Abstract
Objective To determine the effectiveness and cost effectiveness of using information from circulating biomarkers to inform the prioritisation process of patients with stable angina awaiting coronary artery bypass graft surgery.
Design Decision analytical model comparing four prioritisation strategies without biomarkers (no formal prioritisation, two urgency scores, and a risk score) and three strategies based on a risk score using biomarkers: a routinely assessed biomarker (estimated glomerular filtration rate), a novel biomarker (C reactive protein), or both. The order in which to perform coronary artery bypass grafting in a cohort of patients was determined by each prioritisation strategy, and mean lifetime costs and quality adjusted life years (QALYs) were compared.
Data sources Swedish Coronary Angiography and Angioplasty Registry (9935 patients with stable angina awaiting coronary artery bypass grafting and then followed up for cardiovascular events after the procedure for 3.8 years), and meta-analyses of prognostic effects (relative risks) of biomarkers.
Results The observed risk of cardiovascular events while on the waiting list for coronary artery bypass grafting was 3 per 10 000 patients per day within the first 90 days (184 events in 9935 patients). Using a cost effectiveness threshold of £20 000-£30 000 (€22 000-€33 000; $32 000-$48 000) per additional QALY, a prioritisation strategy using a risk score with estimated glomerular filtration rate was the most cost effective strategy (cost per additional QALY was <£410 compared with the Ontario urgency score). The impact on population health of implementing this strategy was 800 QALYs per 100 000 patients at an additional cost of £245 000 to the National Health Service. The prioritisation strategy using a risk score with C reactive protein was associated with lower QALYs and higher costs compared with a risk score using estimated glomerular filtration rate.
Conclusion Evaluating the cost effectiveness of prognostic biomarkers is important even when effects at an individual level are small. Formal prioritisation of patients awaiting coronary artery bypass grafting using a routinely assessed biomarker (estimated glomerular filtration rate) along with simple, routinely collected clinical information was cost effective. Prioritisation strategies based on the prognostic information conferred by C reactive protein, which is not currently measured in this context, or a combination of C reactive protein and estimated glomerular filtration rate, is unlikely to be cost effective. The widespread practice of using only implicit or informal means of clinically ordering the waiting list may be harmful and should be replaced with formal prioritisation approaches.
Introduction
Across clinical medicine there is intense interest in the potential of novel circulating biomarkers to provide additional prognostic information beyond standard clinical measures, which in turn might help to optimise the type, amount, timing, or targeting of subsequent intervention for patients.1 2 3 4 While there is growing appreciation of the need to demonstrate the clinical impact of biomarkers for altered decision making,5 research into the effectiveness and cost effectiveness of different strategies using biomarkers has been lacking. Such evaluations are a particular challenge in the common situation where the effects of biomarkers in terms of both quality adjusted life years (QALYs) and costs are likely to be smaller than those associated with direct interventions. This suggests the importance of considering the impact on the health populations (rather than individual patients) of investing in one biomarker programme compared with alternative investments.
Prioritising the waiting list for coronary artery surgery6 7 8 9 is an example of a common (and politically sensitive) clinical decision in which biomarkers might help select patients who will benefit from an earlier operation. Recent trials confirm that coronary artery bypass grafting remains the standard of care for severe coronary disease,7 and recent national data show that the numbers of procedures remain high.10 Maximum waiting times vary between countries (for example, 14 days for high risk patients in Canada11 and 90 days for all patients in the United Kingdom12), with an appreciable daily risk of death or myocardial infarction.13 14 The effectiveness of biomarkers is determined by their ability to predict such events and they have the potential to change the day on which coronary artery bypass grafting is optimally performed. Scores have been proposed for formal prioritisation of waiting lists15 16 but they do not include information on biomarkers, are not widely used, and their cost effectiveness has not been assessed. Estimated glomerular filtration rate is not only measured routinely but is the first biomarker to be recorded in a national angiographic registry of patients with stable angina. Among the “novel” biomarkers, which are not currently widely measured, the highly sensitive C reactive protein has the largest evidence base (77 prognostic studies).17 Clinical guidelines recommend consideration of glomerular filtration rate and C reactive protein as circulating biomarkers18 19 but do not detail how specific clinical decisions might be influenced in light of the measurement.
This study provides a framework for assessing whether the costs of measuring biomarkers are justified by benefits in terms of QALYs. We determined the effectiveness and cost effectiveness of using estimated glomerular filtration rate and highly sensitive C reactive protein for prioritising patients with stable angina awaiting coronary artery bypass grafting. We used a large registry of patients awaiting coronary artery surgery20 to estimate risk of events on the waiting list, and meta-analyses of the prognostic effect of biomarkers. These were incorporated into a decision analytical model as a framework to compare the cost effectiveness of alternative prioritisation strategies without the use of biomarkers with those including urgency scores,15 16 or risk scores without and with the use of biomarkers (routine, novel, or both).
Methods
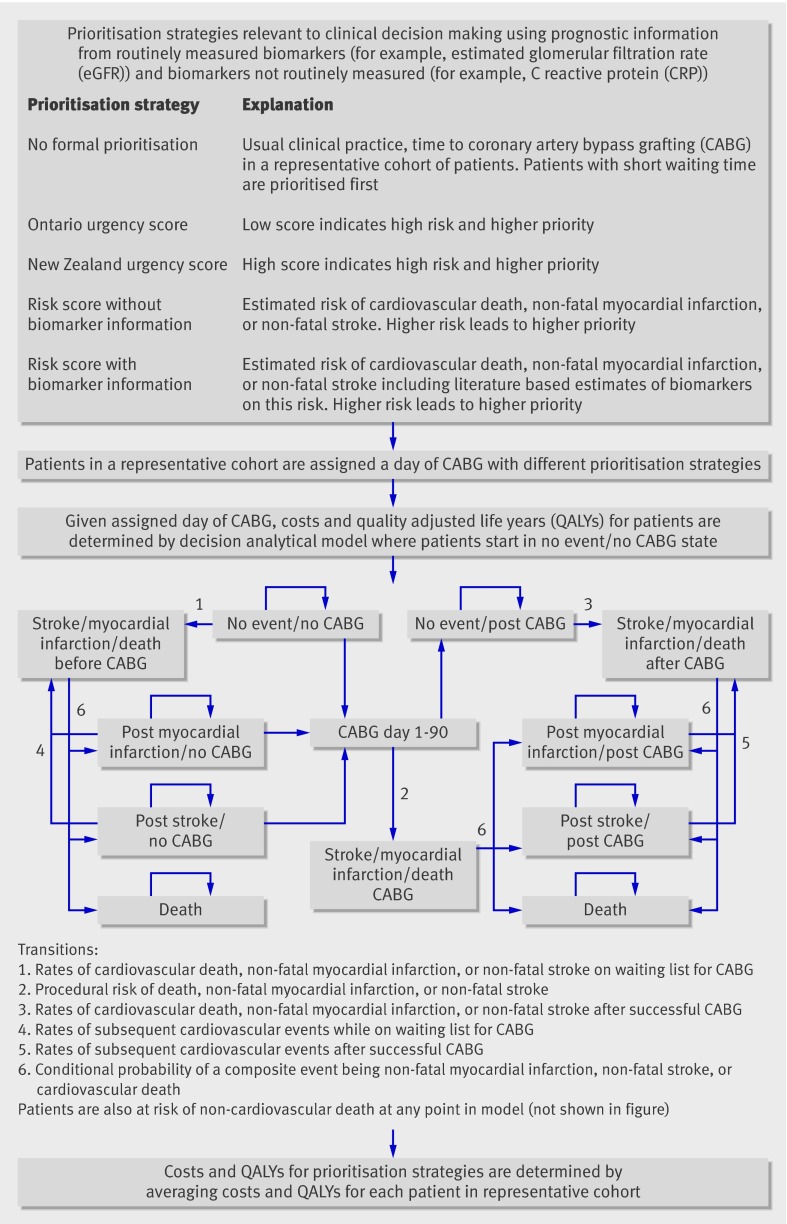
Figure 1 provides an overview of the prioritisation strategies, the analytical approach, and decision analytical model. We compared costs and health outcomes of seven prioritisation strategies: no formal prioritisation (that is, usual clinical practice), urgency scores (Ontario and New Zealand algorithms), risk score without the use of biomarkers, and three approaches using a risk score with biomarkers: use of either a single routinely measured biomarker, estimated glomerular filtration rate (estimated using the Cockroft Gault or modified diet and renal disease equations), or a novel biomarker (highly sensitive C reactive protein), or a combination of these two biomarkers. The strategies were selected for clinical and policy relevance, spanning routine biomarkers the measurement of which has become widespread (for example, estimated glomerular filtration rate) and those not yet routine (for example, highly sensitive C reactive protein). The main outcomes were lifetime costs and QALYs, which were determined by ranking and assigning patients a day of coronary artery bypass grafting according to each prioritisation strategy in a representative cohort; estimating costs and QALYs for each patient in this cohort conditional on the assigned day of coronary artery bypass grafting; and averaging the costs and QALYs across all patients in the cohort for each prioritisation strategy.
Fig 1 Prioritisation strategies
Data sources and evidence synthesis
We estimated the risk of cardiovascular events (defined as death from cardiovascular causes, myocardial infarction, or stroke) while on the waiting list for coronary artery bypass grafting, procedural risk, and risk after coronary artery bypass grafting from 9935 patients in the Swedish Coronary Angiography and Angioplasty Registry20 between 2000 and 2005. This registry includes consecutive patients without exclusion criteria in all 30 centres in Sweden. For the statistical analysis we selected variables based on a combination of statistical significance and clinical a priori importance. We carried out systematic reviews and meta-analyses of the effects (relative risks) on prognosis of estimated glomerular filtration rate and highly sensitive C reactive protein by searching Medline and Embase up to November 2008.17 Eligible studies were defined by a population with stable coronary disease followed up for fatal or non-fatal coronary events. The effect of estimated glomerular filtration rate was determined using the cut point of <60 ml/min at which chronic kidney disease is defined, and because of previous evidence that the relation between renal function and clinical events is not linear.21 The effect of highly sensitive C reactive protein was expressed according to the top and middle of the distribution compared with the bottom third and was assumed to be log-linear. The results from the meta-analysis were incorporated into the risk equations estimated from the dataset of the Swedish Coronary Angiography and Angioplasty Registry. We then applied the risk equations in the study in two ways; as variable estimates for the decision analytical model (in determining transition probabilities), and to define the prioritisation strategies (that includes biomarker information) used to rank patients. Costs and health related quality of life associated with procedures and different health states in the decision analytical model were estimated from the literature (table 1).
Table 1.
Cost and quality of life variables for decision analytical model
| Model variables | Mean value | Source |
|---|---|---|
| Annual costs (£): | ||
| Ischaemic heart disease without an event | 483 | Health Technology Assessment22 |
| First year after myocardial infarction | 2201 | Health Technology Assessment22 |
| Second and subsequent years after myocardial infarction | 774 | Health Technology Assessment22 |
| First year after stroke | 9845 | Health Technology Assessment22 |
| Second and subsequent years after stroke | 2597 | Health Technology Assessment22 |
| Costs: | ||
| Coronary artery bypass grafting* | 8203 | Department of Health23 |
| C reactive protein test | 6 | Research collaborator cost |
| Quality of life weights: | ||
| Ischaemic heart disease | 0.718 | Health Technology Assessment24 |
| First year after myocardial infarction | 0.683 | Health Technology Assessment24 |
| Second and subsequent years after myocardial infarction | 0.718 | Health Technology Assessment24 |
| After stroke (combining disabling and non-disabling stroke) | 0.612 | Health Technology Assessment24 |
The prioritisation strategies were applied to a cohort of patients (n=338) with complete data in the Swedish Coronary Angiography and Angioplasty Registry, including estimated glomerular filtration rate (which was incorporated in 2005) and time to coronary artery bypass grafting (required to implement a strategy of no formal prioritisation). This cohort was representative of the 9935 patients included in the estimation of the risk equations for risk factors and number of diseased vessels. Data on highly sensitive C reactive protein were not, however, available in the Swedish Coronary Angiography and Angioplasty Registry so we used highly sensitive C reactive protein data from a previous study25 to develop imputation algorithms which estimated the probabilities based on observed covariates, that an individual was in the top, middle, or bottom third of the highly sensitive C reactive protein distribution. In the Swedish registry data we then averaged the hazard of events in each third according to this probability. Full details of the estimated risk equations, systematic review, and decision analytical model are available elsewhere.17
Cost effectiveness analysis
Using a lifetime time horizon, we undertook the cost effectiveness analysis from a UK health service perspective, with costs expressed in UK sterling at 2006-7 prices. We discounted costs and QALYs by 3.5% per annum.26 Mean costs and QALYs for the various comparators are presented and their cost effectiveness compared using standard decision rules and estimating incremental cost effectiveness ratios as appropriate.27 The incremental cost effectiveness ratio examines the additional costs that one strategy incurs over another and compares this with the additional health benefits. Results are interpreted using the National Institute for Health and Clinical Excellence (NICE) cost effectiveness threshold range of £20 000-£30 000 per additional QALY—we consider strategies below this threshold to be cost effective.26
Alternative scenarios
All patients in the representative cohort waiting for coronary artery bypass grafting were assumed to have the procedure within a maximum waiting time, but the order in which they had the procedure within this period was determined by the alternative methods of prioritisation. Since the maximum waiting time may vary in different healthcare settings and may affect the estimated cost effectiveness, we assessed the three different maximum waiting times of 15, 40, and 90 days. To assess the robustness of the cost effectiveness results we investigated several alternative scenarios, including the cost of the biomarker tests and upper and lower 95% confidence intervals of the predictive effect of biomarkers on cardiovascular events.
Results
The rate of cardiovascular events while on the waiting list for coronary artery bypass grafting was 3 per 10 000 patients per day within the first 90 days: 184 events in 9935 patients, with a mean 59 days at risk (table 2). Risk factors associated with an increased risk and included in the risk equation were age, diabetes, heart failure, previous myocardial infarction, and involvement of the left main coronary artery or three vessel disease. In the Swedish data the area under the receiver operating characteristic curve (c statistic) was 0.68. External validation of this score on the UK dataset showed a similar c statistic of 0.65.25 The event rate within 30 days of coronary artery bypass grafting was about 5%, and noticeably lower thereafter (0.7 per 10 000 patients per day based on 680 events in 6980 patients over a mean of 3.8 years of follow-up). The biomarker effects from the meta-analyses that were incorporated into the decision models were 1.96 (95% confidence interval 1.76 to 2.17) for top thirds compared with bottom thirds of C reactive protein and 2.00 (1.65 to 2.42) for patients with estimated glomerular filtration rates <60 ml/min compared with those with rates ≥60 ml/min.
Table 2.
Risk of cardiovascular death, non-fatal myocardial infarction, or non-fatal stroke while on the waiting list for CABG, within first 30 days after CABG, and long term
| Risk while on waiting list for CABG* | Procedural risk of CABG† | Long term risk‡ | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No of events or patients/Total No of patients§ | Hazard ratio (95% CI) | Adjusted hazard ratio | No of events or patients/Total No of patients§ | Odds ratio (95% CI) | No of events or patients/Total No of patients§ | Hazard ratio (95% CI) | Adjusted hazard ratio | |||
| Events: | ||||||||||
| Death | 83/9935 | — | — | 90/7375 | — | 478/6980 | — | — | ||
| Myocardial infarction | 84/9935 | — | — | 224/7375 | — | 137/6980 | — | — | ||
| Stroke | 30/9935 | — | — | 106/7375 | — | 161/6980 | — | — | ||
| Death, myocardial infarction or stroke | 184/9935 | — | — | 395/7375 | — | 680/6980 | — | — | ||
| Variables in statistical models | ||||||||||
| Age (per year) | 66.03 | 1.05 (1.03 to 1.06) | 1.05 | 65.71 | 1.04 (1.02 to 1.05) | 65.55 | 1.05 (1.04 to 1.06) | 1.05 | ||
| Heart failure | 816/9935 | 2.43 (1.69 to 3.50) | 2.45 | 554/7375 | 1.82 (1.35 to 2.44) | 485/6980 | 2.23 (1.81 to 2.75) | 2.25 | ||
| Previous myocardial infarction | 2947/9935 | 1.32 (0.97 to 1.80) | 1.29 | 2124/7375 | 1.52 (1.22 to 1.89) | 1957/6980 | 1.15 (0.98 to 1.36) | 1.13 | ||
| Diabetes | 1432/9935 | 1.57 (1.11 to 2.23) | 1.56 | 1015/7375 | 2.00 (1.56 to 2.56) | 912/6980 | 1.68 (1.39 to 2.03) | 1.67 | ||
| Previous stroke | 598/9935 | 1.85 (1.21 to 2.83) | 1.89 | 422/7375 | 2.14 (1.55 to 2.95) | 372/6980 | 2.07 (1.63 to 2.62) | 2.11 | ||
| Left main or three-vessel disease | 7801/9935 | 1.51 (0.99 to 2.31) | 1.51 | 5768/7375 | 1.62 (1.20 to 2.18) | 5426/6980 | 1.22 (1.00 to 1.49) | 1.22 | ||
| CRP third: | ||||||||||
| 2nd third¶ | — | 1.40 (1.33 to 1.47) | 1.40 | — | — | 1.40 (1.33 to 1.47) | 1.40 | |||
| 3rd third¶ | — | 1.96 (1.76 to 2.17) | 1.96 | — | — | 1.96 (1.76 to 2.17) | 1.96 | |||
| eGFR** | — | 2.00 (1.65 to 2.42) | 2.00 | — | — | 2.00 (1.65 to 2.42) | 2.00 | |||
CABG=coronary artery bypass grafting; eGRF estimated glomerular filtration rate. Hazard ratios were adjusted to ensure consistency for adjustment factors across all studies considered in meta-analysis.
*Events occurring within 90 days of assignment of CABG, patients censored at revascularisation or 90 days, mean time at risk=59 days.
†Events occurring within 30 days of procedure.
‡Risk of events in patients without an event on waiting list or an event within 30 days after CABG, mean time at risk=3.8 years.
§Mean for continuous age variable.
¶Relative risks compared with lowest third. Based on 77 studies totalling 56 496 patients and 5798 outcome events
**<60ml/min v ≥60ml/min. Based on 12 studies totalling 31 839 patients and 1639 outcome events.
Cost effectiveness of prioritisation strategies
Three prioritisation strategies (including the use of the risk score with C reactive protein) were excluded as they were dominated (more costly and less effective than one or more of the other strategies) or extendedly dominated (a combination of other strategies being more cost effective). Of the remaining four prioritisation strategies, using the £20 000-£30 000 per additional QALY threshold range, a risk score using estimated glomerular filtration rate seemed cost effective irrespective of maximum waiting time applied (the incremental cost effectiveness ratios compared with Ontario urgency score were <£410 per QALY). A prioritisation strategy with a risk score using information from highly sensitive C reactive protein and estimated glomerular filtration rate is unlikely to be cost effective, as the incremental cost effectiveness ratios were well above the threshold value when compared with a risk score using estimated glomerular filtration rate alone (table 3). The absolute gains in QALYs were small, as were the incremental costs. For example, the incremental gain in outcomes from using estimated glomerular filtration rate was estimated to be about 0.008 QALYs compared with no formal prioritisation, representing an additional three days of perfect health (for an incremental cost of £2.45 per patient).
Table 3.
Costs and health outcomes of prioritisation strategies together with estimated cost effectiveness ratios
| Strategy | Maximum waiting time | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 90 days | 40 days | 15 days | ||||||||||
| Cost | Life years | QALY | ICER | Cost | Life years | QALY | ICER | Cost | Life years | QALY | ICER | |
| No formal prioritisation | 16099.77 | 11.6611 | 8.2796 | 16095.47 | 11.6845 | 8.2973 | 16093.22 | 11.6963 | 8.3062 | |||
| Ontario urgency score | 16100.00 | 11.6646 | 8.2822 | 88 | 16095.53 | 11.6861 | 8.2984 | 55 | 16093.24 | 11.6969 | 8.3066 | 31 |
| New Zealand urgency score | 16100.87 | 11.6663 | 8.2835 | Extendedly dominated* | 16095.91 | 11.6868 | 8.2990 | Extendedly dominated* | 16093.38 | 11.6972 | 8.3068 | Extendedly dominated* |
| Risk score without biomarker | 16101.98 | 11.6713 | 8.2871 | Extendedly dominated* | 16096.37 | 11.6891 | 8.3006 | Extendedly dominated* | 16093.53 | 11.6980 | 8.3074 | Extendedly dominated* |
| Risk score with CRP | 16107.99 | 11.6714 | 8.2872 | Dominated† | 16102.37 | 11.6891 | 8.3007 | Dominated† | 16099.54 | 11.6980 | 8.3074 | Dominated† |
| Risk score with eGFR | 16102.22 | 11.6721 | 8.2877 | 405 | 16096.47 | 11.6894 | 8.3009 | 380 | 16093.57 | 11.6981 | 8.3075 | 362 |
| Risk score with CRP+eGFR | 16108.19 | 11.6723 | 8.2878 | 57 842 | 16102.46 | 11.6895 | 8.3009 | 133 287 | 16099.57 | 11.6982 | 8.3075 | 374 371 |
QALY=quality adjusted life year; ICER=incremental cost effectiveness ratio; CRP=C reactive protein; eGFR=estimated glomerular filtration rate.
ICERs are calculated as cost per QALY.
*Combination of two other comparators has lower costs and better health outcome—for example, a combination of clinical practice and risk stratification with eGFR will always be more cost effective than New Zealand urgency score.
†Comparator strategy has lower cost and better health outcome—for example, risk stratification with CRP is associated with lower mean QALYs and higher mean costs compared with risk stratification with eGFR.
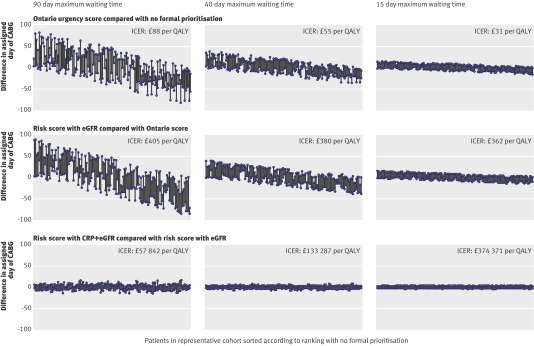
Figure 2 plots the difference in the assigned day of coronary artery bypass grafting between non-dominated prioritisation strategies. When the Ontario urgency score was compared with no formal prioritisation using a 90 day maximum waiting time, 48.6% of patients would undergo surgery on average 28.2 days later and 50% of patients on average 27.4 days sooner. When a risk score with estimated glomerular filtration rate and highly sensitive C reactive protein was compared with a risk score using estimated glomerular filtration rate alone using a 15 day maximum waiting time (fig 2), 23.3% of patients would undergo surgery on average 1.2 days later and 24.2% of patients an average 1.1 days sooner. Figure 2 shows that adding the novel circulating biomarker highly sensitive C reactive protein to the routinely measured estimated glomerular filtration rate has little scope for improved effectiveness (changing the day of coronary artery bypass grafting) with 90 day maximum waits, and none for shorter maximum waiting times.
Fig 2 Impact of different prognostic risk scores on changing assigned day of coronary artery bypass grafting (CABG), with positive (negative) values denoting a patient was operated on later (sooner) for a particular comparison. The impact on quality adjusted life years (QALYs) is derived from these changes in CABG order. ICER=incremental cost effectiveness ratio, CRP=C reactive protein
Alternative scenarios
The findings of the base case scenario were not altered by the results of alternative scenarios based on the 95% confidence intervals for the hazard ratios of estimated glomerular filtration rate and highly sensitive C reactive protein. Using the upper confidence limit (that is, biomarkers carrying more information) and a 90 day maximum waiting time, the incremental cost effectiveness ratio of a prioritisation strategy with a risk score using estimated glomerular filtration rate and highly sensitive C reactive protein compared with a risk score using estimated glomerular filtration rate alone was £39 000 per additional QALY. Using the lower confidence limit (that is, biomarkers carrying less information) and a 90 day maximum waiting time, the incremental cost effectiveness ratio of a prioritisation strategy with a risk score using estimated glomerular filtration rate and highly sensitive C reactive protein compared with a risk score using estimated glomerular filtration rate alone was £79 000 per additional QALY. Comparing a risk score with estimated glomerular filtration rate with the Ontario urgency score yielded an incremental cost effectiveness ratio of £306 with the same scenario, indicating that a risk score with estimated glomerular filtration rate was still the most cost effective prioritisation strategy.
The cost effectiveness of using novel biomarkers is sensitive to the cost of the test itself. For example, considering a waiting time of 90 days, lowering the cost of highly sensitive C reactive protein to £3 (£6 in the base case analysis) reduces the incremental cost effectiveness ratio of the risk score with estimated glomerular filtration rate and highly sensitive C reactive protein compared with the risk score based on estimated glomerluar filtration rate alone to about £29 000 per QALY. At a cost of £2 for highly sensitive C reactive protein, the subsequent incremental cost effectiveness ratio decreases to below the lower bound of the NICE threshold (about £19 000 per QALY).
Discussion
Based on our prospective outcome study of over 9000 patients, we developed a risk score based on age, heart failure, myocardial infarction, diabetes, stroke, and coronary anatomy for predicting cardiovascular events while on the waiting list for coronary artery bypass grafting. In our decision model we found that incorporation of a routinely available biomarker (estimated glomerular filtration rate) to this risk score was associated with changes in the day of assigned coronary artery bypass grafting, leading to higher QALYs at modest additional cost. This explicit strategy of formally prioritising the waiting list was both cost effective and robust to alternative assumptions, including variation in the maximum waiting list times and uncertainty in the prognostic effect of biomarkers. Adding highly sensitive C reactive protein, a more costly biomarker, which is not routinely measured, seemed to add insufficient prognostic information to be cost effective in prioritising the patients.
Interpreting incremental cost effectiveness ratios for biomarkers
Prognostic biomarkers are not interventions themselves, but there are strong parallels with the interpretation of the cost effectiveness of biomarkers and interventions. For example, the average risk is reduced with most commonly used preventive interventions in cardiovascular disease, but the benefits for the individual can vary and for some will be small. Similarly, for an individual patient the additional gains in quality adjusted survival when averaged out across all patients were small. From the perspective of an individual patient this may not seem a worthwhile gain. However, a more policy relevant perspective is that of population health.28 Our findings suggest that implementing the strategy of a risk score that includes estimated glomerular filtration rate could offer important gains in population health—800 QALYs per 100 000 patients at an additional cost of £245 000 to the National Health Service. The critical issue is how much health is forgone by displacing existing services elsewhere in the NHS to generate the funds to meet this additional cost. This is central to the cost effectiveness threshold used by NICE. Recent empirical work on the efficiency of different clinical specialties in the NHS suggests that £245 000 taken from diabetes care would on average displace nine life years, and from respiratory disease 45 life years.29 Although life years are not the same as QALYs, these estimates are considerably lower than the 800 QALYs gained from the use of estimated glomerular filtration rate in prioritising patients awaiting coronary artery bypass grafting. This provides evidence that investing in such a biomarker prioritisation programme would be good value for money.
Strengths of the study
Our study has several strengths. Firstly, we provide an overall framework for assessing the cost effectiveness of prognostic biomarkers and risk scores. Such studies have been called for but remain rare.5 Secondly, we incorporated data from a large national contemporary registry of patients undergoing coronary angiography and followed up for cardiovascular events over three phases: while patients were on the waiting list, immediately after coronary artery bypass grafting, and long term. Thirdly, we used meta-analytical estimates of effect, which are more precise than those from individual studies, for the contribution of individual biomarkers. Although new markers may contribute more prognostic information, there is a potential trade-off against the additional costs of obtaining this information.
Limitations and research implications
Our study has limitations. Firstly, in the absence of a pre-existing risk score for predicting events while patients were on the waiting list, we developed a new risk equation in our Swedish dataset and carried out external validation in a smaller UK dataset; but this risk score requires further validation and refinement.30 This is a challenge given that, to our knowledge, Sweden is currently the only country with a national coronary angiography register—that is, with sufficient patient numbers to estimate event rates. Secondly, datasets recording novel biomarkers at the time of angiography among patients awaiting coronary artery bypass grafting are lacking, and therefore we imputed values for highly sensitive C reactive protein for the patients subjected to the different prioritisation strategies when evaluating the decision analytical model. While the imputation may have diluted the effect of the prioritisation strategies including highly sensitive C reactive protein, the relative risks estimates for C reactive protein are likely to be inflated because of publication bias and inadequate adjustment for the routinely recorded factors known to relate to both highly sensitive C reactive protein and outcome (including smoking, diabetes, obesity, and lipid concentrations).17 Even when using the upper 95% confidence limit for the C reactive protein effect, a prioritisation strategy with a risk score using information from highly sensitive C reactive protein and estimated glomerular filtration rate had an incremental cost effectiveness ratio exceeding £40 000 per QALY, and is thus unlikely to be considered cost effective. Thirdly, ideally meta-analysis using individual participant data31 should be used to estimate the effect of prognostic biomarkers because of the ability to standardise control for confounders. Because of the adjustment biases observed in our literature based meta-analysis,17 were such an individual participant meta-analysis available it is likely to find a weaker C reactive protein effect (that is, C reactive protein would be less effective) in prioritising patients. Fourthly, we did not fully assess uncertainty in the decision model, because such analyses are computationally intensive (may take days to run). Future development of decision models should therefore incorporate probabilistic sensitivity analyses.31 This would also enable value of information analysis to help identify specific areas where further research is most worthwhile to fund.32
Effectiveness of other biomarkers
Our model suggests that emerging biomarkers that are more expensive than highly sensitive C reactive protein would need an even greater effect on assigning day of coronary artery bypass grafting to be cost effective. For example, the cost of brain natriuretic peptide is five times higher than highly sensitive C reactive protein; with the relative risks that have been reported33 it is highly unlikely to have a sufficient impact on quality adjusted survival to be cost effective.
The predictive ability of multiple biomarkers has not been widely assessed, and to date findings are conflicting.34 35 Findings from our model suggest that combinations of costly biomarkers are unlikely to be cost effective. However, information on biomarkers already obtained in clinical practice (that is, at zero marginal cost) might be more promising candidates for cost effectiveness evaluation given evidence that, for example, white cell count34 or haemoglobin concentration might contribute independent prognostic information.
Varying maximum waiting times
Importantly, we found that formal prioritisation with a risk score including estimated glomerular filtration rate had favourable incremental cost effectiveness ratios even when maximum waiting times were reduced to 14 days. The most recent figures (August 2008) from the UK Department of Health suggest that about half of the patients waiting for coronary artery bypass grafting have been doing so for between one and three months, and about half up to one month. Clearly the shorter the waiting time the less scope there is for biomarkers to reorder the waiting list and prove cost effective. A corollary of this is that in those countries or regions with very long waiting times (>90 days), highly sensitive C reactive protein might become cost effective. Our study did not deal with whether it is better to invest healthcare resources in using information on biomarkers to achieve a more efficient prioritisation or on initiatives to shorten overall waiting times. However, our findings suggest that even with short waiting times some formal prioritisation is better than none.
Clinical relevance of coronary artery surgery
Coronary artery surgery plays an important part in the management of angina pectoris because it is the only procedure that has been shown to reduce event rates compared with medical therapy36 or with percutaneous coronary intervention.7 By contrast, percutaneous coronary intervention has been shown to offer no reduction in event rates compared with medical therapy.37 Recent data from the UK suggest that the numbers of coronary artery bypass grafting procedures have increased (20 512 in 2007 and 22 846 in 2008).10 Furthermore, the characteristics of those patients are changing, with evidence of higher risk patients receiving coronary artery bypass grafting (older people and those more likely to have diabetes). Such factors tend to increase the importance of risk scoring and prioritisation because they would be expected to increase the event rate while waiting.
Clinical and policy implications
Specifically our findings lend support to a change in clinical practice, with introduction of formal methods of prioritising patients for coronary artery bypass grafting. We found that usual care, in which the queue for coronary artery bypass grafting is ordered informally,38 might be harmful, being associated with lower QALYs than formal prioritisation methods, and is not cost effective. We propose that formal prioritisation scores are implemented to support (not dictate) the scheduling of coronary artery bypass grafting procedures. Several lines of evidence suggest that barriers to implementation of formal prioritisation scores, while real, may not be large. Firstly, use of routinely collected data on scores for calculating the risk of operative mortality (for example, EuroScore)39 is already widespread; suggesting that the IT infrastructure and clinical culture for implementing scores already exists. Secondly, “formal protocols” for prioritisation have recently been recommended.40
More broadly, across clinical specialties we propose that professional societies and government policy forming bodies such as NICE should consider cost effectiveness in evaluating prognostic biomarker strategies using similar principles as they do for drug, device, and other technologies. This is timely because in 2009 NICE announced plans to coordinate the evaluation of innovative diagnostics used in the NHS, and as we show here markers such as estimated glomerular filtration rate may have both diagnostic and prognostic properties. Against the background of little previous research evaluating the cost effectiveness of prognostic biomarkers in relation to specific clinical decisions, we provide a framework for their evaluation. This is an important issue because of the burgeoning number (>100)4 of prognostic biomarkers in coronary disease alone, which differ in cost of measurement, and evidence of incremental prognostic value.
Conclusion
A widely available biomarker (estimated glomerular filtration rate) is cost effective when combined with other simple risk information in prioritising patients waiting for coronary artery bypass grafting. The prognostic information conferred by highly sensitive C reactive protein, which is not routinely measured, or a combination of highly sensitive C reactive protein and estimated glomerular filtration rate is unlikely to be cost effective. Most importantly, the common practice of informally ordering the waiting for coronary artery bypass grafting, without the aid of any formal prioritisation strategy, is not cost effective and should be replaced.
What is already known on this topic
Circulating prognostic biomarkers have been widely proposed as adjuncts to the management of many diseases, but their costs and any impact on quality adjusted survival are commonly smaller than those associated with interventions
The cost effectiveness of using prognostic biomarkers in any clinical setting has seldom been assessed
An important specific clinical example involves the use of biomarkers to improve prediction of the risk of events in patients on the waiting list for coronary artery bypass grafting, which could help prioritise patients according to clinical need
What this study adds
This study provides empirical evidence of the importance of assessing the cost effectiveness of prognostic biomarkers
Some formal prioritisation of patients awaiting coronary artery bypass grafting is better (more cost effective) than none
Adding C reactive protein, a non-routinely measured and more costly biomarker, may not be cost effective in prioritising patients awaiting coronary artery bypass grafting
Contributors: MS, MH, and SP contributed to the design and implementation of the decision analytical and cost effectiveness models. MH analysed the decision model. HH led and JD and NF carried out the systematic review. MJS generated the scaling factors for the meta-analysis and RC carried out the meta-analyses of the biomarkers. KA supervised the statistical aspects of the meta-analyses and decision analytical modelling. MH and HH wrote the manuscript; SP wrote much of the discussion. AT contributed clinical insights into the design and analysis of the meta-analyses and decision analytical modelling. AH advised on the scope of the biomarkers to include and the interpretation of the biomarker results in biological and clinical context. BK contributed to the design of the project at its inception. MJ obtained clearance for the use of the data from the Swedish Coronary Angiography and Angioplasty Registry and its linkage to the Swedish death and hospital admission registries, and US obtained permission to use the Swedish Coronary Angiography and Angioplasty Registry data for this project and helped interpret the data. J-CK made available for sharing the St George’s angina dataset, which allowed imputation of C reactive protein in the Swedish dataset and the calculation of adjustment factors. HH, MS, SP, AH, J-CK, BK, GF, and AT obtained grant funding. All authors approved the final version submitted for publication. HH is guarantor.
Funding: This study was funded by a grant from the Health Technology Assessment programme, HTA 05-40 and a National Institute for Health Research programme grant (RP-PG-0407-10314). The views expressed here are those of the authors and do not necessarily reflect those of the Department of Health. MJS is supported by the British Heart Foundation (RG/07/007). ADH is supported by a British Heart Foundation senior research fellowship (FS/05/125).
Competing interests: All authors have completed the unified competing interest form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare (1) no financial support for the submitted work from anyone other than their employer; (2) no financial relationships with commercial entities that might have an interest in the submitted work; (3) no spouses, partners, or children with relationships with commercial entities that might have an interest in the submitted work; and (4) no non-financial interests that may be relevant to the submitted work.
Ethical approval: The analysis of the SCA Swedish Coronary Angiography and Angioplasty Registry AR database was approved by the Regionala etikprövningsnämnden, Linköping (M108-07).
Data sharing: Requests for access to the data are welcome.
Cite this as: BMJ 2010;340:b5606
References
- 1.Moons KG, Royston P, Vergouwe Y, Grobbee DE, Altman DG. Prognosis and prognostic research: what, why, and how? BMJ 2009;338:b375. [DOI] [PubMed] [Google Scholar]
- 2.Hemingway H. Prognosis research: why is Dr Lydgate still waiting? J Clin Epidemiol 2006;59:1229-38. [DOI] [PubMed] [Google Scholar]
- 3.Hemingway H, Riley RD, Altman DG. Ten steps to improving prognosis research. BMJ 2009;339:b4184. [DOI] [PubMed] [Google Scholar]
- 4.Vasan RS. Biomarkers of cardiovascular disease: molecular basis and practical considerations. Circulation 2006;113:2335-62. [DOI] [PubMed] [Google Scholar]
- 5.Hlatky MA, Greenland P, Arnett DK, Ballantyne CM, Criqui MH, Elkind MS, et al. Criteria for evaluation of novel markers of cardiovascular risk: a scientific statement from the American Heart Association. Circulation 2009;119:2408-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hemingway H, Crook AM, Feder G, Banerjee S, Dawson JR, Magee P, et al. Underuse of coronary revascularization procedures in patients considered appropriate candidates for revascularization. N Engl J Med 2001;344:645-54. [DOI] [PubMed] [Google Scholar]
- 7.Serruys PW, Morice MC, Kappetein AP, Colombo A, Holmes DR, Mack MJ, et al. Percutaneous coronary intervention versus coronary-artery bypass grafting for severe coronary artery disease. N Engl J Med 2009;360:961-72. [DOI] [PubMed] [Google Scholar]
- 8.Yusuf S, Zucker D, Peduzzi P, Fisher LD, Takaro T, Kennedy JW, et al. Effect of coronary artery bypass graft surgery on survival: overview of 10-year results from randomised trials by the Coronary Artery Bypass Graft Surgery Trialists Collaboration. Lancet 1994;344:563-70. [DOI] [PubMed] [Google Scholar]
- 9.Griffin SC, Barber JA, Manca A, Sculpher MJ, Thompson SG, Buxton MJ, et al. Cost effectiveness of clinically appropriate decisions on alternative treatments for angina pectoris: prospective observational study. BMJ 2007;334:b624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bridgewater B, Keogh B, on behalf of the Society for Cardiothoracic Surgery in Great Britain and Ireland, and Kinsman R, Walton P, Dendrite Clinical Systems. Sixth national adult cardiac surgical database report 2008. Dendrite Clinical Systems, 2009.
- 11.Legare JF, MacLean A, Buth KJ, Sullivan JA. Assessing the risk of waiting for coronary artery bypass graft surgery among patients with stenosis of the left main coronary artery. CMAJ 2005;173:371-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Department of Health. Trust, assurance and safety: the regulation of health professionals in the 21st century. Stationery Office, 2007.
- 13.Morgan CD, Sykora K, Naylor CD. Analysis of deaths while waiting for cardiac surgery among 29,293 consecutive patients in Ontario, Canada. The Steering Committee of the Cardiac Care Network of Ontario. Heart 1998;79:345-9. [PMC free article] [PubMed] [Google Scholar]
- 14.Plomp J, Redekop WK, Dekker FW, van Geldorp TR, Haalebos MM, Jambroes G, et al. Death on the waiting list for cardiac surgery in the Netherlands in 1994 and 1995. Heart 1999;81:593-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hadorn DC, Holmes AC. The New Zealand priority criteria project. Part 2: coronary artery bypass graft surgery. BMJ 1997;314:135-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Naylor CD, Baigrie RS, Goldman BS, Basinski A. Assessment of priority for coronary revascularisation procedures. Revascularisation Panel and Consensus Methods Group. Lancet 1990;335:1070-3. [DOI] [PubMed] [Google Scholar]
- 17.Hemingway H, Henriksson M, Chen R, Damant J, Fitzpatrick NK, Abrams K, et al. The effectiveness and cost-effectiveness of biomarkers for the prioritisation of patients awaiting coronary revascularisation: a systematic review and decision-model. Health Technol Assess 2010;14(In press). [DOI] [PubMed]
- 18.Fox K, Garcia MA, Ardissino D, Buszman P, Camici PG, Crea F, et al. Guidelines on the management of stable angina pectoris: executive summary: the Task Force on the Management of Stable Angina Pectoris of the European Society of Cardiology. Eur Heart J 2006;27:1341-81. [DOI] [PubMed] [Google Scholar]
- 19.Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO 3rd, Criqui M, et al. Markers of inflammation and cardiovascular disease: application to clinical and public health practice—a statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation 2003;107:499-511. [DOI] [PubMed] [Google Scholar]
- 20.Lagerqvist B, James SK, Stenestrand U, Lindback J, Nilsson T, Wallentin L. Long-term outcomes with drug-eluting stents versus bare-metal stents in Sweden. N Engl J Med 2007;356:1009-19. [DOI] [PubMed] [Google Scholar]
- 21.Di Angelantonio E, Danesh J, Eiriksdottir G, Gudnason V. Renal function and risk of coronary heart disease in general populations: new prospective study and systematic review. PLoS Med 2007;4:e270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Robinson M, Palmer S, Sculpher M, Philips Z, Ginnelly L, Bowens A, et al. Cost-effectiveness of alternative strategies for the initial medical management of non-ST elevation acute coronary syndrome: systematic review and decision-analytical modelling. Health Technol Assess 2005;9:1-158. [DOI] [PubMed] [Google Scholar]
- 23.Department of Health. NHS reference costs 2005-06. 2006. www.dh.gov.uk/en/Publicationsandstatistics/Publications/PublicationsPolicyAndGuidance/DH_062884.
- 24.Jones L, Griffin S, Palmer S, Main C, Orton V, Sculpher M, et al. Clinical effectiveness and cost-effectiveness of clopidogrel and modified-release dipyridamole in the secondary prevention of occlusive vascular events: a systematic review and economic evaluation. Health Technol Assess 2004;8(38) iii-iv,1-196. [DOI] [PubMed]
- 25.Cosin-Sales J, Kaski JC, Christiansen M, Kaminski P, Oxvig C, Overgaard MT, et al. Relationship among pregnancy associated plasma protein-A levels, clinical characteristics, and coronary artery disease extent in patients with chronic stable angina pectoris. Eur Heart J 2005;26:2093-8. [DOI] [PubMed] [Google Scholar]
- 26.National Institute for Health and Clinical Excellence (NICE). Guide to the methods of technology appraisal. NICE, 2008. [PubMed]
- 27.Johannesson M, Weinstein MC. On the decision rules of cost-effectiveness analysis. J Health Econ 1993;12:459-67. [DOI] [PubMed] [Google Scholar]
- 28.Diamond G, Kaul S. Cost, effectiveness and cost-effectiveness. Circulation: Cardiovas Qual Outcomes 2009;2:49-54. [DOI] [PubMed] [Google Scholar]
- 29.Martin S, Rice N, Smith P. Does health care spending improve health outcomes? Evidence from English programme budgeting data. J Health Econ 2008;27:826-42. [DOI] [PubMed] [Google Scholar]
- 30.Altman DG, Vergouwe Y, Royston P, Moons KG. Prognosis and prognostic research: validating a prognostic model. BMJ 2009;338:b605. [DOI] [PubMed] [Google Scholar]
- 31.Claxton K, Sculpher M, Drummond M. A rational framework for decision making by the National Institute for Clinical Excellence (NICE). Lancet 2002;360:711-5. [DOI] [PubMed] [Google Scholar]
- 32.Claxton K, Ginnelly L, Sculpher M, Philips Z, Palmer S. A pilot study on the use of decision theory and value of information analysis as part of the NHS health technology assessment programme. Health Technol Assess 2004;8:1-103. [DOI] [PubMed] [Google Scholar]
- 33.Emberson JR, Ng LL, Armitage J, Bowman L, Parish S, Collins R. N-terminal Pro-B-type natriuretic peptide, vascular disease risk, and cholesterol reduction among 20,536 patients in the MRC/BHF heart protection study. J Am Coll Cardiol 2007;49:311-9. [DOI] [PubMed] [Google Scholar]
- 34.Clayton TC, Lubsen J, Pocock SJ, Voko Z, Kirwan BA, Fox KA, et al. Risk score for predicting death, myocardial infarction, and stroke in patients with stable angina, based on a large randomised trial cohort of patients. BMJ 2005;331:869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zethelius B, Berglund L, Sundstrom J, Ingelsson E, Basu S, Larsson A, et al. Use of multiple biomarkers to improve the prediction of death from cardiovascular causes. N Engl J Med 2008;358:2107-16. [DOI] [PubMed] [Google Scholar]
- 36.Yusuf S, Zucker D, Peduzzi P, Fisher LD, Takaro T, Kennedy JW, et al. Effect of coronary artery bypass graft surgery on survival: overview of 10-year results from randomised trials by the Coronary Artery Bypass Graft Surgery Trialists Collaboration. Lancet 1994;344:563-70. Erratum in: Lancet 1994;344:1446. [DOI] [PubMed] [Google Scholar]
- 37.Boden WE, O’Rourke RA, Teo KK, Hartigan PM, Maron DJ, Kostuk WJ, et al: COURAGE Trial Research Group. Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med 2007;356:1503-16. [DOI] [PubMed] [Google Scholar]
- 38.Hemingway H, Crook AM, Feder G, Dawson JR, Timmis A. Waiting for coronary angiography: is there a clinically ordered queue? Lancet 2000;355:985-6. [DOI] [PubMed] [Google Scholar]
- 39.Nashef SA, Roques F, Michel P, Gauducheau E, Lemeshow S, Salamon R. European system for cardiac operative risk evaluation (EuroSCORE). Eur J Cardiothorac Surg 1999;16:9-13. [DOI] [PubMed] [Google Scholar]
- 40.NCEPOD. Death following a first time, isolated coronary artery bypass graft. The heart of the matter. A report of the National Confidential Enquiry into Patient Outcome and Death. 2008. www.ncepod.org.uk/2008report2/Downloads/CABG_report.pdf#search=’bypass’.