Abstract
Growth, differentiation, and programmed cell death (apoptosis) are mainly controlled by cytokines. The Janus kinase–signal transducers and activators of transcription (JAK-STAT) signal pathway is an important component of cytokine signaling. We have previously shown that STAT3 induces a molecule designated as SSI-1, which inhibits STAT3 functions. To clarify the physiological roles of SSI-1 in vivo, we generated, here, mice lacking SSI-1. These SSI-1−/− mice displayed growth retardation and died within 3 weeks after birth. Lymphocytes in the thymus and spleen of the SSI-1−/− mice exhibited accelerated apoptosis with aging, and their number was 20–25% of that in SSI-1+/+ mice at 10 days of age. However, the differentiation of lymphocytes lacking SSI-1 appeared to be normal. Among various pro- and anti-apoptotic molecules examined, an up-regulation of Bax was found in lymphocytes of the spleen and thymus of SSI-1−/− mice. These findings suggest that SSI-1 prevents apoptosis by inhibiting the expression of Bax.
The homeostatic regulation of cell populations is controlled by a balance among proliferation, growth arrest, and apoptosis, and this balance is mainly controlled by cytokines and growth factors. Cytokines act by binding to receptors expressed on the surfaces of responsive cells, which are associated with one or more members of the Janus kinase (JAK) family of cytoplasmic tyrosine kinases. The JAK–signal transducers and activators of transcription (STAT) signal pathway plays an important role in cytokine signaling (1–3), and is unique in that it features a direct linkage of receptor–ligand interaction on the cell surface to gene expression in the nucleus (4–6). However, the mechanism of negative control of cytokine actions involved in limiting their signal transductions is comparatively less well characterized. In 1997, the molecules that were expressed by stimulation of cytokine such as interleukin 6 (IL-6) and inhibited cytokine signal transmission by binding to JAK were isolated [STAT-induced STAT inhibitor-1 (SSI-1), suppressor of cytokine signaling (SOCS-1), Jak-binding protein (JAB)] (7–9). Subsequently, SSI-1 was found to form a family consisting of at least eight molecules, which were structurally characterized by an SH2 domain and a C-terminal conserved region (SC-motif/SOCS-box/CH-domain) (10–12), and it was recently known that SSI-1 inhibits not only IL-6 signaling but also interferon (IFN)-γ, IL-2, IL-3, and growth hormone signaling in vitro (13). It is expected that further study of SSI family molecules engaged in the negative feedback mechanism of cytokines will clarify the control mechanism of cytokines, which have remained obscure.
MATERIALS AND METHODS
Generation of SSI-1-Deficient Mice.
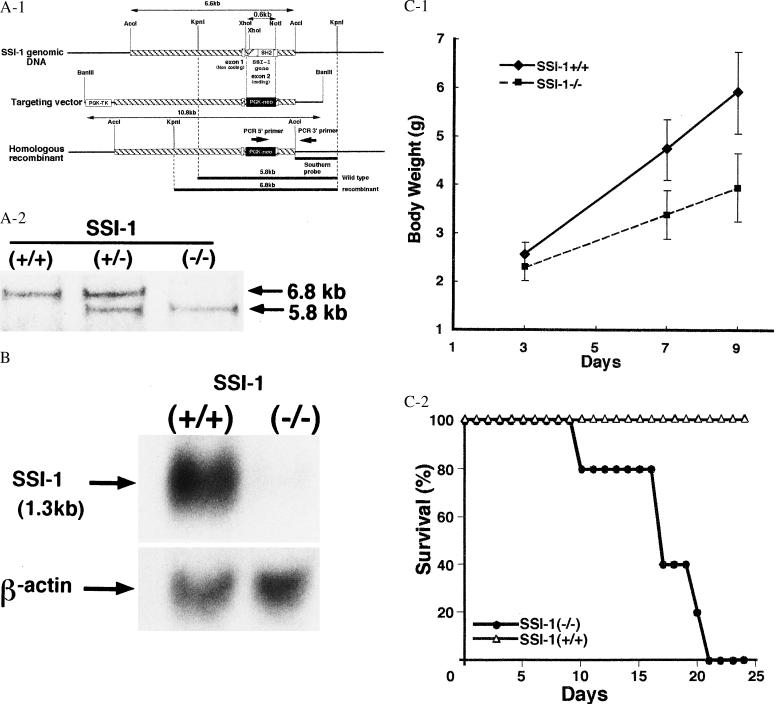
A 129/G mouse genomic library (Stratagene) containing the SSI-1 gene was screened, subcloned into the pBluescript vector, and characterized by restriction endonuclease mapping and DNA sequencing. A targeting vector was designed to replace the SSI-1 intron with phosphoglycerate kinase neo. This targeting vector was flanked by a 5.5-kb fragment at the 5′ end and a 0.8-kb fragment at the 3′ end and contained a PGK-tk cassette at the 5′ end of the vector. It was linearized with BanIII and electroporated into embryonic day 14.1 embryonic stem cells. Clones resistant to G418 and gancyclovir were screened for homologous recombination by PCR with the primers shown in Fig. 1 and confirmed by means of Southern blot analysis with the probe (AccI and KpnI fragment) shown in Fig. 1. The homologous recombinant embryonic stem clones were injected into blastocysts of C57B1/6J mice and transferred into the uteri of pseudopregnant C57B1/6J females. The resulting chimaeric mice were backcrossed to C57B1/6J mice, and heterozygous mutants were identified by means of PCR and confirmed by using Southern blotting from tail DNA. Brother–sister matings of heterozygous mice resulted in homozygous mutants. All animals were housed under specific pathogen-free conditions.
Figure 1.
(A) Homologous recombination of the SSI-1 locus. (Aa) Restriction map of the 129/G murine SSI-1 genomic clone, targeting vector, and homologous recombinant. PGK-neo replaced a 0.6-kb XhoI–NotI fragment containing the great part of exon 2. A herpes simplex virus thymidine kinase (HSV-TK) with a PGK promoter cassette was present on the 5′ flank of the vector. (Ab) Representative southern analysis of progeny from +/− × +/− mating. Tail biopsy genomic DNA was digested with KpnI and probed with a southern probe. The wild-type allele is 5.8 kb, and the homologous recombinant exhibits the predicted 6.8-kb band. (B) SSI-1−/− mice showed no evidence of SSI-1 mRNA. Northern blot analysis of lung tissue lysates from SSI-1+/+ mice with the SSI-1 cDNA probe exhibited expression of 1.3-kb SSI-1 mRNA but SSI-1−/− mice lacked this product. β-actin mRNA was included as a loading control. (Ca) SSI-1 mice showed an ≈40% decrease in body weight by 9 days of age. The mean weight of SSI-1+/+ is shown by the solid line and that of SSI-1−/− by the broken line. Each line represents the mean of seven experiments. (Cb) SSI-1 mice all died between 2 and 3 weeks after birth. Percentage survivals of SSI-1+/+ are shown by ▵ and of SSI-1−/− by •.
Northern Blot Analysis.
Total RNA was extracted from the lung tissues of SSI-1−/− and SSI-1+/+ mice with RNA ZOL B (Tel-Test, Friendswood, TX), and was electrophoresed, transferred to nylon membrane, and hybridized with 32P-labeled mouse SSI-1 cDNA.
The 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyl Tetrazolium Bromide (MTT) Dye Conversion.
Cell viability and number were assessed by MTT staining essentially as described by Mosmann (14).
Flow Cytometry.
Thymocytes and splenocytes were prepared from age-matched SSI-1+/+ or SSI-1−/− mice. Red cell-depleted splenocytes were obtained after treatment with Ack buffer (0.15 M NH4Cl/1.0 mM KHCO3/0.1 mM Na2 EDTA. After a washing with PBS, they were counted and resuspended in a staining medium. A total of 106 cells was stained as described in the protocols by using the following antibodies (Abs): anti-CD4, CD8, CD3, B220, IgM, Mac-1, and Gra-1 antibody (PharMingen). The stained cells were analyzed by means of double-color flow cytometry on a FACScalibur (Becton Dickinson) by using cellquest software (Becton Dickinson).
Terminal Deoxynucleotidyltransferase-Mediated UTP End Labeling (TUNEL) Staining.
For detection of DNA fragmentation in situ, paraffin-embedded sections were tested by the TUNEL method as described elsewhere (15). Terminal deoxynucleotidyltransferase (TdT) was used to incorporate Biotin-16-dUTP (Boehringer Mannheim) into the ends of DNA fragments. TUNEL signals were detected with the aid of Texas Red-conjugated anti-avidin Ab (1:200, Biomeda, Foster City, CA).
Immunohistochemical Staining.
Paraffin-embedded sections were immunostained by a method described elsewhere (16). Sections were treated with anti-Bcl-2 Ab (1:100, Biomol, Plymouth Meeting, PA) or anti-Bax Ab (1:100) and then incubated with fluorescein isothiocyanate-conjugated goat anti-Hamster IgG (1:300, PharMingen) or fluorescein isothiocyanate-conjugated goat anti-Rabbit IgG (1:200, Seikagaku, Kogyo, Tokyo).
In Vitro Thymocyte Culture.
Fresh thymocytes were plated at 2 × 105 cells per 100 μl in a 96-well plate in medium containing 5% fetal calf serum and stimulated with anti-CD3 (1 μg/ml) Ab, anti-CD3 Ab plus IL-2 (20 ng/ml), anti-CD3 Ab plus IL-4 (20 ng/ml), or not stimulated. Cell viability was assayed by means of MTT dye conversion. For each day, triplicate cultures were counted and averaged.
Western Blotting.
Thymocytes were stimulated with 1 μg/ml anti-CD3 Ab (PharMingen) plus 20 ng/ml IL-4 (Pepro Tech EC, Rocky Hill, NJ) at 37°C for 1 hr or 3 hr, or not stimulated. Cells were solubilized with lysis buffer (0.5% Nonidet P-40/10 mM Tris, pH 7.4/150 mM NaCl/1 mM EDTA/1 mM Na2PO4) containing protease inhibitors. Immunoprecipitates obtained with anti-STAT6 Ab (R & D Systems) were blotted with anti-phosphotyrosine Ab (4G10; Upstate Biotechnology) and reblotted with anti-STAT6 Ab after stripping the first blot. Whole cell lysates were blotted with anti-Bax Ab or anti-Bcl-2 Ab (both Santa Cruz Biotechnology).
RESULTS
To clarify the functional and developmental roles of SSI-1, we used homologous recombination in embryonic stem cells to generate mice lacking SSI-1. The disrupted SSI-1 allele was ultimately transmitted through the germ line, as confirmed by Southern blot (DNA) analysis (Fig. 1A). Northern blots confirmed the loss of SSI-1 mRNA in lung tissues from SSI-1−/− mice (Fig. 1B). Homologous mutant mice (SSI-1−/−) were healthy and normal at birth and were initially indistinguishable from control littermates (SSI-1+/+). Although the body weight of SSI-1−/− mice did not differ significantly from that of SSI-1+/+ mice on postnatal day 3, an approximately 40% decrease in body weight was observed on postnatal day 9 (Fig. 1C). Moreover, SSI-1−/− mice all died within 3 weeks after birth (Fig. 1C).
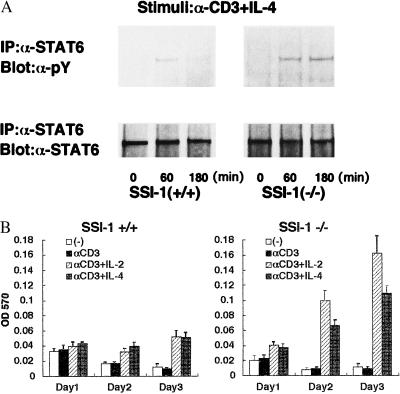
SSI-1 has been shown in vitro to be a negative feedback factor in the JAK-STAT signal pathway. We therefore carried out the following experiment to determine whether lymphocytes lacking SSI-1 exhibited prolonged activation of STAT. We stimulated the thymocytes of 10-day-old mice with anti-CD3 Ab plus IL-4 and examined phosphorylation of STAT6 at 0, 1, and 3 hr after the stimulation. In the thymocytes lacking SSI-1, the phosphorylation of STAT6 was not transient and still detected 3 hr after the stimulation (Fig. 2A). The thymocytes of 10-day-old mice were then stimulated similarly with anti-CD3 Ab, anti-CD3 Ab plus IL-2, and anti-CD3 Ab plus IL-4 and survival of thymocytes on days 1, 2, and 3 was examined by using MTT dye conversion, respectively (14) (Fig. 2B). Without stimulation or with anti-CD3 Ab alone, apoptosis occurred in thymocytes from both SSI-1+/+ and −/− mice, but the addition of IL-2 or IL-4 reduced apoptosis and the number of living thymocytes increased. In the thymocytes of SSI-1−/− mice, IL-2- or IL-4-induced lymphocyte proliferation was significantly augmented compared with SSI-1+/+ mice. These findings suggested that this increase in proliferation could be due to the impaired negative feedback mechanism of cytokine signals.
Figure 2.
(A) The activation of STAT6 continued after stimulation by IL-4 in thymocytes of SSI-1−/− mice. Cells were either nonstimulated or stimulated with IL-4 plus α-CD3. Immunoprecipitated (IP) STAT6 and STAT6 were immunoblotted with anti-phosphotyrosine Ab (α-pY). Blots were reprobed with α-STAT6 Ab. (B) MTT staining was measured on days 1, 2, and 3 after stimulation. Each column represents the mean of seven experiments.
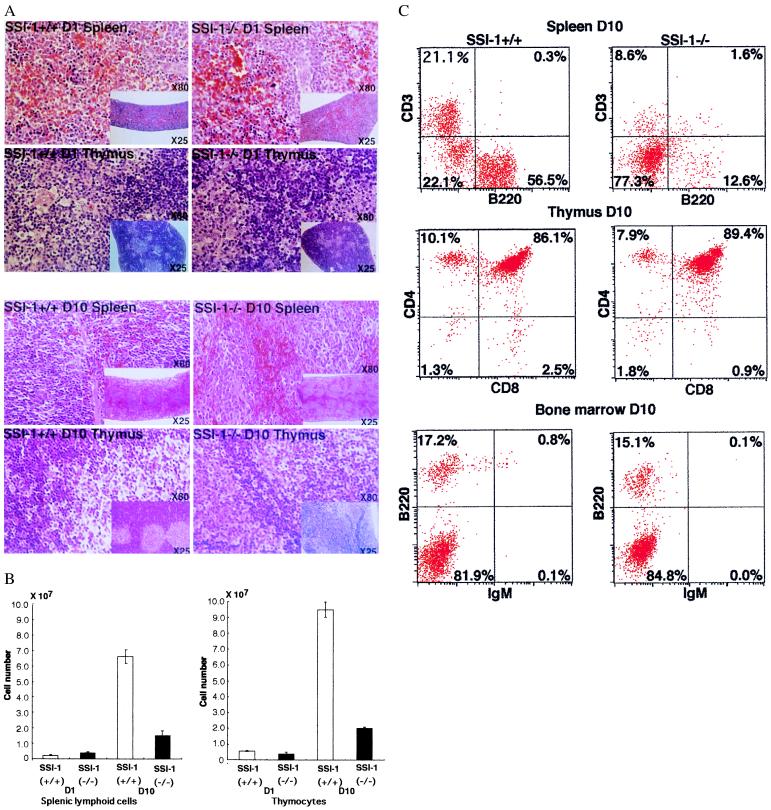
When the spleen and thymus of SSI-1−/− mice were examined histologically by means of hematoxylin/eosin staining, little difference was seen between the number of lymphocytes in SSI-1−/− and SSI-1+/+ mice on postnatal day 1, whereas the numbers of lymphocytes in the spleen and thymus lacking SSI-1 had decreased markedly at 10 days of age. The spleen of SSI-1−/− mice at 10 days of age was distorted in shape and exhibited partial atrophy of the white pulp. In the thymus, moreover, a borderline between cortex and medulla was not visible (Fig. 3A). The number of total thymocytes and splenic lymphoid cells of SSI-1−/− mice markedly decreased and at 10 days of age, approximately 75–80% of lymphocytes had disappeared as compared with SSI-1+/+ (Fig. 3B). However, the number of bone marrow cells did not significantly differ between SSI-1+/+ and SSI-1−/− mice (data not shown).
Figure 3.
(A) Histological appearances of the spleen and thymus in SSI-1−/− mice at 1 and 10 days of age. Hematoxylin/eosin stained sections of the spleen and thymus from SSI-1−/− mice 10-days-old reveal decreased lymphocytes (X80). The low magnification (X25) reveals a distorted shape and partial atrophy of white pulp in the spleen and an indistinct borderline between cortex and medulla in the thymus of SSI-1−/− mice. (B) Total thymocytes and splenic lymphoid cell numbers in SSI-1+/+ mice and −/− mice. The means and standard deviations for 10 +/+ and 10 −/− mice are shown. Numbers of total thymocytes and splenic lymphoid cells for SSI-1+/+ are shown by empty bars, and for SSI-1−/− by filled bars. (C) Flow cytometric analysis of splenocytes, thymocytes and bone marrow cells from SSI-1+/+ and SSI-1−/− mice 10-days-old.
Next, to examine lymphocyte differentiation in the spleen, thymus, and bone marrow, the splenocytes, thymocytes, and bone marrow cells of SSI-1−/− mice at 10 days of age were stained for two-color flow cytofluorometric analysis with Abs to B220, CD3, CD4, CD8, Mac-1, Gra-1, and IgM (Fig. 3C). In the spleen, the number of B220+ or CD3+ cells in SSI-1−/− mice were markedly decreased compared with those in SSI-1+/+ mice. However, the ratio of B220/CD3 splenocytes remained unaffected despite a massive decrease in the number of splenic lymphoid cells in SSI-1−/− mice. The number of Mac-1+ and Gra-1+ splenocytes did not significantly differ between SSI-1−/− and SSI-1+/+ mice (data not shown). The number of thymocytes in SSI-1−/− mice was only 25% of that of control thymocytes (Fig. 3B). However, the percentages of CD4+CD8−, CD4−CD8+, and CD4+CD8+ cells did not differ between SSI-1+/+ and SSI-1−/− mice. Similarly, the percentages of IgM+ and B220+ cells in the bone marrow did not significantly differ between SSI-1+/+ and SSI-1−/− mice. In SSI-1−/− mice, the spleen and thymus appeared to exhibit normal differentiation of lymphocytes despite a massive decrease of the number of lymphocytes. These findings suggested that SSI-1 is not essential for the generation and differentiation of lymphocytes but that it may be important for the survival of lymphocytes in vivo.
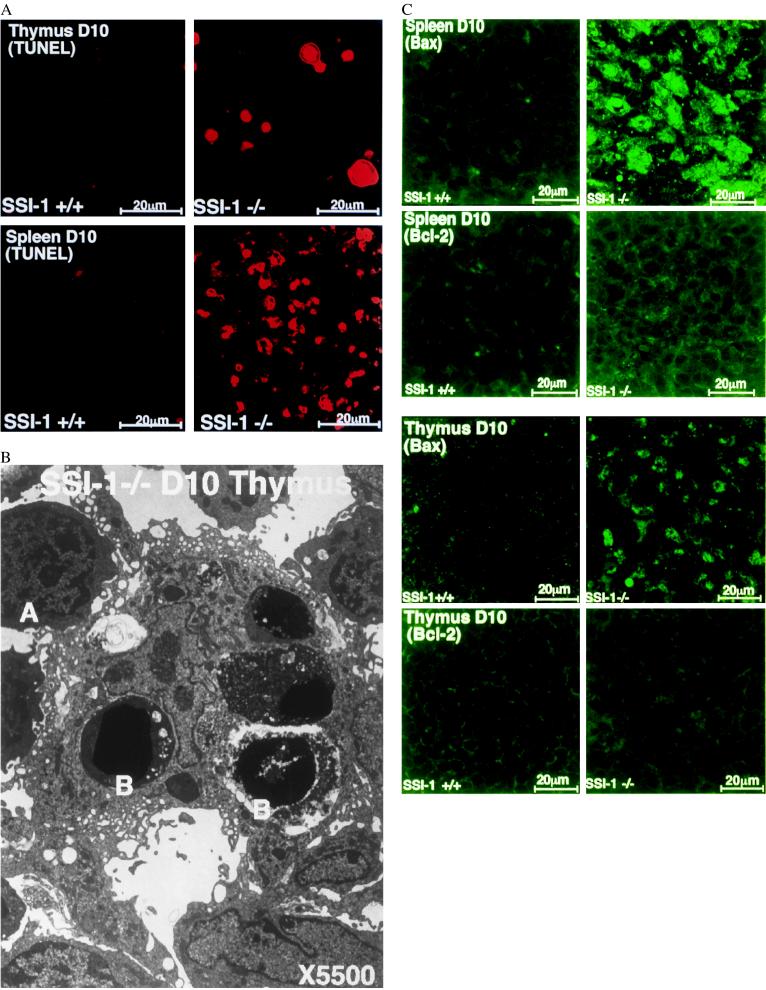
We examined the spleen and thymus of 10-day-old mice immunohistochemically by means of TUNEL staining (15) to determine the cause of the decrease in the number of lymphocytes in SSI-1−/− mice. In the spleen and thymus of SSI-1−/− mice, there were more cells labeled with TUNEL than in those of SSI-1+/+ mice (Fig. 4A). In addition, electron microscopic examination detected a larger number of apoptotic cells with the typical condensation of nuclear chromatin and apoptic bodies in SSI-1−/− mice (Fig. 4B). The spleen and thymus of 10-day-old mice were then stained immunohistologically (16) with anti-Bax Ab or anti-Bcl-2 Ab to clarify the mechanism of accelerated apoptosis of lymphocytes. Compared with SSI-1+/+ mice, the cells expressing Bax had markedly increased in the spleen and thymus of SSI-1−/− mice. However, there was no significant difference in Bcl-2 expression between SSI-1+/+ and SSI-1−/− mice (Fig. 4C). Moreover, no difference in the expression of molecules inducing apoptosis such as Fas, Fas ligand, tumor necrosis factor-α, and glucocortisol, could be detected between SSI-1+/+ and SSI-1−/− mice (data not shown). These findings suggested that accelerated apoptosis of lymphocytes in SSI-1−/− mice may be due to the augmented expression of Bax, resulting in a reduction in the lymphocytes.
Figure 4.
(A) Numerous apoptotic splenocytes and thymocytes in the SSI-1−/− mice 10 days old are demonstrated by TUNEL staining. (B) Electron micrograph of the thymus of SSI-1−/− mice at 10 days old (×5500). Cells (A) are still relatively normal, but the apoptotic cells (B) have lost cell membrane microvilli and clearly exhibit chromatin condensation and apoptic bodies. (C) Immunohistochemical staining of the spleen and thymus. Comparisons of Bax expression in SSI-1+/+ mice (Top Left) and that in −/− mice (Top Right), and of Bcl-2 expression in +/+ mice (Bottom Left) and that in −/− mice (Bottom Right).
DISCUSSION
The cytokine signal pathways that include Ras–MAP and JAK-STAT signal cascades are known to be involved in the generation of an anti-apoptotic signal, most likely through the induction of the Bcl-2 (17–19). Our results showed that in thymocytes lacking SSI-1, cytokine-induced proliferation was augmented by prolonged activation of STAT in vitro (Fig. 2 A and B). This finding indicates that, as expected, SSI-1 plays an important role in the negative feedback mechanism of the cytokine. However, in the thymus and spleen, a large number of lymphocytes lacking SSI-1 showed evidence of apoptosis with aging, which involved an increase in Bax expression in vivo (Fig. 4A–C). These seemingly contradictory findings may indicate the possibility that SSI-1 inhibits not only the JAK-STAT signal pathways, but also other signal pathways, which promote apoptosis.
The balance of interactions between pro- and anti-apoptosis members of the Bcl-2 gene family are believed to regulate apoptosis (20). Bax is one of the genes in this family. A predominance of Bax has been found to accelerate apoptosis in response to cytokine removal (20) and the expression of the Bax transgene in T cells leads to a large reduction in the numbers of mature T cells in vivo (21). In contrast, overexpression of Bcl-2 strongly reduces apoptosis (22). The ratio of Bcl-2 to Bax determines whether a cell will respond to apoptotic stimuli. Although the embryonic development of Bcl-2−/− mice is normal, these mice display growth retardation, shortened lifespan and massive apoptosis in the spleen and thymus (23, 24). These findings are similar to those for SSI-1−/− mice. Nevertheless, the present study did not show any significant difference in the level of Bcl-2 protein between SSI-1−/− and SSI-1+/+ mice (Fig. 4C). Then, it has been recently demonstrated that a death signal generates the activation of Bax, which in turn induces homodimerization, translocation into mitochondrial membrane, mitochondrial dysfunction, and apoptosis (25). SSI-1−/− mice showed an increased level of Bax protein in the spleen and thymus (Fig. 4C). Thus, the apoptosis of lymphocytes lacking SSI-1 may be due to this increase in Bax expression. These findings suggest that, although the relation between Bax expression and the JAK-STAT signal pathway has not been clarified yet, SSI-1 may directly or indirectly inhibit the other signal pathways, except JAK-STAT signal cascades, which induce Bax expression and thus prevent Bax-induced apoptosis in vivo. However, further analysis is required to determine which signal pathway in the expression of Bax is inhibited by SSI-1 and whether SSI-1 inhibits apoptosis through inhibition of JAK activation.
Whereas SSI-1 is strongly expressed in thymus, spleen, and peripheral blood leukocytes, any expression of SSI-2 or -3 is hardly observed in them (3, 9). This difference in distribution suggests that other members of the SSI-family may not able to compensate for the lack of SSI-1 in lymphocytes. Although our analysis mainly focused on the immune system of SSI-1−/− mice in this study, SSI-1−/− mice at 10 days of age surprisingly showed apparent severe cardiac hypertrophy and histological degeneration of the hepatocytes (our unpublished data). The physiological roles of SSI-1 in other tissues have not been clarified for the present. Future analysis will be necessary to clarify them or to determine whether SSI-1 inhibits Bax expression similarly in tissues other than lymphocytes.
Acknowledgments
We wold like to thank T. Tanaka, S. Nagata, and S. Akira for their suggestions, K. Satoh and F. Ishitobi for their technical assistance, and N. Kameoka and M. Tagami for their secretarial assistance. This study was supported by a Grant-in-Aid from the Ministry of Education, Science and Culture, Japan.
ABBREVIATIONS
- STAT
signal transducers and activators of transcription
- SSI-1
STAT-induced STAT inhibitor-1
- SOCS-1
supressor of cytokine signaling
- JAB
Jak-binding protein
- Bax
Bcl-2-associated X protein
- Bcl-2
B cell lymphoma/leukemia-2
- JAK
Janus kinase
- IL
interleukin
- INF
interferon
- TUNEL
terminal deoxynucleotidyltransferase-mediated UTP end labeling
- Ab
antibody
References
- 1.Darnell J E, Jr, Kerr I M, Stark G R. Science. 1994;264:1415–1421. doi: 10.1126/science.8197455. [DOI] [PubMed] [Google Scholar]
- 2.Ihle J N. Nature (London) 1995;377:591–594. doi: 10.1038/377591a0. [DOI] [PubMed] [Google Scholar]
- 3.Kishimoto T, Akira S, Narazaki M, Taga T. Blood. 1995;86:1243–1254. [PubMed] [Google Scholar]
- 4.Taniguchi T. Science. 1995;268:251–255. doi: 10.1126/science.7716517. [DOI] [PubMed] [Google Scholar]
- 5.Ihle J N. Cell. 1996;84:331–334. doi: 10.1016/s0092-8674(00)81277-5. [DOI] [PubMed] [Google Scholar]
- 6.Darnell J E., Jr Science. 1997;277:1630–1635. doi: 10.1126/science.277.5332.1630. [DOI] [PubMed] [Google Scholar]
- 7.Starr R, Willson T A, Viney E M, Murray L J, Rayner J R, Jenkins B J, Gonda T J, Alexander W S, Metcalf D, Nicola N A, et al. Nature (London) 1997;387:917–921. doi: 10.1038/43206. [DOI] [PubMed] [Google Scholar]
- 8.Endo T A, Masuhara M, Yokouchi M, Suzuki R, Sakamoto H, Mitsui K, Matsumoto A, Tanimura S, Ohtsubo M, Misawa H, et al. Nature (London) 1997;387:921–924. doi: 10.1038/43213. [DOI] [PubMed] [Google Scholar]
- 9.Naka T, Narazaki M, Hirata M, Matsumoto T, Minamoto S, Aono A, Nishimoto N, Kajita T, Taga T, Yoshizaki K, et al. Nature (London) 1997;387:924–929. doi: 10.1038/43219. [DOI] [PubMed] [Google Scholar]
- 10.Masuhara M, Sakamoto H, Matsumoto A, Suzuki R, Yasukawa H, Mitsui K, Wakioka T, Tanimura S, Sasaki A, Misawa H, et al. Biochem Biophys Res Commun. 1997;239:439–446. doi: 10.1006/bbrc.1997.7484. [DOI] [PubMed] [Google Scholar]
- 11.Aman M J, Leonard W J. Curr Biol. 1997;7:784–788. doi: 10.1016/s0960-9822(06)00405-2. [DOI] [PubMed] [Google Scholar]
- 12.Hilton D J, Richardson R T, Alexander W S, Viney E M, Willson T A, Sprigg N S, Starr R, Nicholson S E, Metcaf F D, Nicola N A. Proc Natl Acad Sci USA. 1998;95:114–119. doi: 10.1073/pnas.95.1.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adams T E, Hansen J A, Starr R, Nicola N A, Hilton D J, Billestrap N. J Biol Chem. 1998;273:1285–1287. doi: 10.1074/jbc.273.3.1285. [DOI] [PubMed] [Google Scholar]
- 14.Mosmann T. J Immunol Methods. 1983;65:55–58. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 15.Gavrieli Y, Sherman Y, Ben-Sasson S A. J Cell Biol. 1992;119:493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sato K, Waguri S, Ohsawa Y, Nitatori T, Kon S, Kominami E, Watanabe T, Gotow T, Uchiyama Y. Arch Histol Cytol. 1997;60:275–287. doi: 10.1679/aohc.60.275. [DOI] [PubMed] [Google Scholar]
- 17.Fukada T, Hibi M, Takahashi-Tezuka M, Fujitani Y, Yamaguchi T, Nakajima K, Hirano T. Immunity. 1996;5:449–460. doi: 10.1016/s1074-7613(00)80501-4. [DOI] [PubMed] [Google Scholar]
- 18.Quelle F W, Wang J, Feng J, Wang D, Cleveland J L, Ihle J N, Zanbetti G P. Genes Dev. 1998;12:1099–1107. doi: 10.1101/gad.12.8.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kinoshita T, Yokota T, Arai K, Miyajima A. Oncogene. 1995;10:2207–2212. [PubMed] [Google Scholar]
- 20.Oltivai Z N, Milliman C L, Korsmeyer S J. Cell. 1993;74:609–619. doi: 10.1016/0092-8674(93)90509-o. [DOI] [PubMed] [Google Scholar]
- 21.Bardy H J, Gomez G G, Kirberg J K, Berns A J M. EMBO J. 1996;15:6991–7001. [PMC free article] [PubMed] [Google Scholar]
- 22.Katsumata M, Siegel R M, Louie D C, Miyashita T, Tsujimoto Y, Nowell D C, Greene M I, Reed J C. Proc Natl Acad Sci USA. 1992;89:11376–11380. doi: 10.1073/pnas.89.23.11376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Veis D J, Sorenson C M, Shutter J R, Korsmeyer S J. Cell. 1993;75:229–240. doi: 10.1016/0092-8674(93)80065-m. [DOI] [PubMed] [Google Scholar]
- 24.Nakayama K, Nakayama K, Negishi I, Kuida K, Shinkai Y, Louie M C, Fields L E, Lucas P J, Stewart V, Alt F W, et al. Science. 1993;261:1584–1588. doi: 10.1126/science.8372353. [DOI] [PubMed] [Google Scholar]
- 25.Gross A, Jockel, Wei M C, Korsmeyer S J. EMBO J. 1998;17:3878–3885. doi: 10.1093/emboj/17.14.3878. [DOI] [PMC free article] [PubMed] [Google Scholar]