Abstract
Expression of the Na+/glucose cotransporter SGLT1 in Xenopus oocytes is characterized by a phlorizin-sensitive leak current (in the absence of glucose) that was originally called a “Na+ leak” and represents some 5–10% of the maximal Na+/glucose cotransport current. We analyzed the ionic nature of the leak current using a human SGLT1 mutant (C292A) displaying a threefold larger leak current while keeping a reversal potential (VR) of ≈−15 mV as observed for wt SGLT1. VR showed only a modest negative shift when extracellular Na+ concentration ([Na+]o) was lowered and it was completely insensitive to changes in extracellular Cl−. When extracellular pH (pHo) was decreased from 7.5 to 6.5 and 5.5, VR shifted by +15 and +40 mV, respectively, indicating that protons may be the main charge carrier at low pHo but other ions must be involved at pHo 7.5. In the presence of 15 mM [Na+]o (pHo = 7.5), addition of 75 mM of either Na+, Li+, Cs+, or K+ generated similar increases in the leak current amplitude. This observation, which was confirmed with wt SGLT1, indicates a separate pathway for the leak current with respect to the cotransport current. This means that, contrary to previous beliefs, the leak current cannot be accounted for by the translocation of the Na-loaded and glucose-free cotransporter. Using chemical modification and different SGLT1 mutants, a relationship was found between the cationic leak current and the passive water permeability suggesting that water and cations may share a common pathway through the cotransporter.
Introduction
Many ion-coupled cotransporters feature a leak current, i.e., an ion transport activity that can be observed as a specific, inhibitor-sensitive current in the absence of substrate. The exact relationship between these leak currents and the cotransport kinetic mechanism remains not well established. In some cases, the leak current is thought to be mediated by the reaction steps that precede the binding of the cotransported substrate. Indeed, the Na+ leak identified in the rat renal type II Na+/Pi cotransporter (1) is proposed to be the result of the binding of a Na+ ion to the empty carrier facing the extracellular space followed by its translocation inside the cell. Such a mechanism would also explain the Na+ leak observed in the rat thyroid Na+/I− symporter (2) as well as the Na+ leak reported to share the same pathway as the substrate in the human choline transporter (3). In other cases the leak is considered to be the result of a conduction pathway that is independent of the steps leading to cotransport. For example, the GABA transporter GAT1 has been shown to generate a Li+ leak current (4) and even a Cs+ leak (5) in the complete absence of Na+, the cation driving the cotransport mechanism. Another isoform of the same transporter family (GAT4) displays mutually exclusive Li+ and Cl− leaks that also require the absence of external Na+ (6). Nevertheless, in many instances, the experimental evidence favoring a distinct permeation mechanism for the leak current is not really exclusive. An extensive review of the subject is provided in Andrini et al.(7).
In the case of the human Na+/glucose cotransporter (hSGLT1) expressed in Xenopus laevis oocytes, a phlorizin (Pz)-sensitive current averaging 5–10% of the maximal Na+/glucose cotransport current is observed clearly (8,9). This leak current was considered to come from the glucose-independent steps of the cotransport mechanism, i.e., extracellular Na+ binding, a slow reorientation of the loaded cotransporter followed by intracellular Na+ release and return of the Na+ binding site to the extracellular-facing configuration. This current was consequently called a “Na+ leak” (9) in the case of SGLT1 and other related transporters (1–3,10) despite the fact that evidence for the involvement of Na+ is rather weak. Direct measurements comparing the uptake of 22Na with the charge carried by the leak current seemed to support the idea of a leak mediated by Na+ (11) but the small amplitude of the leak current makes the correlation between charge and Na+ transport difficult to measure. The selectivity for Na+ is seriously challenged by the observation that the leak current reverses at a potential ranging from −40 to −10 mV (9) whereas the reversal potential of Na+ in a Xenopus oocyte is ∼+70 mV. In addition, careful reading of the original study (9) indicates that a reduction in [Na+]o produced a positive displacement of the reversal potential instead of the negative displacement that would have been expected for a Na-selective pathway. In fact, the “Nernstian shift” depicted in Fig. 6 B of Umbach et al. (9) is not consistent with the values of VR reported in the text of −42 mV in the presence of 100 mM [Na+]o and +6 mV at 10 mM [Na+]o. Although it was further shown that neither the magnitude nor the reversal potential of the leak current was sensitive to variations in extracellular K+ (up to 25 mM) or to replacement of extracellular Cl− (9), the true ionic nature of the leak current remains to be established.
To investigate the ionic nature of the leak current through human SGLT1, we took advantage of a mutant (C292A) characterized previously in our laboratory (12) that exhibits a leak current three times larger than that of wt SGLT1 at hyperpolarizing potentials. Interestingly, the mutation C292Y in hSGLT1 is known to cause the hereditary disease glucose-galactose malabsorption syndrome (13,14). Our results show that the leak current can be carried by a variety of monovalent cations including some that are incapable of affecting the cotransporter presteady state currents and steady-state cotransport currents. All experiments were repeated with wt SGLT1 and similar results were obtained though with a lower signal/noise ratio. We conclude that the leak current is due to ionic diffusion along a cationic-selective and Pz-sensitive aqueous pathway through the cotransporter. Contrary to previous belief, the leak current is independent of the Na+ binding site that leads to the different conformational states through which Na+/glucose cotransport is mediated.
Materials and Methods
Oocyte preparation and injection
Oocytes were removed surgically from X. laevis frogs and were defolliculated as described previously (15). One or two days after defolliculation, healthy oocytes were injected with 46 nL of water containing mRNA coding for human myc-hSGLT1 (0.1 μg/μL) or the C292A, C610A (0.25 μg/μL in each case) mutants of this protein. As shown previously (16), this N-terminus epitope-tagged version of human SGLT1 displays properties that are indistinguishable from the untagged form.
Molecular biology
The methods used to construct the human SGLT1, C292A, and C610A mutants were explained in detail previously (12). For some control experiments, the myc epitope needed to be removed from the pBS-myc-SGLT1 clones. This was done using the method of Sawano et al. (17) and the oligonucleotide CTAGTAGGATGGACAGTAGCACCTGGAG.
Electrophysiology
When needed, 5 mM α-methyl-D-glucose (αMG, a nonmetabolized glucose analog) or 200 μM Pz were added to the normal saline solution used for electrophysiology (in mM: 90 NaCl, 3 KCl, 0.82 MgCl2, 0.74 CaCl2, 10 HEPES and adjusted to pH 7.5 with Tris). Solutions of different pH were obtained by mixing a normal saline solution buffered with 10 mM Tris with another buffered with 10 mM 2-(N-morpholino)ethanesulfonic acid until the desired pH was reached. Sodium replacement was carried out with N-methyl-D-glucamine (NMDG+) or, when appropriate, with Li+, Cs+, or K+. Chloride replacement was carried out with sodium cyclamate (Fluka, St. Louis, MO). Unless otherwise mentioned, all chemicals were obtained from Sigma-Aldrich (St. Louis, MO).
Two-microelectrode voltage-clamp experiments and data acquisition were carried out as described previously (15).
Electrophysiological measurements were done using a voltage pulse protocol with a holding potential of −50 mV and three sequential repetitions of 300 ms voltage steps ranging from −155 mV to +70 mV in increments of 25 mV. Current versus membrane potential curves (I/V curves) were obtained by averaging the current measured between 250 and 300 ms. Charge transfer curves (Q/V curves) were obtained as described previously (15). Briefly, current in the presence of 200 μM Pz was subtracted from a corresponding current measured in the normal saline solution. The resulting signal was baseline corrected by subtracting the mean of a 20-ms time window positioned at the end of each pulse. Integrating this signal during the off relaxation of the leak current yielded the total charge transferred at each potential.
Volume measurements
Our control solution contained (in mM): 80 NaCl, 3 KCl, 0.82 MgCl2, 0.74 CaCl2, 5 HEPES and 25 mannitol. The hyposmotic solution was made by reducing the mannitol concentration from 25 to 5 mM. The effect of the reducing agent dithiothreitol (DTT) was studied by adding 10 mM DTT to isosmotic and hyposmotic solutions. An oocyte was perfused with isosmotic + DTT solution for at least 20 min before any recordings were carried out. An improved version of the apparatus described in Charron et al. (18) was used for volumetry experiments. The bath was illuminated from above with a light-emitting diode and the cross-section of the oocyte was measured 30 times/s with custom-made pixel counting software using the image obtained from a CCD camera and an inverted microscope with a 3× objective.
The water permeability (LP) of the oocyte was calculated using the swelling rate slope resulting from a hyposmotic shock, as described in Bourgeois et al. (19), according to the relation:
| (1) |
where dV/dt is the water flux after the hyposmotic shock and dV/dto is the water flux before the shock. S is a standard oocyte surface of 0.4 cm2 assuming a membrane infolding factor of 9 (20), vw is the specific volume of water (18.2 cm3/mol) and Δπ represents the size of the hyposmotic shock, 20 mOsm in the case of this study. To obtain the water permeability attributable only to SGLT1, the oocyte was exposed to a hyposmotic solution containing 200 μM Pz after the initial hyposmotic shock. The LP calculated from the measurement in Pz solution was then subtracted from the initial whole oocyte LP to get the water permeability due to SGLT1 expression.
Immunocolorimetric detection of SGLT1 plasma membrane expression
Surface quantification of SGLT1 expression in Xenopus oocytes was carried out as described previously (21) with some modifications. Briefly, each group of oocytes was incubated for 30 min in a blocking solution (normal saline containing 5% bovine serum albumin) that was used also for subsequent steps of the assay unless otherwise noted. The oocytes were then incubated for 60 min with the primary antibody (rabbit anti-myc mAb, 1/500 dilution (Cell Signaling Technology, Danvers, MA)). Oocytes were washed eight times in normal saline solution before being exposed again for 30 min to the blocking solution and then incubated for 60 min with the secondary antibody (goat anti-rabbit HRP conjugated, 1/500 dilution (Millipore, Billerica, MA)). The oocytes were finally washed eight times in normal saline solution. To carry out the colorimetric reaction, oocytes were exposed individually for 60 min to 100 μL of colorimetric reaction solution (composition: 9 mL of 50 mM phosphate/citrate buffer pH 9 + 2 μL of 30% hydrogen peroxide + 1 mg tetramethylbenzidine dissolved in 1 mL DMSO). Then, 50 μL of 1 M sulfuric acid was added to end the reaction and absorbance (λ = 450 nm) was measured in 100 μL of the resulting yellow solution (without oocyte). Control experiments were carried out using the human SGLT1 (wt and mutants) lacking the epitope tag.
Statistics
Data are presented as average ± SE where n is the number of oocytes used, and are compared using Student's t-test. In the figures, error bars are not shown when smaller than symbols.
Results
Fig. 1 A depicts the total currents measured in the presence of a normal saline solution or in a saline solution containing 0.2 mM Pz or 5 mM αMG for a series of oocytes expressing hSGLT1. In Fig. 1 B, the increased current due to addition of 5 mM αMG is depicted together with the leak current obtained by subtraction of the current measured in the presence of 0.2 mM Pz from the current measured in the saline solution. At −155 mV, the leak current averaged −82.3 ± 9.8 nA, displaying an average reversal potential of −14.8 ± 2.7 mV (n = 5). The average leak current at −155 mV represented 5.6% of the cotransport current that was −1460 ± 150 nA for the same oocytes. The C292A mutant, which was partially characterized in a previous study, mediated a much larger leak current (12). The average total currents for oocytes expressing C292A are shown in Fig. 1 C. Fig. 1 D depicts the corresponding leak currents, which averaged −287 ± 19 nA at −155 mV with a mean reversal potential of −13.9 ± 2.3 mV (n = 6). The cotransport current observed on adding 5 mM αMG was very much reduced for the C292A mutant and averaged only −140 ± 17 nA at −155 mV.
Figure 1.
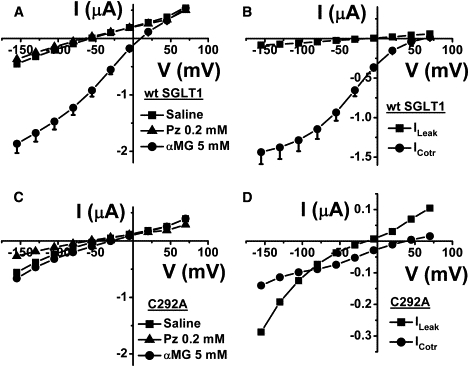
Leak and cotransport currents of wt hSGLT1 and C292A mutant. (A) The total currents of wt SGLT1 for five oocytes coming from three different donor frogs in normal saline solution, with 0.2 mM Pz or with 5 mM αMG. (B) The leak current was obtained by subtracting the current in the presence of 0.2 mM Pz from the current in normal saline solution. The cotransport current represents the increase in wt SGLT1 current on addition of 5 mM αMG. (C) The same solutions used in A were presented to the C292A mutant (n = 6). (D) The leak current and the cotransport current were extracted from the total currents (C) of the C292A mutant. Mean ± SE are shown.
As wt SGLT1 was shown previously to be capable of generating a H+/glucose cotransport when pHo is reduced from 7.5 to 6.5 and 5.5 in the absence of Na+ (22–24), we tested the effect of external pH on C292A. In the presence of Na+, the effect of pHo on the current stimulated by 5 mM αMG was significant. The cotransport current at pH 5.5 averaged −924 ± 65 nA at −155 mV (n = 7) (Fig. 2 A). This indicates that the cotransport capacity of the mutant is rescued by an acidic pH. In the presence of 90 mM Na+ and protons (pHo = 6.5), the apparent affinity of this mutant SGLT1 for αMG was 0.12 ± 0.04 mM at −155 mV (data not shown). In the case of wt SGLT1, a H+/glucose cotransport with a low affinity for glucose can be measured in the presence of an acidified extracellular solution only when Na+ is replaced by an impermeant cation (22,23). If 90 mM Na+ remains in the extracellular solution, acidification of the external solution has a relatively small effect on the cotransport current (n = 5) (Fig. 2 B). This indicates that the C292A mutant is well expressed at the oocyte membrane and can bind αMG with a normal affinity similar to that of wt SGLT1. The difference between the mutant and the wt is that the mutant requires the presence of both Na+ and an acidic pH to generate a cotransport current whereas the wt functions well with Na+ and is not very pH-sensitive.
Figure 2.
Proton-driven cotransport currents of wt SGLT1 and C292A mutant. (A) The cotransport currents of the C292A mutant were obtained by subtracting the currents in the normal saline solution (buffered with Tris and 2-(N-morpholino)ethanesulfonic acid) from the currents in the presence of 5 mM αMG (n = 7). (B) The same data analysis as in A was carried out on the currents of wt SGLT1 (n = 5). Mean ± SE are shown.
We next examined the nature of the large leak current of C292A. Replacing extracellular Na+ by NMDG+ produced a progressive decrease in the leak current (Fig. 3) and a modest shift of the reversal potential (Fig. 3, inset) by +18 mV between 5 mM and 90 mM Na+ (14 mV per decade, n = 8). This shift represents only 24% of the predicted shift in ENa and is indicative of Na+ being only one of several permeant ions present. A portion of the Na+ effect on the leak current amplitude certainly reflects the requirement of Na+ for Pz binding as is observed commonly for wt SGLT1 (25,26).
Figure 3.
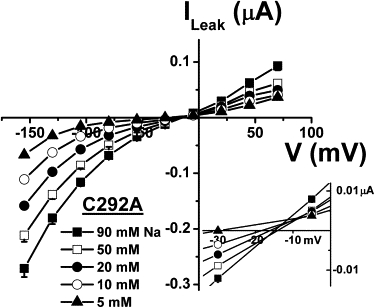
Na+ dependence of the C292A leak current. At each extracellular Na+ concentration, the current in the presence of 0.2 mM Pz was subtracted from the current in saline solution (pH 7.5) to derive the leak current (n = 8). The reversal potentials of the leak currents at each [Na+]o were enlarged (inset). Mean ± SE are shown.
In Fig. 4, the effects of extracellular Cl− replacement on the leak current through C292A are shown. Replacement of Cl− by cylamate− in the external solution, in the presence of 90 mM Na+, produced no significant effect on the leak current or on its reversal potential that remained constant when measured in the presence of Cl− concentrations varying from 6 to 70 mM (n = 6).
Figure 4.
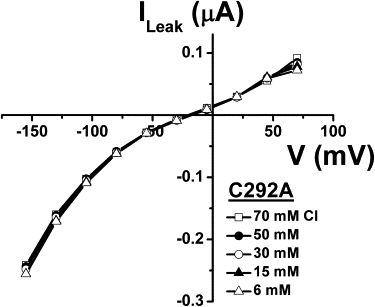
Cl− dependence of the C292A leak current. The leak currents were obtained by subtracting the current in the presence of 0.2 mM Pz from the current in the saline solution at each extracellular Cl− concentration (n = 6). Mean ± SE are shown.
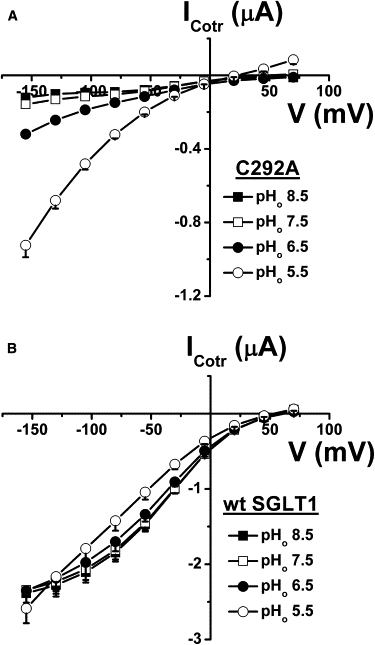
As cationic flux seems responsible for the leak current through C292A, and because proton flux through SGLT1 has been described (22,23), we tested the possibility that protons could mediate the leak current through C292A. When the external solution was acidified to pH 6.5 and pH 5.5, the leak current increased significantly (Fig. 5) and the reversal potential was displaced toward more positive values (Fig. 5, inset). From an external pH of 7.5 to 5.5, the reversal potential changed at a rate of 23 mV/pH unit (n = 12). This is consistent with a large proportion of the leak current being mediated by protons at pHo 5.5. However, it is clear that at pHo 7.5 the role of protons in the leak current is minimal because increasing the pHo to 8.5 did not modulate the leak current or the reversal potential in any significant way.
Figure 5.
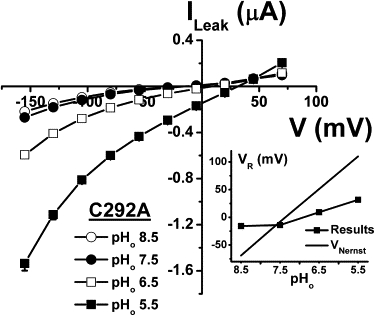
Proton dependence of the C292A leak current. At each pHo, the current in the presence of 0.2 mM Pz was subtracted from the current in saline solution to get the leak current (n = 12). The reversal potentials were extracted from the leak current at each pHo and plotted against pHo (inset). The Nernst equation was used to obtain a theoretical prediction of the behavior of the reversal potential, with pHi = 7.35. Mean ± SE are shown.
As the leak current VR is far from ENa at pH 7.5 (Fig. 3), where proton flux is negligible, and as this VR varies less than would be expected as a function of [Na+]o (Fig. 3, inset), other cations must participate in the leak current. Because external Na+ is required for Pz binding (25,26) (that in turn is required for leak current measurement), we replaced 75 mM Na+ with NMDG+, keeping 15 mM Na+ present in the external solution to permit sufficient Pz binding. With the C292A mutant expressed at the plasma membrane, the leak current averaged −56.9 ± 4.2 nA at −130 mV (Fig. 6). When an additional 75 mM Na+ was present in the external solution the leak, as already seen in Fig. 3, increased to −111.5 ± 7.4 nA at −130 mV. Interestingly, leak currents of similar amplitudes were attained by adding 75 mM Li+, Cs+, or K+ (Fig. 6). Of these monovalent cations, only Na+ can generate a significant cotransport current through wt SGLT1 although Li+ has been reported to support a small cotransport current at very high glucose concentrations (23). Analysis of the presteady-state currents, which are transient, Pz-sensitive currents measured in the absence of substrate as the oocyte is quickly clamped to various membrane potentials, is a useful tool for identifying the cations that can bind to the cotransporter (27–30). Presteady-state currents are associated with the voltage-dependent reorientation of the empty carrier and with extracellular cation binding steps (31–33). The charge corresponding to the area under the presteady-state current is plotted against the membrane potential (Q/V) in Fig. 6 B for the C292A mutant. As expected, changing [Na+]o from 15 to 90 mM was associated with greater charge entry when the membrane potential was hyperpolarized. This was not observed when 75 mM Li+, Cs+, or K+ was added to the 15 mM Na+ solution, thus establishing that extracellular Na+ is the only cation tested that can produce significant charge displacement while interacting with the mutant SGLT1 binding site. This implies that several monovalent cations are equally capable of mediating a leak current even if the conformational changes they generate are distinct from those produced by the interaction of Na+ with the specific cation binding sites of the cotransporter.
Figure 6.
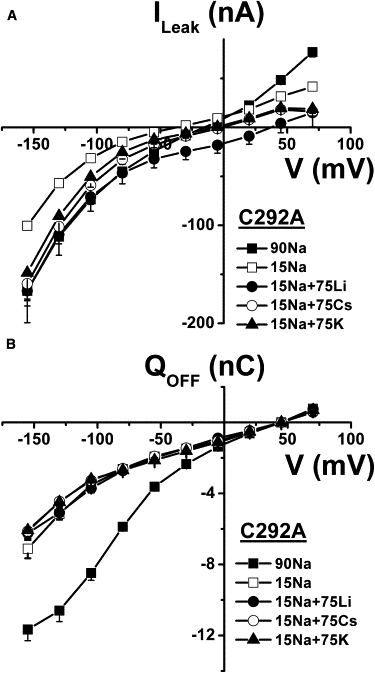
Cationic selectivity of C292A leak current. (A) In the presence of 15 mM Na+, 75 mM of additional Na+ or Li+, Cs+, or K+ was added to the bath and current was measured; current was also measured using the same solutions but in the presence of 0.2 mM Pz, and subtraction of the Pz current from the current in the corresponding saline solution was carried out to derive each leak current (n = 5). (B) Q/V curves associated with the leak currents of A depicting the transient charge movements related to the return to −50 mV after each voltage pulse. Mean ± SE are shown.
To eliminate the possibility that these results might be due to the C292A mutant having perturbed cation binding sites, we repeated these experiments with the small leak current of wt SGLT1. Starting from 15 mM Na+, addition of 75 mM Na+, Li+, Cs+, or K+ stimulated the leak current significantly (Fig. 7 A). The corresponding selectivity seemed to be slightly different from the C292A mutant and displayed the following preference: K+ > Cs+ > Li+ = Na+. For wt SGLT1, the presteady-state charge transfer was larger than for the C292A mutant, but it was clear that only Na+ could generate significant charge movement (Fig. 7 B).
Figure 7.
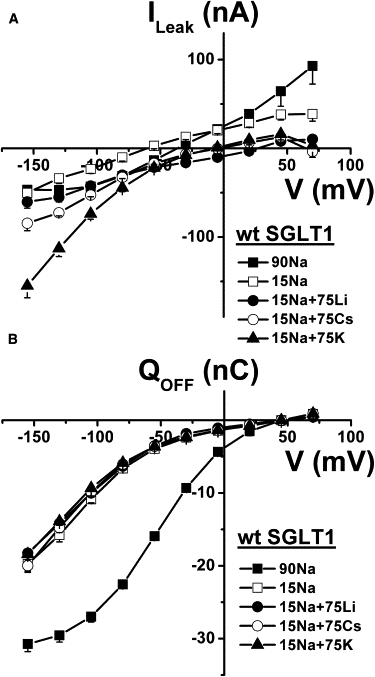
Cationic selectivity of wt SGLT1 leak current. (A) In the presence of 15 mM Na+, 75 mM of additional Na+ or Li+, Cs+, or K+ was added to the bath and the leak current was derived by subtracting current measured in each saline solution containing 0.2 mM Pz from current measured in the corresponding saline solution (n = 4). (B) Q/V curves associated with the leak currents of A depicting the transient charge movements related to the return to −50 mV after each voltage pulse. Mean ± SE are shown.
The results presented so far suggest that the leak current mediated by Li+, Cs+, and K+ ions occur through a Pz-sensitive pathway that does not involve the cotransporter cation binding site. This is reminiscent of the passive water permeability (LP) associated with SGLT1. LP was shown to be Pz-sensitive (34,35) and to not require the presence of either Na+ or glucose (36). LP was also independent of membrane potential, indicating that the pathway used by water through SGLT1 was not influenced by the conformational changes that are required to generate cotransport (37). One interesting possibility is that the cationic leak current could share the pathway with water across the cotransporter. We have shown in a previous study (12) that treating wt SGLT1 with DDT produced a decrease in its leak current. In a series of paired experiments where the leak current and the phlorizin-sensitive Lp were measured before and after a 20-min treatment with 10 mM DTT, the leak current was reduced 3.4-fold for the wt SGLT1 (n = 7) and by 2.6-fold for the mutant C292A (n = 8) (Fig. 8). In both cases, this decrease in the leak current was associated with a small but highly significant decrease in the Pz-sensitive LP (p < 0.0002 for wt and p < 0.0002 for C292A).
Figure 8.
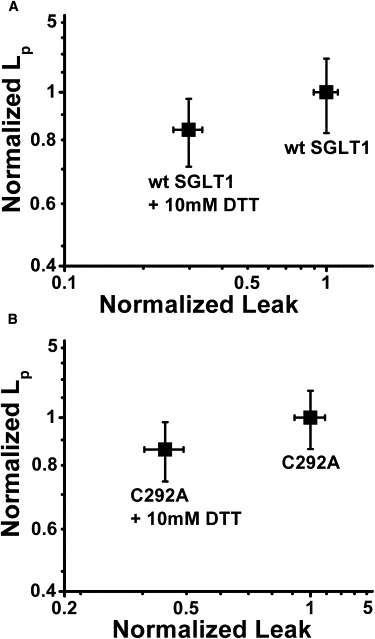
Effect of DTT on water permeability and leak current. (A) Effects of 10 mM DTT on wt SGLT1 leak current (at −130 mV) and LP, where the results obtained using the control saline solution were normalized to a value of 1 for each oocyte. (B) Effect of 10 mM DTT on the leak current (at −130 mV) and LP of the C292A mutant, where the results obtained using the control saline solution were normalized to a value of 1 for each oocyte. In both cases, the effect of DTT on LP was statistically significant (p < 0.0002).
We further examined whether this relationship between Pz-sensitive LP and leak current could hold for different mutants such as C292A and C610A, which are known to display leak currents that are significantly larger than for wt SGLT1 (12). To accurately interpret these results, it was necessary to correct the measured leak and LP for the level of expression of each mutant with respect to wt SGLT1. This was carried out by immunocolorimetric detection on live oocytes. As depicted in Fig. 9 B, the expression levels of C292A (measured on n = 2 different groups of 5–12 oocytes) and of another mutant, C610A (n = 2), and wt SGLT1 (n = 4) were measured. Specificity of the whole method was established by using noninjected oocytes as well as oocytes expressing the nontagged versions of each mutant and of wt SGLT1. In each case, the specific signal was obtained by subtracting the spectrophotometric absorbance of noninjected oocytes and resulted in expression levels relative to wt SGLT1 of 77 ± 13% and 64 ± 13% for C292A and C610A respectively. Once the measured leak currents and LP were corrected for this expression level at the plasma membrane, C292A displayed a leak current 3.6 times larger than for wt SGLT1 (p < 0.001) whereas its LP was 2.1 times larger (p < 0.001). In the case of C610A, a similar correlation was observed (see Fig. 9 A).
Figure 9.
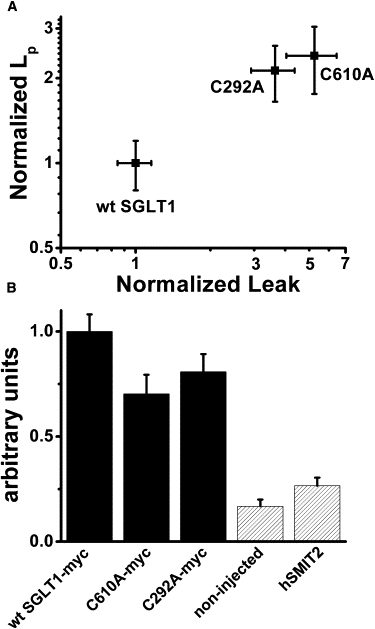
Relationship between water permeability and leak current. (A) Effect of mutations C292A and C610A on LP and the leak current (at −155 mV) relative to wt SGLT1 after correcting for plasma membrane expression level. (B) Immunocolorimetric detection of plasma membrane expression levels of wt SGLT1 (n = 4, where each is a group of 5–12 oocytes), C292A (n = 2) and C610A (n = 2). Noninjected oocytes (n = 4) as well as oocytes expressing the human Na+/myo-inositol cotransporter 2 (hSMIT2) (n = 2) were used as negative controls. Mean ± SE are shown.
Discussion
The large signal/noise ratio of electrophysiological measurements carried out on oocytes expressing SGLT1 and other members of the SLC5A family has allowed the detection of small currents mediated by cotransporters in the absence of substrate. First described in 1990 by Umbach et al. (9) for rabbit SGLT1, the leak current reversal potential was −42 mV in the presence of 100 mM [Na+]o and was reported to move to +6 mV in the presence of 10 mM [Na+]o. Even though the absolute value of VR was clearly different from ENa and the reported positive change on reducing [Na+]o was opposite from the expected change for a Na+ selective pathway, the leak current was assumed to be a Na+ leak and has been modeled by us and others as representing conformations that the cotransporter might adopt in the absence of substrate (8,24,32,38,39). The ionic nature of the leak current associated to SGLT1 has never been revisited. Taking advantage of a mutant displaying a strong leak current, we felt that we had a good opportunity to better understand the nature of this current.
The leak through the C292A mutant
The leak of C292A is clearly cationic selective as reduction of [Cl−]o from 70 to 6 mM did not produce any change in the leak amplitude nor in its reversal potential. In fact, Fig. 4 illustrates the good reproducibility provided by our method to measure Pz-sensitive leak currents in oocytes. The main observation of this study is that a Pz-sensitive leak current can be measured for different monovalent cations provided sufficient Na+ is present to allow Pz to bind to its specific site. Under these conditions, adding Na+, Li+, Cs+, or K+ produced a stimulation of the leak current and the expected positive shift in the reversal potential. For hyperpolarizing potentials where 15 mM Na+ is expected to be sufficient to support efficient Pz binding, it is remarkable to note that the I/V curves of the leak currents observed in the presence of Li+, Cs+, or K+ are all similar (Fig. 6 A). In contrast, only Na+ can affect the Q/V curve (Fig. 6 B) and produce significant conformational changes leading to high affinity glucose cotransport. Although it has been reported that Li+ can bind to SGLT1 and can drive glucose into the cell (23), it must do so through conformational changes that are different from the ones produced by Na+ binding, as can be inferred from Fig. 6 B. Moreover, the fact that Li+, Cs+, and K+ can mediate leak currents that are similar to the leak observed with Na+ suggests that the cationic leak current is present for both Na+ free and Na+ bound cotransporter states. Therefore, because leak currents of similar amplitudes can be observed with cations that do not participate in the conformational changes leading to high affinity substrate cotransport, the translocation of the Na-loaded cotransporter cannot satisfactorily explain the leak current and there is no reason to include it in future modeling of the cotransport mechanism.
The leak current of C292A was increased significantly by external acidification to pHo 6.5 and pHo 5.5, and its reversal potential was shifted toward positive membrane potentials (Fig. 5). Although protons are permeable through the leak pathway, the proton concentration does not seem to be high enough to mediate a significant portion of the leak current at pH 7.5. In consequence, in the presence of the normal saline solution (pH 7.5), the leak current is expected to be mediated mainly by Na+ and K+. Assuming intracellular concentrations of 10 mM for Na+ (18) and 80 mM for K+ (40), the calculated ENa and EK would be +55 mV and −83 mV, respectively. The shifts in VR produced by replacing 75 mM NMDG by the same amount of Na+ or K+ in the external solution (Fig. 6 A) can be accounted for by the Goldman-Hodgkin-Katz equation using permeability ratios of 2:1 for PK/PNa and 0.3 for PNMDG/PNa.
The leak through the wt SGLT1
Even though the wt SGLT1 generates a leak current three times smaller than the C292A mutant, all the experiments carried out in this study were done using both proteins and support the idea that the leaks observed in wt SGLT1 and in the C292A mutant are similar in nature. As was the case for C292A, our experiments showed that the wt SGLT1 leak was insensitive to [Cl−]o (data not shown), a result that was also reported for rabbit SGLT1 (7,9). The leak current of wt SGLT1 could also be stimulated by addition of Na+, Li+, Cs+, or K+ to the external solution. In contrast to C292A, external solution acidification did not produce a significant increase in wt SGLT1 leak current amplitude in the presence of 90 mM [Na+]o as other cations such as Na+ and K+ seem to be preferred. This indicates that the selectivity of the leak current is slightly different for wt SGLT1 than for C292A. In Fig. 7 A, adding Na+, Li+, Cs+, or K+ to a solution already containing 15 mM Na+ stimulated a leak current with the following selectivity (at −130 mV): Na+ ∼ Li+ < Cs+ < K+. This contrasts with C292A where all these four cations had similar permeability.
The leak current and the cotransporter water permeability share a common pathway
We have obtained several lines of evidence that cations and water share a common molecular pathway through the cotransporter. Both permeation pathways are Pz-sensitive and independent of the conformational changes that are specifically triggered by Na+ binding to its transport site (this study and Duquette (37)). A relationship was found between LP and ILeak for wt SGLT1 and two mutants presenting an increased leak current. A relationship was also observed on paired measurements involving the effect of DTT on wt SGLT1 and the C292A mutant. Nevertheless, the pathways used by water and by cations to cross SGLT1 may not be 100% identical and it is possible that a mutation or a chemical modification could affect the movement of water and cations in variable proportions. At this point, the observation that the water permeability and cationic leak currents are affected in the same direction by two mutations and by a DTT treatment is only suggestive that water and cations may share a common pathway.
Interestingly, in 2008, a high-resolution structure of the Vibrio parahaemolyticus Na+/galactose symporter (vSGLT) was published (41). vSGLT has a sequence identity of 32% (60% similarity) with hSGLT1. It is interesting to see that water filled vestibules are present on the intra- and extracellular sides of the cotransporter. It is also notable that the amino acid corresponding to human C292 is located near the water-filled cytosolic vestibule in a short linker disrupting the helix forming the sixth transmembrane segment (according to the nomenclature of Abramson and Wright (42)). Mutating this residue to an alanine is likely to introduce some stiffness in this linker and make the sixth transmembrane segment more rigid. This constraint could be the source of the higher ionic leak and water permeability of this mutant and a cause for the incapacity of the C292A mutant to translocate glucose across the membrane at normal pHs.
Conclusion
The leak current associated with SGLT1 was discovered in 1990 and was wrongly referred to as a Na+ leak. It was considered to involve the cotransporter states associated with the voltage-dependent conformational changes of the free and Na+ bound carrier. This study indicates that the leak current is mediated by a variety of monovalent cations, including cations that do not generate the conformational changes associated to the Na+ binding site used for cotransport. A certain relationship between cation and water flux suggests that cations and water molecules may share some common pathway. We propose that the leak current is mediated by a water-filled crevice that crosses the SGLT1 molecule and that is closed when Pz is bound to the cotransporter.
Acknowledgments
This work was supported by the Canadian Institutes for Health Research (MOP-10580).
References
- 1.Forster I., Hernando N., Murer H. The voltage dependence of a cloned mammalian renal type II Na+/Pi cotransporter (NaPi-2) J. Gen. Physiol. 1998;112:1–18. doi: 10.1085/jgp.112.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eskandari S., Loo D.D., Carrasco N. Thyroid Na+/I− symporter. Mechanism, stoichiometry, and specificity. J. Biol. Chem. 1997;272:27230–27238. doi: 10.1074/jbc.272.43.27230. [DOI] [PubMed] [Google Scholar]
- 3.Iwamoto H., Blakely R.D., De Felice L.J. Na+, Cl−, and pH dependence of the human choline transporter (hCHT) in Xenopus oocytes: the proton inactivation hypothesis of hCHT in synaptic vesicles. J. Neurosci. 2006;26:9851–9859. doi: 10.1523/JNEUROSCI.1862-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.MacAulay N., Zeuthen T., Gether U. Conformational basis for the Li(+)-induced leak current in the rat gamma-aminobutyric acid (GABA) transporter-1. J. Physiol. 2002;544:447–458. doi: 10.1113/jphysiol.2002.022897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mager S., Kleinberger-Doron N., Lester H.A. Ion binding and permeation at the GABA transporter GAT1. J. Neurosci. 1996;16:5405–5414. doi: 10.1523/JNEUROSCI.16-17-05405.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Karakossian M.H., Spencer S.R., Eskandari S. Novel properties of a mouse gamma-aminobutyric acid transporter (GAT4) J. Membr. Biol. 2005;203:65–82. doi: 10.1007/s00232-004-0732-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Andrini O., Ghezzi C., Forster I.C. The leak mode of type II Na(+)-P(i) cotransporters. Channels (Austin). 2008;2:346–357. doi: 10.4161/chan.2.5.6900. [DOI] [PubMed] [Google Scholar]
- 8.Chen X.Z., Coady M.J., Lapointe J.Y. Thermodynamic determination of the Na+: glucose coupling ratio for the human SGLT1 cotransporter. Biophys. J. 1995;69:2405–2414. doi: 10.1016/S0006-3495(95)80110-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Umbach J.A., Coady M.J., Wright E.M. Intestinal Na+/glucose cotransporter expressed in Xenopus oocytes is electrogenic. Biophys. J. 1990;57:1217–1224. doi: 10.1016/S0006-3495(90)82640-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coady M.J., Wallendorff B., Lapointe J.Y. Identification of a novel Na+/myo-inositol cotransporter. J. Biol. Chem. 2002;277:35219–35224. doi: 10.1074/jbc.M204321200. [DOI] [PubMed] [Google Scholar]
- 11.Mackenzie B., Loo D.D., Wright E.M. Relationships between Na+/glucose cotransporter (SGLT1) currents and fluxes. J. Membr. Biol. 1998;162:101–106. doi: 10.1007/s002329900347. [DOI] [PubMed] [Google Scholar]
- 12.Gagnon D.G., Bissonnette P., Lapointe J.Y. Identification of a disulfide bridge linking the fourth and the seventh extracellular loops of the Na+/glucose cotransporter. J. Gen. Physiol. 2006;127:145–158. doi: 10.1085/jgp.200509439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martín M.G., Turk E., Wright E.M. Defects in Na+/glucose cotransporter (SGLT1) trafficking and function cause glucose-galactose malabsorption. Nat. Genet. 1996;12:216–220. doi: 10.1038/ng0296-216. [DOI] [PubMed] [Google Scholar]
- 14.Wright E.M. I. Glucose galactose malabsorption. Am. J. Physiol. 1998;275:G879–G882. doi: 10.1152/ajpgi.1998.275.5.G879. [DOI] [PubMed] [Google Scholar]
- 15.Gagnon D.G., Frindel C., Lapointe J.Y. Effect of substrate on the pre-steady-state kinetics of the Na(+)/glucose cotransporter. Biophys. J. 2007;92:461–472. doi: 10.1529/biophysj.106.092296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bissonnette P., Noël J., Lapointe J.Y. Functional expression of tagged human Na+-glucose cotransporter in Xenopus laevis oocytes. J. Physiol. 1999;520:359–371. doi: 10.1111/j.1469-7793.1999.00359.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sawano A., Miyawaki A. Directed evolution of green fluorescent protein by a new versatile PCR strategy for site-directed and semi-random mutagenesis. Nucleic Acids Res. 2000;28:E78. doi: 10.1093/nar/28.16.e78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Charron F.M., Blanchard M.G., Lapointe J.Y. Intracellular hypertonicity is responsible for water flux associated with Na+/glucose cotransport. Biophys. J. 2006;90:3546–3554. doi: 10.1529/biophysj.105.076745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bourgeois F., Coady M.J., Lapointe J.Y. Determination of transport stoichiometry for two cation-coupled myo-inositol cotransporters: SMIT2 and HMIT. J. Physiol. 2005;563:333–343. doi: 10.1113/jphysiol.2004.076679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zampighi G.A., Kreman M., Wright E.M. A method for determining the unitary functional capacity of cloned channels and transporters expressed in Xenopus laevis oocytes. J. Membr. Biol. 1995;148:65–78. doi: 10.1007/BF00234157. [DOI] [PubMed] [Google Scholar]
- 21.Zerangue N., Schwappach B., Jan L.Y. A new ER trafficking signal regulates the subunit stoichiometry of plasma membrane K(ATP) channels. Neuron. 1999;22:537–548. doi: 10.1016/s0896-6273(00)80708-4. [DOI] [PubMed] [Google Scholar]
- 22.Hirayama B.A., Loo D.D., Wright E.M. Protons drive sugar transport through the Na+/glucose cotransporter (SGLT1) J. Biol. Chem. 1994;269:21407–21410. [PubMed] [Google Scholar]
- 23.Hirayama B.A., Loo D.D., Wright E.M. Cation effects on protein conformation and transport in the Na+/glucose cotransporter. J. Biol. Chem. 1997;272:2110–2115. doi: 10.1074/jbc.272.4.2110. [DOI] [PubMed] [Google Scholar]
- 24.Chen X.Z., Coady M.J., Lapointe J.Y. Sodium leak pathway and substrate binding order in the Na+-glucose cotransporter. Biophys. J. 1997;73:2503–2510. doi: 10.1016/S0006-3495(97)78278-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oulianova N., Falk S., Berteloot A. Two-step mechanism of phlorizin binding to the SGLT1 protein in the kidney. J. Membr. Biol. 2001;179:223–242. doi: 10.1007/s002320010049. [DOI] [PubMed] [Google Scholar]
- 26.Falk S., Oulianova N., Berteloot A. Kinetic mechanisms of inhibitor binding: relevance to the fast-acting slow-binding paradigm. Biophys. J. 1999;77:173–188. doi: 10.1016/S0006-3495(99)76880-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hilgemann D.W., Nicoll D.A., Philipson K.D. Charge movement during Na+ translocation by native and cloned cardiac Na+/Ca2+ exchanger. Nature. 1991;352:715–718. doi: 10.1038/352715a0. [DOI] [PubMed] [Google Scholar]
- 28.Rakowski R.F. Charge movement by the Na/K pump in Xenopus oocytes. J. Gen. Physiol. 1993;101:117–144. doi: 10.1085/jgp.101.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mager S., Naeve J., Lester H.A. Steady states, charge movements, and rates for a cloned GABA transporter expressed in Xenopus oocytes. Neuron. 1993;10:177–188. doi: 10.1016/0896-6273(93)90309-f. [DOI] [PubMed] [Google Scholar]
- 30.Wadiche J.I., Arriza J.L., Kavanaugh M.P. Kinetics of a human glutamate transporter. Neuron. 1995;14:1019–1027. doi: 10.1016/0896-6273(95)90340-2. [DOI] [PubMed] [Google Scholar]
- 31.Chen X.Z., Coady M.J., Lapointe J.Y. Fast voltage clamp discloses a new component of presteady-state currents from the Na(+)-glucose cotransporter. Biophys. J. 1996;71:2544–2552. doi: 10.1016/S0006-3495(96)79447-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Parent L., Supplisson S., Wright E.M. Electrogenic properties of the cloned Na+/glucose cotransporter: II. A transport model under nonrapid equilibrium conditions. J. Membr. Biol. 1992;125:63–79. doi: 10.1007/BF00235798. [DOI] [PubMed] [Google Scholar]
- 33.Loo D.D., Hazama A., Wright E.M. Relaxation kinetics of the Na+/glucose cotransporter. Proc. Natl. Acad. Sci. USA. 1993;90:5767–5771. doi: 10.1073/pnas.90.12.5767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Loo D.D., Zeuthen T., Wright E.M. Cotransport of water by the Na+/glucose cotransporter. Proc. Natl. Acad. Sci. USA. 1996;93:13367–13370. doi: 10.1073/pnas.93.23.13367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Duquette P.P., Bissonnette P., Lapointe J.Y. Local osmotic gradients drive the water flux associated with Na(+)/glucose cotransport. Proc. Natl. Acad. Sci. USA. 2001;98:3796–3801. doi: 10.1073/pnas.071245198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meinild A., Klaerke D.A., Zeuthen T. The human Na+-glucose cotransporter is a molecular water pump. J. Physiol. 1998;508:15–21. doi: 10.1111/j.1469-7793.1998.015br.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Duquette, P.-P. 2000. A study of the driving force involved in the water flux associated to Na+/glucose cotransport. Thesis. University of Montreal, Montreal.
- 38.Falk S., Guay A., Berteloot A. Reduction of an eight-state mechanism of cotransport to a six-state model using a new computer program. Biophys. J. 1998;74:816–830. doi: 10.1016/S0006-3495(98)74006-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Loo D.D., Hirayama B.A., Wright E.M. Conformational changes couple Na+ and glucose transport. Proc. Natl. Acad. Sci. USA. 1998;95:7789–7794. doi: 10.1073/pnas.95.13.7789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cougnon M., Bouyer P., Planelles G. Further investigation of ionic diffusive properties and of NH4+ pathways in Xenopus laevis oocyte cell membrane. Pflugers Arch. 1996;431:658–667. doi: 10.1007/BF02191917. [DOI] [PubMed] [Google Scholar]
- 41.Faham S., Watanabe A., Abramson J. The crystal structure of a sodium galactose transporter reveals mechanistic insights into Na+/sugar symport. Science. 2008;321:810–814. doi: 10.1126/science.1160406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Abramson J., Wright E.M. Structure and function of Na(+)-symporters with inverted repeats. Curr. Opin. Struct. Biol. 2009;19:425–432. doi: 10.1016/j.sbi.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]


