25th and 75th percentiles, bars for 10th and 90th percentiles and values outside 10th and 90th percentiles were plotted as points. Analysis by logistic regression of stroke on plasma cytokine concentrations adjusted for age and gender was followed by Receiver Operating Characteristics (ROC) curve analyses to determine good predictors of stroke based on Area Under Curve (AUC). The level of statistical significance was set at P<0.05 without correction for multiple testing. This approach was used because all the cytokines/chemokines measured represent the single assessment of the level of systemic inflammation in relation to stroke. Hence, the null hypotheses are interrelated to address a single outcome [15]. These data were analyzed using SigmaPlot 2006 (version 10.0) with SigmaStat (version 3.5) integration (Chicago, IL) for windows and STATA (version 10.0, College Station, TX, USA) software.
3. Results
Table 2 shows some demographic characteristics of the STOP study participants. Mean age was similar for the study participants who did not get stroke (HbSS-NS) compared with those who developed stroke (HbSS-S), but the gender distribution differed, with the HbSS-NS group having a majority of males (62%), whereas the HbSS-S group had a majority of females (62%).
Table 2.
Demographics Characteristics of STOP study participants
| Characteristics | Hbss No Stroke | Hbss Stroke |
|---|---|---|
| Number | 13 | 13 |
| Gender (male/female) | 8/5 | 5/8 |
| Mean age (years) | 8.7 | 7.9 |
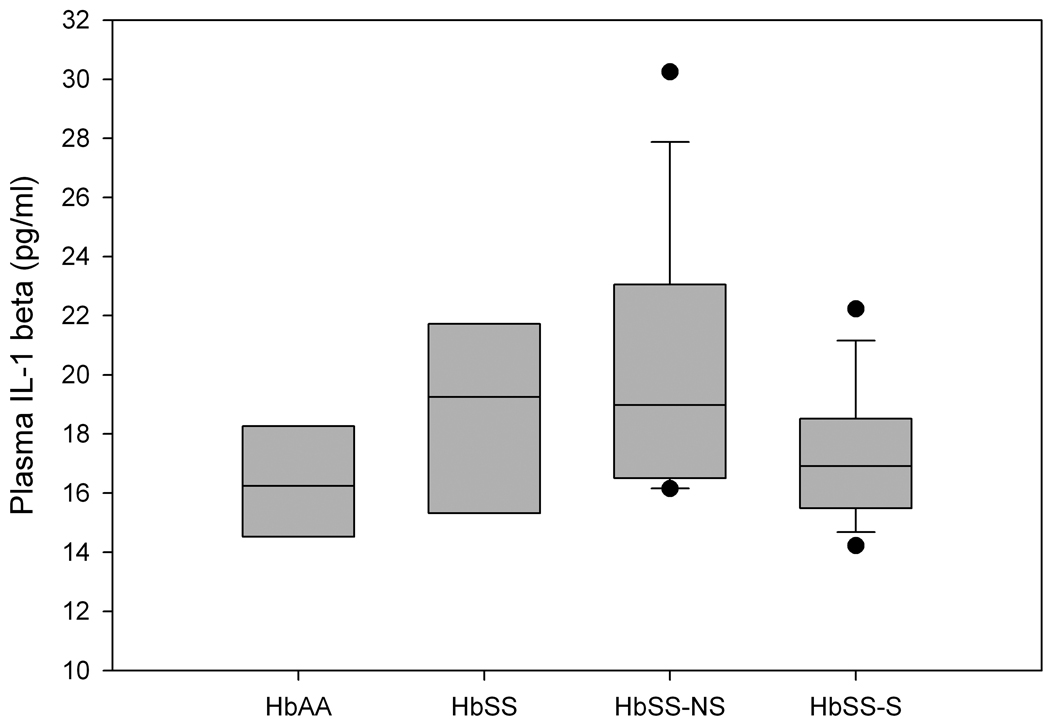
The median concentrations for 22 of the 27 inflammatory proteins measured are shown in Table 3. The concentrations for 5 of the analytes could not be determined at 1 in 4 dilution of samples; IL-2, IL-9, IL-15 and FGF basic protein fell below the minimum concentration detectable by the assay (10 pg/ml) while RANTES was above the maximum concentration detectable (24,512 pg/ml). Of those that were measured, IL-4, IL-5, IL-6, IL-7, IL-10, IL-12, IL-13, IL-17, IFN-γ and G-CSF showed a trend in which the median concentration was highest in the steady-state HbSS group compared with the other test groups. The HbSS-NS group had the highest median compared with the other groups for circulating IL-1RA, VEGF and PDGF-bb levels. The steady-state HbSS and HbSS-NS groups had elevated median concentrations of IL-1β (Figure 1) and MIP-1β (Table 3) compared with the other groups. The median levels of MIP-1α, MCP-1 and IP-10 in the 2 test groups (HbSS-NS and HbSS-S) were similar. The highest median of eotaxin was observed in the HbSS-S group followed by the HbSS-NS, with HbAA control and steady-state HbSS being comparable.
Table 3.
Median plasma cytokine concentration in pg/ml (25th, 75th %ile)
| Cytokine | HbAA Controls | HbSS Controls | HbSS No Stroke | HbSS Stroke |
|---|---|---|---|---|
| L-1β | 16 (15, 18) | 19 (16, 22) | 19 (17, 23) | 17 (16, 18) |
| IL-1RA | 98 (77, 217) | 304 (243, 380) | 941 (260, 1491) | 287 (242, 477) |
| IL-4 | 3 (3, 4) | 6 (3, 6) | 3 (3, 4) | 3 (3, 4) |
| IL-5 | 12 (10, 13) | 22 (15, 22) | 13 (11, 13) | 13 (11, 14) |
| IL-6 | 9 (6, 10) | 10 (7, 16) | 11 (9, 13) | 10 (8, 14) |
| IL-7 | 29 (17, 40) | 44 (26, 56) | 25 (21, 29) | 22.50 (21, 27) |
| IL-8 | 9 (5, 14) | 15 (7, 27) | 12 (10, 29) | 16 (9, 19) |
| IL-10 | 11 (10, 13) | 16 (10, 19) | 12 (10, 16) | 11 (11, 13) |
| IL-12 | 14 (12, 18) | 20 (11, 28) | 14 (12, 19) | 11 (10, 15) |
| IL-13 | 16 (15, 21) | 20 (14, 25) | 15 (12, 21) | 10 (8, 17) |
| IL-17 | 40 (9, 65) | 96 (58, 139) | 6 (5, 19) | 32 (26, 44) |
| Eotaxin | 58 (49, 69) | 58 (38, 147) | 85 (64, 133) | 109 (81, 151) |
| G-CSF | 106 (78, 191) | 252 (97, 369) | 114 (95, 149) | 89 (86, 107) |
| GM-CSF | 43 (43, 43) | 82 (53, 110) | 173 (125, 221) | 10 (7, 77) |
| IFN-γ | 112 (99, 169) | 277 (124, 320) | 149 (115, 179) | 114 (99, 155) |
| IP-10 | 1171 (973, 1292) | 1279 (949, 2036) | 1248 (801, 1724) | 1166 (651, 1957) |
| MCP-1 | 26 (18, 42) | 17 (11, 28) | 33 (16, 116) | 27 (13, 41) |
| MIP-1α | 5 (4, 6) | 26 (12, 329) | 4 (4, 7) | 5 (4, 21) |
| MIP-1β | 96 (66, 150) | 245 (73, 325) | 254 (188, 393) | 205 (152, 367) |
| PDGF-bb | 4697 (2873, 71856) | 3876 (2194, 7100) | 8937 (6022, 13342) | 7960 (5024, 11932) |
| TNF-α | 57 (46, 70) | 124 (62, 148) | 57 (41, 65) | 58 (48, 73) |
| VEGF | 60 (19, 161) | 73 (35, 350) | 243 (78, 394) | 108 (52, 177) |
%ile, percentile
Figure 1.
Comparison of circulating IL-1β for HbAA controls, HbSS steady-state controls, HbSS-NS STOP study subjects with no stroke and HbSS-S STOP study subjects who developed stroke. Values are median plus 25th and 75th percentiles, bars represent 10th and 90th percentiles and points represent values outside of the 10th and 90th percentiles. Using Dunn’s Method for pair-wise comparison there was no statistically significant difference in the median concentration of IL-1β between the groups.
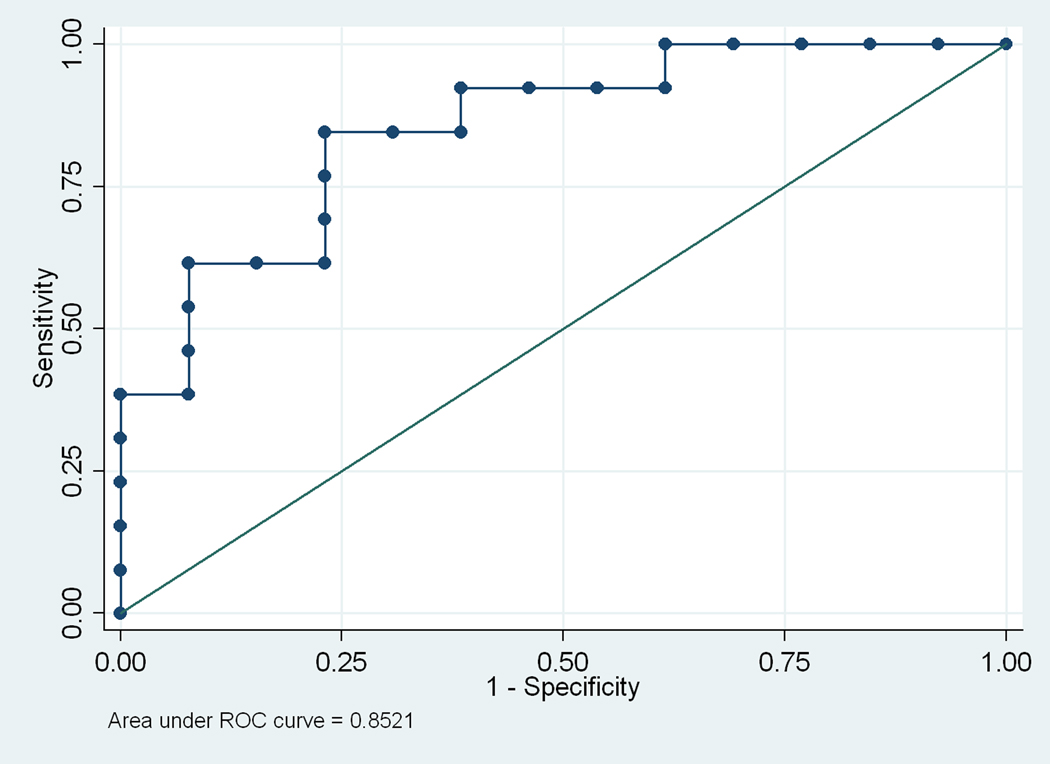
Analysis of the effects of plasma inflammatory protein concentration on the likelihood of a completed stroke revealed that IL-1β and VEGF impacted the outcome of stroke (P<0.05), but the odds ratio (OR) of VEGF (OR, 0.99) indicated that it had little or no effect on the likelihood of developing stroke. These results therefore revealed IL-1β (OR 0.59) as the only inflammatory protein, among those measured, which could protect against stroke development (Table 4). The data also indicate that IL-1β is a good biomarker for predicting the likelihood of completed stroke in HbSS individuals with abnormal cerebral blood flow, as demonstrated by the significant area under the ROC curve (AUC = 0.85), which is just short of 1.00, the maximum attainable area for an ideal biomarker (Figure 2).
Table 4.
AuC post-correlation after logistic regression of stroken on test variable adjusted for age and gender
| Test Variable | Odds Ratio (95% CI) | P-value | AUC |
|---|---|---|---|
| L-1β | 0.59 (0.36–0.96) | 0.034 | 0.852 |
| VEGF | 0.99 (0.98–1.00) | 0.048 | 0.799 |
| IL-5 | 1.59 (0.96–2.63) | 0.074 | 0.799 |
| IL-1RA | 1.00 (0.99–1.00) | 0.161 | 0.755 |
| PDGF-bb | 1.00 (0.99–1.00) | 0.171 | 0.769 |
| G-CSF | 0.99 (0.97–1.01) | 0.252 | 0.757 |
| IFN-γ | 0.99 (0.98–1.01) | 0.411 | 0.746 |
Values are odds ratios (95% confidence intervals, CI) and corresponding p-values. AUC, area under curve. Considering the odds ratio and AUC, IL-1β emerges as the best cytokine, which is associated with a reduced risk and a good predictor of development of HbSS stroke in participants with abnormal TCD. VEGF shows some statistical significance, however the odds ratio is approximately 1, indicating this growth factor is a poor predictor of stroke risk in these HbSS patients.
Figure 2.
Receiver operating characteristics curve of stroke on plasma IL-1β concentration, adjusted for age and gender. This ROC curve shows that IL-1β is a good biomarker for predicting the likelihood of stroke development in HbSS individuals with abnormal cerebral blood flow determined by TCD. The area under the ROC curve of 0.85 is good, since 1.00 is the maximum attainable by an ideal biomarker
4. Discussion
HbSS cerebro-vascular complications involve narrowing of the cerebral blood vessels due to changes in the intima and media of these vessels, induced by inflammatory mediators. This leads to compensatory increase in the velocity of blood flow in narrowed portions of the vessels, based on a derivation of Bernoulli’s principle of fluid dynamics [16]. The detection of an abnormal TCD therefore implies some level of stenosis of cerebral vessels causing increased blood flow velocity in the cerebral vasculature [16, 17]. Effects of the stenosis include cerebral hypoxia and turbulent blood flow, associated with increased inflammation via activation of the vascular endothelium. Animal models of cerebral ischemia disclose a key role for the IL-1 family in regulating blood flow in the presence of cerebral ischemia as well as activating cerebral vascular endothelial cells to produce adhesion molecules and chemokines that increase recruitment of inflammatory cells. Cerebral hypoxia stimulates the production of IL-1β from microglia, astrocytes, neurons and endothelial cells, with some contribution from peripheral immune cells. The IL-1β secretion prompts cerebral vessel endothelial production of chemokine (C-C motif) ligand 2 (CCL2) chemokines as well as increased expression of intercellular adhesion molecule-1 (ICAM-1), E-selectin and P-selectin, in effect promoting acute inflammation [18, 19].
Interestingly, IL-1β has been implicated in both deleterious and beneficial roles in cerebral ischemia. For example, experimental transient global cerebral ischemia in rats leads to increased IL-1β mRNA and protein [16, 20, 21] and increased brain damage occurs when IL-1β is administered to rats prior to inducing cerebral ischemia [20, 22]. Inhibiting IL-1β by its naturally occurring competitive inhibitor, interleukin-1 receptor antagonist (IL-1RA), further elucidates its role. Reduced infarct size is associated with the administration of recombinant IL-1RA or over expression of IL-1RA prior to cerebral ischemia in affected mice compared with controls [23], whereas increased damage is found in IL-1RA deficient IL-1RA knockout mice [24]. Inactivation or knockout of interleukin-1 receptor 1(IL-1R1) also reduced cerebral ischemic damage [25]. Conversely, increased IL-1β following cerebral insult is associated with more mRNA expression of ceruloplasmin in astrocytes. Ceruloplasmin has potent antioxidant properties and catalyzes the dismutation of free radicals, thus affording protection to the brain [26]. Cytokine activated astrocytes produce trophic factors for the maintenance of the cerebro-vascular endothelium, restoration of the blood brain barrier (BBB) and promotion of angiogenesis demonstrated in experiments with IL-1β null mice, where animals lacking IL-1β showed less astrocyte reactivity 2 to 3 days following cortical lesion and increased permeability of the BBB at 7 days post lesion compared with wild-type controls [27]. These results link the early activation of astrocytes and their subsequent role in cerebro-vascular repair to the production of IL-1β.
The results of this study confirm a significant role for IL-1 in HbSS stroke by demonstrating that plasma IL-1β is a good predictor for an outcome of stroke and is associated with protection against stroke development in HbSS children with abnormal TCD. This observation is consistent with available evidence concerning association of systemic inflammation with development of cerebro-vascular disease. It indicates that modestly increased levels of IL-1β may be beneficial in protecting the brain from ischemia. Mild hypoxic insult has been shown to precondition the brain and decrease the extent of damage caused by subsequent severe events [28]. Levels of glycogen in astrocytes are increased when they are activated in the process of ischemic preconditioning under the influence of insulin-like growth factor 1 (IGF-1)[29] which is downstream from IL-1β signaling [28]. Together, these observations support the notion that cytokine activated astrocytes are central to the reported benefits of preconditioning insults [30].
IL-1β and TNF-α can activate astrocytes by crossing the blood brain barrier or when produced by microglia. Cytokines such as IL-1β and ciliary neurotropic factor (CNTF) have been shown to induce astrocyte nuclear hypertrophy which is a sign of activation [31, 32]. Activated astrocytes are considered to be the main source of antioxidant defense in the brain following ischemic reperfusion and are less vulnerable to injury from reactive oxygen species than neurons [31, 33, 34]. The levels of cytosolic proteins with antioxidant properties are increased in activated astrocytes. For example, the multifunctional protein ceruloplasmin in astrocytes is increased after injury due to IL-1β stimulation [26, 31, 33, 35]. An alternate explanation of our data may be that moderate elevation of plasma IL-1β correlates with stroke risk and the lower levels of IL-1β observed in the HbSS-S group who developed stroke in the STOP study, may be a reflection of physiological compensation for processes that ultimately lead to stroke. We intend to explore this question in subsequent studies.
Possible applications for the findings of this study include determining the protective concentration range for the plasma IL-1β and combining this assessment with TCD studies in order to improve evaluation of stroke risk in HbSS and hence, management of the cerebrovascular events. Furthermore, these data stimulate the notion that cytokine activation of astrocytes protects HbSS children with abnormal TCD from developing stroke. This idea is worthy of empirical testing. In addition, there are polymorphisms in the genes regulating the production of IL-1β and other members of the IL-1 family (both ligands and receptors) that may be worth investigating by a genomic study of individuals with impaired TCD, to determine if specific polymorphisms are associated with likelihood of progression to stroke.
There are some practical concerns limiting our ability to fully interpret these results. These plasma measurements represent a single time-point in a cross sectional comparison of these inflammatory markers in HbSS patients with abnormal TCD who did or did not develop subsequent stroke. Although we are aware of temporal variation in concentration of plasma cytokines/chemokines, this was the most practical initial approach for establishing severity related associations. The mean concentration from serial monthly steady-state samples might reflect better the baseline concentrations of inflammatory mediators.
Although degradation of cytokines/chemokines in plasma stored at −70 degrees Celsius has been found to be negligible [36], we based our conclusions on logistic regression analysis of only the HbSS-NS and HbSS-S samples, which were collected over the same period and stored together under the same conditions.
5. Conclusion
This study demonstrates a new important finding that modestly increased plasma IL-1β concentration is associated with protection from stroke development in HbSS children with abnormal TCD and furthermore, that plasma IL-1β is a good predictor of stroke in HbSS. Using plasma IL-1β levels in combination with TCD measurements may improve evaluation of stroke risk in HbSS patients, by early identification of those needing intensive prophylactic interventions. This needs to be confirmed in a larger study and the mechanisms for the IL-1β protection deserve further investigation.
Acknowledgments
Our gratitude goes to all the participants and collaborators in the STOP study.
This work was supported by the National Institutes of Health (NIH) /Fogarty International Center (FIC) postdoctoral training grant in Genomics and Hemoglobinopathies, NIH-FIC- 5R90HG004151-03 (to JKS), NIH grants S06 GM008248 (to JMH) and RR033062, G12-RR03034 and P20-RR11104 (to Morehouse School of Medicine).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Okpala I. The intriguing contribution of white blood cells to sickle cell disease - a red cell disorder. Blood Reviews. 2004;18:65–73. doi: 10.1016/s0268-960x(03)00037-7. [DOI] [PubMed] [Google Scholar]
- 2.Bridgers WA. Cerebral vascular disease accompanying sickle cell anemia. Am J Pathol. 1939;15:353–362. [PMC free article] [PubMed] [Google Scholar]
- 3.Pavlakis SG, Prohovnik I, Piomelli S, DeVivo DC. Neurologic complications of sickle cell disease. Adv Pediatr. 1989;36:247–276. [PubMed] [Google Scholar]
- 4.del ZG, Ginis I, Hallenbeck JM, ladecola C, Wang X, Feuerstein GZ. Inflammation and stroke: putative role for cytokines, adhesion molecules and iNOS in brain response to ischemia. Brain Pathol. 2000;10:95–112. doi: 10.1111/j.1750-3639.2000.tb00247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Archer DR, Stiles JK, Newman GW, Quarshie A, Hsu LL, Sayavongsa P, et al. C-reactive protein and interleukin-6 are decreased in transgenic sickle cell mice fed a high protein diet. J Nutr. 2008;138:1148–1152. doi: 10.1093/jn/138.6.1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hibbert JM, Hsu LL, Bhathena SJ, Irune I, Sarfo B, Creary MS, et al. Proinflammatory cytokines and the hypermetabolism of children with sickle cell disease. Exp Biol Med (Maywood) 2005;230:68–74. doi: 10.1177/153537020523000109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu T, Clark RK, McDonnell PC, Young PR, White RF, Barone FC, et al. Tumor necrosis factor-alpha expression in ischemic neurons. Stroke. 1994;25:1481–1488. doi: 10.1161/01.str.25.7.1481. [DOI] [PubMed] [Google Scholar]
- 8.Sairanen T, Carpen O, Karjalainen-Lindsberg ML, Paetau A, Turpeinen U, Kaste M, et al. Evolution of cerebral tumor necrosis factor-alpha production during human ischemic stroke. Stroke. 2001;32:1750–1758. doi: 10.1161/01.str.32.8.1750. [DOI] [PubMed] [Google Scholar]
- 9.Han HS, Yenari MA. Cellular targets of brain inflammation in stroke. Curr Opin Investig Drugs. 2003;4:522–529. [PubMed] [Google Scholar]
- 10.Allan SM, Rothwell NJ. Cytokines and acute neurodegeneration. Nat Rev Neurosci. 2001;2:734–744. doi: 10.1038/35094583. [DOI] [PubMed] [Google Scholar]
- 11.Adams RJ, McKie VC, Hsu L, Files B, Vichinsky E, Pegelow C, et al. Prevention of a first stroke by transfusions in children with sickle cell anemia and abnormal results on transcranial Doppler ultrasonography. N Engl J Med. 1998;339:5–11. doi: 10.1056/NEJM199807023390102. [DOI] [PubMed] [Google Scholar]
- 12.Ohene-Frempong K, Weiner SJ, Sleeper LA, Miller ST, Embury S, Moohr JW, et al. Cerebrovascular Accidents in Sickle Cell Disease: Rates and Risk Factors. Blood. 1998;91:288–294. [PubMed] [Google Scholar]
- 13.Powars D, Wilson B, Imbus C, Pegelow C, Allen J. The natural history of stroke in sickle cell disease. Am J Med. 1978;65:461–471. doi: 10.1016/0002-9343(78)90772-6. [DOI] [PubMed] [Google Scholar]
- 14.Lee MT, Piomelli S, Granger S, Miller ST, Harkness S, Brambilla DJ, et al. Stroke Prevention Trial in Sickle Cell Anemia (STOP): extended follow-up and final results. Blood. 2006;108:847–852. doi: 10.1182/blood-2005-10-009506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Motulsky H. In Intuitive Biostatistics. New York: Oxford University Press Inc.; 1995. Multiple measurements to answer one question, chapt. 13. (Also available on-line at http://www.graphpad.com/www/book/mulcomp.htm) [Google Scholar]
- 16.Adams RJ. Big Strokes in Small Persons. Arch Neurol. 2007;64:1567–1574. doi: 10.1001/archneur.64.11.1567. [DOI] [PubMed] [Google Scholar]
- 17.Platt OS. Preventing stroke in sickle cell anemia. N Engl J Med. 2005;353:2743–2745. doi: 10.1056/NEJMp058274. [DOI] [PubMed] [Google Scholar]
- 18.Bernardes-Silva M, Anthony DC, Issekutz AC, Perry VH. Recruitment of neutrophils across the blood-brain barrier: the role of E- and P-selectins. J Cereb Blood Flow Metab. 2001;21:1115–1124. doi: 10.1097/00004647-200109000-00009. [DOI] [PubMed] [Google Scholar]
- 19.Proescholdt MG, Chakravarty S, Foster JA, Foti SB, Briley EM, Herkenham M. Intracerebroventricular but not intravenous interleukin-1beta induces widespread vascular-mediated leukocyte infiltration and immune signal mRNA expression followed by brain-wide glial activation. Neuroscience. 2002;112:731–749. doi: 10.1016/s0306-4522(02)00048-9. [DOI] [PubMed] [Google Scholar]
- 20.Davies CA, Loddick SA, Toulmond S, Stroemer RP, Hunt J, Rothwell NJ. The progression and topographic distribution of interleukin-1beta expression after permanent middle cerebral artery occlusion in the rat. J Cereb Blood Flow Metab. 1999;19:87–98. doi: 10.1097/00004647-199901000-00010. [DOI] [PubMed] [Google Scholar]
- 21.Haqqani AS, Nesic M, Preston E, Baumann E, Kelly J, Stanimirovic D. Characterization of vascular protein expression patterns in cerebral ischemia/reperfusion using laser capture microdissection and ICAT-nanoLC-MS/MS. FASEB J. 2005;19:1809–1821. doi: 10.1096/fj.05-3793com. [DOI] [PubMed] [Google Scholar]
- 22.Yamasaki Y, Matsuura N, Shozuhara H, Onodera H, Itoyama Y, Kogure K. Interleukin-1 as a pathogenetic mediator of ischemic brain damage in rats. Stroke. 1995;26:676–680. doi: 10.1161/01.str.26.4.676. [DOI] [PubMed] [Google Scholar]
- 23.Mulcahy NJ, Ross J, Rothwell NJ, Loddick SA. Delayed administration of interleukin-1 receptor antagonist protects against transient cerebral ischaemia in the rat. Br J Pharmacol. 2003;140:471–476. doi: 10.1038/sj.bjp.0705462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pinteaux E, Rothwell NJ, Boutin H. Neuroprotective actions of endogenous interleukin-1 receptor antagonist (IL-1ra) are mediated by glia. Glia. 2006;53:551–556. doi: 10.1002/glia.20308. [DOI] [PubMed] [Google Scholar]
- 25.Basu A, Lazovic J, Krady JK, Mauger DT, Rothstein RP, Smith MB, et al. Interleukin-1 and the interleukin-1 type 1 receptor are essential for the progressive neurodegeneration that ensues subsequent to a mild hypoxic/ischemic injury. J Cereb Blood Flow Metab. 2005;25:17–29. doi: 10.1038/sj.jcbfm.9600002. [DOI] [PubMed] [Google Scholar]
- 26.Kuhlow CJ, Krady JK, Basu A, Levison SW. Astrocytic ceruloplasmin expression, which is induced by IL-1beta and by traumatic brain injury, increases in the absence of the IL-1 type 1 receptor. Glia. 2003;44:76–84. doi: 10.1002/glia.10273. [DOI] [PubMed] [Google Scholar]
- 27.Herx LM, Yong VW. Interleukin-1 beta is required for the early evolution of reactive astrogliosis following CNS lesion. J Neuropathol Exp Neurol. 2001;60:961–971. doi: 10.1093/jnen/60.10.961. [DOI] [PubMed] [Google Scholar]
- 28.Brucklacher RM, Vannucci RC, Vannucci SJ. Hypoxic preconditioning increases brain glycogen and delays energy depletion from hypoxia-ischemia in the immature rat. Dev Neurosci. 2002;24:411–417. doi: 10.1159/000069051. [DOI] [PubMed] [Google Scholar]
- 29.Dringen R, Hamprecht B. Glucose, insulin, and insulin-like growth factor I regulate the glycogen content of astroglia-rich primary cultures. J Neurochem. 1992;58:511–517. doi: 10.1111/j.1471-4159.1992.tb09750.x. [DOI] [PubMed] [Google Scholar]
- 30.Mason JL, Suzuki K, Chaplin DD, Matsushima GK. Interleukin-1{beta} Promotes Repair of the CNS. J Neurosci. 2001;21:7046–7052. doi: 10.1523/JNEUROSCI.21-18-07046.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Albrecht PJ, Dahl JP, Stoltzfus OK, Levenson R, Levison SW. Ciliary neurotrophic factor activates spinal cord astrocytes, stimulating their production and release of fibroblast growth factor-2, to increase motor neuron survival. Exp Neurol. 2002;173:46–62. doi: 10.1006/exnr.2001.7834. [DOI] [PubMed] [Google Scholar]
- 32.Hudgins SN, Levison SW. Ciliary neurotrophic factor stimulates astroglial hypertrophy in vivo and in vitro. Exp Neurol. 1998;150:171–182. doi: 10.1006/exnr.1997.6735. [DOI] [PubMed] [Google Scholar]
- 33.Anderson CM, Nedergaard M. Astrocyte-mediated control of cerebral microcirculation. Trends Neurosci. 2003;26:340–344. doi: 10.1016/S0166-2236(03)00141-3. [DOI] [PubMed] [Google Scholar]
- 34.Swanson RA, Ying W, Kauppinen TM. Astrocyte influences on ischemic neuronal death. Curr Mol Med. 2004;4:193–205. doi: 10.2174/1566524043479185. [DOI] [PubMed] [Google Scholar]
- 35.Chang RC, Chen W, Hudson P, Wilson B, Han DS, Hong JS. Neurons reduce glial responses to lipopolysaccharide (LPS) and prevent injury of microglial cells from over-activation by LPS. J Neurochem. 2001;76:1042–1049. doi: 10.1046/j.1471-4159.2001.00111.x. [DOI] [PubMed] [Google Scholar]
- 36.Kenis G, Teunissen C, De JR, Bosmans E, Steinbusch H, Maes M. Stability of interleukin 6, soluble interleukin 6 receptor, interleukin 10 and CC16 in human serum. Cytokine. 2002;19:228–235. [PubMed] [Google Scholar]
- 37.Barone FC, Arvin B, White RF, Miller A, Webb CL, Willette RN, et al. Tumor necrosis factor-alpha. A mediator of focal ischemic brain injury. Stroke. 1997;28:1233–1244. doi: 10.1161/01.str.28.6.1233. [DOI] [PubMed] [Google Scholar]
- 38.Bruce AJ, Boling W, Kindy MS, Peschon J, Kraemer PJ, Carpenter MK, et al. Altered neuronal and microglial responses to excitotoxic and ischemic brain injury in mice lacking TNF receptors. Nat Med. 1996;2:788–794. doi: 10.1038/nm0796-788. [DOI] [PubMed] [Google Scholar]
- 39.Ginis I, Jaiswal R, Klimanis D, Liu J, Greenspon J, Hallenbeck JM. TNF-alpha-induced tolerance to ischemic injury involves differential control of NF-kappaB transactivation: the role of NF-kappaB association with p300 adaptor. J Cereb Blood Flow Metab. 2002;22:142–152. doi: 10.1097/00004647-200202000-00002. [DOI] [PubMed] [Google Scholar]
- 40.Hallenbeck JM. The many faces of tumor necrosis factor in stroke. Nat Med. 2002;8:1363–1368. doi: 10.1038/nm1202-1363. [DOI] [PubMed] [Google Scholar]
- 41.Murakami Y, Saito K, Hara A, Zhu Y, Sudo K, Niwa M, et al. Increases in tumor necrosis factor-alpha following transient global cerebral ischemia do not contribute to neuron death in mouse hippocampus. J Neurochem. 2005;93:1616–1622. doi: 10.1111/j.1471-4159.2005.03163.x. [DOI] [PubMed] [Google Scholar]
- 42.Offner H, Subramanian S, Parker SM, Afentoulis ME, Vandenbark AA, Hurn PD. Experimental stroke induces massive, rapid activation of the peripheral immune system. J Cereb Blood Flow Metab. 2006;26:654–665. doi: 10.1038/sj.jcbfm.9600217. [DOI] [PubMed] [Google Scholar]
- 43.Pradillo JM, Romera C, Hurtado O, Cardenas A, Moro MA, Leza JC, et al. TNFR1 upregulation mediates tolerance after brain ischemic preconditioning. J Cereb Blood Flow Metab. 2005;25:193–203. doi: 10.1038/sj.jcbfm.9600019. [DOI] [PubMed] [Google Scholar]
- 44.Uno H, Matsuyama T, Akita H, Nishimura H, Sugita M. J Cereb Blood Flow Metab. Vol. 17. 1997. Induction of tumor necrosis factor-alpha in the mouse hippocampus following transient forebrain ischemia; pp. 491–499. [DOI] [PubMed] [Google Scholar]
- 45.Yang GY, Gong C, Qin Z, Ye W, Mao Y, Bertz AL. Inhibition of TNFalpha attenuates infarct volume and ICAM-1 expression in ischemic mouse brain. Neuroreport. 1998;9:2131–2134. doi: 10.1097/00001756-199806220-00041. [DOI] [PubMed] [Google Scholar]
- 46.Emsley HC, Smith CJ, Georgiou RF, Vail A, Hopkins SJ, Rothwell NJ, et al. A randomised phase II study of interleukin-1 receptor antagonist in acute stroke patients. J Neurol Neurosurg Psychiatry. 2005;76:1366–1372. doi: 10.1136/jnnp.2004.054882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ooboshi H, Ibayashi S, Shichita T, Kumai Y, Takada J, Ago T, et al. Postischemic gene transfer of interleukin-10 protects against both focal and global brain ischemia. Circulation. 2005;111:913–919. doi: 10.1161/01.CIR.0000155622.68580.DC. [DOI] [PubMed] [Google Scholar]
- 48.Pelidou SH, Kostulas N, Matusevicius D, Kivisakk P, Kostulas V, Link H. High levels of IL-10 secreting cells are present in blood in cerebrovascular diseases. Eur J Neurol. 1999;6:437–442. doi: 10.1046/j.1468-1331.1999.640437.x. [DOI] [PubMed] [Google Scholar]
- 49.Spera PA, Ellison JA, Feuerstein GZ, Barone FC. IL-10 reduces rat brain injury following focal stroke. Neurosci Lett. 1998;251:189–192. doi: 10.1016/s0304-3940(98)00537-0. [DOI] [PubMed] [Google Scholar]
- 50.Strle K, Zhou JH, Shen WH, Broussard SR, Johnson RW, Freund GG, et al. Interleukin-10 in the brain. Crit Rev Immunol. 2001;21:427–449. [PubMed] [Google Scholar]
- 51.Tarkowski E, Rosengren L, Blomstrand C, Wikkelso C, Jensen C, Ekholm S, et al. Intrathecal release of pro- and anti-inflammatory cytokines during stroke. Clin Exp Immunol. 1997;110:492–499. doi: 10.1046/j.1365-2249.1997.4621483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.van EE, Gussekloo J, de Craen AJ, Bootsma-van der WA, Frolich M, Westendorp RG. Inflammation and stroke: the Leiden 85-Plus Study. Stroke. 2002;33:1135–1138. doi: 10.1161/01.str.0000014206.05597.9e. [DOI] [PubMed] [Google Scholar]
- 53.Benveniste EN. Cytokine actions in the central nervous system. Cytokine Growth Factor Rev. 1998;9:259–275. doi: 10.1016/s1359-6101(98)00015-x. [DOI] [PubMed] [Google Scholar]
- 54.Flanders KC, Ren RF, Lippa CF. Transforming Growth Factor-[beta]s in Neurodegenerative Disease. Progress in Neurobiology. 1998;54:71–85. doi: 10.1016/s0301-0082(97)00066-x. [DOI] [PubMed] [Google Scholar]
- 55.Lu YZ, Lin CH, Cheng FC, Hsueh CM. Molecular mechanisms responsible for microglia-derived protection of Sprague-Dawley rat brain cells during in vitro ischemia. Neurosci Lett. 2005;373:159–164. doi: 10.1016/j.neulet.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 56.Pang L, Ye W, Che XM, Roessler BJ, Betz AL, Yang GY. Reduction of inflammatory response in the mouse brain with adenoviral-mediated transforming growth factor-ss1 expression. Stroke. 2001;32:544–552. doi: 10.1161/01.str.32.2.544. [DOI] [PubMed] [Google Scholar]
- 57.Emsley HC, Tyrrell PJ. Inflammation and infection in clinical stroke. J Cereb Blood Flow Metab. 2002;22:1399–1419. doi: 10.1097/01.WCB.0000037880.62590.28. [DOI] [PubMed] [Google Scholar]
- 58.Soriano SG, Amaravadi LS, Wang YF, Zhou H, Yu GX, Tonra JR, et al. Mice deficient in fractalkine are less susceptible to cerebral ischemia-reperfusion injury. J Neuroimmunol. 2002;125:59–65. doi: 10.1016/s0165-5728(02)00033-4. [DOI] [PubMed] [Google Scholar]
- 59.Tarozzo G, Campanella M, Ghiani M, Bulfone A, Beltramo M. Expression of fractalkine and its receptor, CX3CR1, in response to ischaemia-reperfusion brain injury in the rat. Eur J Neurosci. 2002;15:1663–1668. doi: 10.1046/j.1460-9568.2002.02007.x. [DOI] [PubMed] [Google Scholar]
- 60.Chen Y, Hallenbeck JM, Ruetzler C, Bol D, Thomas K, Berman NE, et al. Overexpression of monocyte chemoattractant protein 1 in the brain exacerbates ischemic brain injury and is associated with recruitment of inflammatory cells. J Cereb Blood Flow Metab. 2003;23:748–755. doi: 10.1097/01.WCB.0000071885.63724.20. [DOI] [PubMed] [Google Scholar]
- 61.Garau A, Bertini R, Colotta F, Casilli F, Bigini P, Cagnotto A, et al. Neuroprotection with the CXCL8 inhibitor repertaxin in transient brain ischemia. Cytokine. 2005;30:125–131. doi: 10.1016/j.cyto.2004.12.014. [DOI] [PubMed] [Google Scholar]