Abstract
Interleukin (IL)-15 is a cytokine that acts on a wide range of cell types but is most crucial for the development, homeostasis, and function of a specific group of immune cells that includes CD8 T cells, NK cells, NKT cells, and CD8αα intraepithelial lymphocytes. IL-15 signals are transmitted through the IL-2/15Rβ and common γ (γC) chains; however, it is the delivery of IL-15 to these signaling components that is quite unique. As opposed to other cytokines that are secreted, IL-15 primarily exists bound to the high affinity IL-15Rα. When IL-15/IL-15Rα complexes are shuttled to the cell surface, they can stimulate opposing cells through the β/γC receptor complex. This novel mechanism of IL-15 delivery has been called trans-presentation. This review discusses how the theory of trans-presentation came to be, evidence that it is the major mechanism of action, the current understanding of the cell types thought to mediate trans-presentation, and possible alternatives for IL-15 delivery.
Keywords: IL-15, IL-15 Receptors, memory CD8 T cells, NK cells, monocytes, dendritic cells, homeostasis, lymphocyte development, trans-presentation, Cis-presentation, cytokine receptor complexes, bone marrow chimeras, transgenic mice
Introduction
Trans-presentation is a mechanism of cytokine delivery that has been described for IL-15, a key factor in the homeostasis and development of certain subsets of lymphocytes. The current theory of trans-presentation proposes that intracellular IL-15 binds to a high affinity IL-15 binding protein (i.e. IL-15Rα) that is shuttled to the cell surface where it stimulates IL-15 signaling components on neighboring cells through a cell-cell interaction (Figure1A). This pathway is of great significance because current evidence points toward trans-presentation as the major mechanism mediating IL-15 responses. Trans-presentation, as a unique mechanism for IL-15 alone, is also interesting since IL-15 shares cytokine receptor subunits with IL-2. Indeed, the preference of IL-15 for trans-presentation rather than conventional soluble cytokine delivery helps explain how such similar cytokines mediate different functions. Trans-presentation of IL-15 is advantageous compared to conventional cytokine secretion as it allows a more directed and controlled delivery to responsive cells. Since elevated IL-15 has detrimental effects on lymphocytes [1,2], the tighter regulation afforded by trans-presentation may be a major reason this mechanism evolved. In this review, we will describe how the theory of trans-presentation came to be, evidence that it is the major mechanism of action, the current understanding of the cell types thought to mediate trans-presentation, and possible alternatives of IL-15 delivery.
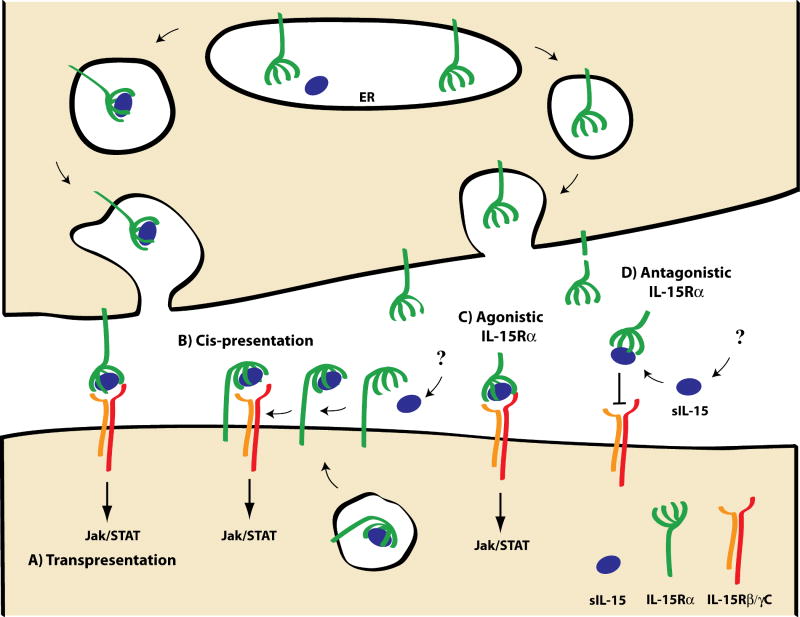
Figure 1. IL-15 Trans-presentation and other speculative mechanisms of IL-15 delivery.
Cartoon depicts the interface between two cell surfaces with possible scenarios mediating (A-C) or inhibiting (D) IL-15 responses. A) For trans-presentation, IL-15Rα and IL-15 encounter each other in the endoplasmic reticulum (ER) and are transported to the cell surface where the cell surface complex can stimulate neighboring cells through the IL-15Rβ/γC. B) Cis-presentation has been suggested where IL-15 is presented by IL-15Rα on the same cell; this mechanisms may utilize IL-15 derived from autocrine or paracrine sources. C) IL-15/IL-15Rα complexes can be generated artificially and act as agonist to stimulate neighboring cells. D) Cleaved, empty IL-15Rα may act as a sink to bind sIL-15 and antagonize IL-15 activity. The depiction of the interaction between IL-15Rα and IL-15 is stylized and not reflective of protein structural studies.
Early Investigations of IL-15 functions
IL-15 is a member of the four α-helix family of cytokines that acts on many cell types but is most important for the development, homeostasis, and function of CD8 T cells, NK, NKT cells, and CD8αα intestinal intraepithelial lymphocytes (iIEL). IL-15 signals through a receptor complex containing the common γ (γC) receptor subunit that is shared by IL-2, IL-4, IL-7, IL-9, and IL-21, other four α-helix cytokines. Whereas IL-4, IL-7, IL-9, and IL-21 signal through a heterodimeric receptor complex composed of a private Rα and the γC subunit, IL-2 signals through a heterotrimeric receptor complex composed of IL-2Rα, a shared IL-2/15Rβ (i.e. CD122), and the γC. As IL-2Rα has no signaling capacity, the IL-2/15Rβ and γC subunits (referred to from here on as the β/γC) mediates all IL-2 signals leading to JAK1 and 3 stimulation and subsequent STAT5 activation [3,4]. Although not involved in IL-2 signaling, IL-2Rα is important for the formation of high affinity heterotrimeric IL-2R complexes. Separately, the β and γC do not bind IL-2 but, when associated (β/γC), have an intermediate affinity for IL-2 (kD∼10-9 M) and thus can mediate IL-2 signals, albeit inefficiently. When IL-2Rα is also present, the IL-2Rα converts the β/γC to a high affinity complex (kD∼10-11 M). The lower affinity of IL-2Rα for IL-2 alone (kD∼10-8 M) compared to the β/γC suggests that IL-2 first binds the β/γC complex and then recruits the IL-2Rα to form the high affinity heterotrimeric complex. While some studies have detected preformed β/γC in the absence of IL-2 [5], other studies have provided evidence that the IL-2Rα can also be found associated with IL-2Rβ without the γC and suggest that IL-2 binds this complex first and then recruits the γC [6,7].
IL-15 also signals through the β/γC complex as wells as utilizes a private receptor α subunit, IL-15Rα that shares structural similarities with IL-2Rα. Both IL-2Rα and IL-15Rα each contain a short cytoplasmic tail, transmembrane domain, a stalk, a hinge region, and cytokine-binding domain called a sushi domain, which is required for cytokine binding [3,8,9]. Whereas IL-2Rα has two sushi domains, IL-15Rα has only one. Regarding cytokine binding, IL-15Rα is quite different from IL-2Rα becuase it already has a very high affinity for IL-15 (kD∼10-11 M), independent of the β/γC [3,8]. Upon binding IL-15, IL-15Rα has been demonstrated to undergo some conformational changes, though the extent to which this occurs and the consequences thereof remain unknown [10-12]. In the absence of IL-15Rα, the β/γC complex binds IL-15 with an intermediate affinity and is capable of mediating responses [4]. Interestingly, the affinity of β/γC for IL-15 is not further increased by the presence of IL-15Rα [3,8] Unlike IL-2Rα, some unique signaling properties have been attributed to IL-15Rα, which are distinct from the JAK/STAT signaling induced by the β/γC[13-16]. These findings have yet to be extended to IL-15-dependent lymphocytes and, consequently, their prevalence and significance is poorly characterized.
Not surprisingly, when IL-15 was first discovered it was found to induce many of the same responses as IL-2 in vitro, such as T cell proliferation (reviewed in [9]). Since all three of its receptor subunits were often detected on responding cells, it was assumed that IL-15 worked in a similar manner as IL-2 but with specificity dictated by the IL-15Rα chain; there were clues that these two cytokines worked in different ways. To start with, the in vivo expression pattern of IL-15Rα is much broader than that of IL-2Rα and overlaps with IL-15 expression, at least at the transcript level. Whereas IL-2Rα expression is mostly restricted to T cells (i.e. the main IL-2 targets), IL-15Rα is expressed in almost every cell and tissue type [3] - this being unusual as the major targets of IL-15 are lymphocytes as determined in IL-15 knockout studies [17]. Furthermore, responses to IL-15 in vitro do not absolutely require IL-15Rα. This could have been an indication that IL-15Rα merely enhanced signaling; however, considering that the presence of IL-15Rα did not further increase the affinity of IL-15 for the β/γC cytokine complex, how this occurred was not clear.
The characterization of IL-15Rα deficient mice reaffirmed the importance of IL-15Rα in IL-15 responses [18]. Similar to IL-15-/- mice [17], IL-15Rα-/- mice are generally healthy but have very specific deficiencies in CD8 T cells (particularly memory phenotype CD8 T cells), NK cells, NKT cells, and CD8αα iIEL[18]. Further characterization of these mice found that deficiencies in these specific lymphocyte subsets were due primarily to defects in development and homeostasis, providing evidence that the most important functions of IL-15 and IL-15Rα are those acting during steady state conditions [19-23]. The fact that the degree of lymphocyte deficiencies between the two mice is similar indicates that in vivo IL-15 responses are heavily dependent on IL-15Rα.
Development of the theory of trans-presentation
One of the first clues that IL-15 works in an unconventional manner was a study by Averil Ma's group showing that IL-15-mediated T cell proliferation induced by poly I:C in mice did not require responding T cells to express IL-15Rα[24]. More surprisingly, IL-15 responses were dependent on IL-15Rα expression by the cells in the surrounding environment. At first glance, this looked like a classic example of a cytokine that has indirect effects, but it was already established that IL-15 directly induced T cells to proliferate [4]. Thus, the results from Ma et al. seemed highly coincidental given that the direct effects of IL-15 (through the β/γC) were so similar to the putative indirect effects. Not long after, Dubois et al. [25] proposed the theory of trans-presentation based on careful manipulations of each of the IL-15R chains on both responding T cells and monocytic cell lines. Their study showed that IL-15 induced a prolonged effect on T cells compared to IL-2 by virtue of IL-15 being bound to IL-15Rα, which allowed for the continued presence of IL-15 on the cell surface of monocytes. In addition, IL-15 and IL-15Rα were found to associate intracellularly and could be followed from the endoplasmic reticulum to the cell surface [25] (Figure 1A). Earlier studies also detected IL-15 on the cell surface of human monocytes [26,27] but suggested that the IL-15 was not bound to its receptor subunits [26]. As distinct protocols have been used to separate IL-15 from cell surface IL-15Rα [25,26], and may therefore be subject to different caveats, this conclusion warrants further investigation. More importantly, both studies were able to show that membrane-associated IL-15 was biologically active and induced proliferation of cocultured T cells in [25,26]. Similar to the in vivo requirements, these T cells required the expression of IL-2/15Rβ and γC but not IL-15Rα [25]. The model of trans-presentation provided a number of answers to prior inconsistencies. For example, the finding that IL-15Rα shuttles IL-15 to the cell surface along with the very high affinity of IL-15Rα for IL-15 suggested that IL-15 need not be secreted; this provided an explanation for why IL-15 is so rarely detected in biological solutions. Overall, trans-presentation was a mechanism that explained how IL-15Rα expression by neighboring cells was crucial while still allowing IL-15 to induce direct effects through the β/γC.
While these elegant studies provided in vitro evidence for trans-presentation, the significance of trans-presentation in vivo was unclear. In addition to the earlier report analyzing poly I:C responses in mice[24], subsequent in vivo studies provided additional evidence for trans-presentation and began to identify the events in which trans-presentation occurs. In general, these studies examining requirements for IL-15Rα in memory CD8 T cell homeostasis and lymphocyte development gave credence for trans-presentation in vivo. For memory CD8 T cells, two separate studies using adoptive transfer models found that IL-15Rα was dispensable in CD8 T cells for the generation of antigen-specific memory CD8 T cells, their subsequent homeostatic proliferation as well as their response to a bolus of soluble IL-15 (sIL-15) [28,29]. Again, consistent with the trans-presentation model, these IL-15-mediated responses by CD8 T cells required IL-15Rα expression by the host cells [28,29]. Analogous conclusions for the requirements of IL-15Rα in development and homeostasis of NK cells were also made in studies using various combinations of cell transfers and BM chimeras. In essence, NK cells require IL-15Rα expression by the cells in their environment but did not need self-expression [30-33]. In a more striking example of the requirements for IL-15Rα, the development of CD8αα iIELs, which includes both TCRγδ and TCRαβ subsets, is completely defective when IL-15Rα expression is restricted to radiation-sensitive (hematopoietic) cells but fully restored when restricted to radiation-resistant (i.e. parenchymal) cells. Furthermore, iIEL development required hematopoietic cells to express IL-15Rβ providing evidence that IL-15 acts directly on the iIELs. This example provided some of the strongest evidence for trans-presentation as an important in vivo mechanism because the IL-15 responding cells were of a completely different origin than the trans-presenting cells.
To investigate whether cells synthesizing IL-15 are the same cells that trans-present IL-15, BM chimeric mice were generated in which the hematopoietic cells expressed either IL-15Rα or IL-15, but not both simultaneously [34,35]. In this circumstance, NK cell development and memory CD8 T cell homeostasis were deficient. IL-15 responses were restored only when a portion of hematopoietic cells expressed both IL-15 and IL-15Rα. For IELs, coordinate expression of IL-15 and IL-15Rα by the same cells was also implied as IL-15 expression by radiation-resistant cells was as crucial for IEL development as IL-15Rα [32]. More recent evidence has also shown that, even under stimulatory conditions such as with poly I:C, IL-15 is not transferred between cell types in vivo [36]. Altogether, these findings demonstrate that coordinate expression of IL-15 and IL-15Rα by the same cell type is needed for IL-15 responses by memory CD8 T cells, NK cells, and IELs as well as give further credence to the model of trans-presentation.
Identifying IL-15 trans-presenting cells
Ever since the description of trans-presentation, the identity of the cells that trans-present IL-15 has been in question. A logical approach would be to identify cells expressing both IL-15 and IL-15Rα. Multiple studies have detected IL-15 and IL-15Rα transcripts in many cell types in both stimulatory and non-stimulatory conditions. Unfortunately, due to the limited number of appropriate reagents as well as the nature of the tight regulation of IL-15, there has been slow progress in characterizing the expression of IL-15 and IL-15Rα protein.
In general, IL-15Rα protein is as ubiquitously expressed as its reported transcript expression [3,8,37]. While many studies have described expression of IL-15Rα protein, a number of IL-15Rα isoforms have been identified whose expression has not been well characterized. These isoforms are generated from alternative splicing of the various exons encoding for the mature protein. Isoforms lacking the sushi domain of IL-15Rα are expectedly unable to stimulate IL-15-responsive cells [38] as this is the cytokine-binding domain [3,8,38]. Most isoforms, however, do contain the sushi domain and possess biological activity [39]. Soluble isoforms exist as well, some consisting only of the leader peptide and the sushi domain. These soluble variants could have agonistic or antagonistic activity [39] and their proposed functions will be discussed below. Future studies will need to determine how the expression of these various IL-15Rα isoforms relates to IL-15 responses.
Unlike IL-15Rα, IL-15 protein expression is much more restricted. This may be in part because mechanisms exist that limit the translation of IL-15 protein, including the presence of multiple translational start sites and negative regulatory elements in the 5′ UTR[1,40,41]. Additionally, two isoforms of IL-15 that differ in the length of the signal peptide have been identified: the long signal peptide (LSP) and short signal peptide (SSP). These isoforms have distinct trafficking properties that promote either transport through the ER (LSP isoform) or to the nucleus and cytoplasm (SSP isoform) [42-44]. Because the SSP isoform would not therefore encounter IL-15R signaling receptors, it has been speculated that this isoform may be regulatory in nature. Although the expression is not well characterized, the LSP isoform is the predominant isoform expressed, with the SSP isoform being expressed in the heart and testis [43]. Additional IL-15 isoforms with alternative exon usage have been described that can inhibit the activity of full length IL-15 [45]. Whereas there are multiple mechanisms to limit IL-15 protein expression, similar regulatory mechanisms have not been observed for IL-15Rα and may explain why IL-15Rα protein expression is more prevalent than IL-15.
Given that trans-presentation requires cell surface expression of IL-15, the mere presence of IL-15 mRNA or cytoplasmic protein may not be sufficient to identify actively trans-presenting cells. As such, detecting cell surface IL-15 associated with IL-15Rα is most meaningful. Unfortunately, detecting cell surface IL-15 has been quite challenging, particularly in mice. This could be in part because the amount of IL-15 present on the cell surface straddles the level of detection. This is not surprising for a cytokine that functions mainly in lymphocyte homeostasis or ontogeny: IL-7 is similarly difficult to detect. In addition to human monocytes[26], other studies have shown surface IL-15 on BM-derived DCs and neutrophils in mice [46,47] but these instances were after TLR stimulation and are less physiologically relevant to situations of normal homeostasis. As a means to limit IL-15 activity, the transport of IL-15 to the cell surface may be tightly regulated and exist only in a subset of cells expressing IL-15 transcripts.
The lack of co-expression of IL-15 and IL-15Rα on the cell surface suggests that much of the cell surface IL-15Rα may be empty. This may be because the ability to trans-present is transiently regulated at the level of IL-15 expression or IL-15Rα is present to carry out other trans-presentation-independent functions of IL-15Rα. Since IL-15Rα expression by lymphocytes is not crucial for IL-15 functions and these cells may not express IL-15, we speculate that IL-15Rα on lymphocytes is more likely to mediate trans-presentation-independent roles of IL-15, which will be discussed later. In contrast, we postulate the IL-15Rα on myeloid cells primarily mediates trans-presentation of IL-15 to lymphocytes as myeloid cells are some of the few cells found to express both cell surface IL-15 and IL-15Rα [25-27,36]. Because many cell types express IL-15Rα but not IL-15, the identification of IL-15Rα+ cells is a poor prognosticator of trans-presentation.
In light of the little knowledge regarding which cells express cell surface IL-15, in vivo models have provided a better understanding of the cells trans-presenting IL-15 by measuring the biological activities of IL-15. For example, early studies using BM chimeras revealed cell types mediating trans-presentation, in addition to providing the first evidence for this mechanism in vivo. From these studies, memory CD8 T cells were found to rely heavily on IL-15 trans-presented by hematopoietically-derived cells for basal proliferation. NK and naïve CD8 T cells receive IL-15 signals from hematopoietic as well as parenchymal cells [29,32]. In stark contrast to memory CD8 T cells, the development of CD8αα iIELs and invariant NKT cells require trans-presentation of IL-15 exclusively by parenchymal cells [32] and (manuscript in preparation). Altogether, these observations point out that different cell types receive their IL-15 signals from varied sources, which may be somewhat determined by their biological niches. The more pertinent implication for these cell-specific preferences is that despite the ubiquitous expression of IL-15Rα, cells that require IL-15 exhibit particular tropisms for the source of the cytokine. Studies using bone marrow chimeras distinguished the roles of hematopoietic and parenchymal cells in IL-15 trans-presentation, but as these are obviously highly heterogeneous compartments, it remains imperative to further identify the cell type(s) within these compartments mediating trans-presentation.
To assess the contribution of specific cell types, our group has generated two lines of transgenic mice that use cell-specific promoters to drive IL-15Rα expression in an environment where all other cells are deficient in IL-15Rα. As DCs have been theorized to be the most likely cell type to trans-present IL-15 to CD8 T cells and NK cells, the first model generated expressed IL-15Rα under the CD11c promoter [37]. Using this model, the role of DCs in CD8 T cell biology and NK cell development has been investigated [37,48]. At the steady state, naïve CD8 T cell numbers are only minimally restored, total NK cell numbers were recovered by ∼50%, while the proportions of memory phenotype CD8 T cells almost reach normal frequencies [37]. Detailed analysis revealed the contraction of the virus-specific CD8 T cells only partially involved IL-15Rα+ DCs, with the surviving cells predominately KLRG-1-, CD27+ T cells (consistent with a memory precursor phenotype), perhaps indicating a preference of this CD8 memory subset for DC trans-presentation. Moreover, homeostatic proliferation of established memory CD8 T cells is very efficiently driven by DCs trans-presenting IL-15. Upon dissecting specific stages of NK cell development, trans-presentation by DCs efficiently mediated the differentiation of immature NK cells involving the acquisition of Ly49 molecules. DCs were less effective in the later maturation events [48]. In contrast, deficiencies in IL-15 dependent-iIELs were present at a level similar to that of IL-15Rα-deficient mice [37], which is not surprising given their absolute predilection for IL-15Rα expression by parenchymal cells. Collectively, these findings are intriguing as they indicate a temporal preference for IL-15 from DCs, which could be dictated by stage-specific localization or molecular programming of the cell.
A truly compelling example of cell specificity in trans-presentation was made in the analysis of our second transgenic mouse, where IL-15Rα expression is driven by an intestinal epithelial cell promoter, Villin [49-51]. IL-15Rα expression restricted to the intestinal epithelium was completely sufficient to restore the deficiencies in CD8αα TCRγδ and TCRαβ iIELs that exists in IL-15Rα-/- mice [49]. Outside the intestines, the phenotype of the lymphocytes showed no evidence of encountering IL-15. In addition to these unique CD8αα T cell populations, memory CD8 T cells also reside in the intestinal epithelium; however, these memory CD8 T cells are mostly IL-15 independent as these cells have down-regulated expression of the IL-2/15Rβ [52]. This study not only demonstrated a temporal preference for trans-presentation but also demonstrated how effectively transpresentation restricts IL-15 responses.
By identifying IL-15 trans-presenting cells, we can begin to ask what cell characteristics make them proficient to trans-present IL-15. Are these cells unique in the ability to trans-present IL-15 simply because of their co-expression of cell surface IL-15Rα/IL-15 and residence in the appropriate location for cell-cell interactions or is trans-presentation of IL-15 more specifically controlled?
Regulation of Trans-presentation
Currently, little is known about the regulation of IL-15 trans-presentation. While highly unconventional, trans-presentation as a method of cytokine delivery affords various means of controlling IL-15 availability. As this is the major mechanism mediating IL-15 responses, it stipulates that certain conditions be met. At the simplest level, transcription of IL-15 and IL-15Rα, within the same cell, is a prerequisite for trans-presentation; however, it is not currently known if up-regulating transcription of IL-15 and IL-15Rα alone is sufficient to increase the amount of IL-15 trans-presented on the cell surface. For example, IL-15 and/or IL-15Rα are up-regulated by TLR ligands and TNF family members [27,53-57], but whether these forms of stimuli enhance IL-15 trans-presentation or promote an alternative delivery of IL-15 is not clear. Since IL-15 trans-presentation is still a relatively new concept, many studies have not directly addressed whether transpresentation was involved. The significance of transcriptional regulation of IL-15 and IL-15Rα is particularly relevant for trans-presentation as multiple layers of post-transcriptional regulation exist that likely dictate whether IL-15 is available for trans-presentation. If IL-15 protein is present in more limited quantities than IL-15Rα, then regulating IL-15 may be the most likely strategy for controlling functional trans-presentation.
After protein expression, the next layer of regulation is the transport of IL-15 to the surface. Recent reports demonstrated that IL-15Rα is required for the transport of IL-15 from the endoplasmic reticulum to the surface of a cell [58]. Through a mechanism, that has yet to be investigated, IL-15Rα with bound IL-15 can be internalized via endosomes and later resurface [25]. This process likely reduces the demand on cytokine production and/or limits cytokine availability but is also another potential level of control. Studies investigating the role of the short cytoplasmic tail of the IL-15Rα found that truncating this portion of the protein prevents the normal expression of the IL-15/IL-15Rα complex on the cell surface and subsequent recycling [25,59]. While this didn't inhibit the cell from trans-presenting IL-15 to neighboring cells, less IL-15/IL-15Rα was available. Regardless of the role of IL-15Rα in shuttling IL-15, it is not clear if this shuttling is constitutive or is subjected to regulation by external stimuli.
Another obvious criterion for receiving trans-presented IL-15 is a cell's proximity to the cellular source of IL-15. As the major IL-15 responding cells are motile lymphocytes, do lymphocytes randomly encounter IL-15 trans-presenting cells? Rather than being stochastic, it would be more logical if lymphocytes were directed to the IL-15 source. Memory CD8 T cells are found in distinct tissues sites, such as the B cell areas of the spleen [60]. The preference for this site within the spleen may be evidence that memory CD8 T cells are directed to areas more ideally suited for receiving homeostatic signals. For NK and NKT cells, little is about whether specific niches exist.
It stands to reason that trans-presentation on its own is a weak basis for intercellular contact so it is feasible that other molecules may be involved in facilitating this interaction. Studies examining the stimulatory properties of IL-15/IL-15Rα complexes found that complexes with an IL-15Rα containing an Fc domain in place of the transmembrane domain were more effective than complexes without an Fc region [61]. The effect disappeared in mice deficient for Fc receptors suggesting that, by virtue of binding to Fc receptors, chimeric IL-15/IL-15Rα complexes are more effective when bound to a cell surface. Whether this is because the active domain is more accessible or whether other cell components enhance this interaction is not known but is indicative that IL-15 delivered via a cell contact is advantageous. Overall, it is possible that other molecules enhance the process of trans-presentation.
Prospective alternatives to trans-presentation
The aforementioned studies support the importance of trans-presentation; yet most of these events occur during the steady state for which trans-presentation is ideally suited. That said, do all IL-15 responses utilize trans-presentation, particularly those not confined by low, controlled levels of IL-15? Whereas there is compelling evidence that trans-presentation is the main mechanism of IL-15 delivery, questions remain regarding its inviolability. Essentially, are there times when IL-15 acts independent of trans-presentation? Other groups have proposed that there are alternative mechanisms for IL-15 responses and delivery. While these alternatives are demonstrably plausible, the physiological significance or prevalence of these mechanisms is presently not clear. Hence, these alternative scenarios are considered here, along with their caveats.
In addition to the functions in development and homeostasis, it is speculated that IL-15 is involved in pathogen responses, in part because IL-15 and IL-15Rα are up-regulated by a number of pathogens and TLR ligands. Presumably, this up-regulation could facilitate the expansion of antigen-responsive T cells and NK cells. If an abundant amount of IL-15 was needed, overriding the mechanism of trans-presentation could facilitate the faster and more plentiful availability of IL-15. In early characterizations of the roles of IL-15 and IL-15Rα in CD8 T cell responses, it was found that CD8 T cell expansion after a VSV infection was partially defective in the absence of IL-15 but was not defective in the absence of IL-15Rα [19]. A similar finding was also observed in an LCMV response but only among the T cell responses against the two more minor epitopes, not with the dominant epitopes [20]. This suggested that IL-15 potentiates weaker T cell responses in an IL-15Rα-independent manner, possibly because an abundance of sIL-15 is available to act through the β/γC. In normal, IL-15Rα-sufficient mice, IL-15 would likely bypass binding to IL-15Rα only if the amount of IL-15 secreted was abundant enough to occupy all IL-15Rα molecules; this could be one instance when sIL-15 is detected in biological fluids and trans-presentation is not involved in an IL-15 response.
Whereas the last scenario described an IL-15Rα-independent response, other mechanisms utilizing IL-15Rα, which are distinct from trans-presentation, have also been proposed. Despite the number of studies that have definitively shown IL-15Rα expression is not required by IL-15-dependent cells (i.e. CD8 T cells, NK cells, and iIELs)[28-32], it is unclear why these lymphocytes express some of the highest levels of IL-15Rα. The expression of IL-15Rα on these lymphocytes could be left over from an earlier evolutionary mechanism of IL-15 delivery that predates trans-presentation; however, there must have been pressure to maintain it. In analysis of the crystal structure of IL-15Rα, the threonine/proline rich region, which is between the transmembrane domain and the IL-15-binding domain, was found to possess potential flexibility. This implied that IL-15Rα, in addition to trans-presenting IL-15, could also present IL-15 to an adjacent β/γC on the same surface [11]; this would constitute cis-presentation (Figure 1B) as opposed to trans-presentation.
Cis-presentation of IL-15 by IL-15Rα is clearly feasible, but the source of IL-15 or the situations when this might occur have not been identified. If sIL-15 is not present, cis-presentation would require IL-15 to be cell intrinsic. As T cells and NK cells are not thought to be a source of IL-15 [26,62], cis-presentation of IL-15 for these cells would not be likely. In spite of the common belief that these lymphocytes do not express IL-15, this issue has recently been challenged as Miranda-Carús et al reported that human T cells express cell surface IL-15 ex vivo [63]. In this study, IL-15 expression increased with culturing and enhanced T cell proliferation [63]. While this finding provides evidence that IL-15 can signal in an autocrine manner, it is difficult to differentiate trans-presentation between T cells and cis-presentation using in vitro cultures; however, the degree of proliferation was enhanced by increased cell density, suggesting trans-presentation was likely involved at some level [63]. In general, more evidence is needed to determine whether human T cells respond to IL-15 by cis-presentation and if T cells indeed express IL-15 protein.
Despite the source of IL-15 being in question, naïve murine CD8 T cells transfected with IL-15Rα were shown to display an enhanced survival and proliferation in response to recombinant IL-15 versus their non-transfected counterparts [64]. A similar finding was also observed when a mutated form of IL-15Rα containing an alternative cytoplasmic region was used to replace the endogenous IL-15Rα locus and knock-in T cells transferred into normal hosts. The wildtype and knock-in T cells had slightly increased levels of homeostatic proliferation compared to IL-15Rα-/- T cells [59]. Altogether, these findings demonstrate a small advantage of expressing IL-15Rα and thus provide support for the concept of cis-presentation but, again, the source of IL-15 has yet to be identified. Overall, we speculate that cis-presentation is more likely to occur when abundant sIL-15 is present or when IL-15 is acquired in an autocrine manner. Regarding T cells, this may be more feasible for humans than mice. While these issues are beginning to be addressed for T cells, these questions have not been addressed in the other major IL-15 target cells (i.e. NK, NKT cells, and iIELs).
Unlike lymphocytes, DCs are a more definitive source of IL-15, express all the IL-15R subunits, and are IL-15 responsive. Specifically, IL-15 exerts effects in DC differentiation [31,65] as well as function [66-69]. The method of IL-15 delivery in these situations is not well investigated and thus whether DCs obtain IL-15 in a trans-presentation-dependent or -independent fashion is not clear. Because DCs are a source of IL-15, we think DCs are a very logical cell type to utilize cis-presentation.
Naturally-occurring soluble cytokine receptors are found with other cytokines and can act as agonists, antagonists, or chaperones. Soluble IL-15Rα proteins can be generated by proteolytic cleavage or through the expression of an alternatively spliced variant of IL-15Rα lacking the transmembrane domain [70,71]. If IL-15Rα is shed while binding IL-15, this complex would likely be stimulatory and could represent another possibility for IL-15 delivery that is still mediated by IL-15Rα but is nonetheless distinct from genuine trans-presentation (Figure 1C, agonistic IL-15Rα). In this way, IL-15 could be delivered to responding cells without the requirements and ramifications for cell-cell contact and so represents a less restricted source of IL-15. Indeed, there is much evidence that artificially-generated sIL-15Rα/IL-15 complexes act as potent agonists [61,72-74]. Similarly, IL-6 is known to bind a naturally-occurring soluble form of IL-6Rα that can stimulate the signal-transducing protein gp130 expressed on cell surfaces to mediate IL-6 signals; this is a well-recognized mechanism for IL-6 termed trans-signaling [75]. In this situation, the responding cell only requires expression of gp130, thus lifting a restriction on cells' responsiveness to IL-6. In light of the differences that exist between contact-dependent and soluble trans-presentation, we contend that distinctions be made regarding these two methods of IL-15 stimulation and suggest that the term trans-presentation be reserved for circumstances that involve cell-cell contact. To date, two studies have detected sIL-15Rα/IL-15 complexes in mouse serum [36,39]; however, these endogenously-derived sIL-15Rα/IL-15 complexes where not as effective in stimulating T cells and NK cells as their membrane-bound counterparts [36,39].
Despite the uncertain activity of endogenous sIL-15Rα/IL-15 complexes, it is clear that IL-15 bound to IL-15Rα is more potent than sIL-15 [61,72-74]. These findings demonstrating the enhanced potency of IL-15/IL-15Rα complexes not only provide an explanation for the effectiveness of transpresentation as a mechanism but also have important implications for the use of IL-15Rα/IL-15 complexes as therapeutic agents. As the use of IL-15 clinically has been hampered by production difficulties, the use of sIL-15Rα/IL-15 complexes could reduce production demands and increase cytokine stability. Furthermore, strategies can be developed to direct the activity of the sIL-15Rα/IL-15 complexes to specific cells. IL-15 is highly interesting clinically as it is theorized to mediate many of the same responses as IL-2 with increased efficacy and possibly lower toxicity. High dose IL-2 therapy is currently approved for renal carcinoma and metastatic melanoma treatment to enhance anti-tumor responses and is effective in a small subset of patients [76]. In addition to promoting CD8 T cell and NK cell responses, IL-2 also stimulates CD4+ regulatory T cells in an IL-2Rα-dependent manner, which would inhibit anti-tumor responses. As IL-15 trans-presentation is not specific for IL-2Rα-bearing cells, IL-15 responses would be more directed towards CD8 T cells and NK cells than IL-2.
Contrary to the studies showing sIL-15Rα/IL-15 is an agonist, other studies indicate that sIL-15Rα acts as a sink for sIL-15, inhibiting sIL-15 function [36,71] (Figure 1D, antagonistic IL-15Rα). Indeed, sIL-15Rα molecules have been detected in culture supernatants and mouse serum and have antagonistic activities [70,71]. It is difficult to envision how virtually the same protein complex (i.e. IL-15/IL-15Rα) can have little effect in one system and be stimulatory in another. An explanation may be derived from a similar phenomenon that has been observed in the IL-4Rα system. In addition to being expressed on the cell surface, IL-4Rα is also expressed in a soluble form that stabilizes binding of IL-4 to membrane IL-4R complexes (IL-4Rα/γC) when IL-4 is in excess; however, when sIL-4Rα is in excess, sIL-4Rα sequesters IL-4 thus preventing activity [77]. Alternatively, the different activities of sIL-15Rα, agonist versus antagonist, may lie in the source of the protein. The studies reporting sIL-15Rα to be agonistic used recombinant IL-15Rα [11,72,74], whereas antagonistic or ineffective sIL-15Rα were cell-derived [37,71].
The alternative mechanisms of IL-15 delivery presented herein are intriguing possibilities but likely represent minor functions and in general, less evidence presently supports their existence. Considering that the most crucial functions of IL-15 regulate development and homeostasis and a large amount of data supports trans-presentation in these activities, trans-presentation is still likely the dominant mechanism used in IL-15 responses. Since there is still much unresolved, further investigations are needed to better distinguish between these different mechanisms but will surely lead to a better understanding of IL-15 responses.
Concluding Remarks
A significant amount of evidence exists that supports trans-presentation as a major mechanism mediating IL-15 responses; this may be in part because it is a logical form of cytokine delivery that provides a low level delivery of IL-15 in a very controlled manner. In contrast, other mechanisms mediating IL-15 responses have been suggested but will require further investigation to determine their prevalence in biologically relevant IL-15 responses. Elucidating the mechanism by which IL-15 acts will require more stringent analysis than typically used for other cytokines. For example, evidence of cell surface IL-15 rather than cytoplasmic protein or mRNA will be more pertinent for identifying cells that trans-present IL-15. Surely, it will be more challenging to distinguish the mechanisms in human systems versus murine models. Overall, determining the mechanisms utilized by IL-15 in future studies will increase our understanding of how IL-15 functions in vivo. Regardless, the success of IL-15 in a clinical setting may or may not depend on whether therapeutic applications replicate the normal cytokine's actions. From what we have learned so far, IL-15 complexed to IL-15Rα is more effective than sIL-15 and may become more potent if bound to an artificial cell. Furthermore, future studies will need to investigate the other side of coin, which is to determine whether IL-15-responding cell possess characteristics that make them more or less receptive to receiving IL-15 signals delivered via trans-presentation.
Acknowledgments
We thank Stephanie Watowich, Eliseo Castillo, and Lisa Ma for critical reading of the manuscript. S.W.S acknowledges support from a NIH predoctoral training grant in Cancer Immunology (CA009598). Research in K.S.S. laboratory is supported by NIH grant AI070910 and the MD Anderson Trust Fellowship.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Fehniger TA, Suzuki K, Ponnappan A, VanDeusen JB, Cooper MA, Florea SM, et al. Fatal leukemia in interleukin 15 transgenic mice follows early expansions in natural killer and memory phenotype CD8+ T cells. J Exp Med. 2001 Jan 15;193(2):219–31. doi: 10.1084/jem.193.2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nishimura H, Yajima T, Naiki Y, Tsunobuchi H, Umemura M, Itano K, et al. Differential roles of interleukin 15 mRNA isoforms generated by alternative splicing in immune responses in vivo. J Exp Med. 2000 Jan 3;191(1):157–70. doi: 10.1084/jem.191.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Giri JG, Kumaki S, Ahdieh M, Friend DJ, Loomis A, Shanebeck K, et al. Identification and cloning of a novel IL-15 binding protein that is structurally related to the alpha chain of the IL-2 receptor. EMBO J. 1995 Aug 1;14(15):3654–63. doi: 10.1002/j.1460-2075.1995.tb00035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Giri JG, Ahdieh M, Eisenman J, Shanebeck K, Grabstein K, Kumaki S, et al. Utilization of the beta and gamma chains of the IL-2 receptor by the novel cytokine IL-15. EMBO J. 1994 Jun 15;13(12):2822–30. doi: 10.1002/j.1460-2075.1994.tb06576.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Takeshita T, Ohtani K, Asao H, Kumaki S, Nakamura M, Sugamura K. An associated molecule, p64, with IL-2 receptor beta chain. Its possible involvement in the formation of the functional intermediate-affinity IL-2 receptor complex. J Immunol. 1992 Apr 1;148(7):2154–8. [PubMed] [Google Scholar]
- 6.Stauber DJ, Debler EW, Horton PA, Smith KA, Wilson IA. Crystal structure of the IL-2 signaling complex: paradigm for a heterotrimeric cytokine receptor. Proc Natl Acad Sci U S A. 2006 Feb 21;103(8):2788–93. doi: 10.1073/pnas.0511161103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bodnar A, Nizsaloczki E, Mocsar G, Szaloki N, Waldmann TA, Damjanovich S, et al. A biophysical approach to IL-2 and IL-15 receptor function: localization, conformation and interactions. Immunol Lett. 2008 Mar 15;116(2):117–25. doi: 10.1016/j.imlet.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 8.Anderson DM, Kumaki S, Ahdieh M, Bertles J, Tometsko M, Loomis A, et al. Functional characterization of the human interleukin-15 receptor alpha chain and close linkage of IL15RA and IL2RA genes. J Biol Chem. 1995 Dec 15;270(50):29862–9. doi: 10.1074/jbc.270.50.29862. [DOI] [PubMed] [Google Scholar]
- 9.Waldmann TA. The biology of interleukin-2 and interleukin-15: implications for cancer therapy and vaccine design. Nat Rev Immunol. 2006 Aug;6(8):595–601. doi: 10.1038/nri1901. [DOI] [PubMed] [Google Scholar]
- 10.Hanick NA, Rickert M, Varani L, Bankovich AJ, Cochran JR, Kim DM, et al. Elucidation of the interleukin-15 binding site on its alpha receptor by NMR. Biochemistry. 2007 Aug 21;46(33):9453–61. doi: 10.1021/bi700652f. [DOI] [PubMed] [Google Scholar]
- 11.Olsen SK, Ota N, Kishishita S, Kukimoto-Niino M, Murayama K, Uchiyama H, et al. Crystal Structure of the interleukin-15.interleukin-15 receptor alpha complex: insights into trans and cis presentation. J Biol Chem. 2007 Dec 21;282(51):37191–204. doi: 10.1074/jbc.M706150200. [DOI] [PubMed] [Google Scholar]
- 12.Lorenzen I, Dingley AJ, Jacques Y, Grotzinger J. The structure of the interleukin-15 alpha receptor and its implications for ligand binding. J Biol Chem. 2006 Mar 10;281(10):6642–7. doi: 10.1074/jbc.M513118200. [DOI] [PubMed] [Google Scholar]
- 13.Bulfone-Paus S, Bulanova E, Pohl T, Budagian V, Durkop H, Ruckert R, et al. Death deflected: IL-15 inhibits TNF-alpha-mediated apoptosis in fibroblasts by TRAF2 recruitment to the IL-15Ralpha chain. FASEB J. 1999 Sep;13(12):1575–85. doi: 10.1096/fasebj.13.12.1575. [DOI] [PubMed] [Google Scholar]
- 14.Budagian V, Bulanova E, Orinska Z, Thon L, Mamat U, Bellosta P, et al. A promiscuous liaison between IL-15 receptor and Axl receptor tyrosine kinase in cell death control. EMBO J. 2005 Dec 21;24(24):4260–70. doi: 10.1038/sj.emboj.7600874. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 15.Bulanova E, Budagian V, Pohl T, Krause H, Durkop H, Paus R, et al. The IL-15R alpha chain signals through association with Syk in human B cells. J Immunol. 2001 Dec 1;167(11):6292–302. doi: 10.4049/jimmunol.167.11.6292. [DOI] [PubMed] [Google Scholar]
- 16.Pereno R, Gaggero A, Scudeletti M, Lanza L, Meazza R, Mishal Z, et al. IL-15/IL-15R alpha intracellular trafficking in human cells and protection from apoptosis. Ann N Y Acad Sci. 1999 Jun 22;876:236–45. doi: 10.1111/j.1749-6632.1999.tb07644.x. [DOI] [PubMed] [Google Scholar]
- 17.Kennedy MK, Glaccum M, Brown SN, Butz EA, Viney JL, Embers M, et al. Reversible defects in natural killer and memory CD8 T cell lineages in interleukin 15-deficient mice. J Exp Med. 2000 Mar 6;191(5):771–80. doi: 10.1084/jem.191.5.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lodolce JP, Boone DL, Chai S, Swain RE, Dassopoulos T, Trettin S, et al. IL-15 receptor maintains lymphoid homeostasis by supporting lymphocyte homing and proliferation. Immunity. 1998 Nov;9(5):669–76. doi: 10.1016/s1074-7613(00)80664-0. [DOI] [PubMed] [Google Scholar]
- 19.Schluns KS, Williams K, Ma A, Zheng XX, Lefrancois L. Cutting edge: Requirement for IL-15 in the generation of primary and memory antigen-specific CD8 T cells. Journal of Immunology. 2002 May 15;168(10):4827–31. doi: 10.4049/jimmunol.168.10.4827. [DOI] [PubMed] [Google Scholar]
- 20.Becker TC, Wherry EJ, Boone D, Murali-Krishna K, Antia R, Ma A, et al. Interleukin 15 is required for proliferative renewal of virus-specific memory CD8 T cells. J Exp Med. 2002 Jun 17;195(12):1541–8. doi: 10.1084/jem.20020369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ranson T, Vosshenrich CA, Corcuff E, Richard O, Laloux V, Lehuen A, et al. IL-15 availability conditions homeostasis of peripheral natural killer T cells. Proc Natl Acad Sci U S A. 2003 Mar 4;100(5):2663–8. doi: 10.1073/pnas.0535482100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ranson T, Vosshenrich CA, Corcuff E, Richard O, Muller W, Di Santo JP. IL-15 is an essential mediator of peripheral NK-cell homeostasis. Blood. 2003 Jun 15;101(12):4887–93. doi: 10.1182/blood-2002-11-3392. [DOI] [PubMed] [Google Scholar]
- 23.Matsuda JL, Gapin L, Sidobre S, Kieper WC, Tan JT, Ceredig R, et al. Homeostasis of V alpha 14i NKT cells. Nat Immunol. 2002 Oct;3(10):966–74. doi: 10.1038/ni837. [DOI] [PubMed] [Google Scholar]
- 24.Lodolce JP, Burkett PR, Boone DL, Chien M, Ma A. T cell-independent interleukin 15Ralpha signals are required for bystander proliferation. J Exp Med. 2001 Oct 15;194(8):1187–94. doi: 10.1084/jem.194.8.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dubois S, Mariner J, Waldmann TA, Tagaya Y. IL-15Ralpha recycles and presents IL-15 In trans to neighboring cells. Immunity. 2002 Nov;17(5):537–47. doi: 10.1016/s1074-7613(02)00429-6. [DOI] [PubMed] [Google Scholar]
- 26.Musso T, Calosso L, Zucca M, Millesimo M, Ravarino D, Giovarelli M, et al. Human monocytes constitutively express membrane-bound, biologically active, and interferon-gamma-upregulated interleukin-15. Blood. 1999 May 15;93(10):3531–9. [PubMed] [Google Scholar]
- 27.Neely GG, Robbins SM, Amankwah EK, Epelman S, Wong H, Spurrell JC, et al. Lipopolysaccharide-stimulated or granulocyte-macrophage colony-stimulating factor-stimulated monocytes rapidly express biologically active IL-15 on their cell surface independent of new protein synthesis. J Immunol. 2001 Nov 1;167(9):5011–7. doi: 10.4049/jimmunol.167.9.5011. [DOI] [PubMed] [Google Scholar]
- 28.Burkett PR, Koka R, Chien M, Chai S, Chan F, Ma A, et al. IL-15R alpha expression on CD8+ T cells is dispensable for T cell memory. Proc Natl Acad Sci U S A. 2003 Apr 15;100(8):4724–9. doi: 10.1073/pnas.0737048100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schluns KS, Klonowski KD, Lefrancois L. Transregulation of memory CD8 T-cell proliferation by IL-15R alpha(+) bone marrow-derived cells. Blood. 2004 Feb 1;103(3):988–94. doi: 10.1182/blood-2003-08-2814. [DOI] [PubMed] [Google Scholar]
- 30.Koka R, Burkett PR, Chien M, Chai S, Chan F, Lodolce JP, et al. Interleukin (IL)-15R[alpha]-deficient natural killer cells survive in normal but not IL-15R[alpha]-deficient mice. J Exp Med. 2003 Apr 21;197(8):977–84. doi: 10.1084/jem.20021836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kawamura T, Koka R, Ma A, Kumar V. Differential roles for IL-15R alpha-chain in NK cell development and Ly-49 induction. J Immunol. 2003 Nov 15;171(10):5085–90. doi: 10.4049/jimmunol.171.10.5085. [DOI] [PubMed] [Google Scholar]
- 32.Schluns KS, Nowak EC, Cabrera-Hernandez A, Puddington L, Lefrancois L, Aguila HL. Distinct cell types control lymphoid subset development by means of IL-15 and IL-15 receptor alpha expression. Proceedings of the National Academy of Sciences of the United States of America. 2004 Apr 13;101(15):5616–21. doi: 10.1073/pnas.0307442101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koka R, Burkett P, Chien M, Chai S, Boone DL, Ma A. Cutting edge: murine dendritic cells require IL-15Ralpha to prime NK cells. J Immunol. 2004 Sep 15;173(6):3594–8. doi: 10.4049/jimmunol.173.6.3594. [DOI] [PubMed] [Google Scholar]
- 34.Burkett PR, Koka R, Chien M, Chai S, Boone DL, Ma A. Coordinate Expression and Trans Presentation of Interleukin (IL)-15R{alpha} and IL-15 Supports Natural Killer Cell and Memory CD8+ T Cell Homeostasis. J Exp Med. 2004 Sep 27;200(7):825–34. doi: 10.1084/jem.20041389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sandau MM, Schluns KS, Lefrancois L, Jameson SC. Cutting edge: transpresentation of IL-15 by bone marrow-derived cells necessitates expression of IL-15 and IL-15R alpha by the same cells. J Immunol. 2004 Dec 1;173(11):6537–41. doi: 10.4049/jimmunol.173.11.6537. [DOI] [PubMed] [Google Scholar]
- 36.Mortier E, Woo T, Advincula R, Gozalo S, Ma A. IL-15Ralpha chaperones IL-15 to stable dendritic cell membrane complexes that activate NK cells via trans presentation. J Exp Med. 2008 May 12;205(5):1213–25. doi: 10.1084/jem.20071913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stonier SW, Ma LJ, Castillo EF, Schluns KS. Dendritic cells drive memory CD8 T cell homeostasis via IL-15 trans-presentation. Blood. 2008 Sep;23:4546–54. doi: 10.1182/blood-2008-05-156307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dubois S, Magrangeas F, Lehours P, Raher S, Bernard J, Boisteau O, et al. Natural splicing of exon 2 of human interleukin-15 receptor alpha-chain mRNA results in a shortened form with a distinct pattern of expression. J Biol Chem. 1999 Sep 17;274(38):26978–84. doi: 10.1074/jbc.274.38.26978. [DOI] [PubMed] [Google Scholar]
- 39.Bulanova E, Budagian V, Duitman E, Orinska Z, Krause H, Ruckert R, et al. Soluble Interleukin IL-15Ralpha is generated by alternative splicing or proteolytic cleavage and forms functional complexes with IL-15. J Biol Chem. 2007 May 4;282(18):13167–79. doi: 10.1074/jbc.M610036200. [DOI] [PubMed] [Google Scholar]
- 40.Bamford RN, Battiata AP, Waldmann TA. IL-15: the role of translational regulation in their expression. J Leukoc Biol. 1996 Apr;59(4):476–80. doi: 10.1002/jlb.59.4.476. [DOI] [PubMed] [Google Scholar]
- 41.Bamford RN, DeFilippis AP, Azimi N, Kurys G, Waldmann TA. The 5′ untranslated region, signal peptide, and the coding sequence of the carboxyl terminus of IL-15 participate in its multifaceted translational control. J Immunol. 1998 May 1;160(9):4418–26. [PubMed] [Google Scholar]
- 42.Kurys G, Tagaya Y, Bamford R, Hanover JA, Waldmann TA. The long signal peptide isoform and its alternative processing direct the intracellular trafficking of interleukin-15. J Biol Chem. 2000 Sep 29;275(39):30653–9. doi: 10.1074/jbc.M002373200. [DOI] [PubMed] [Google Scholar]
- 43.Tagaya Y, Kurys G, Thies TA, Losi JM, Azimi N, Hanover JA, et al. Generation of secretable and nonsecretable interleukin 15 isoforms through alternate usage of signal peptides. Proc Natl Acad Sci U S A. 1997 Dec 23;94(26):14444–9. doi: 10.1073/pnas.94.26.14444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gaggero A, Azzarone B, Andrei C, Mishal Z, Meazza R, Zappia E, et al. Differential intracellular trafficking, secretion and endosomal localization of two IL-15 isoforms. Eur J Immunol. 1999 Apr;29(4):1265–74. doi: 10.1002/(SICI)1521-4141(199904)29:04<1265::AID-IMMU1265>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 45.Tan X, Lefrancois L. Novel IL-15 isoforms generated by alternative splicing are expressed in the intestinal epithelium. Genes Immun. 2006 Jul;7(5):407–16. doi: 10.1038/sj.gene.6364314. [DOI] [PubMed] [Google Scholar]
- 46.Oh S, Perera LP, Terabe M, Ni L, Waldmann TA, Berzofsky JA. IL-15 as a mediator of CD4+ help for CD8+ T cell longevity and avoidance of TRAIL-mediated apoptosis. Proc Natl Acad Sci U S A. 2008 Apr 1;105(13):5201–6. doi: 10.1073/pnas.0801003105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Miyazaki S, Ishikawa F, Shimizu K, Ubagai T, Edelstein PH, Yamaguchi K. Gr-1high polymorphonuclear leukocytes and NK cells act via IL-15 to clear intracellular Haemophilus influenzae in experimental murine peritonitis and pneumonia. J Immunol. 2007 Oct 15;179(8):5407–14. doi: 10.4049/jimmunol.179.8.5407. [DOI] [PubMed] [Google Scholar]
- 48.Castillo EF, Stonier SW, Frasca L, Schluns KS. DCs direct the in vivo development and maintenance of NK cells via IL-15 trans-presentation. J Immunol. 2009 doi: 10.4049/jimmunol.0900719. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ma LJ, Acero LF, Zal T, Schluns KS. Trans-presentation of IL-15 by intestinal epithelial cells drives development of CD8alphaalpha IELs. J Immunol. 2009 Jul 15;183(2):1044–54. doi: 10.4049/jimmunol.0900420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pinto D, Robine S, Jaisser F, El Marjou FE, Louvard D. Regulatory sequences of the mouse villin gene that efficiently drive transgenic expression in immature and differentiated epithelial cells of small and large intestines. J Biol Chem. 1999 Mar 5;274(10):6476–82. doi: 10.1074/jbc.274.10.6476. [DOI] [PubMed] [Google Scholar]
- 51.Robine S, Jaisser F, Louvard D. Epithelial cell growth and differentiation. IV. Controlled spatiotemporal expression of transgenes: new tools to study normal and pathological states. Am J Physiol. 1997 Oct;273(4 Pt 1):G759–G762. doi: 10.1152/ajpgi.1997.273.4.G759. [DOI] [PubMed] [Google Scholar]
- 52.Masopust D, Vezys V, Wherry EJ, Barber DL, Ahmed R. Cutting edge: gut microenvironment promotes differentiation of a unique memory CD8 T cell population. J Immunol. 2006 Feb 15;176(4):2079–83. doi: 10.4049/jimmunol.176.4.2079. [DOI] [PubMed] [Google Scholar]
- 53.Zhang X, Sun S, Hwang I, Tough DF, Sprent J. Potent and selective stimulation of memory-phenotype CD8+ T cells in vivo by IL-15. Immunity. 1998 May;8(5):591–9. doi: 10.1016/s1074-7613(00)80564-6. [DOI] [PubMed] [Google Scholar]
- 54.Ostrowski MA, Justement SJ, Ehler L, Mizell SB, Lui S, Mican J, et al. The role of CD4+ T cell help and CD40 ligand in the in vitro expansion of HIV-1-specific memory cytotoxic CD8+ T cell responses. J Immunol. 2000 Dec 1;165(11):6133–41. doi: 10.4049/jimmunol.165.11.6133. [DOI] [PubMed] [Google Scholar]
- 55.Rappl G, Kapsokefalou A, Heuser C, Rossler M, Ugurel S, Tilgen W, et al. Dermal fibroblasts sustain proliferation of activated T cells via membrane-bound interleukin-15 upon long-term stimulation with tumor necrosis factor-alpha. J Invest Dermatol. 2001 Jan;116(1):102–9. doi: 10.1046/j.1523-1747.2001.00239.x. [DOI] [PubMed] [Google Scholar]
- 56.Park CS, Yoon SO, Armitage RJ, Choi YS. Follicular dendritic cells produce IL-15 that enhances germinal center B cell proliferation in membrane-bound form. J Immunol. 2004 Dec 1;173(11):6676–83. doi: 10.4049/jimmunol.173.11.6676. [DOI] [PubMed] [Google Scholar]
- 57.Ferlazzo G, Pack M, Thomas D, Paludan C, Schmid D, Strowig T, et al. Distinct roles of IL-12 and IL-15 in human natural killer cell activation by dendritic cells from secondary lymphoid organs. Proc Natl Acad Sci U S A. 2004 Nov 23;101(47):16606–11. doi: 10.1073/pnas.0407522101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Duitman EH, Orinska Z, Bulanova E, Paus R, Bulfone-Paus S. How a cytokine is chaperoned through the secretory pathway by complexing with its own receptor: lessons from interleukin-15 (IL-15)/IL-15 receptor alpha. Mol Cell Biol. 2008 Aug;28(15):4851–61. doi: 10.1128/MCB.02178-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wu Z, Xue HH, Bernard J, Zeng R, Issakov D, Bollenbacher-Reilley J, et al. The IL-15 receptor {alpha} chain cytoplasmic domain is critical for normal IL-15Ralpha function but is not required for trans-presentation. Blood. 2008 Dec 1;112(12):4411–9. doi: 10.1182/blood-2007-03-080697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Khanna KM, McNamara JT, Lefrancois L. In situ imaging of the endogenous CD8 T cell response to infection. Science. 2007 Oct 5;318(5847):116–20. doi: 10.1126/science.1146291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dubois S, Patel HJ, Zhang M, Waldmann TA, Muller JR. Preassociation of IL-15 with IL-15R alpha-IgG1-Fc enhances its activity on proliferation of NK and CD8+/CD44high T cells and its antitumor action. J Immunol. 2008 Feb 15;180(4):2099–106. doi: 10.4049/jimmunol.180.4.2099. [DOI] [PubMed] [Google Scholar]
- 62.Grabstein KH, Eisenman J, Shanebeck K, Rauch C, Srinivasan S, Fung V, et al. Cloning of a T cell growth factor that interacts with the beta chain of the interleukin-2 receptor. Science. 1994 May 13;264(5161):965–8. doi: 10.1126/science.8178155. [DOI] [PubMed] [Google Scholar]
- 63.Miranda-Carus ME, ito-Miguel M, Llamas MA, Balsa A, Martin-Mola E. Human T cells constitutively express IL-15 that promotes ex vivo T cell homeostatic proliferation through autocrine/juxtacrine loops. J Immunol. 2005 Sep 15;175(6):3656–62. doi: 10.4049/jimmunol.175.6.3656. [DOI] [PubMed] [Google Scholar]
- 64.Rowley J, Monie A, Hung CF, Wu TC. Expression of IL-15RA or an IL-15/IL-15RA fusion on CD8+ T cells modifies adoptively transferred T-cell function in cis. Eur J Immunol. 2009 Feb;39(2):491–506. doi: 10.1002/eji.200838594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Regamey N, Obregon C, Ferrari-Lacraz S, van LC, Chanson M, Nicod LP, et al. Airway epithelial IL-15 transforms monocytes into dendritic cells. Am J Respir Cell Mol Biol. 2007 Jul;37(1):75–84. doi: 10.1165/rcmb.2006-0235OC. [DOI] [PubMed] [Google Scholar]
- 66.Jinushi M, Takehara T, Tatsumi T, Kanto T, Groh V, Spies T, et al. Autocrine/paracrine IL-15 that is required for type I IFN-mediated dendritic cell expression of MHC class I-related chain A and B is impaired in hepatitis C virus infection. J Immunol. 2003 Nov 15;171(10):5423–9. doi: 10.4049/jimmunol.171.10.5423. [DOI] [PubMed] [Google Scholar]
- 67.Feau S, Facchinetti V, Granucci F, Citterio S, Jarrossay D, Seresini S, et al. Dendritic cell-derived IL-2 production is regulated by IL-15 in humans and in mice. Blood. 2005 Jan 15;105(2):697–702. doi: 10.1182/blood-2004-03-1059. [DOI] [PubMed] [Google Scholar]
- 68.Ohteki T, Suzue K, Maki C, Ota T, Koyasu S. Critical role of IL-15-IL-15R for antigen-presenting cell functions in the innate immune response. Nat Immunol. 2001 Dec;2(12):1138–43. doi: 10.1038/ni729. [DOI] [PubMed] [Google Scholar]
- 69.Ku CC, Kappler J, Marrack P. The growth of the very large CD8+ T cell clones in older mice is controlled by cytokines. J Immunol. 2001 Feb 15;166(4):2186–93. doi: 10.4049/jimmunol.166.4.2186. [DOI] [PubMed] [Google Scholar]
- 70.Budagian V, Bulanova E, Orinska Z, Ludwig A, Rose-John S, Saftig P, et al. Natural Soluble Interleukin-15R{alpha} Is Generated by Cleavage That Involves the Tumor Necrosis Factor-{alpha}-converting Enzyme (TACE/ADAM17) J Biol Chem. 2004 Sep 24;279(39):40368–75. doi: 10.1074/jbc.M404125200. [DOI] [PubMed] [Google Scholar]
- 71.Mortier E, Bernard J, Plet A, Jacques Y. Natural, proteolytic release of a soluble form of human IL-15 receptor alpha-chain that behaves as a specific, high affinity IL-15 antagonist. J Immunol. 2004 Aug 1;173(3):1681–8. doi: 10.4049/jimmunol.173.3.1681. [DOI] [PubMed] [Google Scholar]
- 72.Rubinstein MP, Kovar M, Purton JF, Cho JH, Boyman O, Surh CD, et al. Converting IL-15 to a superagonist by binding to soluble IL-15R{alpha} Proc Natl Acad Sci U S A. 2006 Jun 13;103(24):9166–71. doi: 10.1073/pnas.0600240103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Stoklasek TA, Schluns KS, Lefrancois L. Combined IL-15/IL-15Ralpha immunotherapy maximizes IL-15 activity in vivo. J Immunol. 2006 Nov 1;177(9):6072–80. doi: 10.4049/jimmunol.177.9.6072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mortier E, Quemener A, Vusio P, Lorenzen I, Boublik Y, Grotzinger J, et al. Soluble interleukin-15 receptor alpha (IL-15R alpha)-sushi as a selective and potent agonist of IL-15 action through IL-15R beta/gamma. Hyperagonist IL-15 × IL-15R alpha fusion proteins. J Biol Chem. 2006 Jan 20;281(3):1612–9. doi: 10.1074/jbc.M508624200. [DOI] [PubMed] [Google Scholar]
- 75.Rose-John S, Scheller J, Elson G, Jones SA. Interleukin-6 biology is coordinated by membrane-bound and soluble receptors: role in inflammation and cancer. J Leukoc Biol. 2006 Aug;80(2):227–36. doi: 10.1189/jlb.1105674. [DOI] [PubMed] [Google Scholar]
- 76.Overwijk WW, Schluns KS. Functions of gammaC cytokines in immune homeostasis: Current and potential clinical applications. Clin Immunol. 2009 May 8; doi: 10.1016/j.clim.2009.03.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fernandez-Botran R, Chilton PM, Ma Y, Windsor JL, Street NE. Control of the production of soluble interleukin-4 receptors: implications in immunoregulation. J Leukoc Biol. 1996 Apr;59(4):499–504. doi: 10.1002/jlb.59.4.499. [DOI] [PubMed] [Google Scholar]