Abstract
Human low density lipoprotein (LDL) undergoes oxidation and glycation in vivo. By themselves, oxidized LDL (oxLDL) and AGE-LDL have proinflammatory properties and are considered atherogenic. But the atherogenicity of these lipoproteins are significantly increased as a consequence of the formation of immune complexes (IC) involving autoantibodies spontaneously formed. OxLDL and AGE antibodies have been shown to be predominantly of the IgG1 and IgG3 isotypes. OxLDL antibodies are able to activate the complement system by the classical pathway and to induce FcR-mediated phagocytosis. In vitro and ex vivo studies performed with modified LDL-IC have proven their pro-inflammatory and atherogenic properties. Clinical studies have demonstrated that the levels of circulating modified LDL-IC correlate with parameters indicative of cardiovascular and renal disease in diabetic patients and other patient populations. The possibility that spontaneously formed or induced modified LDL antibodies (particularly IgM oxLDL antibodies) may have a protective effect has been suggested, but the data is unclear and needs to be further investigated.
Keywords: Atherosclerosis, cardiovascular disease, immunopathology, autoimmunity, modified LDL, oxidized LDL, AGE-LDL, LDL antibodies, autoantibodies
Introduction
While there is general consensus about the inflammatory nature of atherosclerosis [1; 2; 3], understanding the multitude of insults that initiate and perpetuate the inflammatory process is far from clear and very much in its infancy. This review will focus on the proposed and still controversial role of modified LDL antibodies (mLDL Abs), which have been characterized in great detail from both the structural and biological points of view. The involvement of humoral immune processes in atherosclerosis is not likely to be limited to mLDL Abs. In this issue of CI others will discuss the role of antiphosphorylcholine antibodies, and special mention needs to be made of the literature suggesting that heat shock proteins and their corresponding antibodies may also play a significant role in cardiovascular disease [4; 5; 6].
The immunogenicity of modified lipoproteins
In vitro-modified human lipoproteins are immunogenic when injected into rabbits and rodents [7; 8; 9; 10]. They are also recognized by autoantibodies that seem to exist virtually in every single human adult [10; 11]. It is possible that the antibodies that are detected in normolipemic and hyperlipemic individuals recognize LDL with different degrees of modification [12] but the precise nature of the modifications and epitopes recognized by human antibodies is not totally clear. According to Tertov et al. the LDL isolated from circulating immune complexes formed by LDL and corresponding antibodies (human LDL-IC) is partially denatured and desyalated [13]. However, the main LDL modifications used to detect human mLDL Abs and to immunize rabbits and other laboratory animals are based on oxidative reactions [copper-oxidized LDL (oxLDL), MDA-modified LDL (MDA-LDL)] or on advanced glycation [AGE-modified LDL (AGE-LDL)].
As determined by analyzing LDL fractionated from immune complexes precipitated from human sera using polyethylene glycol (PEG) [14], such LDL was enriched in malondialdehyde (MDA)-lysine, Ne(carboxyethyl) lysine (CEL) and Ne (carboxymethyl) lysine (CML) [10]. However, inhibition studies performed with IgG purified from precipitated IC showed that the reactivity with immobilized oxLDL was only partially inhibited with MDA-LDL, meaning that oxLDL contains additional epitopes besides MDA-lysine. Similarly, the reactivity with immobilized AGE-modified LDL was not completely inhibited with CML, indicating that AGE-LDL contains other epitopes in addition to CML. Also of interest was the fact that neither MDA-modified bovine serum albumin (BSA) nor CML-modified BSA were effective inhibitors of the reactivity of IgG antibodies to oxLDL or AGE-LDL. Thus, the human immune system can discriminate MDA-lysine and CML epitopes in different proteins without detectable cross-reactivity [10].
While most of the research concerning oxidized LDL antibodies has been centered on epitopes derived from aminoacid modification, Frostegård and co-workers have proposed that antibodies to phosphatidylcholine and phosphorylcholine are also generated by oxidative processes [15; 16]. IgM antibodies to phosphorylcholine have been proposed to protect against atherosclerosis in patients with hypertension [15]. IgG antibodies to phosphorylcholine have been isolated from commercially available intravenous immunoglobulin (IVIg) and partially characterized [17], but their subclass distribution and basic biological properties (opsonizing capacity, complement activation) have not been described and their potential role in atherosclerosis not fully elucidated. The same problems exist in relation to IgG antibodies to oxLDL-beta2GPI that have been proposed to be proatherogenic [18].
Antibodies to AGE-modified LDL have also been isolated and characterized [19]. The main modification recognized by purified AGE-LDL antibodies is CML, but the presence of CEL adducts in the LDL isolated from IC suggests that CEL is also involved in the formation of epitopes recognized by the human immune system.
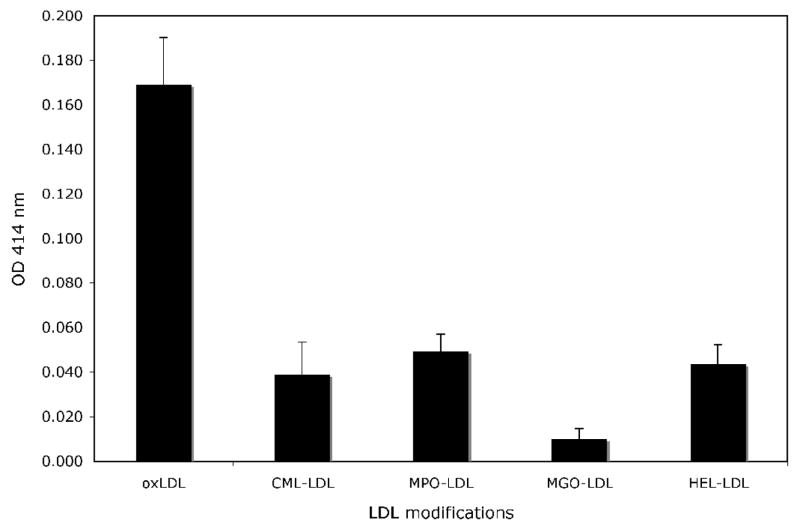
To what extent in vitro modification of LDL reproduces the epitopes generated during in vivo modification remains unclear. Ylä-Herttuala and co-workers published the only direct study of the immunogenicity of LDL isolated from human atherosclerotic lesions and demonstrated their reactivity with antibodies to MDA-lysine and 4-hydroxynonenal (HNE) lysine adducts, both present in oxidized LDL [20]. Several groups have focused their attention on the possible role of myeloperoxidase (MPO)-generated oxidants in vivo [21; 22; 23; 24]. The main arguments favoring this pathway of lipid oxidation are the presence of phagocytic cells able to generate MPO-generated oxidative radicals in the atherosclerotic lesions [25], as well as the observed co-localization of MPO and oxidized LDL [24; 25; 26; 27] in atheroma lesions. In a study examining the reactivity of IgG isolated from IC precipitated from the serum of 37 patients with diabetes (unpublished data), we found that CML-LDL, MPO-LDL, and HEL-LDL were recognized by this purified IgG (Figure 1), while methylglyoxal-modified LDL (MGO-LDL) was not. These data support the postulated generation of MPO-LDL in vivo and its recognition by the immune system. It also confirms the immunogenicity of HEL adducts, while it seems to disprove the immunogenicity of MGO, an intermediate in the pathway that leads to the formation of CML [28].
Figure 1.
Reactivity of human IgG isolated from PEG-precipitated IC obtained from 27 patients with diabetes (14 with type 1 and 13 with type 2 diabetes) with different LDL modifications: copper-oxidized LDL (oxLDL), (carboxymethyl) lysine-modified LDL (CML), myeloperoxidase-modified LDL (MPO-LDL), methylglyoxal-modified LDL (MGO-LDL), and (hexanoyl) lysine-LDL (HEL-LDL). The different forms of modified LDL were coated in enzymoimunoassay plates at identical dilutions, and identical dilutions of sera from the 27 patients were added to the coated plates. The bound antibody was revealed using rabbit anti-human oxLDL antibody[66]. The data is presented as the mean + s.e.m.
Structural and biological characteristics of modified LDL antibodies
Circulating human oxLDL antibodies were the first to be purified by affinity chromatography using immobilized oxLDL [29]. By 2002 we had isolated oxLDL antibodies from 46 patients with type 1 diabetes and found that IgG was the predominant isotype, followed by IgM and IgA. Within the IgG isotype, ox LDL antibodies were predominantly of subclasses 1 and 3 [19; 29; 30]. The predominance of IgG over IgM in isolated circulating oxLDL antibodies is not a characteristic of diabetic patients; it was also observed in antibodies isolated from healthy volunteers and from non-diabetic patients [29]. In a more recent study we tried to compare the concentrations of IgM and IgG oxLDL antibodies in IgG isolated from circulating IC and we confirmed the predominance of IgG antibodies over IgM, by an average 8:1 ratio [31]. A similar predominance of IgG antibodies of subclasses 1 and 3 has been demonstrated in purified AGE-LDL antibodies [19]. Our data contradicts the isotypic distribution of oxLDL antibodies reported by Wu and Lefvert [32], particularly in what concerns the subclass distribution. It must be noted that these authors did not test purified antibodies, and the mouse monoclonal anti-IgG2 antiserum they used is known to have significant issues of specificity and accuracy [33] and to yield higher values for IgG2 than methods using radial immunodiffusion and polyclonal antibodies [11].
IgG1 and IgG3 antibodies are considered pro-inflammatory, based on their ability to activate the complement system by the classical pathway and to interact with Fcγ receptors in phagocytic cells [34]. The involvement of IgG1 and IgG3 antibodies in immune complex disease is also well recognized [35]. To further determine whether oxLDL antibodies expressed those same biological properties, as expected from the predominance of IgG1 and IgG3 isotypes, we carried out extensive in vitro studies, initially using rabbit antibodies to human LDL and later using IC isolated from patient sera and IC prepared with human oxLDL and purified human antibodies to human oxLDL.
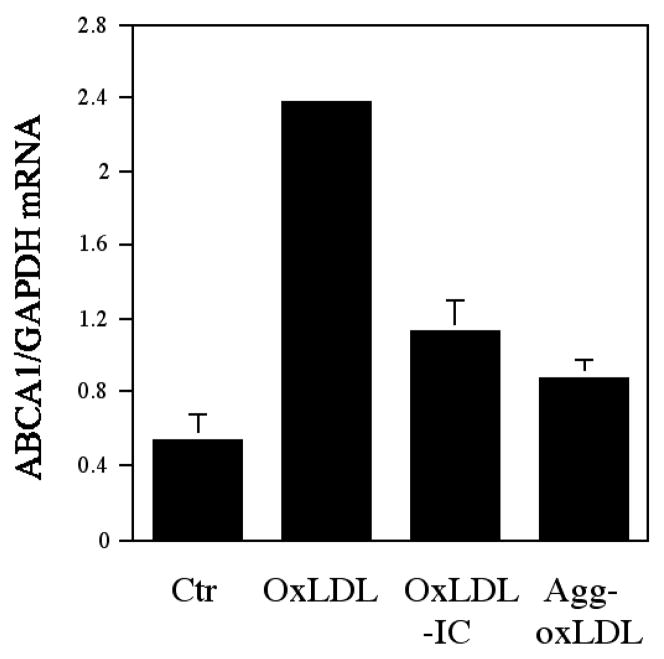
Insoluble IC prepared with human LDL and rabbit LDL antibody have been known to promote the transformation of macrophages into foam cells [36; 37; 38]. The accumulation of cholesterol by macrophages incubated with LDL-IC was a consequence of the IC uptake through Fcγ receptors, primarily the high affinity FcγRI [39] and appears to be a consequence of the delayed degradation of ingested LDL [40] as well as of LDL-IC induced abnormalities in receptor-mediated uptake of native and modified lipoproteins and altered reverse cholesterol transport. LDL-IC-induced foam cells show a paradoxical overexpression of the LDL receptor [38; 40], and, at least in the case of THP-1 cells, increased expression of scavenger receptors for acetylated LDL [41]. These interesting experiments could have possible translational limitations since LDL-IC were prepared with rabbit IgG antibodies, which not only are from a different species, but have a significantly higher affinity than human oxLDL antibodies (Kd values of 9.3×10−11 vs. 1,02 ± 1,1 × 10−8, respectively) [11]. Arguing against a possible difference in the biological behavior of IC formed with human antibodies against modified lipoproteins instead of rabbit antibodies, are studies showing that IC isolated from the serum of patients with type 1 diabetes and healthy controls had the same atherogenic properties as LDL-IC prepared with rabbit antibodies [42]. However, the question was addressed directly after we scaled up our method for the isolation of human oxLDL antibodies by affinity chromatography so that we could obtain the necessary quantities of human antibody to prepare IC containing both human oxLDL and human oxLDL antibodies [43]. Insoluble IC prepared with oxLDL and purified human oxLDL antibodies were equally able to promote CE accumulation in macrophages and induce foam cell formation; the degree of CE accumulation was 10 times higher with human oxLDL-IC than with the same concentration of oxLDL alone [43]. Interestingly human oxLDL-IC are not only able to induce the transformation of macrophages into foam cells but also to markedly affect receptors involved in cholesterol transport and homeostasis. LDL-IC-induced foam cells show a paradoxical over-expression of the LDL receptor in contrast to what happen with oxLDL-induced foam cells which show LDL-R down regulation [38; 40]. The impact of oxLDL and oxLDL-IC in reverse cholesterol transport, specifically in the ABCA1 transporter, shows also marked differences. Regardless of the considerably larger amount of CE deposition induced by oxLDL-IC when compared with oxLDL, oxLDL leads to higher expression of ABCA1 in human macrophages than oxLDL-IC (Figure 2, unpublished data).
Figure 2.
Regulation of ABCA1 gene expression by oxLDL-IC. Human THP-1 monocytes were treated with 160 nM of PMA for 2 days for macrophage transformation and then exposed to 100 μg/ml of oxLDL, 150 μg/ml of oxLDL-IC, and 150 μg/ml of agg-oxLDL for 24 h. After the treatment, RNA was isolated and converted to cDNA. Quantitative real-time PCR was conducted with the cDNA to quantify ABCA1 and GAPDH cDNA. The quantity of ABCA1 cDNA was normalized to GAPDH cDNA. The data is presented as the mean + s.e.m.
All these mechanisms can certainly contribute to the markedly higher intracellular accumulation of cholesteryl esters in presence of oxLDL-IC when compared with oxLDL. The paradoxical overexpression of LDL receptors after exposure of human macrophages to LDL-IC is however extremely interesting because it suggests that ingestion of LDL as part of an IC leads to an unregulated metabolic processing pathway that it is totally independent of the cell cholesterol content [44]. The stimulation of LDL-R expression is mediated not via SREBP but via the ERK signaling pathway and AP1 motif-dependent transcriptional activation [45].
The relevance of LDL-IC to the development of atherosclerosis is shown not only by the ability of the IC to promote the transformation of macrophages into foam cells but for their ability to trigger inflammation. The uptake of LDL-IC prepared with rabbit antibodies through the FcγRI [39], specific for IgG1 antibodies, is associated with activation of human monocyte-derived macrophages and THP-1 cells, inducing the respiratory burst and the release of proinflammatory cytokines [41; 46]. Other groups have reported FcγRIIa-mediated activation of U937 and THP-1 cells incubated with oxLDL-IC [47; 48]. Work by Huang and collaborators demonstrated that LDL-IC activated the MAPK path-way in THP-1 cells [49] and stimulated matrix metalloproteinase-1 expression through the same pathway in U937 [47] and human vascular endothelial cells [50], irrespectively of the type of FcγR engaged.
Using oxLDL IC prepared with human oxLDL and corresponding human antibody, we have proven two critical points concerning the proinflammatory properties of oxLDL-IC. First, as expected from the predominance of IgG1 and IgG3 isotypes in oxLDL antibodies, human oxLDL-IC activate complement through the classical pathway [51]; Also in agreement with the isotype distribution, human oxLDL-IC were shown to activate MonoMac cells, THP-1 cells, and human monocyte-derived macrophages leading to the release of IL-1β, IL-6, IL-10, IL-12, and TNF [43; 51], and this activation is a consequence of the engagement of the FcγRI [51]. With regard to macrophage activation, it is important to note that equivalent concentrations of IC prepared with keyhole lympet hemocyanin (KLH) and human KLH antibodies are significantly less efficient than oxLDL-IC [51]. More recently, a comparative study of the effects of oxLDL, oxLDL IC and KLH IC (both types of IC prepared with human antibodies) on gene expression in U937 cells showed that oxLDL-IC had unique activating effects (not shared by oxLDL or by KLH-IC) on genes believed to enhance cell survival, inhibit cell growth, regulate oxireductase activity, and control transport of fatty acids and aminoacids [52]. The cell survival enhancement effect of oxLDL-IC has been also reported by Okskoji et al.[53] using human monocytes as targets. This anti-apoptotic effect has significant implications in the perpetuation of a chronic inflammatory reaction. Furthermore, in common with KLH-IC, oxLDL-IC enhanced the expression of a number of genes involved in the inflammatory response, including the HSP70 6 gene product, a member of the heat shock 70 kD family [52]. The increased expression of HSP70 6 opens implies the activation of an alternative proinflammatory pathway, given the reported ability of the HSP70 family of gene products to induce the release of proinflammatory cytokines from naïve macrophages [54]. In conclusion, the proinflammatory properties of human oxLDL IC have been extensively documented in vitro and ex vivo experiments.
Pathogenicity of modified LDL antibodies in humans
Initially, the attention of clinical investigators concentrated on finding evidence supporting a pathogenic role for oxLDL and MDA-LDL antibodies, using them as a surrogate measurement of LDL with different degrees of modification. The results of these studies were rather disappointing, because the results were often conflicting and failed to produce a clear cut indication of the clinical value of modified lipoprotein antibody assays as biomarkers for the development and/or progression of atherosclerosis. While some groups reported a positive correlation between the levels of oxLDL antibodies and different endpoints considered as evidence of atherosclerotic vascular disease, such as progression of carotid atherosclerosis or risk for the future development of myocardial infarction [55; 56; 57; 58; 59; 60; 61], others failed to show such correlation or showed an inverse correlation [62; 63; 64; 65; 66; 67; 68; 69; 70; 71; 72; 73; 74].
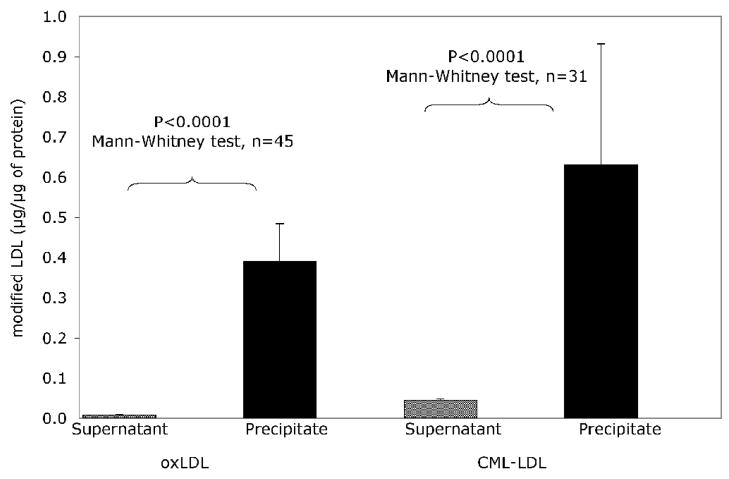
The reasons for these discrepancies have been discussed elsewhere [11]. Basically the assays used by different groups are rather heterogeneous, none of them has risen to the “gold standard” status, and the interference of circulating modified lipoprotein complexes [42; 72] has rarely been considered in the design of these assays, in spite of the fact that IC formation is the most logical explanation for the inverse relationship between serum oxLDL and oxLDL antibody levels that was documented almost a decade ago [75]. The significance of oxLDL-IC as interfering factors in the assay of ox LDL antibodies is strongly supported by recent observations in our laboratory that demonstrate that the vast majority of circulating modified LDL is complexed with the corresponding antibodies (Figure 3, unpublished data). But, in reality, even assuming that the interference of LDL IC could be eliminated, the measurement of modified LDL antibodies in circulation may be more informative of the degree of immunogenicity of modified lipoproteins, which depends probably on the concentration and degree of modification of LDL and on the immune reactivity of the individual, than of the pathogenic potential of modified LDL antibodies.
Figure 3.
Results of capture assays for oxLDL and AGE-LDL on Apo B-rich lipoproteins purified from precipitated mLDL-IC (precipitate) and from supernatant obtained after precipitation of IC (Supernatant). IC were run on a Protein G column to separate IgG from the antigen moiety. The washout containing the antigen moiety as well as the serum obtained after precipitation of IC were run on a heparin-agarose column to isolate Apo B-containing lipoproteins. Capture assays were performed in these fractions (LDL in IC and free LDL). Goat anti-human ApoB:HRP diluted 1:1000 was used to detect captured LDL [123]. The data is presented as the mean + s.e.m.
The pathogenic potential of any type of antibodies depends on their isotype, and, with the exception of anti-receptor antibodies, is fully expressed only after formation of antigen-antibody complexes [34]. As mentioned earlier, oxLDL and AGE-LDL antibodies are predominantly IgG1 and IgG3, considered pro-inflammatory [34]. The involvement of LDL-IC in the pathogenesis of atherosclerosis was suggested over a decade ago by the elegant studies of Yla-Herttuala and co-workers [20; 76]. These authors purified oxLDL and the corresponding IgG antibodies from atheromatous lesions of humans and Watanabe hyperlipidemic rabbits, thus demonstrating that the ingredients necessary for the formation of LDL-IC are present in the damaged arterial wall. Several groups, including ours, have reported a significant correlation between soluble LDL-IC and the presence of clinical and/or laboratorial evidence of cardiovascular disease [71; 72; 77; 78; 79; 80]. Lefvert et al. have reported association between circulating IC measured by non-specific assays and myocardial infarction in individuals with less that 45 yrs of age [81] and found, in a prospective study, that such IC were “a strong and independent risk factor for myocardial infarction” in 50-yr-old men [61].
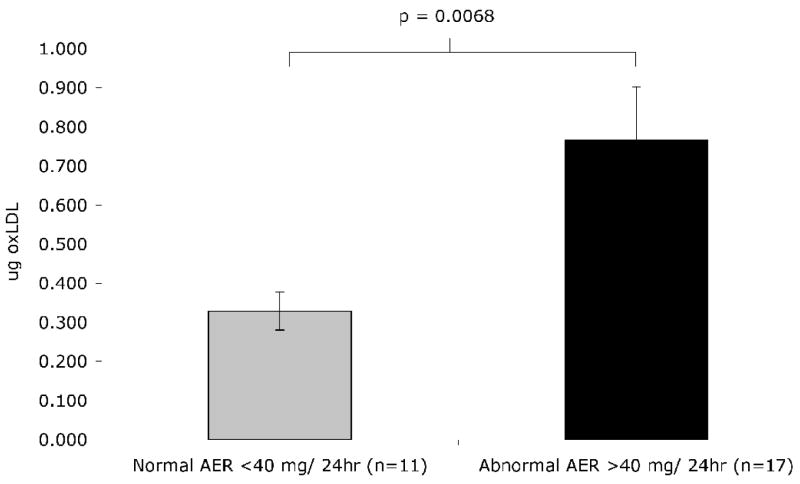
In a recently reported study, we screened 1050 diabetic patients from the DCCT/EDIC cohort for mLDL-IC and evaluated the impact of these IC in progression of carotid intima-media thickness [IMT]. After adjustment for a variety of factors, including age, gender, IMT at year 1, ultrasonography equipment used to measure IMT, DCCT randomization group, smoking, hypertension, HbA1c, logarithm of the albumin excretion rate [AER] and C-Reactive Protein [CRP] levels, mLDL-IC levels were good predictors of progression of internal carotid IMT [82]. We also found significant correlations between the levels of LDL-IC and those of soluble ICAM-1, CRP, and fibrinogen [82]. Furthermore, in another study of the same patient cohorts, we found that the levels of modified LDL-IC were higher in patients with abnormal albumin excretion rate [AER] [83]. In a smaller series of cases of the same cohorts we also demonstrated that patients with AER ≥40mg/24 h have higher concentrations of oxLDL in their isolated circulating IC than those with AER <40 mg/24 h (unpublished data, Figure 4). In a different patient cohort studied in collaboration with Orchard and others, we obtained data confirming that mLDL-IC is a risk factor for incident atherosclerosis and nephropathy [84; 85]. hese clinical and epidemiological studies strongly support the atherogenic potential of oxLDL-IC defined by in vitro and ex vivo studies.
Figure 4.
Comparison of oxLDL levels in ApoB/E-rich lipoproteins purified from PEG-precipitated IC from patients with AER<40 mg/24h and AER ≥40 mg/24 h. Details concerning the isolation of lipoproteins from precipitated IC and the capture of oxLDL in the IC fractions have been published elsewhere [123]. The data is presented as the mean ± s.e.m.
The major factor determining the formation of LDL appears to be the availability of modified LDL, which seems to be directly related to the levels of LDL. Evidence supporting this postulate was obtained in a study performed by our group in which we measured the levels of LDL-IC longitudinally in 26 patients with type 2 diabetes, enrolled before receiving lipid-lowering medications or CYP 344 inhibitors. The patients were first instructed to follow a lipid-lowering diet and exercise, and two weeks later started statin therapy (Simvastatin, 20 mg/day). Simvastatin dosage was adjusted as needed to attain a level of LDL-cholesterol of ≤100 mg/dl. Blood samples were collected at baseline, 3 and 6 months after reaching the target level for cholesterol, and 3 months after stopping Simvastatin. The patients showed a significant reduction in their levels of circulating LDL-IC during therapy, which returned to near baseline levels after discontinuation of therapy [86]. Tsimikas et al. similarly reported that 16 weeks of therapy with Atorvastatin reduced the levels of circulating apoB-containing IC by 29.5% although those changes, in their experience, did not correlate with changes in quantitative coronary arteriography after 18 months of therapy [87].
The flip side: is the humoral immune response to modified lipoproteins protective against the development of atherosclerosis?
In 1995 Palinski et al reported that the immunization of LDL-receptor-deficient [LDLr−/−] rabbits with homologous MDA-LDL reduced atherogenesis [88]. This report triggered multiple animal studies in rabbits and mice, well summarized by Binder et al. [89]. Apo-E deficient mice immunized with homologous MDA-LDL also showed a reduction in the development of atheromatous lesions[88; 90]. Similar observations were reported in hypercholesterolemic rabbits immunized with autologous oxLDL [91]. However, there are significant issues concerning the extrapolation of the data obtained in these animals to our understanding of the role of the humoral immune response in human atherosclerosis. First, antibodies resulting from deliberate immunization have very different characteristics from those emerging spontaneously [10]. Second, there are significant species-related differences in lipoprotein metabolism and adaptive immune responses to modified lipoproteins, and the extrapolation of data obtained on these animal models may be quite irrelevant for the understanding of human atherosclerosis [92]. Third, the reported data from animal experiments is not as clearly supportive of a protective role of oxLDL antibodies as some claim. For example, the studies carried out immunizing LDLr−/− mice with MDA-LDL showed the same “protective” effect after immunization with unmodified LDL, and the reduction in atherosclerosis development was seen in the absence of antibodies to modified LDL [93]. In hypercholesterolemic rabbits immunized with autologous oxLDLantibodies to oxLDL developed spontaneously in non-immunized hypercholesterolemic control rabbits, as well as in rabbits immunized with native LDL [which actually showed the greatest “protective” effect of immunization] [91]. Also in contradiction with the proposed protective role of oxLDL and MDA-LDL antibodies was the observation of Palinski et al. who reported increased oxLDL autoantibody titers in LDLr −/− mice that were significantly correlated with the extent of atherosclerosis [94].
Other groups have reported data supporting the protective role of modified LDL antibodies, using a variety of different experimental strategies. Shaw et al. reported that a human-derived IgG oxLDL monoclonal antibody inhibited the uptake of oxLDL by macrophages [95]. A significant flaw in this study was the fact that the recombinant mono-clonal antibody was synthesized as Fab fragments [95] which cannot activate complement or interact with Fcγ receptors [FcγR] [34; 96], therefore unable to induce an inflammatory reaction, but perfectly able to block the formation of opsonizing IC with complete IgG antibody molecules. More recently, the emphasis has shifted to the possible protective role of IgM antibodies. That IgM antibodies may be protective is logical, given their low affinity, predominant intravascular distribution, and lack of interaction with Fc receptors of phagocytic cells [34; 97; 98]. In some mouse models oxLDL antibodies are predominantly of the IgM isotype, and this led to several postulates, such as that that protective IgM antibodies to modified LDL may exist as “natural” antibodies, a protective form of innate immunity [99]. In humans, as pointed earlier, the predominant isotype of both oxLDL and AGE-LDL antibodies is IgG, and the parallelism with mouse models does not exist. Another line of research supporting a protective role of IgM antibodies is based on the existence and/or induction of polyreactive antibodies [100; 101] that can be induced, for example, by immunization of hypercholesterolemic mice with Streptococcus pneumoniae. This results in the synthesis of protective IgM antibodies directed against phosphorylcholine epitopes shared with oxLDL [102]. A recent report by Yamashita et al. showed that the immunization of hypercholesterolemic pregnant NZW rabbits and LDL receptor-deficient mice with oxLDL resulted in cross-immunization of the progeny, which responded with the synthesis of IgM antibodies and formation of IgM-LDL immune complexes, which in turn appeared to have a protective effect against the development of atherosclerosis [103]. Interestingly, in rabbits, the increased synthesis of IgM antibodies could also be induced with adjuvant alone, and the resulting IgM antibodies reacted not only with oxLDL but also with native LDL. The cross reactivity of rabbit anti-human oxLDL with human native LDL has also been observed by us, and does not appear just a consequence of spontaneous oxidative processes affecting native LDL because human oxLDL antibodies show very limited cross-reactivity with native LDL [10].
IgM antibodies to oxLDL are detectable in humans, as previously stated. Su et al. reported that high levels of IgM ox LDL antibodies, as well as IgM phosphorylcholine antibodies, are related with a slower progression of atherosclerosis in patients with hypertension [15]. Karvonen et al. reported that the titers of IgM oxLDL antibodies are inversely correlated to intima-media thickness [104]. Tsimikas et al. reported that IgM oxLDL antibodies and LDL-IC containing IgM were inversely related with CAD (as detected by angiography), while IgG oxLDL antibodies and LDL-IC containing IgG were directly related to CAD [6; 105]. However, the associations with lower or higher risk of CAD did not hold in multivariate analysis, therefore the immunoglobulin isotype of LDL antibodies did not appear to be an independent predictor of CAD. It also needs to be pointed out that Fredrikson et al published conflicting results, reporting that IgM antibodies to oxLDL correlate with a more rapid progression of carotid disease, as judged by IMT measurements [106].
Having reported that oxLDL-IC were associated with evidence of nephropathy in diabetic patients [83] we have recently carried out a study in which we measured IgM and IgG oxLDL antibodies isolated for PEG-precipitated immune complexes and found that while IgG antibody concentrations were positively correlated with serum creatinine levels and urinary albumin excretion rate, and negatively correlated with estimated glomerular filtration rate. In contrast, no correlation was observed between those parameters and IgM antibody concentrations [107].
In spite of all this conflicting data, several authors considered the evidence supporting a protective role of oxLDL IgM antibodies sufficient to justify the possibility of inducing protection against atherosclerosis by deliberate immunization protocols [108]. However, further illustrating the difficulty in translating animal data to human interventions, a recent report on the effects of pneumococcal vaccination in humans failed to demonstrate the induction of circulating IgM antibodies to oxLDL [109].
Conclusions
The immunogenicity of autologous modified LDL in humans has been established by numerous studies summarized in this review. There is also considerable and conclusive evidence suggesting that the humoral immune response to modified LDL is pathogenic in humans. The predominant isotypes of modified LDL antibodies are IgG, of subclasses 1 and 3, classically considered as proinflammatory [34; 35]. More important is the fact that in vitro and ex vivo data have proven that IC containing oxidized LDL have both atherogenic and proinflammatory properties [38; 41; 42; 43; 45; 47; 49; 52; 110; 111; 112; 113]. It has also been demonstrated that the proinflammatory and proatherogenic properties of oxLDL-IC are significantly more powerful than those of oxLDL [43; 51].
Clinical studies are considerably more difficult to perform, particularly if the objective is to study a cohort sufficiently large as to reach conclusive results. It may be argued that totally conclusive data has not yet been published, but it should be recognized that several groups have reported data that support the association of LDL-IC with manifestations of atherosclerosis, such as coronary artery disease [72; 79; 80; 114], myocardial infarction [81], intima-media thickening [82], and diabetic nephropathy [83; 85]. There is a need for reliable and simple assays so that the predictive value of oxLDL can be adequately assessed.
In any case, there is sufficient evidence supporting the pathogenic potential of autoantibodies to oxLDL and other LDL modifications in humans. The goals of either reducing the immune response to modified LDL or modulating the immune response to favor IgM antibody synthesis are certainly worth attention. Of the two, modulation of the immune response appears as most difficult. Blocking CD40-CD40 ligand interactions could have such an effect [115], but at the potential high cost of interfering with secondary immune responses and development of memory against common pathogens. Alternative interventions could involve the upregulation of T regulatory cells [116; 117]. This intervention appears to be effective in the therapeutic manipulation of transplant rejection responses [118], but at this time can only be considered as a distant and uncertain target. Currently, the only practical approach is to reduce the antigenic load, either by dietary modification, or by pharmacological means. Statins have been shown to reduce the levels of circulating modified LDL-IC [86; 87], and have also anti-inflammatory and anti-oxidant effects [119; 120; 121]. Their effects on modified LDL-IC levels should be investigated in a well designed trial with sufficient numbers of patients and methodological rigor, designed to determine how much of the benefits attributed to statins can be correlated with the reduction of the major pro-inflammatory insult that mLDL-IC appear to represent. Also of note is preliminary data suggesting that dietary modification may reduce the levels of oxLDL and increase the levels of IgM phosphorylcholine antibodies [122]. While the significance of phosphorylcholine antibodies seems clearer in patients with autoimmune disorders, this report certainly opens the door to studies designed to determine whether the same or similar dietary interventions could result in upregulation of the levels of IgM antibodies to modified LDL.
Finally, given that atherosclerosis is a chronic inflammatory disease, one needs to consider whether the use of anti-inflammatory agents, beyond the currently available statins and aspirin, could be clinically useful. The development of statins with enhanced anti-inflammatory properties is certainly an interesting possibility that should be explored. The use of more potent and proven anti-inflammatory agents, such as TNF blockers and phospholipase 2 inhibitors (ref), needs to be carefully considered. Concern about possible adverse effects caused by these blockers used over long periods of time needs to be carefully balanced with the possible benefit of their anti-inflammatory effects.
Acknowledgments
This work was supported by the Research Service of the Ralph H. Johnson Department of Veteran Affairs Medical Center, by a Program Project Grant funded by the National Institutes of Health/NHLBI (PO1-HL55782), by a grant funded by NIH/NIDDK (R01 DK081352), and by a grant from the Juvenile Diabetes Research Foundation (1-2006-49).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–74. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 2.Ross R. Atherosclerosis-An inflammatory disease. N Eng J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 3.Steinberg D. Atherogenesis in perspective: Hypercholesterolemia and Inflammation as Partners in Crime. Nature Medicine. 2002;8:1211–1217. doi: 10.1038/nm1102-1211. [DOI] [PubMed] [Google Scholar]
- 4.Mayr M, Metzler B, Kiechl S, Willeit J, Schett G, Xu Q, Wick G. Endothelial cyto-toxicity mediated by serum antibodies to heat shock proteins of Escherichia coli and Chlamydia pneumoniae: immune reactions to heat shock proteins as a possible link between infection and atherosclerosis. Circulation. 1999;99:1560–6. doi: 10.1161/01.cir.99.12.1560. [DOI] [PubMed] [Google Scholar]
- 5.Xu Q. Role of heat shock proteins in atherosclerosis. Arterioscler Thromb Vasc Biol. 2002;22:1547–59. doi: 10.1161/01.atv.0000029720.59649.50. [DOI] [PubMed] [Google Scholar]
- 6.Mayr M, Kiechl S, Tsimikas S, Miller E, Sheldon J, Willeit J, Witztum JL, Xu Q. Oxidized low-density lipoprotein autoantibodies, chronic infections, and carotid atherosclerosis in a population-based study. J Am Coll Cardiol. 2006;47:2436–43. doi: 10.1016/j.jacc.2006.03.024. [DOI] [PubMed] [Google Scholar]
- 7.Steinbrecher UP, Fisher M, Witztum JL, Curtiss LK. Immunogenicity of homologous low density lipoprotein after methylation, ethylation, acetylation, or carbamylation: generation of antibodies specific for derivatized lysine. Journal of Lipid Research. 1984;25:1109–16. [PubMed] [Google Scholar]
- 8.Palinski W, Yla-Herttuala S, Rosenfeld ME, Butler SW, Socher SA, Parthasarathy S, Curtiss LK, Witztum JL. Antisera and monoclonal antibodies specific for epitopes generated during oxidative modification of low density lipoprotein. Arteriosclerosis. 1990;10:325–35. doi: 10.1161/01.atv.10.3.325. [DOI] [PubMed] [Google Scholar]
- 9.Steinbrecher UP. Oxidation of human low density lipoprotein results in derivatization of lysine residues of apolipoprotein B by lipid peroxide decomposition products. J Biol Chem. 1987;262:3603–8. [PubMed] [Google Scholar]
- 10.Virella G, Thorpe S, Alderson NL, Derrick MB, Chassereau C, Rhett JM, Lopes-Virella MF. Definition of the immunogenic forms of modified human LDL recognized by human autoantibodies and by rabbit hyperimmune antibodies. J Lipid Research. 2004;45:1859–67. doi: 10.1194/jlr.M400095-JLR200. [DOI] [PubMed] [Google Scholar]
- 11.Virella G, Lopes-Virella MF. Lipoprotein autoantibodies: measurement and significance. Clinical and Diagnostic Laboratory Immunology. 2003;10:499–505. doi: 10.1128/CDLI.10.4.499-505.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fernvik EC, Ketelhuth DF, Russo M, Gidlund M. The autoantibody repertoire against copper- or macrophage-modified LDL differs in normolipidemics and hypercho-lesterolemic patients. J Clin Immunol. 2004;24:170–6. doi: 10.1023/B:JOCI.0000019782.67993.0b. [DOI] [PubMed] [Google Scholar]
- 13.Tertov VV, Sobenin IA, Orekhov AN, Jaakkola O, Solakivi T, Nikkari T. Characteristics of low density lipoprotein isolated from circulating immune complexes. Atherosclerosis. 1996;122:191–9. doi: 10.1016/0021-9150(95)05737-4. [DOI] [PubMed] [Google Scholar]
- 14.Zubler RH, Perin LH, Creighton WD, Lambert PH. Use of polyethylene glycol (PEG) to concentrate immune complexes from serum or plasma samples. Ann rheum Dis. 1977;36(Suppl):23– 26. [Google Scholar]
- 15.Su J, Georgiades A, Wu R, Thulin T, de Faire U, Frostegard J. Antibodies of IgM subclass to phosphorylcholine and oxidized LDL are protective factors for atherosclerosis in patients with hypertension. Atherosclerosis. 2006;188:160–6. doi: 10.1016/j.atherosclerosis.2005.10.017. [DOI] [PubMed] [Google Scholar]
- 16.Wu R, Huang YH, Elinder LS, Frostegard J. Lysophosphatidylcholine is involved in the antigenicity of oxidized LDL. Arterioscler Thromb Vasc Biol. 1998;18:626–30. doi: 10.1161/01.atv.18.4.626. [DOI] [PubMed] [Google Scholar]
- 17.Su J, Hua X, Concha H, Svenungsson E, Cederholm A, Frostegard J. Natural antibodies against phosphorylcholine as potential protective factors in SLE. Rheumatology (Oxford) 2008;47:1144–50. doi: 10.1093/rheumatology/ken120. [DOI] [PubMed] [Google Scholar]
- 18.Lopez LR, Kobayashi K, Matsunami Y, Matsuura E. Immunogenic Oxidized Low-density Lipoprotein/beta2-glycoprotein I Complexes in the Diagnostic Management of Atherosclerosis. Clin Rev Allergy Immunol. 2008 doi: 10.1007/s12016-008-8096-8. [DOI] [PubMed] [Google Scholar]
- 19.Virella G, Thorpe SR, Alderson NL, Stephan EM, Atchley DH, Wagner F, Lopes-Virella MF, Group DER. Autoimmune response to advanced glycosylation end-products of human Low Density Lipoprotein. J Lipid Res. 2003;443:487–493. doi: 10.1194/jlr.M200370-JLR200. [DOI] [PubMed] [Google Scholar]
- 20.Yla-Herttuala S, Palinski W, Rosenfeld ME, Parthasarathy S, Carew TE, Butler S, Witztum JL, Steinberg D. Evidence for the presence of oxidatively modified low density lipoprotein in atherosclerotic lesions of rabbit and man. J Clin Invest. 1989;84:1086–95. doi: 10.1172/JCI114271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carr AC, McCall MR, Frei B. Oxidation of LDL by myeloperoxidase and reactive nitrogen species: reaction pathways and antioxidant protection. Arterioscler Thromb Vasc Biol. 2000;20:1716–23. doi: 10.1161/01.atv.20.7.1716. [DOI] [PubMed] [Google Scholar]
- 22.Malle E, Hazell L, Stocker R, Sattler W, Esterbauer H, Waeg G. Immunologic detection and measurement of hypochlorite-modified LDL with specific monoclonal antibodies. Arterioscler Thromb Vasc Biol. 1995;15:982–9. doi: 10.1161/01.atv.15.7.982. [DOI] [PubMed] [Google Scholar]
- 23.Moguilevsky N, Zouaoui Boudjeltia K, Babar S, Delree P, Legssyer I, Carpentier Y, Vanhaeverbeek M, Ducobu J. Monoclonal antibodies against LDL progressively oxidized by myeloperoxidase react with ApoB-100 protein moiety and human atherosclerotic lesions. Biochem Biophys Res Commun. 2004;323:1223–8. doi: 10.1016/j.bbrc.2004.08.220. [DOI] [PubMed] [Google Scholar]
- 24.Podrez EA, Abu-Soud HM, Hazen SL. Myeloperoxidase-generated oxidants and atherosclerosis. Free Radic Biol Med. 2000;28:1717–25. doi: 10.1016/s0891-5849(00)00229-x. [DOI] [PubMed] [Google Scholar]
- 25.Daugherty A, Dunn JL, Rateri DL, Heinecke JW. Myeloperoxidase, a catalyst for lipoprotein oxidation, is expressed in human atherosclerotic lesions. J Clin Invest. 1994;94:437–44. doi: 10.1172/JCI117342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hazell LJ, Arnold L, Flowers D, Waeg G, Malle E, Stocker R. Presence of hypo-chlorite-modified proteins in human atherosclerotic lesions. J Clin Invest. 1996;97:1535–44. doi: 10.1172/JCI118576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Malle E, Waeg G, Schreiber R, Grone EF, Sattler W, Grone HJ. Immunohistochemical evidence for the myeloperoxidase/H2O2/halide system in human atherosclerotic lesions: colocalization of myeloperoxidase and hypochlorite-modified proteins. Eur J Biochem. 2000;267:4495–503. doi: 10.1046/j.1432-1327.2000.01498.x. [DOI] [PubMed] [Google Scholar]
- 28.Nagai R, Matsumoto K, Ling X, Suzuki H, Araki T, Horiuchi S. Glycolaldehyde, a reactive intermediate for advanced glycation end products, plays an important role in the generation of an active ligand for the macrophage scavenger receptor. Diabetes. 2000;49:1714–23. doi: 10.2337/diabetes.49.10.1714. [DOI] [PubMed] [Google Scholar]
- 29.Mironova M, Virella G, Lopes-Virella MF. Isolation and characterization of human antioxidized LDL autoantibodies. Arterioscler Thromb Vasc Biol. 1996;16:222–229. doi: 10.1161/01.atv.16.2.222. [DOI] [PubMed] [Google Scholar]
- 30.Virella G, Koskinen S, Krings G, Onorato JM, Thorpe SR, Lopes-Virella M. Immunochemical characterization of purified human oxidized low-density lipoprotein antibodies. Clinical Immunology. 2000;95:135–44. doi: 10.1006/clim.2000.4857. [DOI] [PubMed] [Google Scholar]
- 31.Virella G, Carter RE, Saad A, Crosswell EG, Game BA, Study DE, Lopes-Virella MF. Distribution of IgM and IgG antibodies to oxidized LDL in immune complexes isolated from patients with type 1 diabetes and its relationship with nephropathy. Clinical Immunology in print. 2008 doi: 10.1016/j.clim.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu R, Lefvert AK. Autoantibodies against oxidized low density lipoproteins (oxLDL): characterization of antibody isotype, subclass, affinity and effect on the macrophage uptake of oxLDL. Clin Exp Immunol. 1995;102:174–80. doi: 10.1111/j.1365-2249.1995.tb06652.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jefferis R. Polyclonal and monoclonal antibody reagents specific for IgG subclasses. Monogr Allergy. 1986;19:71–85. [PubMed] [Google Scholar]
- 34.Virella G. Biosynthesis, metabolism and biological properties of immunoglobulins. In: Virella G, editor. Medical Immunology. Informa; N.Y. and London: 2007. pp. 65–72. [Google Scholar]
- 35.Virella G, Tsokos G. Immune complex diseases. In: Virella G, editor. Medical Immunology. Informa; N.Y. and London: 2007. pp. 323–334. [Google Scholar]
- 36.Klimov AN, Denisenko AD, Popov AV, Nagornev VA, Pleskov VM, Vinogradov AG, Denisenko TV, Magracheva E, Kheifes GM, Kuznetzov AS. Lipoprotein-antibody immune complexes: Their catabolism and role in foam cell formation. Atherosclerosis. 1985;58:1–15. doi: 10.1016/0021-9150(85)90051-6. [DOI] [PubMed] [Google Scholar]
- 37.Klimov AN, Denisenko AD, Vinogradov AG, Nagornev VA, Pivovarova YI, Sitnikova OD, Pleskov VM. Accumulation of cholesteryl esters in macrophages incubated with human lipoprotein-antibody autoimmune complex. Atherosclerosis. 1988;74:41–6. doi: 10.1016/0021-9150(88)90189-x. [DOI] [PubMed] [Google Scholar]
- 38.Griffith RL, Virella GT, Stevenson HC, Lopes-Virella MF. Low density lipoprotein metabolism by human macrophages activated with low density lipoprotein immune complexes. A possible mechanism of foam cell formation. Journal of Experimental Medicine. 1988;168:1041–59. doi: 10.1084/jem.168.3.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lopes-Virella MF, Binzafar N, Rackley S, Takei A, La Via M, Virella G. The uptake of LDL-IC by human macrophages: predominant involvement of the Fc gamma RI receptor. Atherosclerosis. 1997;135:161–70. doi: 10.1016/s0021-9150(97)00157-3. [DOI] [PubMed] [Google Scholar]
- 40.Lopes-Virella MF, Griffith RL, Shunk KA, Virella GT. Enhanced uptake and impaired intracellular metabolism of low density lipoprotein complexed with anti-low density lipoprotein antibodies. Arteriosclerosis and Thrombosis. 1991;11:1356–1367. doi: 10.1161/01.atv.11.5.1356. [DOI] [PubMed] [Google Scholar]
- 41.Kiener PA, Rankin BM, Davis PM, Yocum SA, Warr GA, Grove RI. Immune complexes of LDL induce atherogenic responses in human monocytic cells. Arteriosclerosis, Thrombosis & Vascular Biology. 1995;15:990–9. doi: 10.1161/01.atv.15.7.990. [DOI] [PubMed] [Google Scholar]
- 42.Mironova M, Virella G, Virella-Lowell I, Lopes-Virella MF. Anti-modified LDL antibodies and LDL-containing immune complexes in IDDM patients and healthy controls. Clinical Immunology & Immunopathology. 1997;85:73–82. doi: 10.1006/clin.1997.4404. [DOI] [PubMed] [Google Scholar]
- 43.Virella G, Atchley DH, Koskinen S, Zheng D, Lopes-Virella M. Pro-atherogenic and pro-inflammatory properties of immune complexes prepared with purified human oxLDL antibodies and human oxLDL. Clinical Immunology. 2002;105:81–92. doi: 10.1006/clim.2002.5269. [DOI] [PubMed] [Google Scholar]
- 44.Huang Y, Ghosh MJ, Lopes-Virella MF. Transcriptional and post-transcriptional regulation of LDL receptor gene expression in PMA-treated THP-1 cells by LDL-containing immune complexes. Journal of Lipid Research. 1997;38:110–20. [PubMed] [Google Scholar]
- 45.Fu Y, Huang Y, Bandyopadhyay S, Virella G, Lopes-Virella MF. LDL immune complexes stimulate LDL receptor expression in U937 histiocytes via extracellular signal-regulated kinase and AP-1. J Lipid Res. 2003;44:1315–21. doi: 10.1194/jlr.M200415-JLR200. Epub 2003 May 1. [DOI] [PubMed] [Google Scholar]
- 46.Virella G, Munoz JF, Galbraith GM, Gissinger C, Chassereau C, Lopes-Virella MF. Activation of human monocyte-derived macrophages by immune complexes containing low-density lipoprotein. Clinical Immunology & Immunopathology. 1995;75:179–89. doi: 10.1006/clin.1995.1069. [DOI] [PubMed] [Google Scholar]
- 47.Huang Y, Fleming AJ, Wu S, Virella G, Lopes-Virella MF. Fc-gamma receptor cross-linking by immune complexes induces matrix metalloproteinase-1 in U937 cells via mitogen-activated protein kinase. Arterioscler Thromb Vasc Biol. 2000;20:2533–8. doi: 10.1161/01.atv.20.12.2533. [DOI] [PubMed] [Google Scholar]
- 48.Nagarajan S. Anti-OxLDL IgG blocks OxLDL interaction with CD36, but promotes FcgammaR, CD32A-dependent inflammatory cell adhesion. Immunol Lett. 2007;108:52–61. doi: 10.1016/j.imlet.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 49.Huang Y, Jaffa A, Koskinen S, Takei A, Lopes-Virella MF. Oxidized LDL-containing immune complexes induce Fc gamma receptor I-mediated mitogen-activated protein kinase activation in THP-1 macrophages. Arteriosclerosis, Thrombosis & Vascular Biology. 1999;19:1600–7. doi: 10.1161/01.atv.19.7.1600. [DOI] [PubMed] [Google Scholar]
- 50.Huang Y, Mironova M, Lopes-Virella MF. Oxidized LDL stimulates matrix metallo-proteinase-1 expression in human vascular endothelial cells. Arterioscler Thromb Vasc Biol. 1999;19:2640–7. doi: 10.1161/01.atv.19.11.2640. [DOI] [PubMed] [Google Scholar]
- 51.Saad AF, Virella G, Chassereau C, Boackle RJ, Lopes-Virella MF. OxLDL immune complexes activate complement and induce cytokine production by MonoMac 6 cells and human macrophages. J Lipid Res. 2006;47:1975–83. doi: 10.1194/jlr.M600064-JLR200. Epub 2006 Jun 27. [DOI] [PubMed] [Google Scholar]
- 52.Hammad SM, Twal WO, Barth JL, Smith KJ, Saad AF, Virella G, Argraves WS, Lopes-Virella MF. Oxidized LDL immune complexes and oxidized LDL differentially affect the expression of genes involved with inflammation and survival in human U937 monocytic cells. Atherosclerosis. 2009;202:394–404. doi: 10.1016/j.atherosclerosis.2008.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Oeal, Kovanen PT, Lindstedt KA, Jansson B, Pentikainen MO. OxLDL-IgG immune complexes induce survival of human monocytes. Arterioscler Thromb Vasc Biol. 2006;26:576–83. doi: 10.1161/01.ATV.0000201041.14438.8d. [DOI] [PubMed] [Google Scholar]
- 54.Svensson PA, Asea A, Englund MC, Bausero MA, Jernas M, Wiklund O, Ohlsson BG, Carlsson LM, Carlsson B. Major role of HSP70 as a paracrine inducer of cytokine production in human oxidized LDL treated macrophages. Atherosclerosis. 2006;185:32–8. doi: 10.1016/j.atherosclerosis.2005.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bellomo G, Maggi E, Poli M, Agosta FG, Bollati P, Finardi G. Autoantibodies against oxidatively modified low-density lipoproteins in NIDDM. Diabetes. 1995;44:60–66. doi: 10.2337/diab.44.1.60. [DOI] [PubMed] [Google Scholar]
- 56.Erkkilä AT, Närvänen O, Lehto S, Uusitupa MIJ, Ylä-Herttuala S. Autoantibodies against oxidized low-density lipoprotein and cardiolipin in patients with coronary heart disease. Arterioscl Thromb Vasc Biol. 2000;20:204–209. doi: 10.1161/01.atv.20.1.204. [DOI] [PubMed] [Google Scholar]
- 57.Lehtimaki T, Lehtinen S, Solakivi T, Nikkila M, Jaakkola O, Jokela H, Yla-Herttuala S, Luoma JS, Koivula T, Nikkari T. Autoantibodies against oxidized low density lipoprotein in patients with angiographically verified coronary artery disease. Arteriosclerosis, Thrombosis & Vascular Biology. 1999;19:23–7. doi: 10.1161/01.atv.19.1.23. [DOI] [PubMed] [Google Scholar]
- 58.Maggi E, Chiesa R, Melissano G, Castellano R, Astore D, Grossi A, Finardi G, Bellomo G. LDL oxidation in patients with severe carotid atherosclerosis. A study of in vitro and in vivo oxidation markers. Arterioscler Thromb. 1994;14:1892–1899. doi: 10.1161/01.atv.14.12.1892. [DOI] [PubMed] [Google Scholar]
- 59.Palinski W, Yla-Herttuala S, Rosenfeld ME, Butler SW, Socher SA, Parthasarathy S, Curtiss LK, Witztum JL. Antisera and monoclonal antibodies specific for epitopes generated during oxidative modification of low density lipoprotein. Arteriosclerosis. 1990;10:325–35. doi: 10.1161/01.atv.10.3.325. [DOI] [PubMed] [Google Scholar]
- 60.Salonen JT, Yla-Herttuala S, Yamamoto R, Butler S, Korpela H, Salonen R, Nyyssonen K, Palinski W, Witztum JL. Autoantibody against oxidised LDL and progression of carotid atherosclerosis. Lancet. 1992;339:883–7. doi: 10.1016/0140-6736(92)90926-t. [DOI] [PubMed] [Google Scholar]
- 61.Mustafa A, Nityanand S, Berglund L, Lithell H, Lefvert AK. Circulating immune complexes in 50-year-old men as a strong and independent risk factor for myocardial infarction. Circulation. 2000;102:2576–81. doi: 10.1161/01.cir.102.21.2576. [DOI] [PubMed] [Google Scholar]
- 62.Boullier A, Hamon M, Walters-Laporte E, Martin-Nizart F, Mackereel R, Fruchart JC, Bertrand M, Duriez P. Detection of autoantibodies against oxidized low-density lipoproteins and of IgG-bound low density lipoproteins in patients with corocnary artery disease. Clinica Chimica Acta. 1995;238:1–10. doi: 10.1016/0009-8981(95)06054-h. [DOI] [PubMed] [Google Scholar]
- 63.Festa A, Kopp HP, Schernthaner G, Menzel EJ. Autoantibodies to oxidised low density lipoproteins in IDDM are inversely related to metabolic control and microvascular complications. Diabetologia. 1998;41:350–6. doi: 10.1007/s001250050914. [DOI] [PubMed] [Google Scholar]
- 64.Hulthe J, Wiklund O, Hurt-Camejo E, Bondjers G. Antibodies to oxidized LDL in relation to carotid atherosclerosis, cell adhesion molecules, and phospholipase A(2) Arterioscler Thromb Vasc Biol. 2001;21:269–74. doi: 10.1161/01.atv.21.2.269. [DOI] [PubMed] [Google Scholar]
- 65.Hulthe J, Bokemark L, Fagerberg B. Antibodies to oxidized LDL in relation to intima-media thickness in carotid and femoral arteries in 58-year-old subjectively clinically healthy men. Arterioscler Thromb Vasc Biol. 2001;21:101–7. doi: 10.1161/01.atv.21.1.101. [DOI] [PubMed] [Google Scholar]
- 66.Virella G, Virella I, Leman RB, Pryor MB, Lopes-Virella MF. Anti-oxidized low-density lipoprotein antibodies in patients with coronary heart disease and normal healthy volunteers. International Journal of Clinical & Laboratory Research. 1993;23:95–101. doi: 10.1007/BF02592290. [DOI] [PubMed] [Google Scholar]
- 67.van de Vijver LP, Steyger R, van Poppel G, Boer JM, Kruijssen DA, Seidell JC, Princen HM. Autoantibodies against MDA-LDL in subjects with severe and minor atherosclerosis and healthy population controls. Atherosclerosis. 1996;122:245–53. doi: 10.1016/0021-9150(95)05759-5. [DOI] [PubMed] [Google Scholar]
- 68.Wu R, de Faire U, Lemne C, Witztum JL, Frostegard J. Autoantibodies to OxLDL are decreased in individuals with borderline hypertension. Hypertension. 1999;33:53–9. doi: 10.1161/01.hyp.33.1.53. [DOI] [PubMed] [Google Scholar]
- 69.Leinonen JS, Rantalaiho V, Laippala P, Wirta O, Pasternack A, Alho H, Jaakkola O, Yla-Herttuala S, Koivula T, Lehtimaki T. The level of autoantibodies against oxidized LDL is not associated with the presence of coronary heart disease or diabetic kidney disease in patients with non-insulin-dependent diabetes mellitus. Free Radic Res. 1998;29:137–41. [PubMed] [Google Scholar]
- 70.Uusitupa MIJ, Niskanen L, Luoma J, Vilja P, Rauramaa R, Ylä-Herttula S. Autoantibodies against oxidized LDL do not predict atherosclerosis vascular disease in non-insulin-dependent diabetes mellitus. Arterioscl Thromb Vasc Biol. 1996;16:1236–42. doi: 10.1161/01.atv.16.10.1236. [DOI] [PubMed] [Google Scholar]
- 71.Orchard TJ, Virella G, Forrest KY, Evans RW, Becker DJ, Lopes-Virella MF. Antibodies to oxidized LDL predict coronary artery disease in type 1 diabetes: a nested case-control study from the Pittsburgh Epidemiology of Diabetes Complications Study. Diabetes. 1999;48:1454–8. doi: 10.2337/diabetes.48.7.1454. [DOI] [PubMed] [Google Scholar]
- 72.Lopes-Virella MF, Virella G, Orchard TJ, Koskinen S, Evans RW, Becker DJ, Forrest KY. Antibodies to oxidized LDL and LDL-containing immune complexes as risk factors for coronary artery disease in diabetes mellitus. Clinical Immunology. 1999;90:165–72. doi: 10.1006/clim.1998.4631. [DOI] [PubMed] [Google Scholar]
- 73.Wilson PW, Ben-Yehuda O, McNamara J, Massaro J, Witztum J, Reaven PD. Autoantibodies to oxidized LDL and cardiovascular risk: the Framingham Offspring Study. Atherosclerosis. 2006;189:364–8. doi: 10.1016/j.atherosclerosis.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 74.Hulthe J, Wikstrand J, Lidell A, Wendelhag I, Hansson GK, Wiklund O. Antibody titers against oxidized LDL are not elevated in patients with familial hypercholesterolemia. Arterioscler Thromb Vasc Biol. 1998;18:1203–1211. doi: 10.1161/01.atv.18.8.1203. [DOI] [PubMed] [Google Scholar]
- 75.Shoji T, Nishizawa Y, Fukumoto M, Shimamura K, Kimura J, Kanda H, Emoto M, Kawagishi T, Morii H. Inverse relationship between circulating oxidized low density lipoprotein (oxLDL) and anti-oxLDL antibody levels in healthy subjects. Atherosclerosis. 2000;148:171–7. doi: 10.1016/s0021-9150(99)00218-x. [DOI] [PubMed] [Google Scholar]
- 76.Yla-Herttuala S, Palinski W, Butler S, Picard S, Steinberg D, Witztum JL. Rabbit and human atherosclerotic lesions contain IgG that recognizes epitopes of oxidized LDL. Arterioscler Thromb. 1994;14:32–40. doi: 10.1161/01.atv.14.1.32. [DOI] [PubMed] [Google Scholar]
- 77.Orekhov AN, Kalenich OS, Tertov VV, Novikov ID. Lipoprotein immune complexes as markers of atherosclerosis. International Journal of Tissue Reactions. 1991;13:233–6. [PubMed] [Google Scholar]
- 78.Orekhov AN, Kalenich OS, Tertov VV, Perova NV, Novikov Iy D, Lyakishev AA, Deev AD, Ruda M. Diagnostic value of immune cholesterol as a marker for atherosclerosis. J Cardiovasc Risk. 1995;2:459–66. doi: 10.1177/174182679500200511. [DOI] [PubMed] [Google Scholar]
- 79.Turk Z, Sesto M, Skodlar J, Ferencak G, Turk N, Stavljenic-Rukavina A. Soluble LDL-Immune Complexes in Type 2 Diabetes and Vascular Disease. Horm Metab Res. 2002;34:196–201. doi: 10.1055/s-2002-26706. [DOI] [PubMed] [Google Scholar]
- 80.Tsimikas S, Bergmark C, Beyer RW, Patel R, Pattison J, Miller E, Juliano J, Witztum JL. Temporal increases in plasma markers of oxidized low-density lipoprotein strongly reflect the presence of acute coronary syndromes. J Am Coll Cardiol. 2003;41:360–70. doi: 10.1016/s0735-1097(02)02769-9. [DOI] [PubMed] [Google Scholar]
- 81.Lefvert AK, Hamsten A, Holm G. Association between circulating immune complexes, complement C4 null alleles, and myocardial infarction before age 45 years. Arterioscler Thromb Vasc Biol. 1995;15:665–8. doi: 10.1161/01.atv.15.5.665. [DOI] [PubMed] [Google Scholar]
- 82.Lopes-Virella MF, McHenry MB, Lipsitz S, Yim E, Wilson PF, Lackland DT, Lyons T, Jenkins AJ, Virella G. Immune complexes containing modified lipoproteins are related to the progression of internal carotid intima-media thickness in patients with type 1 diabetes. Atherosclerosis. 2007;190:359–69. doi: 10.1016/j.atherosclerosis.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 83.Atchley DH, Lopes-Virella MF, Zheng D, Virella G. Oxidized LDL-Anti-Oxidized LDL immune complexes and diabetic nephropathy. Diabetologia. 2002;45:1562–1571. doi: 10.1007/s00125-002-0962-y. [DOI] [PubMed] [Google Scholar]
- 84.Costacou T, Lopes-Virella MF, Zgibor JC, Virella G, Otvos J, Walsh M, Orchard TJ. Markers of endothelial dysfunction in the prediction of coronary artery disease in type 1 diabetes. The Pittsburgh Epidemiology of Diabetes Complications Study. J Diabetes Complications. 2005;19:183–93. doi: 10.1016/j.jdiacomp.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 85.Yishak AA, Costacou T, Virella G, Zgibor J, Fried L, Walsh M, Evans RW, Lopes-Virella M, Kagan VE, Otvos J, Orchard TJ. Novel predictors of overt nephropathy in subjects with type 1 diabetes. A nested case control study from the Pittsburgh Epidemiology of Diabetes Complications cohort. Nephrol Dial Transplant. 2006;21:93–100. doi: 10.1093/ndt/gfi103. Epub 2005 Sep 6. [DOI] [PubMed] [Google Scholar]
- 86.Lopes-Virella MF, Mironova M, Stephan E, Durazo-Arvizu R, Virella G. Role of simvastatin as an immunomodulator in type 2 diabetes. Diabetes Care. 2004;27:908–13. doi: 10.2337/diacare.27.4.908. [DOI] [PubMed] [Google Scholar]
- 87.Tsimikas S, Witztum JL, Miller ER, Sasiela WJ, Szarek M, Olsson AG, Schwartz GG. High-dose atorvastatin reduces total plasma levels of oxidized phospholipids and immune complexes present on apolipoprotein B-100 in patients with acute coronary syndromes in the MIRACL trial. Circulation. 2004;110:1406–12. doi: 10.1161/01.CIR.0000141728.23033.B5. Epub 2004 Sep 7. [DOI] [PubMed] [Google Scholar]
- 88.Palinski W, Miller E, Witztum JL. Immunization of low density lipoprotein (LDL) receptor deficient rabbits with homologous malondialdehyde-modified LDL reduces atherogenesis. Proc Natl Acad Sci USA. 1995;92:821–825. doi: 10.1073/pnas.92.3.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Binder CJ, Chang MK, Shaw PX, Miller YE, Hartvigsen K, Dewan A, WJL Innate and Acquired Immunity in Atherogenesis. Nature Medicine. 2002;8:1218–1226. doi: 10.1038/nm1102-1218. [DOI] [PubMed] [Google Scholar]
- 90.George J, Afek A, Gilburd B, Levkovitz H, Shaish A, Goldberg I, Kopolovic Y, Wick G, Shoenfeld Y, Harats D. Hyperimmunization of apo-E-deficient mice with homologous malondialdehyde low-density lipoprotein suppresses early atherogenesis. Atherosclerosis. 1998;138:147–52. doi: 10.1016/s0021-9150(98)00015-x. [DOI] [PubMed] [Google Scholar]
- 91.Ameli S, Hultgardh-Nilsson A, Regnstrom J, Calara F, Yano J, Cercek B, Shah PK, Nilsson J. Effect of immunization with homologous LDL and oxidized LDL on early atherosclerosis in hypercholesterolemic rabbits. Arterioscler Thromb Vasc Biol. 1996;16:1074–9. doi: 10.1161/01.atv.16.8.1074. [DOI] [PubMed] [Google Scholar]
- 92.Virella G, Lopes-Virella MF. Humoral immunity and atherosclerosis. Nature Medicine. 2003;9:243–244. doi: 10.1038/nm0303-243. [DOI] [PubMed] [Google Scholar]
- 93.Freigang S, Horkko S, Miller E, Witztum JL, Palinski W. Immunization of LDL receptor-deficient mice with homologous malondialdehyde-modified and native LDL reduces progression of atherosclerosis by mechanisms other than induction of high titers of antibodies to oxidative neoepitopes. Arteriosclerosis, Thrombosis & Vascular Biology. 1998;18:1972–82. doi: 10.1161/01.atv.18.12.1972. [DOI] [PubMed] [Google Scholar]
- 94.Palinski W, Tangirala RK, Miller E, Young SG, Witztum JL. Increased autoantibody titers against epitopes of oxidized LDL in LDL receptor-deficient mice with increased atherosclerosis. Arterioscler Thromb Vasc Biol. 1995;15:1569–76. doi: 10.1161/01.atv.15.10.1569. [DOI] [PubMed] [Google Scholar]
- 95.Shaw PX, Horkko S, Tsimikas S, Chang MK, Palinski W, Silverman GJ, Chen PP, Witztum JL. Human-derived anti-oxidized LDL autoantibody blocks uptake of oxidized LDL by macrophages and localizes to atherosclerotic lesions in vivo. Arteriosclerosis, Thrombosis & Vascular Biology. 2001;21:1333–9. doi: 10.1161/hq0801.093587. [DOI] [PubMed] [Google Scholar]
- 96.Virella G. Immunoglobulin structure. In: Virella G, editor. Medical Immunology. Informa; NY and London: 2007. pp. 55–63. [Google Scholar]
- 97.Virella G. Humoral immune response and its induction by active immunization. In: Virella G, editor. Medical Immunology. Informa; N.Y. and London: 2007. pp. 159–172. [Google Scholar]
- 98.Mariani G, Strober W. Immunoglobulin Metabolism. In: Metzger H, editor. Fc Receptors and the Action of Antibodies. American Society for Microbiology Press; Washington, DC: 1990. pp. 94–180. [Google Scholar]
- 99.Binder CJ, Shaw PX, Chang MK, Boullier A, Hartvigsen K, Horkko S, Miller YI, Woelkers DA, Corr M, Witztum JL. The role of natural antibodies in atherogenesis. J Lipid Res. 2005;46:1353–63. doi: 10.1194/jlr.R500005-JLR200. Epub 2005 May 16. [DOI] [PubMed] [Google Scholar]
- 100.Cohn M. Degeneracy, mimicry and crossreactivity in immune recognition. Mol Immunol. 2005;42:651–5. doi: 10.1016/j.molimm.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 101.Notkins AL. Polyreactivity of antibody molecules. Trends Immunol. 2004;25:174–9. doi: 10.1016/j.it.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 102.Binder CJ, Horkko S, Dewan A, Chang MK, Kieu EP, Goodyear CS, Shaw PX, Palinski W, Witztum JL, Silverman GJ. Pneumococcal vaccination decreases athero-sclerotic lesion formation: molecular mimicry between Streptococcus pneumoniae and oxidized LDL. Nat Med. 2003;9:736–43. doi: 10.1038/nm876. Epub 2003 May 12. [DOI] [PubMed] [Google Scholar]
- 103.Yamashita T, Freigang S, Eberle C, Pattison J, Gupta S, Napoli C, Palinski W. Maternal immunization programs postnatal immune responses and reduces atherosclerosis in offspring. Circ Res. 2006;99:e51–64. doi: 10.1161/01.RES.0000244003.08127.cc. [DOI] [PubMed] [Google Scholar]
- 104.Karvonen J, Paivansalo M, Kesaniemi YA, Horkko S. Immunoglobulin M type of autoantibodies to oxidized low-density lipoprotein has an inverse relation to carotid artery atherosclerosis. Circulation. 2003;108:2107–12. doi: 10.1161/01.CIR.0000092891.55157.A7. Epub 2003 Oct 6. [DOI] [PubMed] [Google Scholar]
- 105.Tsimikas S, Brilakis ES, Lennon RJ, Miller ER, Witztum JL, McConnell JP, Kornman KS, Berger PB. Relationship of IgG and IgM autoantibodies to oxidized low density lipoprotein with coronary artery disease and cardiovascular events. J Lipid Res. 2007;48:425–33. doi: 10.1194/jlr.M600361-JLR200. [DOI] [PubMed] [Google Scholar]
- 106.Fredrikson GN, Hedblad B, Berglund G, Alm R, Nilsson JA, Schiopu A, Shah PK, Nilsson J. Association between IgM against an aldehyde-modified peptide in apolipoprotein B-100 and progression of carotid disease. Stroke. 2007;38:1495–500. doi: 10.1161/STROKEAHA.106.474577. [DOI] [PubMed] [Google Scholar]
- 107.Virella G, Carter RE, Saad A, Croswell EG, Game A, Lopes-Virella MF, Group DES. Distribution of IgM and IgG antibodies to oxidized LDL in immune complexes isolated from patients with type 1 diabetes and its relationship with nephropathy. Clinical Immunology. 2008 doi: 10.1016/j.clim.2008.02.005. in print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hansson GK. Vaccination against atherosclerosis: science or fiction? Circulation. 2002;106:1599–601. doi: 10.1161/01.cir.0000035275.64667.a3. [DOI] [PubMed] [Google Scholar]
- 109.Damoiseaux J, Rijkers G, Tervaert JW. Pneumococcal vaccination does not increase circulating levels of IgM antibodies to oxidized LDL in humans and therefore precludes an anti-atherogenic effect. Atherosclerosis. 2007;190:10–1. doi: 10.1016/j.atherosclerosis.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 110.Ghosh M, Huang Y, Lopes-Virella MF. Modified LDL-immune Complexes Upregulates Macrophage Scavenger Receptors in THP-1 Cells. Council on Arteriosclerosis for the 69th Scientific Sessions of the AHA; 1996. p. A1060. [Google Scholar]
- 111.Gisinger C, Virella GT, Lopes-Virella MF. Erythrocyte-bound low-density lipoprotein immune complexes lead to cholesteryl ester accumulation in human monocyte-derived macrophages. Clinical Immunology & Immunopathology. 1991;59:37–52. doi: 10.1016/0090-1229(91)90080-t. [DOI] [PubMed] [Google Scholar]
- 112.Lopes-Virella MF, Huang Y. Oxidized LDL-Containing Immune Complexes Stimulate Mitogen-Activated Protein (MAP) Kinase in Macrophages. Journal of Investigative Medicine. 1998;46:232A. [Google Scholar]
- 113.Mironova MA, Klein RL, Virella GT, Lopes-Virella MF. Anti-modified LDL antibodies, LDL-containing immune complexes, and susceptibility of LDL to in vitro oxidation in patients with type 2 diabetes. Diabetes. 2000;49:1033–41. doi: 10.2337/diabetes.49.6.1033. [DOI] [PubMed] [Google Scholar]
- 114.Tertov VV, Orekhov AN, Kacharava AG, Sobenin IA, Perova NV, Smirnov VN. Low Density Lipoprotein-Containing Circulating Immune Complexes and Coronary Atherosclerosis. Experimental and Molecular Pathology. 1990;52:300–308. doi: 10.1016/0014-4800(90)90071-k. [DOI] [PubMed] [Google Scholar]
- 115.Farber DL, Virella G. Cell-Mediated Immunity. In: Virella G, editor. Medical Immunology. Informa; New York and London: 2007. pp. 135–158. [Google Scholar]
- 116.Raimondi G, Turner MS, Thomson AW, Morel PA. Naturally occurring regulatory T cells: recent insights in health and disease. Crit Rev Immunol. 2007;27:61–95. doi: 10.1615/critrevimmunol.v27.i1.50. [DOI] [PubMed] [Google Scholar]
- 117.Yang XF. Factors regulating apoptosis and homeostasis of CD4+ CD25(high) FOXP3+ regulatory T cells are new therapeutic targets. Front Biosci. 2008;13:1472–99. doi: 10.2741/2775. [DOI] [PubMed] [Google Scholar]
- 118.Tao R, Hancock WW. Regulating regulatory T cells to achieve transplant tolerance. Hepatobiliary Pancreat Dis Int. 2007;6:348–57. [PubMed] [Google Scholar]
- 119.Bocan TM. Pleiotropic effects of HMG-CoA reductase inhibitors. Curr Opin Investig Drugs. 2002;3:1312–7. [PubMed] [Google Scholar]
- 120.Endres M. Statins: potential new indications in inflammatory conditions. Atheroscler Suppl. 2006;7:31–5. doi: 10.1016/j.atherosclerosissup.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 121.Patel TN, Shishehbor MH, Bhatt DL. A review of high-dose statin therapy: targeting cholesterol and inflammation in atherosclerosis. Eur Heart J. 2007;28:664–72. doi: 10.1093/eurheartj/ehl445. [DOI] [PubMed] [Google Scholar]
- 122.Elkan AC, Sjoberg B, Kolsrud B, Ringertz B, Hafstrom I, Frostegard J. Gluten-free vegan diet induces decreased LDL and oxidized LDL levels and raised atheroprotective natural antibodies against phosphorylcholine in patients with rheumatoid arthritis: a randomized study. Arthritis Res Ther. 2008;10:R34. doi: 10.1186/ar2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Virella G, Derrick MB, Pate V, Chassereau C, Thorpe SR, Lopes-Virella MF. Development of capture assays for different modifications of human low-density lipoprotein. Clin Diagn Lab Immunol. 2005;12:68–75. doi: 10.1128/CDLI.12.1.68-75.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]