Abstract
Recent neuropathological studies have shown that Fluoro-Jade C (FJC), an anionic fluorescent dye, is a good marker of degenerating neurons. However, those studies have mostly examined acute rather than chronic models of neurodegeneration. We therefore compared FJC staining using the intrastriatal 6-hydroxydopamine (6-OHDA)-injected rat as an acute model and the zitter rat as a chronic model, as both show dopaminergic (DA) neurodegeneration. In the 6-OHDA-injected rat, FJC-positive neurons were found in the substantia nigra pars compacta (SNc) before the loss of tyrosine hydroxylase (TH)-positive DA neurons. In the zitter rat, FJC-labeled fibers were first detected at 1 month old (1M) and were considerably increased in the striatum at 4M, whereas FJC-labeled cell bodies were found at 4M, but not at 1M in the SNc. Furthermore, FJC-labeled neurons of the zitter rat showed TH-immunoreactivity in fibers, but little in cell bodies, while those from the 6-OHDA-injected rat showed TH-immunoreactivity even in the cell bodies. These results demonstrate that FJC is a useful tool for detecting chronically degenerating neurons, and suggest that intracellular substances bound to FJC may accumulate in the cell bodies from fibers at a slower rate in the chronic model than in the acute model.
Keywords: Fluoro-Jade C, zitter rat, 6-OHDA, dopaminergic neurons, chronic neurodegenerative model
I. Introduction
One of the central questions in the neuropathology of neurodegenerative disease is how to detect differences in the vulnerability of neuronal populations to cell death. In particular, identifying the onset of this irreversible process is crucial to the development of therapeutic interventions for neurodegenerative disease. One putative marker of degenerating cells that is increasingly being used for studies of neuronal degeneration in the brain is Fluoro-Jade C (FJC) [20]. FJC, a fluorescent anionic dye, shows greater morphological detail of degenerating neurons than its predecessors, Fluoro-Jade and Fluoro-Jade B [18, 19]. Furthermore, the dye is reportedly compatible with several histological processing and staining protocols. Recent studies using double labeling with FJC staining and immunofluorescence (IF) have shown the degenerating cell types [1, 3, 20, 25]. Concerning the procedure for double labeling, Schmued et al. (2005) performed IF first followed by FJC staining (IF-FJC) to detect degenerating neurons in the cerebral cortex and hippocampus of the kainic acid (KA)-treated rat [20]. Thereafter, many researchers using the Schmued method of IF-FJC have investigated several neurodegeneration models, including models of Parkinson’s disease (PD). However, the double-labeling method has varied considerably among researchers. Even in the same laboratory, Wang et al. (2008) performed IF-FJC, whereas Chen et al. (2008) performed the converse procedure with FJC staining followed by IF (FJC-IF) [3, 25].
Evaluation of previous methods has shown problems involving three processes: 1) procedure; 2) photobleaching; and 3) pretreatment. In terms of procedure, whether FJC staining or IF should be performed first for optimal results remains unclear. With photobleaching, although an acidic glycerol medium is used for double labeling, little is known about the stability of either staining in the medium under photo-irradiation. As for pretreatment, pretreatment with potassium permanganate results in high-contrast FJC staining, but is known to decrease the intensity of IF. The present study initially investigated the optimal double-labeling method using the KA-treated rat model of acute neurodegeneration in the hippocampus and cerebral cortex [18, 20].
Next, to compare acute and chronic neurodegeneration, we used two different animal models for PD: the 6-hydroxydopamine (6-OHDA)-injected rat as a model of acute neuronal degeneration [5, 14, 17] and the zitter mutant rat as a model of chronic neuronal degeneration. Intrastriatal injection of 6-OHDA results in the death of dopaminergic (DA) neurons in the ipsilateral substantia nigra pars compacta (SNc), which progresses over the first few weeks after injection. The zitter rat is an autosomal recessive spontaneous mutant with an 8-bp deletion at a splice donor site of the attractin gene [13], and displays progressive and selective nigrostriatal DA neuron death [22]. In the zitter rat, the number of tyrosine hydroxylase (TH)-positive neurons resembles that of the Sprague-Dawley (SD) rat until 1 month old (1M), but greatly decreases from 2M to 12M [15, 23]. However, little is known about when and where the DA neurons are first damaged and how degeneration proceeds in the nigrostriatal pathway of this mutant rat. To address these issues, FJC may be useful to investigate the spatiotemporal distribution of damaged neurons in the zitter rat.
In the present study, to investigate the utility of FJC as a marker of degenerating neurons in the acute or chronic degenerative model, we first examined the optimal double-labeling method with FJC and IF, and then demonstrated FJC staining using SD rats 3 days after 6-OHDA injection or zitter rats. In the zitter rat, staining was observed at time-points of 1M, 4M, and 12M with a focus on the dynamic time-course patterns of degenerating neurons. Furthermore, double-labeling studies were carried out in brain sections to analyze the properties of dying DA neurons by combing FJC staining with immunofluorescence to neuron-specific nuclear antigen (NeuN), a neuronal marker), glial fibrillary acidic protein (GFAP), an astrocyte marker, and tyrosine hydroxylase (TH), a DA neuronal marker.
II. Materials and Methods
Animals
Male homozygous zitter mutant (zi/zi) rats and male age-matched SD rats at ages of 1M, 4M, and 12M were used in this study. Animals were provided ad libitum access to food and water, and maintained on a 12-hr light/12-hr dark cycle. Rats were handled in accordance with the National Institutes of Health “Guide for the care and use of laboratory animals” and the experimental protocol was approved by the Animal Research Committees of Dokkyo Medical University School of Medicine.
Pharmacological treatment
KA (10 mg/kg body weight; Sigma, St. Louis, MO, USA) dissolved in physiological saline was injected intraperitoneally into SD rats, then tissues were examined 2 days after injection [20]. Rats in the control group were injected with solvent alone.
The 6-OHDA (6 mg/ml; Sigma, St. Louis, MO, USA) was dissolved in saline containing 0.2 mg/ml ascorbic acid. Rats were anaesthetized using a mixture of ketamine hydrochloride and xylazine, and received a single injection of 24 µg (4 µl) of 6-OHDA into the right striatum. The toxin was injected stereotaxically through a 30-gauge cannula fitted to a microsyringe at a rate of 1 µl/min. The cannula was slowly withdrawn 3 min after the end of infusion. Stereotaxic coordinates were taken from an atlas [16] and were the following distances from the bregma: A, 0.6 mm; L, 2.5 mm; and V, 5.0 mm. Tissues were examined 3 days after lesion creation.
All rats were anaesthetized with pentobarbital and perfused transcardially with 0.9% saline followed by 4% paraformaldehyde and 0.2% picric acid in 0.1 M phosphate buffer (PB) (pH 7.4). The brains were immediately removed, then postfixed with the same fixative for 2 days at 4°C, and cryoprotected for an additional 2 days by immersion in 0.1 M PB containing 30% sucrose at 4°C. After that, brain samples were cut into serial sections of 30 µm thickness using a cryostat.
Immunohistochemistry
Immunolabeling with a mouse anti-tyrosine hydroxylase (TH) antibody (a marker of DA neurons) was performed using the avidin-biotin-peroxidase technique (Vectastain ABC kit, Vector Laboratories, Burlingame, CA) according to a previous study [22]. Free-floating sections were quenched for endogenous peroxidase activity by treating them with 1% H2O2 in phosphate-buffered saline (PBS) for 1 hr at room temperature (RT), blocked for 1 hr at RT in 1.5% normal horse serum and PBS containing 0.3% Triton X-100 (0.3% TPBS; Immunostar, Hudson, WI, USA), and then incubated with a mouse anti-TH antibody (1:5000 diluted in TPBS) for 3 days at 4°C. Sections were placed in an appropriate biotinylated secondary antibody diluted in TPBS for 2 hr at RT followed by incubation with avidin-biotin-peroxidase complex (Vector Laboratories) for 2 hr at RT. Immunoreactivities were visualized using 0.2 mg/ml 3-3-diaminobenzidine tetrahydrochloride (DAB; Dojindo, Kumamoto, Japan) and 0.006% H2O2 at RT. The sections were then mounted on gelatin-coated slides, dehydrated through graded concentrations of ethanol, cleared in xylene, and coverslipped with Entellan New (Merck KGaA, Darmstadt, Germany).
FJC staining
FJC, a polyanionic fluorescein derivative that can sensitively and selectively bind to degenerative neurons [20], was applied to examine dynamic time-course changes of dying neurons in brains from the above animal models.
To visualize degenerative neurons, brain sections were mounted on gelatin-coated slides, air-dried, and subjected to FJC staining. The slides were first immersed in a solution containing 1% NaOH in 80% ethanol for 5 min. They were rinsed for 2 min in 70% ethanol and for 2 min in distilled water, then incubated in 0.06% potassium permanganate solution for 10 min. Following a water rinse for 2 min, slides were transferred to the FJC staining solution and stained for 10 min. The proper dilution was accomplished by first making a 0.01% stock solution of FJC dye (Chemicon, Temecula, CA, USA) in distilled water and then adding 1 ml of the stock solution to 99 ml of 0.1% acetic acid. Slides were washed three times each for 1 min and then air-dried on a slide warmer at 50°C for 30 min. After clearing in xylene and coverslipping with Entellan New, sections were examined under an epifluorescence microscope (BX51; Olympus, Tokyo, Japan). The fluorescein/FITC filter system was used for visualizing FJC staining and images were captured for demonstration.
Double labeling with FJC and immunofluorescence
Double-labeling experiments were performed to confirm neuronal properties of FJC-positive cells in the sections by combining IF to NeuN (a neuronal cell marker), TH (a DA neuron marker), or GFAP (an astrocytic cell marker) antisera. We used three different procedures: FJC-IF; FJC-NaBH4-IF; and IF-FJC.
FJC-IF
Sections were first treated as for single FJC staining, and then IF staining was performed. Sections were washed in 0.1% TPBS, blocked for 1 hr in 0.1% TPBS containing 1.5% normal goat serum, washed in 0.1% TPBS, and incubated in mouse anti-NeuN (1:500 dilution; Chemicon, CA, USA) overnight at 4°C. After washing, sections were incubated with Alexa568 conjugated goat anti-mouse IgG (1:500 dilution; Molecular Probes, Eugene, OR, USA) for 1 hr at RT, washed in PBS and coverslipped in acidic mount media (pH 4.5) containing 0.1% acetic acid and 80% glycerin.
FJC-NaBH4-IF
Sodium borohydride (NaBH4) has been suggested to change fixative-induced double-bonded protein crosslinking into single bonds, to reduce non-specific binding of antibodies to sections, and thus to allow partial restoration of the tertiary structures of antigens [8, 11, 26]. Pretreatment of sections with NaBH4 appears to activate immunoreactivity. We therefore applied NaBH4 treatment after FJC staining. After single FJC staining, sections were incubated in 0.1% sodium borohydride for 2 min followed by the aforementioned IF staining.
IF-FJC
Sections were blocked in 0.1% TPBS containing 1.5% normal goat serum and incubated in mouse anti-NeuN, rabbit anti-GFAP (1:2000 dilution, Sigma-Aldrich, St. Louis, MO, USA), or mouse anti-TH (1:1000 dilution) antisera in 0.1% TPBS overnight at 4°C. Subsequently, sections were rinsed in PBS, and incubated with Alexa568-conjugated goat anti-mouse IgG for 1 hr at 37°C. After washing, sections were mounted on gelatin-coated slides, dried for 30 min at 50°C, and subjected to FJC staining. They were rinsed for 5 min in distilled water, then incubated in 0.06% potassium permanganate solution for 2–10 min. Following a 2-min water rinse, slides were transferred to the FJC staining solution and stained for 10 min. The slides were washed and then coverslipped in acidic mount media and examined under an epifluorescence microscope. The photo-irradiation was performed for 0, 3, 5, and 10 min.
Data analysis
Images were captured with an Olympus DP72 system and processed using DP2-BSW software (Olympus) and Adobe Photoshop version 6.0 software (Adobe Systems, San Jose, CA, USA). These images were recorded under the same imaging conditions throughout the same analysis. Intensity of fluorescence was measured and quantified using Image J software (National Institutes of Health, Bethesda, MD, USA) Intensity was calculated and expressed as the average pixel intensity of the region of interest (ROI) subtracted from the value of the background. For each experiment, fluorescence was calculated from at least three different ROIs. These values were averaged and divided into five categories ranging from background intensity to highest intensity (categories: –; +; ++; +++; ++++).
III. Results
Analysis of the optimal method to detect double labeling with FJC and IF
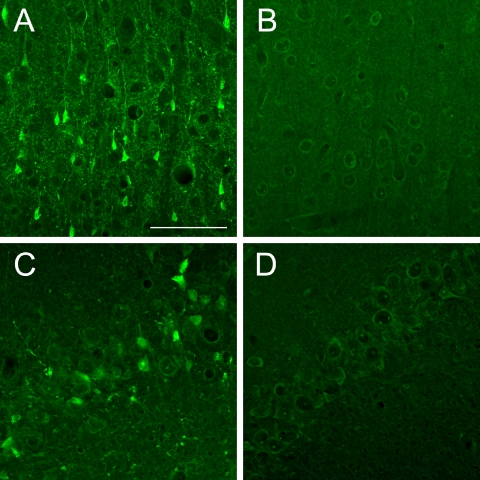
The pattern of FJC staining resulting from KA exposure was consistent with a previous report [20]. Cell bodies and processes of most FJC-positive cells were bright green and exhibited neuronal profiles. These cells were extensively distributed in the cerebral cortex (layer V) and hippocampus (CA3) (Fig. 1A, C). Little staining was found in the control tissues (Fig. 1B, D).
Fig. 1.
Comparison of FJC staining between KA-treated samples (A and C) and saline-treated control samples (B and D). FJC staining in the cerebral cortex, layer V (A and B) and hippocampus, CA3 (C and D). Bar=50 µm.
In these cortical samples, to determine the optimal method of double labeling with FJC and IF using NeuN antibody as a neuronal marker and Alexa568-conjugated secondary antibody, we investigated three processes. These were the FJC/IF procedure, photobleaching time, and potassium permanganate pretreatment time. The results are summarized in Tables 1–3. First, we examined three procedures: FJC-IF; FJC-NaBH4-IF; and IF-FJC (Table 1). FJC-IF caused low intensity of FJC staining and little intensity of IF staining. Even when slides were pretreated with NaBH4 prior to IF in order to increase the intensity of IF (FJC-NaBH4-IF), intensities of both FJC and IF staining were low. In contrast, IF-FJC showed high intensity of both types of staining. In particular, FJC staining was found not only in specific cells, but also in background tissue. Consequently, IF-FJC was the procedure that yielded the best results from double labeling with FJC and IF.
Table 1.
Comparison of the intensity of FJC and immunofluorescence by different staining procedures
| Protocol | FJC-IF | FJC-NaBH4-IF | IF-FJC |
|---|---|---|---|
| FJC | + | + | ++++ |
| IF | – | + | ++ |
Note: Intensity of FJC or IF (NeuN): ++++, highest intensity; ++, moderate intensity; +, low intensity; –, background intensity. FJC-IF, FJC was followed by IF; FJC-NaBH4-IF, FJC was followed by sodium borohydride and then IF; IF-FJC, IF was followed by FJC.
Table 3.
Comparison of potassium permanganate-pretreatment times in the intensity of FJC and immunofluorescence
| Pretreatment time (min) | 2 | 5 | 7 | 10 |
|---|---|---|---|---|
| FJC | *++++ | +++ | +++ | ++ |
| IF | ++++ | +++ | ++ | – |
Note: Intensity of FJC or IF (NeuN): ++++, highest intensity; +++, high intensity; ++, moderate intensity; –; background intensity.
*The intensity of the FJC background was so high that the sample showed a low stain-to-noise ratio.
Next, we checked the duration of stability of both signals on sections coverslipped in acidic mount media under photo-irradiation. Intensity of FJC staining was stable for at least 10 min, whereas that of IF staining decreased with time and had diminished by 10 min (Table 2). Considering that a few minutes was required to observe the sections, the next experiment was treated after photo-irradiation for 3 min.
Table 2.
Comparison of time differences in photobleaching of FJC and immunofluorescence stainings
| Time (min) | 0 | 3 | 5 | 10 |
|---|---|---|---|---|
| FJC | ++++ | ++++ | ++++ | ++++ |
| IF | +++ | ++ | ++ | + |
Note: Intensity of FJC or IF (NeuN): ++++, highest intensity; +++, high intensity; ++, moderate intensity; +, low intensity.
Finally, to detect high-contrast double labeling, we evaluated pretreatment time (2, 5, 7, and 10 min) with potassium permanganate. FJC background gradually decreased with the length of pretreatment. Pretreatment for 2 min yielded intense labeling with high background activity. Pretreatment for 5 or 7 min resulted in high to moderate intensity and low background activity for both stainings (Table 3), while pretreatment for 10 min resulted in very clear identification of FJC staining cells within low background but diminished IF staining. We therefore used 5-min potassium permanganate pretreatment in the following experiments.
FJC staining in an acute PD model
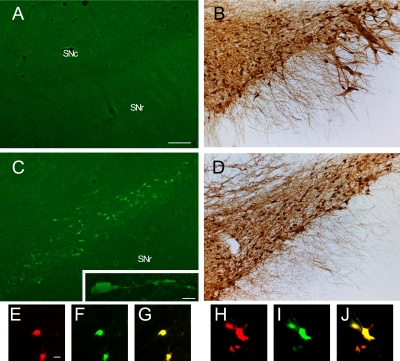
After intrastriatal 6-OHDA injection, substantial DA fiber loss was seen in the striatum as well as neuronal loss in the SN. Progression of DA degeneration over time on the injected side resembled that described in previous reports [5, 14, 17]. In the SN at day 3 after 6-OHDA injection, some FJC-positive cell bodies and processes with neuronal profiles were clearly visualized on the ipsilateral side, but not contralaterally (Fig. 2A, C). In adjacent sections treated with TH antibody, TH-positive neurons were found in almost equal numbers on both sides (Fig. 2B, D). On the ipsilateral side, the ratio of FJC-positive cells to TH-positive cells was approximately 1:5 (Fig. 2C, D).
Fig. 2.
Comparison of FJC staining (A and C) and TH immunostaining (B and D) between the contralateral side (A and B) and ipsilateral side (C and D) in the SN at 3 days after intrastriatal 6-OHDA injection. High magnification view of the highlighted box in C (inset). Cell bodies of the degenerating neurons induced by 6-OHDA were double labeled with IF using TH (E and H) and FJC (F and I) in the SNc and were round (E–G) or shrunken (H–J). G and J represent mergers of E and F, and H and I respectively. SN, substantia nigra; SNc, substantia nigra pars compacta; SNr, substantia nigra pars reticularis. Bars=100 µm (A–D) and 10 µm (inset in C and E–J).
Double labeling with FJC and TH showed that a few FJC-positive neurons were immune-positive for TH. These neurons were round and had shrunken cell bodies (Fig. 2E–J).
FJC staining in a chronic PD model
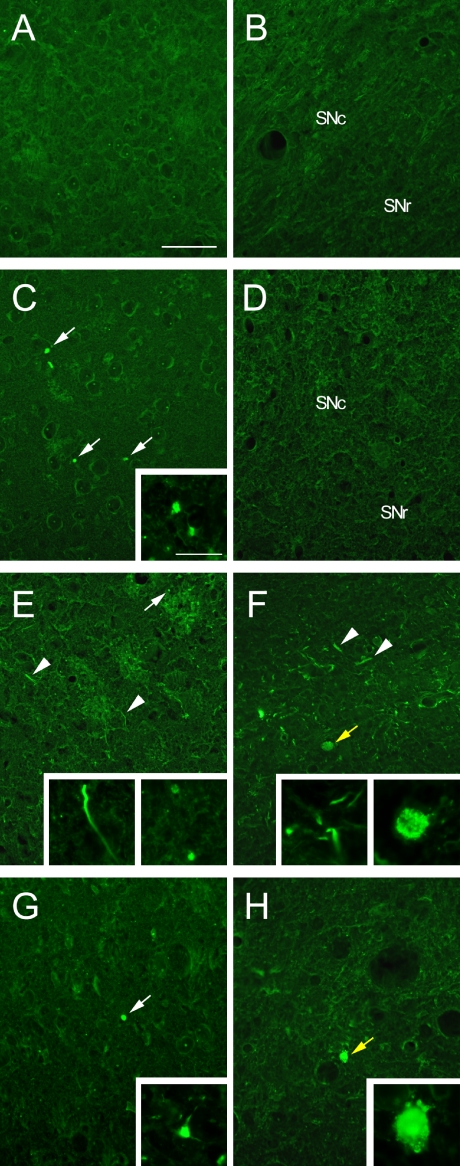
Previous studies from our laboratory have reported age-dependent degeneration of the nigrostriatal DA neuron system [22, 23]. In the present study, spatiotemporal changes in the pattern of FJC-positive cells were examined in the striatum and SN of zi/zi rats at time-points of 1M, 4M, and 12M. At 1M, FJC-positive structures were barely observed in either area in both zi/zi and control SD rats (Fig. 3A–D). However, a few dot-shaped FJC-positive structures were found in the striatum of zi/zi rats (Fig. 3C). At 4M, a large number of FJC-positive structures, some of which were linear and others dot-shaped, were found in the lateral part of the striatum, whereas only a few FJC-positive structures were found in the SN, appearing larger than the dots found in the striatum and seeming to represent shrunken cell bodies without cellular processes (Fig. 3E, F). At 12M, FJC-positive dot-shaped structures were still observed within the striatum and SN. However, these structures were far fewer in number than at 4M, whereas very few FJC-positive structures resembling cell bodies were detected in the SN, as had been observed at 4M (Fig. 3G, H).
Fig. 3.
In the SD rat, no FJC-positive neurons are seen in the striatum (A) or SN (B) at 1M. Distribution of FJC-positive structures in the striatum (C, E, and G) and SN (D, F, and H) of the zi/zi rat at 1M (C and D), 4M (E and F), and 12M (G and H). Staining shows dot-shaped structures (white arrows), fiber-like structures (arrowheads) and cell body-like structures (yellow arrows). In the zi/zi rat, high magnification views show the distinct shaped fibers stained by FJC of the striatum at 1M (inset in C), 4M (inset in E), and 12M (inset in G) and those of the SN at 4M (left-side inset in F), and the cell bodies of the SN at 4M (right-side inset in F) and 12M (inset in H). SN, substantia nigra; SNc, substantia nigra pars compacta; SNr, substantia nigra pars reticularis. Bars=50 µm (A–H) and 10 µm (inset in C and E–H).
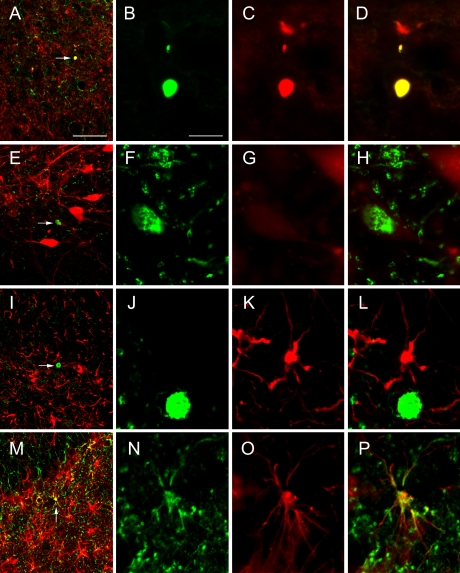
To determine the type of cells that labeled with FJC in the SN and striatum, double labeling was performed for FJC and TH or GFAP in the zi/zi rat brain at 4M, because considerable FJC-labeling was found at this age. Dot-shaped FJC-positive structures in the striatum were labeled with TH (Fig. 4A–D). This meant that the dot-shaped co-labeling structures were part of the fibers of DA neurons. In the SN, FJC-positive cell bodies were weakly labeled or not labeled with TH (Fig. 4E–H). In both areas, FJC-positive cells were not double-labeled with GFAP (Fig. 4I–L).
Fig. 4.
Double labeling of FJC and IF using TH (A–H) or GFAP (I–P) in the striatum (A–D), SN (E–L), and hilus (M–P) of the zi/zi rat at 4M. Each high magnification view of the structure (arrow) in A, E, I and M is shown in B–D, F–H, J–L and N–P; double labeling of FJC (B, F, J and N) and IF using TH (C and G) or GFAP (K and O). D, H, L and P represent mergers of B and C, F and G, J and K, and N and O, respectively. Bars=50 µm (A, E, I and M) and 10 µm (B–D, F–H, J–L and N–P).
Interestingly, small FJC-positive cells were also observed within the hippocampal formation. So far, no reports have described cell death in the hippocampal formation of the zi/zi rat. We therefore tried to elucidate what types of cells these were. The double-labeling study revealed that in the hilus of the hippocampus, FJC-positive cells showed small cell bodies with several short processes and were co-localized with GFAP (Fig. 4M–P).
IV. Discussion
FJC is a novel fluorescent dye introduced in 2005 that has been used to selectively stain degenerating neurons [20]. Compared with its predecessors, Fluoro-Jade and Fluoro-Jade B, FJC exhibits the greatest signal-to-noise ratio, as well as the highest resolution labeling not only of cell bodies but also of dendrites, axons and terminals of degenerating neurons. An additional feature of FJC is its compatibility with several histological processing and staining protocols such as 4'-6-diamidino-2-phenylindole staining, terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling assay and IF [4, 20, 25]. Although studies have evaluated double labeling with FJC and IF, methods have varied and technical problems have been encountered related to procedures, photobleaching, and pretreatment, resulting from susceptibilities of antigenic epitopes. Using the KA-induced neuronal degeneration model, we therefore investigated these three issues and recommend the following modifications of the double-labeling method: 1) IF should be followed by FJC; 2) microscopy of double-labeled slides should be performed within 5 min after photo-irradiation; and 3) pretreatment time with potassium permanganate should be shortened to 5 min rather than the 10 min used in FJC single staining.
The acute neurodegeneration induced by 6-OHDA has previously been detectable using Fluoro-Jade or Fluoro-Jade B in the SN in the acute phase when the cell bodies of affected TH-positive neurons have still been alive [21, 27]. Similarly, we found FJC-positive degenerating neurons at 3 days after intrastriatal 6-OHDA injection. Although Fluoro-Jade staining is confined to cell bodies [27], FJC staining was located in both the processes and cell bodies, indicating that FJC is more sensitive in detecting degenerative processes than Fluoro-Jade in this model. Furthermore, TH-positive neurons were similarly found on both the ipsilateral and contralateral sides at 3 days after injection, but some TH-positive neurons only on the ipsilateral side were also stained with FJC (Fig. 3). It has been demonstrated that intrastriatal 6-OHDA injection results in a partial progressive loss of nigral TH-positive neurons with an onset at about 1 week after injection; however, the cell death-related factors Bcl-2, Bax, and caspase 3 have already changed the level of mRNA or proteins in damaged DA neurons by 4 days after injection [9, 12]. Even though DA neurons still show TH-immunoreactivity, the neurons may already be damaged. TH-positivity in cells double labeled with FJC may thus represent a sign of impending cell death.
Our results showed that FJC-positive neurons were detected in zi/zi rats, in which a progressive neurodegenerative process has been observed in the nigrostriatal system [15, 22, 24]. The FJC-positive cell bodies were shrunken and their fibers were swollen (Fig. 3). Such morphological abnormalities in the zi/zi rat brain were also reported by histological and electronmicroscopic studies [15, 22, 23]. Confirming these previous reports, FJC-positive fibers were first found in the striatum of 1M zi/zi rats but not in the SN, indicating that the death of DA neurons in the zi/zi rats is preceded by a degeneration of DA terminals in the striatum. The number of FJC-positive neurons subsequently increased until 4M, at which point TH-positive neurons were reduced dramatically. At 12M, TH-positive neurons were rare in the SN [23]. The present study demonstrates that FJC is useful for detecting degenerating neurons in the chronic neurodegeneration model. Contrasting with the acute neurodegenerative model using 6-OHDA (Fig. 2), we could not detect FJC-positive cells labeled with TH in zi/zi rats at 4M (Fig. 4); at this age TH-positive cell loss had already occurred [23]. In addition, FJC staining was detected in damaged short processes of the neuronal cell bodies in 6-OHDA-treated rats, but not in zi/zi rats. This discrepancy may be attributable to the rate of accumulation of the substance that binds to FJC in damaged neurons. Although the intracellular molecules binding to FJC remain obscure, candidate molecules would include those with multiple positively charged groups such as poly-amines, as all Fluoro-Jade dyes share the property of possessing multiple acidic substitutions and are known as poly-anionic fluorescein derivatives [20]. Acutely degenerating neurons may accumulate these molecules faster than chronically degenerating neurons. Further studies are needed to determine the biochemical properties of FJC.
Regarding the ability of FJC to label glial cells, our double labeling study of the zi/zi rat hippocampus provides new evidence. The present study showed that FJC and GFAP were co-localized in the hilus, but not in the SN or striatum (Fig. 4), indicating that specific substances interacting with this anionic fluorescein derivative may exist in the astrocytes in the zi/zi rat hippocampal formation and lead to a change in homeostasis, which is a primary role of astrocytes. To date, studies using acute insult models have shown that FJC-positive cells were not co-localized with GFAP immunoreactivity [1, 3]. However, some authors have observed FJC-positive cells exhibiting astroglial morphology in the white matter or gray matter located near the ventricular and meningeal surfaces and the co-localization of FJC and GFAP in some astrocytes of rat retinas [2, 4, 20]. Furthermore, a recent study using a transgenic chronic model of Alzheimer’s disease showed that Fluoro-Jade B labels activated astrocytes, which were identified as an increased intensity of GFAP-immunoreaction [6]. In astrocytes, GFAP upregulation is a central feature of astrogliosis after any preceding injury to the CNS [7]. Since the zi/zi rat is a spontaneous chronic neurodegenerative model and exhibits activated astrocytes [10], FJC may label glial cells in the zi/zi brain. Although we cannot assert that FJC-positive astrocytes are dying, the cells may be damaged and show GFAP upregulation with the resulting co-localization with GFAP in the zi/zi rat. In the microglia, Fluoro-Jade B reportedly co-localizes with a lysosomal membrane protein (CD68) within the microglia or with amyloid-beta protein in glia-like cells, and candidate molecules binding with Fluoro-Jade B could thus exist in lysosomal vacuoles and/or in the core of amyloid deposits [6].
Under neurodegeneration, the differences in the mechanisms of degradation between astrocytes and neurons may create differences in the accumulation rate of substances reacting to FJC. This may result in the difference between FJC co-localizing with one cellular marker or another. We thus cannot rule out the possibility that the substance that binds to FJC may differ between stained astrocytes and stained neurons.
V. Acknowledgments
The authors would like to thank Ms. Y. Yamada and Ms. S. Nihei for technical assistance and Ms. F. Terauchi for her assistance in manuscript preparation. This work was supported in part by grants-in-aid from Dokkyo Medical University, Investigator-Initiated Research Grant (No. 2009-01-2), and the “Academic Frontier” Project from the Ministry of Education, Culture, Sports, Science and Technology, Japan.
VI. References
- 1.Bian G. L., Wei L. C., Shi M., Wang Y. Q., Cao R., Chen L. W. Fluoro-Jade C can specifically stain the degenerative neurons in the substantia nigra of the 1-methyl-4-phenyl-1,2,3,6-tetrahydro pyridine-treated C57BL/6 mice. Brain Res. 2007;1150:55–61. doi: 10.1016/j.brainres.2007.02.078. [DOI] [PubMed] [Google Scholar]
- 2.Brown D. A., Sawchenko P. E. Time course and distribution of inflammatory and neurodegenerative events suggest structural bases for the pathogenesis of experimental autoimmune encephalomyelitis. J. Comp. Neurol. 2007;502:236–260. doi: 10.1002/cne.21307. [DOI] [PubMed] [Google Scholar]
- 3.Chen L. W., Wang Y. Q., Bian G. L., Wei L. C., Yung K. L. Neurokinin-3 peptide instead of neurokinin-1 synergistically exacerbates kainic acid-inducing degeneration of neurons in the substantia nigra of mice. J. Neurochem. 2008;105:203–216. doi: 10.1111/j.1471-4159.2007.05132.x. [DOI] [PubMed] [Google Scholar]
- 4.Chidlow G., Wood J. P., Sarvestani G., Manavis J., Casson R. J. Evaluation of Fluoro-Jade C as a marker of degenerating neurons in the rat retina and optic nerve. Exp. Eye Res. 2009;88:426–437. doi: 10.1016/j.exer.2008.10.015. [DOI] [PubMed] [Google Scholar]
- 5.Cicchetti F., Brownell A. L., Williams K., Chen Y. I., Livni E., Isacson O. Neuroinflammation of the nigrostriatal pathway during progressive 6-OHDA dopamine degeneration in rats monitored by immunohistochemistry and PET imaging. Eur. J. Neurosci. 2002;15:991–998. doi: 10.1046/j.1460-9568.2002.01938.x. [DOI] [PubMed] [Google Scholar]
- 6.Damjanac M., Rioux Bilan A., Barrier L., Pontcharraud R., Anne C., Hugon J., Page G. Fluoro-Jade B staining as useful tool to identify activated microglia and astrocytes in a mouse transgenic model of Alzheimer’s disease. Brain Res. 2007;1128:40–49. doi: 10.1016/j.brainres.2006.05.050. [DOI] [PubMed] [Google Scholar]
- 7.Eddleston M., Mucke L. Molecular profile of reactive astrocytes-implications for their role in neurologic disease. Neuroscience. 1993;54:15–36. doi: 10.1016/0306-4522(93)90380-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eldred W. D., Zucker C., Karten H. J., Yazulla S. Comparison of fixation and penetration enhancement techniques for use in ultrastructural immunocytochemistry. J. Histochem. Cytochem. 1983;31:285–292. doi: 10.1177/31.2.6339606. [DOI] [PubMed] [Google Scholar]
- 9.Hanrott K., Murray T. K., Orfali Z., Ward M., Finlay C., O’Neill M. J., Wonnacott S. Differential activation of PKC delta in the substantia nigra of rats following striatal or nigral 6-hydroxydopamine lesions. Eur. J. Neurosci. 2008;27:1086–1096. doi: 10.1111/j.1460-9568.2008.06097.x. [DOI] [PubMed] [Google Scholar]
- 10.Kondo A., Sendoh S., Miyata K., Takamatsu J. Spongy degeneration in the zitter rat: ultrastructural and immunohistochemical studies. J. Neurocytol. 1995;24:533–544. doi: 10.1007/BF01179978. [DOI] [PubMed] [Google Scholar]
- 11.Kosaka T., Nagatsu I., Wu J. Y., Hama K. Use of high concentrations of glutaraldehyde for immunocytochemistry of transmitter-synthesizing enzymes in the central nervous system. Neuroscience. 1986;18:975–990. doi: 10.1016/0306-4522(86)90112-0. [DOI] [PubMed] [Google Scholar]
- 12.Kramer B. C., Mytilineou C. Alterations in the cellular distribution of bcl-2, bcl-x and bax in the adult rat substantia nigra following striatal 6-hydroxydopamine lesions. J. Neurocytol. 2004;33:213–223. doi: 10.1023/b:neur.0000030696.62829.ec. [DOI] [PubMed] [Google Scholar]
- 13.Kuramoto T., Kitada K., Inui T., Sasaki Y., Ito K., Hase T., Kawagachi S., Ogawa Y., Nakao K., Barsh G. S., Nagao M., Ushijima T., Serikawa T. Attractin/mahogany/zitter plays a critical role in myelination of the central nervous system. Proc. Natl. Acad. Sci. U S A. 2001;98:559–564. doi: 10.1073/pnas.98.2.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee C. S., Sauer H., Bjorklund A. Dopaminergic neuronal degeneration and motor impairments following axon terminal lesion by intrastriatal 6-hydroxydopamine in the rat. Neuroscience. 1996;72:641–653. doi: 10.1016/0306-4522(95)00571-4. [DOI] [PubMed] [Google Scholar]
- 15.Nakadate K., Noda T., Sakakibara S., Kumamoto K., Matsuura T., Joyce J. N., Ueda S. Progressive dopaminergic neurodegeneration of substantia nigra in the zitter mutant rat. Acta Neuropathol. 2006;112:64–73. doi: 10.1007/s00401-006-0058-8. [DOI] [PubMed] [Google Scholar]
- 16.Paxinos G., Watsons C. The Rat Brain in Stereotaxic Coordinates. Academic Press Inc.; New York, USA: 1986. [Google Scholar]
- 17.Sauer H., Oertel W. H. Progressive degeneration of nigrostriatal dopamine neurons following axon terminal lesion by intrastriatal 6-hydroxydopamine in the rat. Neuroscience. 1994;59:401–415. doi: 10.1016/0306-4522(94)90605-x. [DOI] [PubMed] [Google Scholar]
- 18.Schmued L. C., Albertson C., Slikker W., Jr. Fluoro-Jade: a novel fluorochrome for the sensitive and reliable histochemical localization of neuronal degeneration. Brain Res. 1997;751:37–46. doi: 10.1016/s0006-8993(96)01387-x. [DOI] [PubMed] [Google Scholar]
- 19.Schmued L. C., Hopkins K. J. Fluoro-Jade: novel fluorochromes for detecting toxicant-induced neuronal degeneration. Toxicol. Pathol. 2000;28:91–99. doi: 10.1177/019262330002800111. [DOI] [PubMed] [Google Scholar]
- 20.Schmued L. C., Stowers C. C., Scallet A. C., Xu L. Fluoro-Jade C results in ultra high resolution and contrast labeling of degenerating neurons. Brain Res. 2005;1035:24–31. doi: 10.1016/j.brainres.2004.11.054. [DOI] [PubMed] [Google Scholar]
- 21.Smith L. K., Jadavji N. M., Colwell K. L., Katrina Perehudoff S., Metz G. A. Stress accelerates neural degeneration and exaggerates motor symptoms in a rat model of Parkinson’s disease. Eur. J. Neurosci. 2008;27:2133–2146. doi: 10.1111/j.1460-9568.2008.06177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ueda S., Aikawa M., Ishizuya-Oka A., Yamaoka S., Koibuchi N., Yoshimoto K. Age-related dopamine deficiency in the mesostriatal dopamine system of zitter mutant rats: regional fiber vulnerability in the striatum and the olfactory tubercle. Neuroscience. 2000;95:389–398. doi: 10.1016/s0306-4522(99)00451-0. [DOI] [PubMed] [Google Scholar]
- 23.Ueda S., Sakakibara S., Watanabe E., Yoshimoto K., Koibuchi N. Vulnerability of monoaminergic neurons in the brainstem of the zitter rat in oxidative stress. Prog. Brain Res. 2002;136:293–302. doi: 10.1016/s0079-6123(02)36025-4. [DOI] [PubMed] [Google Scholar]
- 24.Ueda S., Sakakibara S., Nakadate K., Noda T., Shinoda M., Joyce J. N. Degeneration of dopaminergic neurons in the substantia nigra of zitter mutant rat and protection by chronic intake of Vitamin E. Neurosci. Lett. 2005;380:252–256. doi: 10.1016/j.neulet.2005.01.053. [DOI] [PubMed] [Google Scholar]
- 25.Wang L., Liu Y. H., Huang Y. G., Chen L. W. Time-course of neuronal death in the mouse pilocarpine model of chronic epilepsy using Fluoro-Jade C staining. Brain Res. 2008;1241:157–167. doi: 10.1016/j.brainres.2008.07.097. [DOI] [PubMed] [Google Scholar]
- 26.Weber E., Voigt K. H., Martin R. Pituitary somatotrophs contain [Met]enkephalin-like immunoreactivity. Proc. Natl. Acad. Sci. U S A. 1978;75:6134–6138. doi: 10.1073/pnas.75.12.6134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zuch C. L., Nordstroem V. K., Briedrick L. A., Hoernig G. R., Granholm A. C., Bickford P. C. Time course of degenerative alterations in nigral dopaminergic neurons following a 6-hydroxydopamine lesion. J. Comp. Neurol. 2000;427:440–454. doi: 10.1002/1096-9861(20001120)427:3<440::aid-cne10>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]