Abstract
Toll-like receptors (TLRs) are important sensors of foreign microbial components as well as products of damaged or inflamed self tissues. Upon sensing these molecules, TLRs initiate a series of downstream signaling events that drive cellular responses including the production of cytokines, chemokines and other inflammatory mediators. This outcome results from the intracellular assembly of protein complexes that drive phosphorylation and other signaling cascades ultimately leading to chromatin remodeling and transcription factor activation. In addition to driving inflammatory responses, TLRs also regulate cell proliferation and survival which serves to expand useful immune cells and integrate inflammatory responses and tissue repair processes. In this context, central TLR signaling molecules, such as the mitogen-activated protein kinases (MAPK) and phosphoinositide 3-kinase (PI3K), play key roles. In addition, four major groups of transcription factors which are targets of TLR activation also control cell fate. This review focuses on the role of TLR signaling as it relates to cell proliferation and survival. This topic not only has important implications for understanding host defense and tissue repair, but also cancer which is often associated with conditions of chronic inflammation.
Keywords: Toll-like receptor, signaling, proliferation, survival, cancer
1. Introduction
The regulation of cell proliferation and survival is critical to mammalian development as well as tissue regeneration and repair. Uncontrolled cell proliferation and survival lead to cancer, a life threatening disease. There are numerous endogenous and exogenous signals moderating cell proliferation and survival, including mitogens, growth factors and hormones.
Host immune responses must also induce cell proliferation and survival. These responses are necessary for host defense following infection as they serve to stimulate leukocyte proliferation in the bone marrow, induce the clonal expansion of lymphocytes in secondary lymphoid tissues and ensure the survival of long-lived memory cells. Additionally, immune mediated inflammatory responses serve to initiate tissue repair processes following local infection. Many of these events are induced by the direct recognition of conserved structural components of viruses, bacteria, fungi, and protozoans, often called PAMPs (pathogen associated molecular patterns), by pattern recognition molecules of the host innate immune system. Prominent among these host pattern recognition molecules are the family of Toll-like receptors (TLRs).
TLRs are type I transmembrane receptors which are structurally characterized by extracellular leucine rich repeats (LRRs) and an intracellular Toll/IL-1 receptor (TIR) signaling domain. TLRs are activated following direct recognition of a wide range of PAMPs that include bacterial lipopolysacharides (LPS), lipoproteins, flagellin as well as viral and bacterial nucleic acids [1]. Upon sensing these molecules TLRs are best known for initiating signaling events that trigger the expression of inflammatory mediators including many cytokines, chemokines and cell adhesion molecules [2]. In addition to PAMPs, TLRs also sense a variety of endogenous molecules that are released as a result of cellular or tissue damage. These events can trigger sterile inflammation without detectable infection [3].
In addition to initiating inflammatory responses, TLRs have been shown to directly regulate cell proliferation and survival in a variety of biological settings [4-6]. Through regulation of compensatory proliferative responses [7] and suppression of apoptosis [8], TLRs have been proposed to protect against injury and initiate tissue repair processes. Many tumor cells appear to express their own set of TLRs, suggesting a role for these receptors in the regulation of tumor growth [9]. Indeed, TLR signaling has been associated with tumorigenesis in several disease models [10-12]. Molecular pathways linking TLR activity to cell proliferation and survival are not fully understood although progress is currently being made due to increased interest in this area. Notably, some TLR signaling pathways, such as the MAPK signaling cascades and the PI3K signaling pathway, have been well studied in cell proliferation and/or survival. In this review, we will focus on TLR signaling pathways involved in driving cell proliferation and survival as well as recent advances in our understanding of the role of TLRs in tissue repair and tumorigenesis.
2. The TLR signaling pathway
2.1 The core TLR signaling cascade
Following receptor ligation with an appropriate agonist, TLRs recruit proximal cytoplasmic adaptor proteins to the receptor signaling domain. This initial complex acts as a scaffold for specific signaling molecules [13]. The selective use of adaptor proteins is one of the main mechanisms of differential signaling downstream of TLRs. There are five such adaptor proteins in mammals, all of which possess the conserved TIR domain which upon TLR stimulation undergo homotypic interactions with the cytosolic TIR domain of the receptor [13]. Myeloid differentiation antigen 88 (MyD88) is a central adaptor required by all TLRs except TLR3 [14]. MyD88 deficient mice fail to respond to TLR2, TLR5, TLR7, TLR8 and TLR9 ligands [15,16] however, responses to TLR4 ligands are only partially impaired. This is due to the existence of alternative adaptor proteins for TLR4 (described below). While MyD88 can be directly recruited to most TLRs, an additional TIR domain containing adaptor called MyD88 adaptor like (Mal) or TIR domain containing protein (TIRAP) is required for MyD88 recruitment to TLRs 2 and 4 [17]. Mal contains a phosphatidylinositol-(4,5)-biphosphate (PIP2) binding domain at its C terminus that serves to localize this adaptor and recruit MyD88 to lipid rafts [18].
Recruitment of TLR adaptor proteins generates a platform for the downstream signaling components which include IL-1 receptor associated kinases (IRAKs), TNF receptor-associated factor 6 (TRAF6), TGF-β activated kinase (TAK1) and TAK1-binding proteins (TABs). IRAKs are a family of serine/threonine kinases required by both the IL-1 receptor and the TLRs to activate nuclear factor kappa-B (NF-κB). There are four IRAKs involved in TLR signaling, IRAK1, IRAK2, IRAK4 and IRAK-M [19]. In a typical ligand-induced signaling cascade, IRAK1 is recruited to the receptor via MyD88 and is activated through phosphorylation by IRAK4 [20]. Activated IRAK1 undergoes autophosphorylation which facilitates TRAF6 recruitment. TRAF6 is an E3 ubiquitin ligase which stimulates the IkappaB kinase (IKK) complex leading to inhibitor of kappa B (IκB) degradation and release of NF-κB to the nucleus [21] (Figure 1). TRAF6 activation of IKK requires two protein complexes: the Ubc13-Uev1A complex and the TAK1-TABs complex [22,23]. Ubc13-Uev1A is a dimeric ubiquitin conjugation enzyme complex that cooperates with TRAF6 in K63 ubiquitination of a number or signaling proteins in the complex and the activation of IKK. TAK1 is a MAPK kinase kinase (MAP3K/MEKK) involved in IKK and c-jun N-terminal kinase (JNK)-p38 activation. TAK1 associates with TAB1, TAB2, TAB3 and a recently discovered TAB4 protein [23-26]. TAK1 is activated by autophosphorylation and this event is facilitated by TAB1 [27]. TAB2 and TAB3 bind K63 linked polyubiquitin chains by their highly conserved zinc finger domain and they serve to link TAK1 to TRAF6 [25,28]. The ability to bind polyubiquitin chains is essential for their ability to activate TAK1 [28]. TAB4 is a phosphatase 2A interacting protein which also binds polyubiquitin and stimulates TAK1-TAB1 phosphorylation [26]. In addition to mediating NF-κB activation through the IKK complex, the MyD88-IRAK-TRAF6 signaling complex also activates interferon response factors (IRFs) following TLR7 or TLR9 stimulation ([29,30]). Similar to the IKK pathway, the activation of IRFs appears to occur through TRAF6-mediated K-63 polyubiquitination [31]. The phosphorylated form of IRFs homodimerize and enter the nucleus to regulate the expression of type 1 intererons (IFNs) and other genes [32,33].
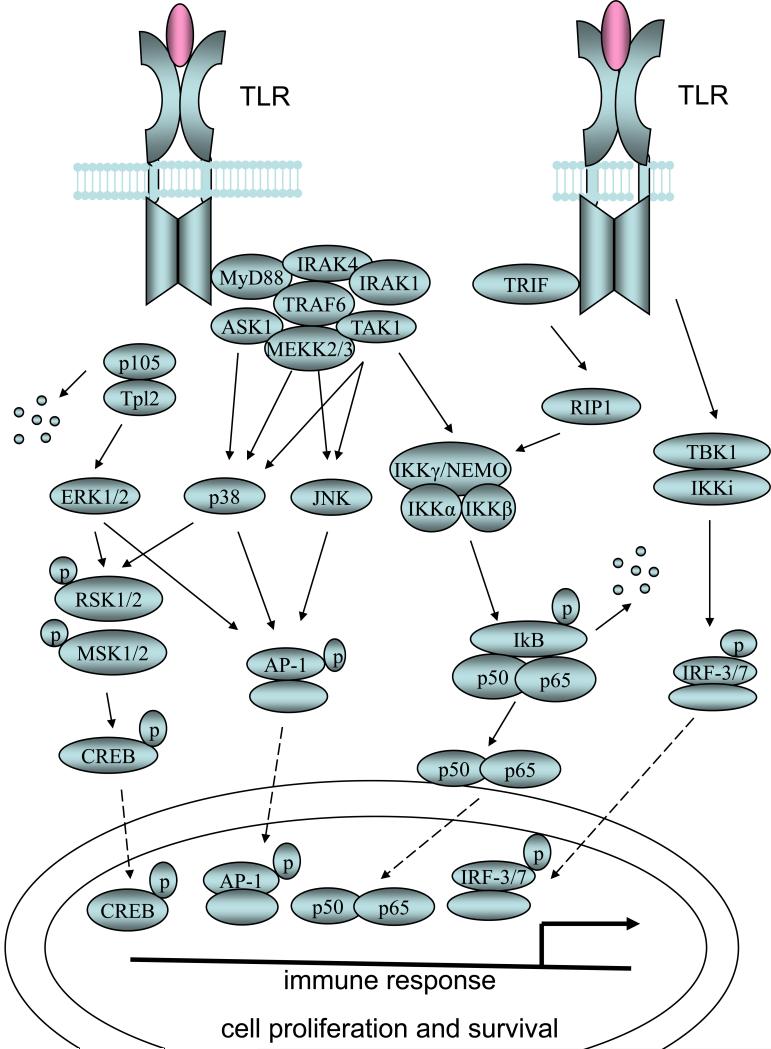
Figure 1. MyD88 dependent and independent TLR signaling pathways.
Activation of TLRs though ligand binding leads to the recruitment of the adaptor protein MyD88 or TRIF. In the MyD88 dependent pathway, activation of IRAK1 by IRAK4 leads to IRAK1 autophosphorylation which facilitates TRAF6 recruitment. TRAF6 then recruits MAP3Ks such as TAK1, MEKK2/3 and ASK1. TAK1 activation leads to the activation of IKK complex and the subsequent IkB degradation and NF-kB nucleus translocation. TAK1 also activates the p38-JNK signaling pathway. Tpl2, MEKK2/3 and ASK1 activate ERK1/2, p38-JNK and p38 respectively. In the MyD88-independent pathway TRIF activates TBK1 as well as IKKi which turns on the IRF-3 transcription factor. TRIF also activates NFkB signaling through RIP1. One of the most important consequences of TLR signaling is the transcriptional regulation of inflammatory genes as well as genes involved in cell proliferation and survival carried out by four groups of transcription factors.
TIR domain containing adaptor protein inducing IFN-β (TRIF), also known as Toll/IL-1R domain containing adaptor molecule 1 (TICAM1), acts as an additional proximal adaptor for TLR3 and TLR4 signaling. TRIF is recruited to the cytosolic domain of TLR4 by TRIF related adaptor molecule (TRAM), also known as TICAM2, which serves as a bridging adaptor [29]. TRIF is responsible for the MyD88-independent responses to LPS and, as expected, mice deficient in both adaptors exhibit no response to LPS at all [30]. TRIF is also required by TLR3 to mediate IRF-3 activation and type 1 IFN production [31]. There are two protein kinases involved in TRIF-mediated activation of IRF-3; TRAF family member-associated NF-κB activator (TANK) binding kinase (TBK1) and inducible IkappaB kinase (IKKi). [1]. Both kinases directly phosphorylate IRF-3 as well as IRF-7 which are responsible for type I IFN induction.
In addition to type I IFNs, IRFs also regulate genes associated with cell proliferation and/or survival (Figure 1). IRF-3 and IRF-5 are potent tumor suppressors, whereas IRF-7 has both oncogenic and anti tumor potential [32]. Interestingly, TBK1 and IKKi have also been associated with oncogenesis and appear to promote cancer cell proliferation and survival. Knocking down the expression of both IKKi and TBK1 diminishes the viability of MCF-7 cells. Whether or not the oncogenic role of TBK1 and IKKi is mediated by IRFs remains unclear. IKKi and TBK1 also target NF-κB, which is responsible for the uncontrolled proliferation of cells overexpressing IKKi [32].
2.2 NF-κB activation in TLR signaling and cell proliferation/survival
NF-κB was originally identified as a transcription factor that binds to the intronic enhancer of the kappa light chain gene (the κB site) expressed in B cells [34]. There are five members in the NF-κB transcription factor family: p65 (REL-A), REL-B, cytoplasmic REL (c-REL), p50 and p52. All five members possess an N-terminal REL homology domain which is responsible for homo- or heterodimerization and sequence-specific DNA binding. However, only REL-A, REL-B and c-REL have a C-terminal transcription activation domain, which means that p50 and p52 need to interact with other members in order to regulate gene expression. The homo or heterodimers of NF-κB are usually sequestered in the cytosol by IκB family proteins. Phosphorylation of IκB by the trimeric IKK complex and degradation of IκB releases NF-κB and enables it to translocate to the nucleus and initiate the transcription of downstream target genes (Figure 1). NF-κB is a central regulator of immune responses and induces the expression of many cytokine genes including IL-2, IL-6, MCP-1 and CD40-L; all of which possess NF-κB binding sites in their promoter regions. NF-κB also targets genes involved in cell proliferation or survival including cyclin D1, cyclin D2, c-Myc, c-Myb, cyclooxygenase 2 (COX-2), BCL-2 and BCL-XL [32,35]. Given this latter function, it is not surprising that NF-κB is considered a tumor promoter [36]. Activation of NF-κB has been observed in chronic myologenous leukemia, prostate cancer, multiple myeloma and hepatocellular carcinoma [37].
With the exception of TLR3, all TLRs activate NF-κB through a canonical pathway involving MyD88, TRAF6, TAK1 and IKK complex (described above). The activation of NF-κB by TLR3 also requires TRAF6, TAK1 and the IKK complex however, TRIF, instead of MyD88, recruits TRAF6 to the cytosolic domain. TRIF is also required for LPS induced late phase activation of NF-κB [31]. In addition to the above canonical pathway, activation of TLR2 by heat-killed S. aureus transactivates NF-κB through a Rac1-PI3K-Akt signaling pathway that does not involve IκB degradation and DNA binding. The molecular mechanism of this transactivation is not clear, however, it is likely that Rac1-PI3K-Akt signaling regulates the p65 transcription complex through phosphorylation-dependent mechanisms [38]
2.3 MAPKs in TLR signaling and cell proliferation and survival
In addition to NF-κB driven transactivation, the expression of IL6, IL8, IL12p40 and MCP-1 is regulated by chromatin remodeling that is induced by MAPK signaling [39]. There are four groups of distinctly regulated MAPKs in mammals: extracellular signal-regulated kinase 1/2 (ERK1/2), p38 proteins (p38α/β/γ/δ), c-Jun N-terminal kinases (JNKs) and ERK5. Their upstream MAPK kinases (MAPKKs or MEKs) are MEK1/2, MKK3/6, MKK4/7 and MEK5, respectively [40]. ERK1/2, p38 and JNK are activated by various TLR ligands including LPS, peptidoglycan, polyI:C and unmethylated CpG DNA [19]. MAPKs regulate numerous cellular events associated with the inflammatory response as well as cell proliferation and survival [40]. For example, ERK1/2 promotes cell cycle progression through inactivation of membrane associated tyrosine- and threonine-specific Cdc2 inhibitory kinase (MYT1) and subsequent activation of the cyclin-dependent kinase p34cdc2 [41]. ERK1/2 can also stimulate cell proliferation through activation of the AP-1 family of dimeric basic region-leucine zipper (bZIP) transcription factors which subsequently act on the cyclin D1 promoter [42]. JNK appears to play a dual role in apoptosis, cell proliferation and survival. Its positive role in regulating cell proliferation and survival is largely mediated through phosphorylation of c-Jun [43].
In contrast to ERK1/2 and JNK, p38 kinase is considered a negative regulator of cell proliferation and survival as reflected by its ability to antagonize JNK/c-Jun [44] and ERK1/2 [45] activities. The balance between ERK1/2, JNK and p38 is believed to play a crucial role in the regulation of cell cycle. Interestingly, both p38 and JNK are activated by TAK1 [22], a MAP3K involved in TLR signaling (see above). Besides TAK1, other MAP3Ks such as tumor progression locus 2 (Tpl2) [46], MEKK1 [47], MEKK2/3 [48,49], and apoptosis-signal regulating kinase 1 (ASK1) [50] have also been implicated in TLR signaling.
Tpl2 was reported to activate ERK1/2 through phosphorylation of MEK3/6 [2]. This event has been shown to regulate TNFα mRNA transport from the nucleus to the cytoplasm in response to LPS stimulation [46]. Tpl2 is also responsible for LPS induced COX-2 expression through ERK1/2 dependent phosphorylation of the transcription factor cAMP response element binding protein (CREB) [51]. MEKK1 has been shown to directly phosphorylate IκBα thus activate NF-κB following TNFα treatment [47], which makes it a potential modulator of TLR signaling. However, mice deficient in MEKK1 show normal responses to both TLR stimuli and TNFα, indicating that other MAP3Ks are involved [52]. Indeed, MEKK3 [48] is required for LPS inducible JNK-p38 and NF-κB activation as well as IL-6 production [48]. MEKK3 deficient cells fail to respond to TLR4 stimulation and show delayed and weak NF-κB DNA binding activitity [53]. Another MAP3K, ASK1, appears to regulate p38 activation and reactive oxygen species production in response to LPS [50]. Taken together, TLRs can regulate a specific set of downstream genes by selectively activating specific MAPK cascades. While the molecular mechanism mediating TLR selectivity on individual MAP3Ks is not clear, adaptors such as TRAF6 are likely to play a key role in their recruitment [50,53] (Figure 1).
As already indicated, the main transcription factors affected by TLR signaling through MAPKs include CREB, AP-1 and NF-κB. While many studies have focused on NF-κB and IRF transcription factors as targets of TLR activation far less attention has been paid to CREB and AP-1, both of which comprise significant regulators of cell proliferation and survival. CREB is a bZIP transcription factor which binds to the cAMP response element (CRE) of numerous genes involved in metabolism, transcription, immune regulation, cell proliferation and survival. CREB activity is enhanced by its coactivators CREB binding protein (CBP) and p300 [54]. AP-1 is a dimeric bZIP protein composed of the Jun, Fos, Maf and ATF subfamilies. Among these, c-Jun is considered the most potent transcriptional activator. AP-1 recognizes the 12-o-tetradecanoylphorbol-13-acetate (TPA) response element or CRE in the promoter region of many genes including cyclin D1 and p53 [55]. CREB and AP-1 can be phosphorylated and regulated by MAPKs either directly or indirectly by downstream MAPK-activated protein kinases (MKs) in response to TLR stimuli. For example, ERKs and JNK phosphorylate Fos and Jun, respectively to activate their transcriptional function [55].
The MKs which signal downstream of the TLRs include the mitogen- and stress-activated kinases (MSKs), the p90 ribosomal S6 kinases (RSKs) and the MAPK-interacting kinases (MNKs). The phosphorylation of H3 by MSK1 and MSK2 serves to recruit and activate several histone acetyl-transferases which increases the transcription of target genes by inducing chromatin remodeling [39]. Through this mechanism, MSK1 and MSK2 promote the binding of phosphorylated CREB and ATF1 to the promoters of IL-10 and dual specificity phosphatase 1 (DUSP1), two negative regulators of TLR mediated inflammatory responses [56]. RSK1 and 2 are required for both TLR2 and TLR4 mediated endocytic responses in dendritic cells [57]. The RSKs have been linked to cell cycle control by activation of the cyclin-dependent kinase p34cdc2 downstream of ERK1/2 [45]. Moreover, RSKs transmit cell survival signals through phosphorylation of Bcl-xL/Bcl-2-associated death promoter (BAD), which suppresses BAD-mediated apoptosis in neurons, and phosphorylation of CREB [58] (Figure 1). Besides MSKs and RSKs, MNKs also mediate inflammatory cytokine production by multiple TLRs [59,60]. Activation of MNK1 and MNK2 in primary mouse macrophages by LPS leads to increased phosphorylation of eukaryotic initiation factor 4E (eIF4E) and increased production of TNFα [60]. MSKs, RSKs and MNKs are all phosphorylated downstream of either p38 or ERK1/2. In dendritic cells, RSKs phosphorylation is mediated by two MKs which are targets of p38 activation [57].
2.4 PI3K in TLR signaling and cell survival
Although there are four classes of PI3Ks, designated by their structure and substrate specificity, only class IA PI3Ks have been shown to play a role in TLR signaling. Class IA PI3Ks are heterodimeric enzymes composed of a regulatory subunit and a catalytic subunit. In mammals, there are five regulatory subunits (p85α, p55α, p50α, p85β or p85γ) and three catalytic subunits (p110α, p110β, p110δ). Most of these subunits are ubiquitously expressed, except p110δ which is found primarily in leukocytes [61]. Class IA PI3Ks are activated by tyrosine-kinase-associated receptors, including TLRs and cytokine receptors [61]. During this event, the phosphotyrosine motifs (pYxxM) of the activated receptors induce binding and conformational changes in PI3K which activate the catalytic subunit. Activated PI3Ks then convert phosphatidylinositol-(4,5)-biphosphate (PIP2) to phosphatidylinositol-(3,4,5)-triphosphate (PIP3). PIP3 binds and activates downstream kinases such as PDK1 and AKT/PKB to regulate cell proliferation, metabolism, survival and cytokine production [62].
The cytosolic domains of TLRs 2, 3 and 5 all bear a conserved YxxM PI3K binding motif which suggests a role for PI3K in their signaling. Indeed, a direct interaction between PI3K and TLRs or their adaptors, such as MyD88, have been reported. Additionally, YxxM motifs in TLRs and MyD88 are required for their interaction with p85 [38,63,64]. TLR2 mediated activation of NF-κB also requires YxxM motifs [38], suggesting that recruitment of PI3K to the cytosolic domain of TLR2 is important for this downstream event. Phosphorylation of Akt, a downstream kinase activated by PI3K, is detected upon stimulation of most TLRs [65]. Although PI3K activation is clearly dependent upon TLRs and/or MyD88, the mechanism by which this event occurs is not clear [64].
Once activated, PI3K regulates TLR signaling in both negative and positive ways. Inhibition of PI3K during TLR2 stimulation, through use of either Ly294002 or dominant-negative Akt, has been shown to greatly reduced NF-κB activation [38]. The fact that other studies have found that PI3K inhibitors enhance pro-inflammatory gene expression, has led to speculation that these inhibitors have additional targets [66]. Studies on PI3K knock out mice however, support the idea that PI3K negatively regulates TLR activation as signaling by TLR2, TLR4, TLR5 and TLR9 is elevated in p85α deficient mice [67,68]. Additionally, LPS-induced IL-12 and nitrite production is increased in p110β deficient cells [69]. PI3K appears to inhibit proinflammatory cytokine production via glycogen synthase kinase 3 (GSK3), a serine-threonine kinase that inhibits the activity of Cyclin D1, β-catenin, c-JUN and Myc transcription factors through phosphorylation at specific serine residues [70]. Recent research suggests that GSK3 can modulate cytokine production downstream of PI3K in response to TLR stimulation. Inhibition of GSK3 activity by either chemical inhibition or Akt mediated phosphorylation results in increased IL-10 production due to enhanced DNA binding activity of CREB as well as enhanced association between CREB and its co-activator CBP. In contrast, IL-12 production is decreased because the amount of nuclear CBP that is accessible to NF-κB is limited [71] (Figure 2). GSK3 also inhibits AP-1 DNA binding activity which could affect IL-10 expression [72].
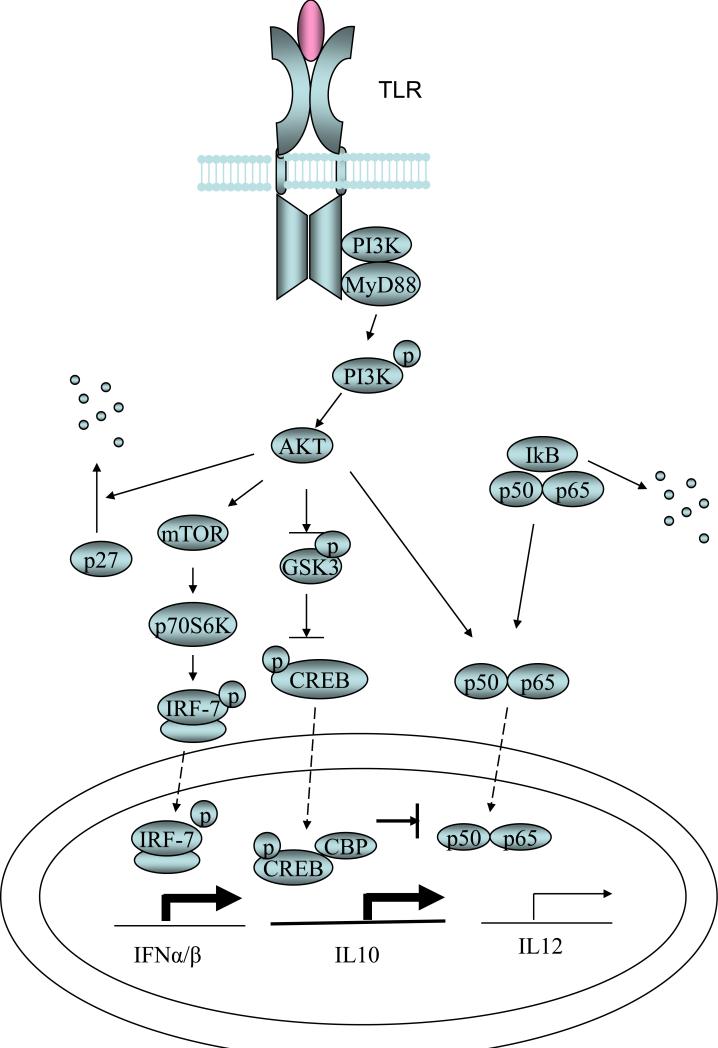
Figure 2. PI3K pathway in TLR signaling.
PI3K physically interacts with multiple TLRs as well as MyD88. Activated PI3K regulates TLR signaling in both positive and negative ways. Inactivation of GSK3 by PI3K-Akt enhances the transcription of IL10 by allowing CREB DNA binding as well as CREB-CBP association. IL-12 transcription is inhibited due to decreased association of NF-kB with CBP. PI3K-Akt also phosphorylates mTOR which leads to the phosphorylation and activation of IRF-7 as well as enhanced production of type I IFNs. PI3K-Akt pathway is also involved in cell proliferation or survival induced by multiple TLR ligands through degradation of the cell cycle inhibitor p27.
Through the use of pharmacologic inhibitors, a recent report found that PI3K is a positive regulator of IRF-7 nuclear translocation and type I IFN production in human primary plasmacytoid DCs stimulated with unmethylated CpG DNA [73]. These results are supported by reports that two kinases downstream of PI3K, mTOR and p70S6K, are positive regulators of TLR induced type I interferon production in human primary plasmacytoid DCs. Either the mTOR inhibitor, rapamycin, or the PI3K inhibitor, LY294002, was shown to suppress CpG-A induced IFN-α secretion in a dose dependent manner. This study also showed that the PI3K-mTOR-p70S6K complex directly affected the interaction between TLR9-MyD88 and thus impaired downstream NF-κB and IRF7 activity [74] (Figure 2). In summary, it appears that PI3K is an important TLR activation component that affects signaling in different ways depending on cell type and readout. As the PI3K pathway is an established key regulator of cell proliferation and survival, future studies will likely reveal interesting examples of crosstalk between TLR and cell proliferation/survival signals.
2.5 Other components in TLR signaling
Bruton's tyrosine kinase (Btk) is an additional enzyme whose activity has been linked to TLR signaling. Btk has long been recognized as a critical player in B cell development, activation and survival [75]. However, in addition to the predicted effects on adaptive immunity, Btk deficiency in both humans and mice also affects innate immune function. Btk interacts with the cytosolic domain of TLRs 4, 6, 8 and 9 and mediates downstream activation of NF-κB. Btk has also been reported to associate with the adaptor proteins MyD88, Mal and IRAK-1 [75-77] and was found to be a component of the activated TLR2 signaling complex [78]. Btk phosphorylation is detected within 5 minutes following stimulation by LPS in human myelomonocytic THP-1 cells [75], indicating that it functions in the early steps of TLR signaling. Indeed, Btk is responsible for Mal phosphorylation shortly after TLR2 or TLR4 stimulation, an event which targets this adaptor for suppressor of cytokine signaling-1 (SOCS1) dependent polyubiquitination and degradation [79,80].
Rho GTPases, a subgroup within the Ras superfamily, have also been shown to affect TLR signaling in multiple cell types [38,81,82]. RhoA, Rac1 and Cdc42 are rapidly activated by TLR stimulation [65]. Inhibition of Rho GTPase, by the Clostridium difficile toxin B-10643, results in reduced NF-κB activity as well as diminished IL-8 and COX-2 expression [81,83,84] however, TNF-α production in response to LPS appears to be elevated [85]. It is likely that Rho GTPase mediates cross-talk or regulation of other signaling pathways during TLR stimulation. Indeed, the elevated TNF-α production resulting from Rho GTPase inhibition in human macrophages is due to prolonged activation of ERK [85]. Cross talk between Rho GTPase and the PI3K pathway has also been implicated [38,86,87].
3. TLRs in tissue repair and tumorigenesis
In a landmark study, MyD88 deficient mice were found to be more susceptible to both radiation and dextran sulfate sodium (DSS) induced intestinal and colonic epithelial injury [7]. This protective role for the TLRs was surprising as, up until that time, their activity was widely associated with the induction of inflammatory processes leading to tissue damage. Importantly, MyD88 deficient mice treated with DSS exhibited impaired crypt repopulation and compensatory proliferation required for tissue repair and regeneration [7]. It was proposed that the components of the host microflora facilitated the tissue repair process by triggering TLR signaling. In support of this, both LPS and LTA mimicked the protection effect of microflora in a TLR2 and TLR4 dependent manner.
Recently, the injection of a flagellin-based polypeptide TLR5 agonist was found to protect mice and rhesus monkeys from an otherwise lethal radiation dose. As tumor cells were not protected, the therapeutic benefits of this peptide are being explored in the protection of cancer patients undergoing radiation treatment [88]. It is now recognized that in addition to PAMPs, a variety of endogenous molecules released from damaged tissue can act to trigger TLR activation leading to tissue repair processes. For example, epithelial hyaluronan, a high molecular weight polymer released from the extracellular matrix upon tissue damage, is recognized by TLR2 and TLR4 which protect against acute lung injury induced by bleomycin. The protection effect has been attributed to NF-κB mediated suppression of apoptosis [8].
Since TLRs appear to promote cell proliferation and tissue repair, their role in tumorigenesis and cancer is now being widely explored. In this regard, MyD88 has been found to play a critical tumorigenic role in a mouse model of intestinal adenoma by upregulating the expression of tumor promoting genes, growth factors and various cytokines and chemokines [11]. Through its effects on IL-6 production, MyD88 has also been found to be a determining factor of gender disparity in the most common form of liver cancer, hepatocellular carcinoma [10]. Chemically induced skin papillomas and sarcomas have also been associated with MyD88 activity [12]. It remains to be determined whether these tumor promoting effects are mediated by specific TLRs. Interestingly, single nucleotide polymorphisms (SNPs) in several TLR loci seem to associate with higher cancer risk. For example, polymorphic variants of TLR4, as well as TLR1, 6 and 10, have been associated with increased prostate cancer risk [89-92]. Other studies have linked TLR2 and TLR4 polymorphisms to increased cervical cancer and TLR2 polymorphism to gastric cancer risk [93].
There are a variety of mechanisms by which TLRs are thought to regulate tumor growth. First, TLRs can directly regulate tumor cell growth by modulating cell proliferation or survival signaling pathways. TLRs are well expressed in various tumor cell types and are able to activate downstream signaling upon stimulation [9]. For example, TLR4 is over expressed in human colon cancers associated with chronic ulcerative colitis and is also highly expressed in an animal model of inflammation-induced colon tumorigenesis. Moreover, TLR4 stimulation appears to directly promote tumorigenesis through upregulation of proto-oncogene expression [94]. TLR4 is also required for COX-2 expression and EGFR signaling, both of which have been previously linked to colon tumor development. Importantly, mice genetically lacking TLR4 are protected against colon tumorigenesis [94,95].
TLRs can also indirectly promote tumor growth by facilitating the creation of an inflammatory microenvironment. For example, versican, an extracellular matrix proteoglycan from Lewis lung carcinoma (LLC), stimulates TLR2 on macrophages to produce tumor promoting cytokines such as TNFα, IL-1β and IL-6 [96]. Finally, TLRs may modulate the microenvironment in a way that enables tumor cells to escape host surveillance. Stimulation of the M26 mouse colon cancer cell line with LPS leads to the induction of iNOS and IL-6 which appear to inhibit T cell proliferation and NK cell cytotoxicity [97]. A similar immunosuppressive effect of nitric oxide and IL-6 was observed following the TLR2-mediated stimulation of the mouse hepatocarcinoma cell line H22. TLR2 was also found to promote cell proliferation in this cell line through JNK and ERK phosphorylation [98].
4. TLR signaling in cell proliferation and survival
A variety of mechanisms are involved in TLR mediated cell proliferation or survival. Unmethylated CpG DNA serves as a growth factor for human myeloma cell lines and rescues these cells from serum-deprivation or dexamethasone-induced apoptosis. The growth/survival promoting effect is due to autocrine secretion of IL-6 induced by TLR9 stimulation [5,6]. ERK1/2 is activated by Chlamydia hsp60 through TLR4 and is responsible for the enhanced proliferation of human vascular smooth muscle cells [99]. The PI3K/Akt pathway is activated in mesenchymal stem cells upon LPS stimulation and mediates the protection effect against oxidative stress and apoptosis induced by serum deprivation [100].
To further investigate the molecular mechanisms by which TLRs promote cell proliferation, Hasan et al. assessed whether TLR signaling would affect cell cycle progression in Rat1 cells. In this study, flagellin, but not polyI:C and LPS, induced cell cycle entry and prevented cell cycle exit when serum was deprived. Interestingly, an IFN receptor neutralizing antibody enabled polyI:C and LPS to induce cell cycle entry, suggesting that type I IFNs antagonize the proliferation promoting effect of TLR ligands. Further investigation revealed that TLR ligands triggered degradation of p27, a cell cycle inhibitor, in a MyD88 and Akt dependent manner and that this event was prevented by IFN-β [4] (Figure 2). These results are consistent with the observation that type I IFNs inhibit mesangial cell proliferation [101]. Most TLR agonists tend to promote survival of human monocyte derived dendritic cells. However, poly I:C and LPS, both of which induce type I IFNs, promote apoptosis which is consistent with the role of p27 in triggering this event [102]. The ability of IFN to prevent p27 degradation may also underlie its ability to synergize with polyI:C in triggering apoptosis in melanoma cell lines [103].
The observation that polyI:C triggers apoptosis in IFN-insensitive human prostate cancer cells suggests that there are IFN-independent mechanisms of TLR-induced apoptosis. In fact, protein kinase C alpha (PKCα)-induced JNK and p38 activation is required in triggering apoptosis in these IFN-insensitive cells [104]. TLRs also appear to drive apoptosis through the Fas pathway. For example, the lipopeptide mediated apoptosis of HEK 293 cells is dependent on the TLR2-MyD88 pathway which appears to involve Fas-associated death domain protein (FADD) and caspase 8 [105,106] Interestingly, suppression of the NF-κB pathway by a dominant-negative form of NF-κB inducing kinase (NIK) facilitates lipoprotein induced apoptosis, indicating that NF-κB signaling normally protects cells from lipoprotein induced apoptosis [106] (Figure 3). Additionally, LPS-mediated activation of caspase 8 triggers cell death in human THP-1 cells, however the involvement of FADD in this event remains to be determined [107]. The function of FADD seems not limited to caspase 8 dependent apoptosis. FADD is not only required for Fas-induced apoptosis, but is also required for TCR induced proliferative responses in T cells [108] as well as TLR-induced proliferative responses in B cells [109]. These observations indicate that FADD may function as a convergent point in the regulation of apoptosis and proliferation.
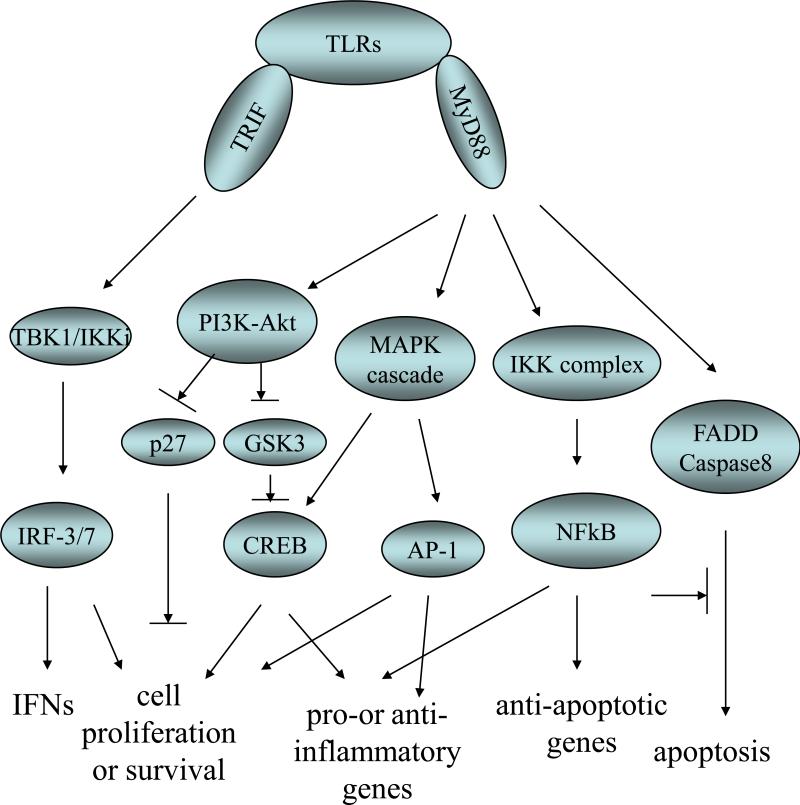
Figure 3. TLR signaling in cell proliferation and survival.
TLR signaling engages two important adaptor proteins MyD88 and TRIF. MyD88 is required for the activation of downstream signaling pathways involving PI3K, MAP3Ks, IKK complex and FADD/caspases 8. PI3K activation leads to CREB activation and p27 degradation, both of which contribute to cell proliferation and/or survival. Activation of MAP3Ks induces the MAPK signaling cascades which eventually activate transcription factors including AP-1 and CREB. Activation of IKK complex results in NFkB nucleus translocation. All these three groups of transcription factors are engaged in the transcription of genes involved in cell fate control as well as inflammation. FADD/caspase8 is responsible for TLR2 induced apoptosis which is antagonized by NF-kB signaling. TRIF is involved in IRF-3 activation and the production of type I IFNs. IRF-3, 5 and 7 are all potential regulators of cell proliferation and survival.
5. Conclusions and future directions
TLRs play a crucial role in innate immunity and guard the host against pathogen invasion. TLR stimulation leads to prompt and tightly regulated inflammatory responses through the induction of pro- and anti-inflammatory cytokines and chemokines. A newly appreciated role of TLRs in cell proliferation, cell survival, and tissue repair greatly expands our general understanding of TLR function. Many TLR signaling components include molecules of the MAPK cascades and PI3K-Akt pathway which are established as key regulators of cell proliferation and survival. The four major groups of transcription factors targeted in TLR signaling also activate downstream genes regulating cell proliferation and cell survival or apoptosis (Figure 3). This extended function for the TLR family makes biologic sense given that the host immune response must expand populations of useful immune cells and also integrate inflammatory responses with tissue repair processes.
The mechanism by which TLR and growth control signals are integrated in different cell types is of considerable importance especially as a number of TLR agonists are being investigated as tumor immunotherapeutics. Care should be exercised when using these agonists as TLR stimulation can clearly promote cell proliferation and, somewhat ironically, tumor growth in various settings. New therapeutic strategies must be designed based on a thorough investigation of tumor responses to TLR stimuli and a deeper understanding of the role of TLRs in tumorigenesis.
Acknowledgements
Research in our laboratory is generously supported by the National Institute of Health, NIAID.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Akira S, Takeda K. Toll-like receptor signalling. Nat.Rev.Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 2.Banerjee A, Gerondakis S. Coordinating TLR-activated signaling pathways in cells of the immune system. Immunol.Cell Biol. 2007;85:420–4. doi: 10.1038/sj.icb.7100098. [DOI] [PubMed] [Google Scholar]
- 3.Wagner H. Endogenous TLR ligands and autoimmunity. Adv.Immunol. 2006;91:159–73. doi: 10.1016/S0065-2776(06)91004-9. [DOI] [PubMed] [Google Scholar]
- 4.Hasan UA, Trinchieri G, Vlach J. Toll-like receptor signaling stimulates cell cycle entry and progression in fibroblasts. J.Biol.Chem. 2005;280:20620–7. doi: 10.1074/jbc.M500877200. [DOI] [PubMed] [Google Scholar]
- 5.Jego G, Bataille R, Geffroy-Luseau A, Descamps G, Pellat-Deceunynck C. Pathogen-associated molecular patterns are growth and survival factors for human myeloma cells through Toll-like receptors. Leukemia. 2006;20:1130–7. doi: 10.1038/sj.leu.2404226. [DOI] [PubMed] [Google Scholar]
- 6.Bohnhorst J, Rasmussen T, Moen SH, Flottum M, Knudsen L, Borset M, et al. Toll-like receptors mediate proliferation and survival of multiple myeloma cells. Leukemia. 2006;20:1138–44. doi: 10.1038/sj.leu.2404225. [DOI] [PubMed] [Google Scholar]
- 7.Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118:229–41. doi: 10.1016/j.cell.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 8.Jiang D, Liang J, Fan J, Yu S, Chen S, Luo Y, et al. Regulation of lung injury and repair by Toll-like receptors and hyaluronan. Nat.Med. 2005;11:1173–9. doi: 10.1038/nm1315. [DOI] [PubMed] [Google Scholar]
- 9.Huang B, Zhao J, Unkeless JC, Feng ZH, Xiong H. TLR signaling by tumor and immune cells: a double-edged sword. Oncogene. 2008;27:218–24. doi: 10.1038/sj.onc.1210904. [DOI] [PubMed] [Google Scholar]
- 10.Naugler WE, Sakurai T, Kim S, Maeda S, Kim K, Elsharkawy AM, et al. Gender disparity in liver cancer due to sex differences in MyD88-dependent IL-6 production. Science. 2007;317:121–4. doi: 10.1126/science.1140485. [DOI] [PubMed] [Google Scholar]
- 11.Rakoff-Nahoum S, Medzhitov R. Regulation of spontaneous intestinal tumorigenesis through the adaptor protein MyD88. Science. 2007;317:124–7. doi: 10.1126/science.1140488. [DOI] [PubMed] [Google Scholar]
- 12.Swann JB, Vesely MD, Silva A, Sharkey J, Akira S, Schreiber RD, et al. Demonstration of inflammation-induced cancer and cancer immunoediting during primary tumorigenesis. Proc.Natl.Acad.Sci.U.S.A. 2008;105:652–6. doi: 10.1073/pnas.0708594105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O'Neill LA, Bowie AG. The family of five: TIR-domain-containing adaptors in Toll-like receptor signalling. Nat.Rev.Immunol. 2007;7:353–64. doi: 10.1038/nri2079. [DOI] [PubMed] [Google Scholar]
- 14.Medzhitov R, Preston-Hurlburt P, Kopp E, Stadlen A, Chen C, Ghosh S, et al. MyD88 is an adaptor protein in the hToll/IL-1 receptor family signaling pathways. Mol.Cell. 1998;2:253–8. doi: 10.1016/s1097-2765(00)80136-7. [DOI] [PubMed] [Google Scholar]
- 15.Kawai T, Adachi O, Ogawa T, Takeda K, Akira S. Unresponsiveness of MyD88-deficient mice to endotoxin. Immunity. 1999;11:115–22. doi: 10.1016/s1074-7613(00)80086-2. [DOI] [PubMed] [Google Scholar]
- 16.Takeuchi O, Takeda K, Hoshino K, Adachi O, Ogawa T, Akira S. Cellular responses to bacterial cell wall components are mediated through MyD88-dependent signaling cascades. Int.Immunol. 2000;12:113–7. doi: 10.1093/intimm/12.1.113. [DOI] [PubMed] [Google Scholar]
- 17.Fitzgerald KA, Palsson-McDermott EM, Bowie AG, Jefferies CA, Mansell AS, Brady G, et al. Mal (MyD88-adapter-like) is required for Toll-like receptor-4 signal transduction. Nature. 2001;413:78–83. doi: 10.1038/35092578. [DOI] [PubMed] [Google Scholar]
- 18.Kagan JC, Medzhitov R. Phosphoinositide-mediated adaptor recruitment controls Toll-like receptor signaling. Cell. 2006;125:943–55. doi: 10.1016/j.cell.2006.03.047. [DOI] [PubMed] [Google Scholar]
- 19.Kawai T, Akira S. TLR signaling. Cell Death Differ. 2006;13:816–25. doi: 10.1038/sj.cdd.4401850. [DOI] [PubMed] [Google Scholar]
- 20.Cao Z, Henzel WJ, Gao X. IRAK: a kinase associated with the interleukin-1 receptor. Science. 1996;271:1128–31. doi: 10.1126/science.271.5252.1128. [DOI] [PubMed] [Google Scholar]
- 21.Deng L, Wang C, Spencer E, Yang L, Braun A, You J, et al. Activation of the IkappaB kinase complex by TRAF6 requires a dimeric ubiquitin-conjugating enzyme complex and a unique polyubiquitin chain. Cell. 2000;103:351–61. doi: 10.1016/s0092-8674(00)00126-4. [DOI] [PubMed] [Google Scholar]
- 22.Wang C, Deng L, Hong M, Akkaraju GR, Inoue J, Chen ZJ. TAK1 is a ubiquitin-dependent kinase of MKK and IKK. Nature. 2001;412:346–51. doi: 10.1038/35085597. [DOI] [PubMed] [Google Scholar]
- 23.Ninomiya-Tsuji J, Kishimoto K, Hiyama A, Inoue J, Cao Z, Matsumoto K. The kinase TAK1 can activate the NIK-I kappaB as well as the MAP kinase cascade in the IL-1 signalling pathway. Nature. 1999;398:252–6. doi: 10.1038/18465. [DOI] [PubMed] [Google Scholar]
- 24.Shibuya H, Yamaguchi K, Shirakabe K, Tonegawa A, Gotoh Y, Ueno N, et al. TAB1: an activator of the TAK1 MAPKKK in TGF-beta signal transduction. Science. 1996;272:1179–82. doi: 10.1126/science.272.5265.1179. [DOI] [PubMed] [Google Scholar]
- 25.Ishitani T, Takaesu G, Ninomiya-Tsuji J, Shibuya H, Gaynor RB, Matsumoto K. Role of the TAB2-related protein TAB3 in IL-1 and TNF signaling. EMBO J. 2003;22:6277–88. doi: 10.1093/emboj/cdg605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Prickett TD, Ninomiya-Tsuji J, Broglie P, Muratore-Schroeder TL, Shabanowitz J, Hunt DF, et al. TAB4 stimulates TAK1-TAB1 phosphorylation and binds polyubiquitin to direct signaling to NF-kappaB. J.Biol.Chem. 2008;283:19245–54. doi: 10.1074/jbc.M800943200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sakurai H, Miyoshi H, Mizukami J, Sugita T. Phosphorylation-dependent activation of TAK1 mitogen-activated protein kinase kinase kinase by TAB1. FEBS Lett. 2000;474:141–5. doi: 10.1016/s0014-5793(00)01588-x. [DOI] [PubMed] [Google Scholar]
- 28.Kanayama A, Seth RB, Sun L, Ea CK, Hong M, Shaito A, et al. TAB2 and TAB3 activate the NF-kappaB pathway through binding to polyubiquitin chains. Mol.Cell. 2004;15:535–48. doi: 10.1016/j.molcel.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 29.Yamamoto M, Sato S, Hemmi H, Uematsu S, Hoshino K, Kaisho T, et al. TRAM is specifically involved in the Toll-like receptor 4-mediated MyD88-independent signaling pathway. Nat.Immunol. 2003;4:1144–50. doi: 10.1038/ni986. [DOI] [PubMed] [Google Scholar]
- 30.Hirotani T, Yamamoto M, Kumagai Y, Uematsu S, Kawase I, Takeuchi O, et al. Regulation of lipopolysaccharide-inducible genes by MyD88 and Toll/IL-1 domain containing adaptor inducing IFN-beta. Biochem.Biophys.Res.Commun. 2005;328:383–92. doi: 10.1016/j.bbrc.2004.12.184. [DOI] [PubMed] [Google Scholar]
- 31.Yamamoto M, Sato S, Hemmi H, Hoshino K, Kaisho T, Sanjo H, et al. Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway. Science. 2003;301:640–3. doi: 10.1126/science.1087262. [DOI] [PubMed] [Google Scholar]
- 32.Clement JF, Meloche S, Servant MJ. The IKK-related kinases: from innate immunity to oncogenesis. Cell Res. 2008;18:889–99. doi: 10.1038/cr.2008.273. [DOI] [PubMed] [Google Scholar]
- 33.Chen W, Lam SS, Srinath H, Jiang Z, Correia JJ, Schiffer CA, et al. Insights into interferon regulatory factor activation from the crystal structure of dimeric IRF5. Nat.Struct.Mol.Biol. 2008;15:1213–20. doi: 10.1038/nsmb.1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sen R, Baltimore D. Multiple nuclear factors interact with the immunoglobulin enhancer sequences. Cell. 1986;46:705–16. doi: 10.1016/0092-8674(86)90346-6. [DOI] [PubMed] [Google Scholar]
- 35.Vallabhapurapu S, Karin M. Regulation and function of NF-kappaB transcription factors in the immune system. Annu.Rev.Immunol. 2009;27:693–733. doi: 10.1146/annurev.immunol.021908.132641. [DOI] [PubMed] [Google Scholar]
- 36.Pikarsky E, Porat RM, Stein I, Abramovitch R, Amit S, Kasem S, et al. NF-kappaB functions as a tumour promoter in inflammation-associated cancer. Nature. 2004;431:461–6. doi: 10.1038/nature02924. [DOI] [PubMed] [Google Scholar]
- 37.Wolska A, Lech-Maranda E, Robak T. Toll-like receptors and their role in hematologic malignancies. Curr.Mol.Med. 2009;9:324–35. doi: 10.2174/156652409787847182. [DOI] [PubMed] [Google Scholar]
- 38.Arbibe L, Mira JP, Teusch N, Kline L, Guha M, Mackman N, et al. Toll-like receptor 2-mediated NF-kappa B activation requires a Rac1-dependent pathway. Nat.Immunol. 2000;1:533–40. doi: 10.1038/82797. [DOI] [PubMed] [Google Scholar]
- 39.Saccani S, Pantano S, Natoli G. p38-Dependent marking of inflammatory genes for increased NF-kappa B recruitment. Nat.Immunol. 2002;3:69–75. doi: 10.1038/ni748. [DOI] [PubMed] [Google Scholar]
- 40.Chang L, Karin M. Mammalian MAP kinase signalling cascades. Nature. 2001;410:37–40. doi: 10.1038/35065000. [DOI] [PubMed] [Google Scholar]
- 41.Palmer A, Gavin AC, Nebreda AR. A link between MAP kinase and p34(cdc2)/cyclin B during oocyte maturation: p90(rsk) phosphorylates and inactivates the p34(cdc2) inhibitory kinase Myt1. EMBO J. 1998;17:5037–47. doi: 10.1093/emboj/17.17.5037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Treinies I, Paterson HF, Hooper S, Wilson R, Marshall CJ. Activated MEK stimulates expression of AP-1 components independently of phosphatidylinositol 3-kinase (PI3-kinase) but requires a PI3-kinase signal To stimulate DNA synthesis. Mol.Cell.Biol. 1999;19:321–9. doi: 10.1128/mcb.19.1.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sancho R, Nateri AS, de Vinuesa AG, Aguilera C, Nye E, Spencer-Dene B, et al. JNK signalling modulates intestinal homeostasis and tumourigenesis in mice. EMBO J. 2009 doi: 10.1038/emboj.2009.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hui L, Bakiri L, Stepniak E, Wagner EF. P38alpha: a Suppressor of Cell Proliferation and Tumorigenesis. Cell.Cycle. 2007;6:2429–33. doi: 10.4161/cc.6.20.4774. [DOI] [PubMed] [Google Scholar]
- 45.Zhang W, Liu HT. MAPK signal pathways in the regulation of cell proliferation in mammalian cells. Cell Res. 2002;12:9–18. doi: 10.1038/sj.cr.7290105. [DOI] [PubMed] [Google Scholar]
- 46.Dumitru CD, Ceci JD, Tsatsanis C, Kontoyiannis D, Stamatakis K, Lin JH, et al. TNF-alpha induction by LPS is regulated posttranscriptionally via a Tpl2/ERK-dependent pathway. Cell. 2000;103:1071–83. doi: 10.1016/s0092-8674(00)00210-5. [DOI] [PubMed] [Google Scholar]
- 47.Lee FS, Hagler J, Chen ZJ, Maniatis T. Activation of the IkappaB alpha kinase complex by MEKK1, a kinase of the JNK pathway. Cell. 1997;88:213–22. doi: 10.1016/s0092-8674(00)81842-5. [DOI] [PubMed] [Google Scholar]
- 48.Huang Q, Yang J, Lin Y, Walker C, Cheng J, Liu ZG, et al. Differential regulation of interleukin 1 receptor and Toll-like receptor signaling by MEKK3. Nat.Immunol. 2004;5:98–103. doi: 10.1038/ni1014. [DOI] [PubMed] [Google Scholar]
- 49.Zhang D, Facchinetti V, Wang X, Huang Q, Qin J, Su B. Identification of MEKK2/3 serine phosphorylation site targeted by the Toll-like receptor and stress pathways. EMBO J. 2006;25:97–107. doi: 10.1038/sj.emboj.7600913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Matsuzawa A, Saegusa K, Noguchi T, Sadamitsu C, Nishitoh H, Nagai S, et al. ROS-dependent activation of the TRAF6-ASK1-p38 pathway is selectively required for TLR4-mediated innate immunity. Nat.Immunol. 2005;6:587–92. doi: 10.1038/ni1200. [DOI] [PubMed] [Google Scholar]
- 51.Eliopoulos AG, Dumitru CD, Wang CC, Cho J, Tsichlis PN. Induction of COX-2 by LPS in macrophages is regulated by Tpl2-dependent CREB activation signals. EMBO J. 2002;21:4831–40. doi: 10.1093/emboj/cdf478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hayden MS, Ghosh S. Signaling to NF-kappaB. Genes Dev. 2004;18:2195–224. doi: 10.1101/gad.1228704. [DOI] [PubMed] [Google Scholar]
- 53.Huang Q, Yang J, Lin Y, Walker C, Cheng J, Liu ZG, et al. Differential regulation of interleukin 1 receptor and Toll-like receptor signaling by MEKK3. Nat.Immunol. 2004;5:98–103. doi: 10.1038/ni1014. [DOI] [PubMed] [Google Scholar]
- 54.Mayr B, Montminy M. Transcriptional regulation by the phosphorylation-dependent factor CREB. Nat.Rev.Mol.Cell Biol. 2001;2:599–609. doi: 10.1038/35085068. [DOI] [PubMed] [Google Scholar]
- 55.Shaulian E, Karin M. AP-1 as a regulator of cell life and death. Nat.Cell Biol. 2002;4:E131–6. doi: 10.1038/ncb0502-e131. [DOI] [PubMed] [Google Scholar]
- 56.Ananieva O, Darragh J, Johansen C, Carr JM, McIlrath J, Park JM, et al. The kinases MSK1 and MSK2 act as negative regulators of Toll-like receptor signaling. Nat.Immunol. 2008;9:1028–36. doi: 10.1038/ni.1644. [DOI] [PubMed] [Google Scholar]
- 57.Zaru R, Ronkina N, Gaestel M, Arthur JS, Watts C. The MAPK-activated kinase Rsk controls an acute Toll-like receptor signaling response in dendritic cells and is activated through two distinct pathways. Nat.Immunol. 2007;8:1227–35. doi: 10.1038/ni1517. [DOI] [PubMed] [Google Scholar]
- 58.Bonni A, Brunet A, West AE, Datta SR, Takasu MA, Greenberg ME. Cell survival promoted by the Ras-MAPK signaling pathway by transcription-dependent and -independent mechanisms. Science. 1999;286:1358–62. doi: 10.1126/science.286.5443.1358. [DOI] [PubMed] [Google Scholar]
- 59.Rowlett RM, Chrestensen CA, Nyce M, Harp MG, Pelo JW, Cominelli F, et al. MNK kinases regulate multiple TLR pathways and innate proinflammatory cytokines in macrophages. Am.J.Physiol.Gastrointest.Liver Physiol. 2008;294:G452–9. doi: 10.1152/ajpgi.00077.2007. [DOI] [PubMed] [Google Scholar]
- 60.Andersson K, Sundler R. Posttranscriptional regulation of TNFalpha expression via eukaryotic initiation factor 4E (eIF4E) phosphorylation in mouse macrophages. Cytokine. 2006;33:52–7. doi: 10.1016/j.cyto.2005.11.017. [DOI] [PubMed] [Google Scholar]
- 61.Okkenhaug K, Vanhaesebroeck B. PI3K in lymphocyte development, differentiation and activation. Nat.Rev.Immunol. 2003;3:317–30. doi: 10.1038/nri1056. [DOI] [PubMed] [Google Scholar]
- 62.Koyasu S. The role of PI3K in immune cells. Nat.Immunol. 2003;4:313–9. doi: 10.1038/ni0403-313. [DOI] [PubMed] [Google Scholar]
- 63.Ojaniemi M, Glumoff V, Harju K, Liljeroos M, Vuori K, Hallman M. Phosphatidylinositol 3-kinase is involved in Toll-like receptor 4-mediated cytokine expression in mouse macrophages. Eur.J.Immunol. 2003;33:597–605. doi: 10.1002/eji.200323376. [DOI] [PubMed] [Google Scholar]
- 64.Laird MH, Rhee SH, Perkins DJ, Medvedev AE, Piao W, Fenton MJ, et al. TLR4/MyD88/PI3K interactions regulate TLR4 signaling. J.Leukoc.Biol. 2009;85:966–77. doi: 10.1189/jlb.1208763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ruse M, Knaus UG. New players in TLR-mediated innate immunity: PI3K and small Rho GTPases. Immunol.Res. 2006;34:33–48. doi: 10.1385/IR:34:1:33. [DOI] [PubMed] [Google Scholar]
- 66.Gunzl P, Schabbauer G. Recent advances in the genetic analysis of PTEN and PI3K innate immune properties. Immunobiology. 2008;213:759–65. doi: 10.1016/j.imbio.2008.07.028. [DOI] [PubMed] [Google Scholar]
- 67.Fukao T, Tanabe M, Terauchi Y, Ota T, Matsuda S, Asano T, et al. PI3K-mediated negative feedback regulation of IL-12 production in DCs. Nat.Immunol. 2002;3:875–81. doi: 10.1038/ni825. [DOI] [PubMed] [Google Scholar]
- 68.Yu Y, Nagai S, Wu H, Neish AS, Koyasu S, Gewirtz AT. TLR5-mediated phosphoinositide 3-kinase activation negatively regulates flagellin-induced proinflammatory gene expression. J.Immunol. 2006;176:6194–201. doi: 10.4049/jimmunol.176.10.6194. [DOI] [PubMed] [Google Scholar]
- 69.Tsukamoto K, Hazeki K, Hoshi M, Nigorikawa K, Inoue N, Sasaki T, et al. Critical roles of the p110 beta subtype of phosphoinositide 3-kinase in lipopolysaccharide-induced Akt activation and negative regulation of nitrite production in RAW 264.7 cells. J.Immunol. 2008;180:2054–61. doi: 10.4049/jimmunol.180.4.2054. [DOI] [PubMed] [Google Scholar]
- 70.Doble BW, Woodgett JR. GSK-3: tricks of the trade for a multi-tasking kinase. J.Cell.Sci. 2003;116:1175–86. doi: 10.1242/jcs.00384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Martin M, Rehani K, Jope RS, Michalek SM. Toll-like receptor-mediated cytokine production is differentially regulated by glycogen synthase kinase 3. Nat.Immunol. 2005;6:777–84. doi: 10.1038/ni1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hu X, Paik PK, Chen J, Yarilina A, Kockeritz L, Lu TT, et al. IFN-gamma suppresses IL-10 production and synergizes with TLR2 by regulating GSK3 and CREB/AP-1 proteins. Immunity. 2006;24:563–74. doi: 10.1016/j.immuni.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 73.Guiducci C, Ghirelli C, Marloie-Provost MA, Matray T, Coffman RL, Liu YJ, et al. PI3K is critical for the nuclear translocation of IRF-7 and type I IFN production by human plasmacytoid predendritic cells in response to TLR activation. J.Exp.Med. 2008;205:315–22. doi: 10.1084/jem.20070763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cao W, Manicassamy S, Tang H, Kasturi SP, Pirani A, Murthy N, et al. Toll-like receptor-mediated induction of type I interferon in plasmacytoid dendritic cells requires the rapamycin-sensitive PI(3)K-mTOR-p70S6K pathway. Nat.Immunol. 2008;9:1157–64. doi: 10.1038/ni.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jefferies CA, Doyle S, Brunner C, Dunne A, Brint E, Wietek C, et al. Bruton's tyrosine kinase is a Toll/interleukin-1 receptor domain-binding protein that participates in nuclear factor kappaB activation by Toll-like receptor 4. J.Biol.Chem. 2003;278:26258–64. doi: 10.1074/jbc.M301484200. [DOI] [PubMed] [Google Scholar]
- 76.Lee KG, Xu S, Wong ET, Tergaonkar V, Lam KP. Bruton's tyrosine kinase separately regulates NFkappaB p65RelA activation and cytokine interleukin (IL)-10/IL-12 production in TLR9-stimulated B Cells. J.Biol.Chem. 2008;283:11189–98. doi: 10.1074/jbc.M708516200. [DOI] [PubMed] [Google Scholar]
- 77.Sochorova K, Horvath R, Rozkova D, Litzman J, Bartunkova J, Sediva A, et al. Impaired Toll-like receptor 8-mediated IL-6 and TNF-alpha production in antigen-presenting cells from patients with X-linked agammaglobulinemia. Blood. 2007;109:2553–6. doi: 10.1182/blood-2006-07-037960. [DOI] [PubMed] [Google Scholar]
- 78.Liljeroos M, Vuolteenaho R, Morath S, Hartung T, Hallman M, Ojaniemi M. Bruton's tyrosine kinase together with PI 3-kinase are part of Toll-like receptor 2 multiprotein complex and mediate LTA induced Toll-like receptor 2 responses in macrophages. Cell.Signal. 2007;19:625–33. doi: 10.1016/j.cellsig.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 79.Mansell A, Smith R, Doyle SL, Gray P, Fenner JE, Crack PJ, et al. Suppressor of cytokine signaling 1 negatively regulates Toll-like receptor signaling by mediating Mal degradation. Nat.Immunol. 2006;7:148–55. doi: 10.1038/ni1299. [DOI] [PubMed] [Google Scholar]
- 80.Gray P, Dunne A, Brikos C, Jefferies CA, Doyle SL, O'Neill LA. MyD88 adapter-like (Mal) is phosphorylated by Bruton's tyrosine kinase during TLR2 and TLR4 signal transduction. J.Biol.Chem. 2006;281:10489–95. doi: 10.1074/jbc.M508892200. [DOI] [PubMed] [Google Scholar]
- 81.Chen LY, Zuraw BL, Liu FT, Huang S, Pan ZK. IL-1 receptor-associated kinase and low molecular weight GTPase RhoA signal molecules are required for bacterial lipopolysaccharide-induced cytokine gene transcription. J.Immunol. 2002;169:3934–9. doi: 10.4049/jimmunol.169.7.3934. [DOI] [PubMed] [Google Scholar]
- 82.Manukyan M, Nalbant P, Luxen S, Hahn KM, Knaus UG. RhoA GTPase activation by TLR2 and TLR3 ligands: connecting via Src to NF-kappa B. J.Immunol. 2009;182:3522–9. doi: 10.4049/jimmunol.0802280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hippenstiel S, Soeth S, Kellas B, Fuhrmann O, Seybold J, Krull M, et al. Rho proteins and the p38-MAPK pathway are important mediators for LPS-induced interleukin-8 expression in human endothelial cells. Blood. 2000;95:3044–51. [PubMed] [Google Scholar]
- 84.Schmeck B, Brunsch M, Seybold J, Krull M, Eichel-Streiber C, Suttorp N, et al. Rho protein inhibition blocks cyclooxygenase-2 expression by proinflammatory mediators in endothelial cells. Inflammation. 2003;27:89–95. doi: 10.1023/a:1023278600596. [DOI] [PubMed] [Google Scholar]
- 85.Monick MM, Powers LS, Butler NS, Hunninghake GW. Inhibition of Rho family GTPases results in increased TNF-alpha production after lipopolysaccharide exposure. J.Immunol. 2003;171:2625–30. doi: 10.4049/jimmunol.171.5.2625. [DOI] [PubMed] [Google Scholar]
- 86.Harokopakis E, Albzreh MH, Martin MH, Hajishengallis G. TLR2 transmodulates monocyte adhesion and transmigration via Rac1- and PI3K-mediated inside-out signaling in response to Porphyromonas gingivalis fimbriae. J.Immunol. 2006;176:7645–56. doi: 10.4049/jimmunol.176.12.7645. [DOI] [PubMed] [Google Scholar]
- 87.Chen BC, Kang JC, Lu YT, Hsu MJ, Liao CC, Chiu WT, et al. Rac1 regulates peptidoglycan-induced nuclear factor-kappaB activation and cyclooxygenase-2 expression in RAW 264.7 macrophages by activating the phosphatidylinositol 3-kinase/Akt pathway. Mol.Immunol. 2009;46:1179–88. doi: 10.1016/j.molimm.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 88.Burdelya LG, Krivokrysenko VI, Tallant TC, Strom E, Gleiberman AS, Gupta D, et al. An agonist of toll-like receptor 5 has radioprotective activity in mouse and primate models. Science. 2008;320:226–30. doi: 10.1126/science.1154986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cheng I, Plummer SJ, Casey G, Witte JS. Toll-like receptor 4 genetic variation and advanced prostate cancer risk. Cancer Epidemiol.Biomarkers Prev. 2007;16:352–5. doi: 10.1158/1055-9965.EPI-06-0429. [DOI] [PubMed] [Google Scholar]
- 90.Song J, Kim DY, Kim CS, Kim HJ, Lee DH, Lee HM, et al. The association between Toll-like receptor 4 (TLR4) polymorphisms and the risk of prostate cancer in Korean men. Cancer Genet.Cytogenet. 2009;190:88–92. doi: 10.1016/j.cancergencyto.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 91.Chen YC, Giovannucci E, Kraft P, Lazarus R, Hunter DJ. Association between Toll-like receptor gene cluster (TLR6, TLR1, and TLR10) and prostate cancer. Cancer Epidemiol.Biomarkers Prev. 2007;16:1982–9. doi: 10.1158/1055-9965.EPI-07-0325. [DOI] [PubMed] [Google Scholar]
- 92.Stevens VL, Hsing AW, Talbot JT, Zheng SL, Sun J, Chen J, et al. Genetic variation in the toll-like receptor gene cluster (TLR10-TLR1-TLR6) and prostate cancer risk. Int.J.Cancer. 2008;123:2644–50. doi: 10.1002/ijc.23826. [DOI] [PubMed] [Google Scholar]
- 93.Pandey S, Mittal RD, Srivastava M, Srivastava K, Singh S, Srivastava S, et al. Impact of Toll-like receptors [TLR] 2 (-196 to -174 del) and TLR 4 (Asp299Gly, Thr399Ile) in cervical cancer susceptibility in North Indian women. Gynecol.Oncol. 2009 doi: 10.1016/j.ygyno.2009.05.032. [DOI] [PubMed] [Google Scholar]
- 94.Fukata M, Chen A, Vamadevan AS, Cohen J, Breglio K, Krishnareddy S, et al. Toll-like receptor-4 promotes the development of colitis-associated colorectal tumors. Gastroenterology. 2007;133:1869–81. doi: 10.1053/j.gastro.2007.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Fukata M, Chen A, Klepper A, Krishnareddy S, Vamadevan AS, Thomas LS, et al. Cox-2 is regulated by Toll-like receptor-4 (TLR4) signaling: Role in proliferation and apoptosis in the intestine. Gastroenterology. 2006;131:862–77. doi: 10.1053/j.gastro.2006.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kim S, Takahashi H, Lin WW, Descargues P, Grivennikov S, Kim Y, et al. Carcinoma-produced factors activate myeloid cells through TLR2 to stimulate metastasis. Nature. 2009;457:102–6. doi: 10.1038/nature07623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Huang B, Zhao J, Li H, He KL, Chen Y, Chen SH, et al. Toll-like receptors on tumor cells facilitate evasion of immune surveillance. Cancer Res. 2005;65:5009–14. doi: 10.1158/0008-5472.CAN-05-0784. [DOI] [PubMed] [Google Scholar]
- 98.Huang B, Zhao J, Shen S, Li H, He KL, Shen GX, et al. Listeria monocytogenes promotes tumor growth via tumor cell toll-like receptor 2 signaling. Cancer Res. 2007;67:4346–52. doi: 10.1158/0008-5472.CAN-06-4067. [DOI] [PubMed] [Google Scholar]
- 99.Sasu S, LaVerda D, Qureshi N, Golenbock DT, Beasley D. Chlamydia pneumoniae and chlamydial heat shock protein 60 stimulate proliferation of human vascular smooth muscle cells via toll-like receptor 4 and p44/p42 mitogen-activated protein kinase activation. Circ.Res. 2001;89:244–50. doi: 10.1161/hh1501.094184. [DOI] [PubMed] [Google Scholar]
- 100.Wang ZJ, Zhang FM, Wang LS, Yao YW, Zhao Q, Gao X. Lipopolysaccharides can protect mesenchymal stem cells (MSCs) from oxidative stress-induced apoptosis and enhance proliferation of MSCs via Toll-like receptor(TLR)-4 and PI3K/Akt. Cell Biol.Int. 2009;33:665–74. doi: 10.1016/j.cellbi.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 101.Chow EK, O'connell RM, Schilling S, Wang XF, Fu XY, Cheng G. TLR agonists regulate PDGF-B production and cell proliferation through TGF-beta/type I IFN crosstalk. EMBO J. 2005;24:4071–81. doi: 10.1038/sj.emboj.7600867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hasan UA, Caux C, Perrot I, Doffin AC, Menetrier-Caux C, Trinchieri G, et al. Cell proliferation and survival induced by Toll-like receptors is antagonized by type I IFNs. Proc.Natl.Acad.Sci.U.S.A. 2007;104:8047–52. doi: 10.1073/pnas.0700664104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Salaun B, Lebecque S, Matikainen S, Rimoldi D, Romero P. Toll-like receptor 3 expressed by melanoma cells as a target for therapy? Clin.Cancer Res. 2007;13:4565–74. doi: 10.1158/1078-0432.CCR-07-0274. [DOI] [PubMed] [Google Scholar]
- 104.Paone A, Starace D, Galli R, Padula F, De Cesaris P, Filippini A, et al. Toll-like receptor 3 triggers apoptosis of human prostate cancer cells through a PKC-alpha-dependent mechanism. Carcinogenesis. 2008;29:1334–42. doi: 10.1093/carcin/bgn149. [DOI] [PubMed] [Google Scholar]
- 105.Aliprantis AO, Yang RB, Mark MR, Suggett S, Devaux B, Radolf JD, et al. Cell activation and apoptosis by bacterial lipoproteins through toll-like receptor-2. Science. 1999;285:736–9. doi: 10.1126/science.285.5428.736. [DOI] [PubMed] [Google Scholar]
- 106.Aliprantis AO, Yang RB, Weiss DS, Godowski P, Zychlinsky A. The apoptotic signaling pathway activated by Toll-like receptor-2. EMBO J. 2000;19:3325–36. doi: 10.1093/emboj/19.13.3325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lehner M, Bailo M, Stachel D, Roesler W, Parolini O, Holter W. Caspase-8 dependent apoptosis induction in malignant myeloid cells by TLR stimulation in the presence of IFN-alpha. Leuk.Res. 2007;31:1729–35. doi: 10.1016/j.leukres.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 108.Zhang J, Cado D, Chen A, Kabra NH, Winoto A. Fas-mediated apoptosis and activation-induced T-cell proliferation are defective in mice lacking FADD/Mort1. Nature. 1998;392:296–300. doi: 10.1038/32681. [DOI] [PubMed] [Google Scholar]
- 109.Imtiyaz HZ, Rosenberg S, Zhang Y, Rahman ZS, Hou YJ, Manser T, et al. The Fas-associated death domain protein is required in apoptosis and TLR-induced proliferative responses in B cells. J.Immunol. 2006;176:6852–61. doi: 10.4049/jimmunol.176.11.6852. [DOI] [PMC free article] [PubMed] [Google Scholar]