Abstract
13-Hydroxyoctadecadienoic acid (13-HODE) is a major component of oxidized low density lipoprotein (OxLDL), which has been shown to have a crucial role in atherogenesis. Of the 13-HODE stereoisomers, 13(S)-HODE and 13(R)-HODE, little is known about the latter in contrast to the former. To detect 13(R)-HODE in atherosclerotic lesions, we prepared a mouse monoclonal antibody against 13(R)-HODE. Competitive enzyme-linked immunosorbent assay clarified the selective reaction of a clone mAb 13H1 with both free and bovine serum albumin-conjugated forms of 13(R)-HODE but not other oxidized lipids including 13(S)-HODE. Immunohistochemical analysis revealed the colocalization of 13(R)-HODE immunoreactivity with the OxLDL marker oxidized phophatidylcholine immunoreactivity in vascular endothelial cells, macrophages and migrating vascular smooth muscle cells in atherosclerotic plaques of human carotid arteries. The present results provide in vivo evidence for the formation of 13(R)-HODE in atherosclerotic lesions of carotid arteries.
Keywords: carotid atherosclerosis, enzyme-linked immunosorbent assay, 13(R)-hydroxyoctadecadienoic acid, immunohistochemistry, monoclonal antibody
I. Introduction
Emerging evidence suggests a pivotal role for oxidized low density lipoprotein (OxLDL) in atherogenesis [2, 15, 22]. It has been shown that OxLDL contains a variety of oxidized lipids including oxidized fatty acids, oxidized free cholesterols and cholesteryl esters, and oxidized phospholipids and hydroxyalkenals. 13-Hydroxyoctadecadienoic acid (13-HODE) is one of the major oxidized fatty acids [11, 23] and a major component of OxLDL detected in atherosclerotic lesions [6, 21]. Several studies have demonstrated that 13-HODE exerts anti-inflammatory effects as a ligand of peroxisome proliferator-activated receptor-gamma (PPARγ) [17], promotes macrophage OxLDL uptake [24], and inhibits endothelial cell/platelet interaction [4, 12], cancer cell metastasis and proliferation [18, 20] and triacylglycerol-rich lipoprotein secretion [16].
There are two stereoisomers of 13-HODE, 13-sinister-HODE [13(S)-HODE] and 13-rectus-HODE [13(R)-HODE]. While 13(S)-HODE is a 15-lipoxygenase (15-LOX) metabolite of linoleic and arachidonic acids [3, 13, 25], 13(R)-HODE is nonenzymatically formed in the presence of oxidative stress [1, 8, 19]. Several studies have suggested implications for 13(S)-HODE in a variety of disorders such as atherosclerosis, thrombosis, inflammation, and malignancies [3, 13, 25]. However, little is known about the biological actions of 13(R)-HODE. It has been shown that atherosclerotic plaque possesses increased 15-LOX activity but contain substantial levels of 13(R)-HODE rather than 13(S)-HODE [6]. Given that 15-LOX has a dual roles in atherogenesis because of the anti- and pro-inflammatory effects of its metabolites in OxLDL [25], it is controversial that 15-LOX and 13(S)-HODE play a critical role in the pathological processes of atherosclerosis. Thus, it is predicted that 13(R)-HODE may be involved in the pathomechanism of atherosclerosis. In the present study, we developed a monoclonal antibody (mAb) against 13(R)-HODE to detect this substance in vivo. This is the first report showing immunohistochemical localization of 13(R)-HODE in the atherosclerotic lesions of human carotid arteries.
II. Materials and Methods
Materials
13(R)-HODE, 13(S)-HODE, 9(R)-HODE, 9(S)-HODE, 13-oxooctadecadienoic acid (13-oxo-ODE) and linoleic acid (Fig. 1A) were purchased from Cayman Chemical Co. (Ann Arbor, MI, USA). Keyhole limpet hemocyanin (KLH), 1-ethyl-3-(dimethylaminopropyl)carbodiimide (EDC), and sulfo-N-hydroxysuccimide (sulfo-NHS) were obtained from Pierce (Rockford, IL, USA). Horse radish peroxidase (HRP)-conjugated goat anti-mouse IgG was purchased from MPBiomedicals, LLC (Solon, OH, USA).
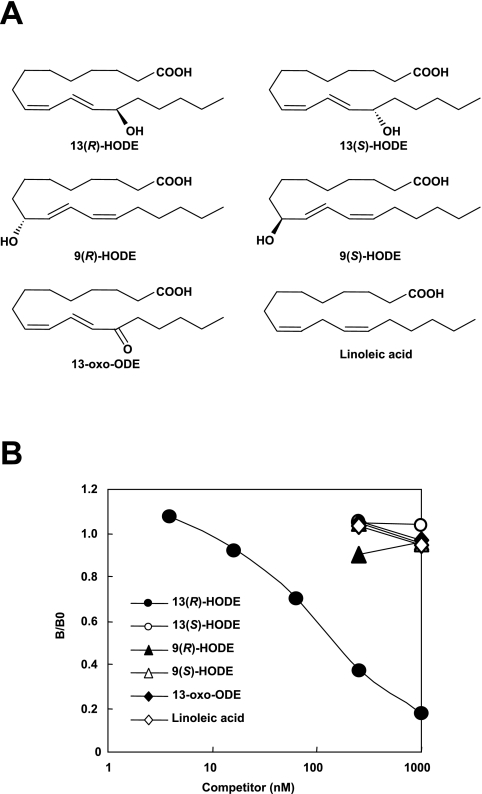
Fig. 1.
Comparision of reactivity of mAb 13H1 with oxidized lipids. (A) Molecular structures of oxidized lipids examined. (B) Results of competitive ELISA on microtiter plate wells coated with 13(R)-HODE-BSA. Abbreviations: BSA, bovine serum albumin; ELISA, enzyme-linked immunosorbent assay; HODE, hydroxyoctadecadienoic acid; oxo-ODE, oxooctadecadienoic acid.
Development of a monoclonal antibody directed to 13(R)-HODE
To evaluate the contribution of 13(R)-HODE to mechanisms of pathological conditions including atherosclerosis, we attempted to develop an mAb specific for this substance. The hapten used in the present study was 13(R)-HODE that was covalently attached to the carrier protein KLH using EDC and sulfo-NHS as described previously [7]. Female BALB/c mice were immunized three times with the 13(R)-HODE-KLH conjugates. We observed an enhanced immune response to the 13(R)-HODE-conjugated bovine serum albumin (BSA) in the immunized BALB/c mice (data not shown). Splenocytes derived from the immunized mice were fused with P3U1 murine myeloma cells and cultured in hypoxanthine/aminopterin/thymidine selection medium.
Culture media of the hybridoma were screened using enzyme-linked immunosorbent assay (ELISA), employing pairs of wells of microtiter plates coated with 13(R)-HODE-BSA conjugates (0.5 µg of protein/well). After incubation with 100 µL of hybridoma supernatants, and with intervening washes with phosphate-buffered saline (PBS), pH 7.6, containing 0.05% Tween 20 (PBS-T), the wells were incubated with HRP-conjugated goat anti-mouse IgG followed by a substrate solution containing 0.5 mg/mL 1,2-phenylenediamine. The affinity of supernatants from obtained clones with oxidized lipids was determined by competitive ELISA. A competitor was incubated with the antibody for 20 hr at 4°C to yield competitor/antibody mixtures at variable concentrations of the competitor. A 100 µL aliquot of the mixtures was added to each well and incubated for 1 hr at 37°C. After discarding the supernatants and washing three times with Tris-buffered saline (TBS), pH 7.5, containing 0.05% Tween 20 (TBS-T), wells were filled with the secondary antibody followed by peroxidase substrate. Results were expressed as the ratio, B/B0 (B=absorbance in the presence of competitor; B0=absorbance in the absence of competitor).
Immunohistochemical analysis of 13(R)-HODE in carotid arteries
The primary antibodies used for immunohistochemistry were 13H1 as well as mouse anti-CD34 IgG1 (Clone No. QBEnd/10; diluted 1:100; Novocastra Laboratories, Newcastle upon Tyne, UK), mouse anti-CD68 IgG1 (Clone No. KP-1; diluted 1:10,000; Dako Cytomation, Kyoto, Japan), mouse anti-α-smooth muscle actin (SMA) IgG1 (Clone No. 1A4; diluted 1:1,000; Dako), mouse anti-oxidized phosphatidylcholine (OxPC) IgM (Clone No. DLH3; diluted 1:3,000) [9], rabbit anti-PPARγ IgG (Cat. No. sc-7196; diluted 1:500; Santa Cruz Biotechnology, Santa Cruz, CA, USA), and rabbit anti-CD36 IgG (Cat. No. sc-9154; diluted 1:300; Santa Cruz). CD34, CD68, SMA and OxPC were used as markers of vascular endothelial cells (VECs), macrophages, vascular smooth muscle cells (VSMCs) and OxLDL, respectively.
This investigation was approved by the local ethics comission in accordance with the Declaration of Helsinki, revised 2008 and carried out on six carotid atherosclerosis patients who underwent carotid endoarterectomy (CEA). Multiple 10-µm-thick sections of freshly frozen CEA specimens embedded in optimal cutting temperature (OCT) compound (Sakura Fine Chemical, Tokyo, Japan) were used for hematoxylin-eosin (H&E) staining and immunohistochemical staining. Sections were air-dried, postfixed for 10 min at 4°C in 100% acetone, rehydrated, quenched for 10 min at 4°C with 3% hydrogen peroxide, rinsed in PBS, pretreated for 20 min at room temperature with 3% skim milk in PBS, and subsequently incubated overnight at 4°C with the primary antibodies. Sections processed with omission of the primary antibodies or incubated with nonimmune serum derived from the same animal species as those producing the antibodies served as negative reaction controls. Antibody binding was visualized by the polymer-immunocomplex (PIC) method using the appropriate Envision system (Dako), according to the manufacturer’s instructions. 3,3'-Diaminobenzidine tetrahydrochloride (DAB) was the chromogen, and hematoxylin, the counterstain. To verify the specificity of 13H1, preabsorption test was performed as the following; some sections were incubated with the antibody preabsorbed with 1.0 mg/mL 13(R)-HODE-BSA conjugates in PBS. Immunohistochemical localization of 13(R)-HODE was verified by comparison with consecutive sections stained with H&E or immunostained for targeted antigens such as CD34, CD68, SMA, OxPC, PPARγ and CD36.
III. Results
Characterization of anti-13(R)-HODE antibody
Hybridomas that produced and released antibodies against 13(R)-HODE were detected by ELISA on plates coated with the 13(R)-HODE-coupled BSA, and five clones reactive with the coated antigen were obtained. Among them, two clones named 13H1 and 13H2 showed relatively high reactivity with coated antigen (data not shown). Finally, one clone, 13H1, associated with good growth and distinctive recognition of 13(R)-HODE was selected and used in the present study. In spite of an extensive screening of hybridomas that produced monoclonal antibodies specific for 13(R)-HODE, it is still unknown whether the antibody may recognize epitopes originating from other oxidized lipids. To answer this question, the specificity of mAb 13H1 was verified by competitive ELISA (Fig. 1B). The antibody 13H1 selectively recognized the coated antigen 13(R)-HODE-BSA as well as the competitor antigen 13(R)-HODE. By contrast, the antibody showed no significant crossreactivity with 13(S)-HODE, 9(R)-HODE, 9(S)-HODE, 13-oxo-ODE, or linoleic acid. These results suggest that mAb 13H1 exclusively recognizes 13(R)-HODE.
Immunohistochemical localization of 13(R)-HODE in atherosclerotic lesions
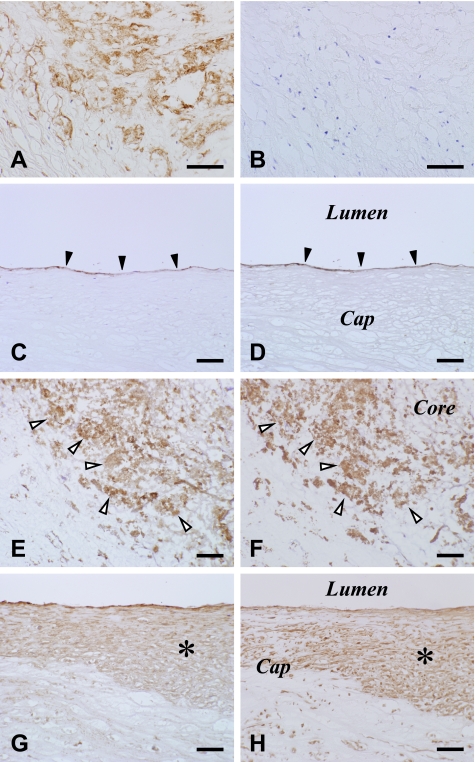
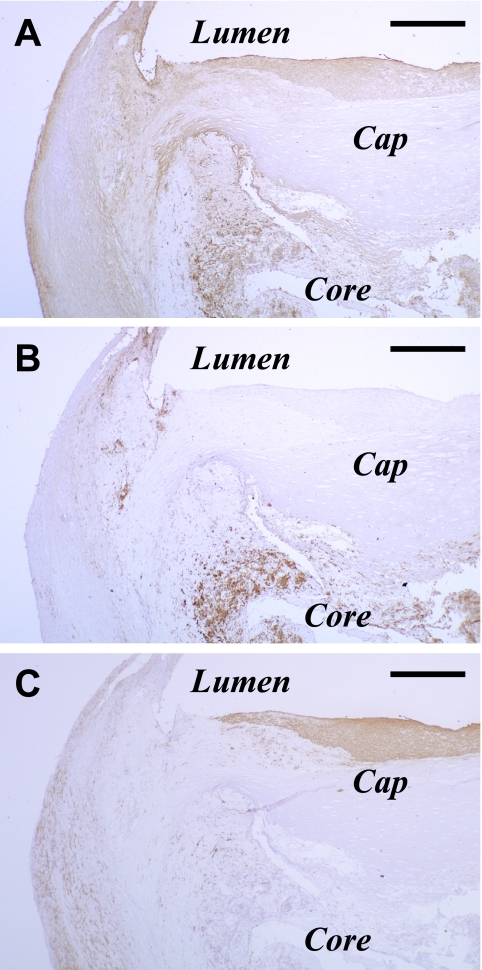
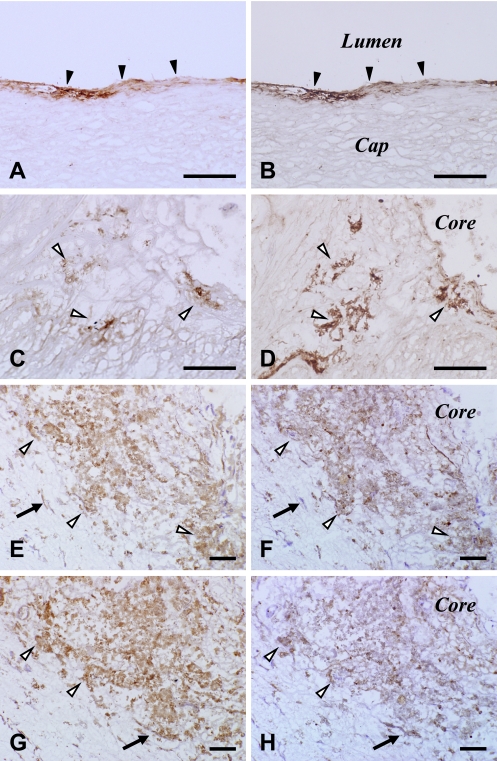
No immunoreaction product deposit was detectable on negative reaction control sections (data not shown) or 13(R)-HODE-preabsorbed sections (Fig. 3A, B). At a low magnification, areas immunoreactive for 13(R)-HODE included those for CD68 and SMA (Fig. 2). At a higher magnification, 13(R)-HODE immunoreactivity was localized in the cytoplasm of CD34-immunoreactive VECs lining luminal surface of carotid arteries (Fig. 3C, D) and neovasculatures, CD68-immunoreactive macrophages (Fig. 3E, F), and SMA-immunoreactive migrating VSMCs (Fig. 3G, H). The determinants of 13(R)-HODE were colocalized and superimposed with those of OxPC, PPARγ and CD36 in these lesional cells (Fig. 4). These findings were observed in fatty streaks as initial lesions as well as advanced lesions. The immunoreactivities for the examined substances including 13(R)-HODE in the lesional cells were readily detectable and pronounced in macrophage-enriched plaque (Figs. 2–4), but by contrast were only very weak or undetectable in macrophage-lacking plaque (data not shown). There was no significant difference in immunostaining patterns for 13(R)-HODE between superficial and deep layers of atherosclerotic plaque as shown at a low magnification view.
Fig. 3.
Negative staining on a section incubated with 13H1 preabsorbed with excess 13(R)-HODE-BSA conjugates (B) and identification of 13(R)-HODE immunoreactivity (A, C, E, G) in CD34-immunoreactive VECs (D), CD68-immunoreactive macrophages (F) and SMA-immunoreactive migrating VSMCs (H) in human atherosclerotic carotid arteries. Areas of panels (A, C, E, G) are the same as those of panels (B, D, F, H) on consecutive sections, respectively. Panels (A, B) show staining on a fatty streak. Solid and blank arrowheads and asterisks indicate VECs, macrophages and migrating VSMCs, respectively. PIC method using DAB (A–H). Bar=100 µm. Abbreviations: BSA, bovine serum albumin; DAB, 3,3'-diaminobenzidine tetrahydrochloride; HODE, hydroxyoctadecadienoic acid; PIC, polymer-immunocomplex; SMA, α-smooth muscle actin; VECs, vascular endothelial cells; VSMCs, vascular smooth muscle cells.
Fig. 2.
Immunohistochemical observations at a low magnification of consecutive sections immunostained for 13(R)-HODE (A), CD68 (B) and SMA (C) in an atherosclerotic plaque of a human carotid artery. PIC method using DAB (A–C). Bar=1 mm. Abbreviations: DAB, 3,3'-diaminobenzidine tetrahydrochloride; HODE, hydroxyoctadecadienoic acid; PIC, polymer-immunocomplex.
Fig. 4.
Comparison of immunohistochemical distribution of 13(R)-HODE (A, C, E, G) with OxPC (B, D), PPARγ (F) and CD36 (H) in lesional cells of human atherosclerotic carotid arteries. Areas of panels (A, C, E, G) are the same as those of panels (B, D, F, H) on consecutive sections, respectively. Solid and blank arrowheads and arrows indicate VECs, macrophages and migrating VSMCs, respectively. PIC method using DAB (A–H). Bar=100 µm. Abbreviations: DAB, 3,3'-diaminobenzidine tetrahydrochloride; HODE, hydroxyoctadecadienoic acid; OxPC, oxidized phosphatidylcholine; PIC, polymer-immunocomplex; PPARγ, peroxisome proliferator-activated receptor-gamma; VECs, vascular endothelial cells; VSMCs, vascular smooth muscle cells.
IV. Discussion
In the present study, we produced mAb 13H1 that selectively recognized 13(R)-HODE and distinguished it from its isomer 13(S)-HODE and other oxidized lipids. Given that there is only a little alteration in molecular structures between 13(R)-HODE and 13(S)-HODE, it is evident that the epitope that 13H1 recognizes exists in a limited part of this substance including the 13(R)-OH group. Moreover, competitive ELISA revealed the reactivity of mAb 13H1 with the coated antigen BSA-conjugated 13(R)-HODE as well as the competitor antigen 13(R)-HODE. Our immunohistochemical finding of the colocalization of the 13(R)-HODE determinants with the OxPC determinants in the atherosclerotic lesions of carotid arteries indicates that OxLDL contains 13(R)-HODE as both the free and protein-bound forms in atherosclerotic lesions. The fact that 13(R)-HODE immunoreactivity was more intense in the macrophage-enriched plaque as compared with the macrophage-lacking plaque shows that tissue levels of this substance depends on those of OxLDL. Also the fact that 13(R)-HODE immunoreactivity was less intense in the extracellular matrix of plaque neointima, as compared with VECs, macrophages, and migrating VSMCs, suggests that the lesional cells took up extracellular OxLDL containing 13(R)-HODE and concentrated them in these cells. This is in keeping with an in vitro study showing 13-HODE uptake induced by cultured VECs under increased permeability conditions [4]. However, it is to be considered with the caveat that 13(R)-HODE could be nonenzymatically formed in these cells after uptake. This is attributable to evidence for increased oxidative stress in VECs, macrophages, and VSMCs, all of which show activation of NADPH oxidase, phospholipase A2, xanthine oxidase, and nitric oxide synthases as well as accumulation of oxidative modification products of nucleic acids, lipids, and proteins [5].
There have been only a few reports showing the biological actions of 13(R)-HODE. A previous study indicated that 13(R)-HODE exerts an antagonizing effect on thromboxane A2-induced platelet aggregation, and that this effect was stronger than that of 13(S)-HODE [12]. This may conflict with another fact that PPARγ agonists including 13-HODE upregulate plasminogen activator inhibitor type-1 (PAI-1) in cultured human endothelial cells [14]. These observations suggest that 13(R)-HODE regulates a balance between coagulative and fibrinolytic activities. Additionally, it has been shown that 13-HODE upregulates the class B scavenger receptor CD36, which we detected in the lesional cells of atherosclerotic plaques in this study, in macrophages via a PPARγ-dependent mechanism [10]. Thus, as an earlier report showing the greater levels of the nonenzymatically formed 13(R)-HODE as compared with the enzymatically produced 13(S)-HODE in atherosclerotic lesions [6], it is likely that 13(R)-HODE as well as 13(S)-HODE acts as an agonist of PPARγ, which is an anti-inflammatory transcription factor.
Collectively, we successfully demonstrated immunohistochemical localization of 13(R)-HODE in VECs, macrophages, and migrating VSMCs in atherosclerotic lesions of human carotid arteries, using a novel specific monoclonal antibody. The colocalization of 13(R)-HODE immunoreactivity with immunoreactivities for OxPC, PPARγ, and CD36 in the lesional cells could indicate that 13(R)-HODE is a major component of OxLDL, and points to the possibility that 13(R)-HODE may induce CD36 via a PPARγ-dependent mechanism. However, since OxLDL contains both proinflammatory and antiinflammatory lipid mediators [2, 15, 22], it remains to be determined whether 13-HODE in OxLDL may exert its antiinflammatory effects on atherosclerosis. For elucidating this issue, it is necessary to obtain direct evidence that 13(R)-HODE derived from athreosclerotic lesions activates PPARγ and consequently upregulates PPARγ-dependent anti-inflammatory gene products.
V. Acknowledgements
The authors wish to thank N. Sakayori for technical assistance and Dr. Y. Kato (Department of Pathology, Tokyo, Women’s Medical University) for valuable suggestions.
VI. References
- 1.Baer A. N., Costello P. B., Green F. A. Stereospecificity of the hydroxyeicosatetraenoic and hydroxyoctadecadienoic acids produced by cultured bovine endothelial cells. Biochim. Biophys. Acta. 1991;1085:45–52. doi: 10.1016/0005-2760(91)90230-f. [DOI] [PubMed] [Google Scholar]
- 2.Birukov K. G. Oxidized lipids: the two faces of vascular inflammation. Curr. Atheroscler. Rep. 2006;8:223–231. doi: 10.1007/s11883-006-0077-x. [DOI] [PubMed] [Google Scholar]
- 3.Buchanan M. R., Haas T. A., Lagarde M., Guichardant M. 13-Hydroxyoctadecadienoic acid is the vessel wall chemorepellant factor, LOX. J. Biol. Chem. 1985;260:16056–16059. [PubMed] [Google Scholar]
- 4.Buchanan M. R., Bertomeu M. C., Haas T. A., Orr W., Eltringham-Smith L. L. Localization of 13-hydroxyoctadecadienoic acid and the vitronectin receptor in human endothelial cells and endothelial cell/platelet interactions in vitro. Blood. 1993;81:3303–3312. [PubMed] [Google Scholar]
- 5.Fearon I. M., Faux S. P. Oxidative stress and cardiovascular disease: novel tools give (free) radical insight. J. Mol. Cell. Cardiol. 2009;47:372–381. doi: 10.1016/j.yjmcc.2009.05.013. [DOI] [PubMed] [Google Scholar]
- 6.Feinmark S. J., Cornicelli J. A. Is there a role for lipoxygenase in atherogenesis? Biochem. Pharmacol. 1997;54:953–959. doi: 10.1016/s0006-2952(97)00135-4. [DOI] [PubMed] [Google Scholar]
- 7.Grabarek Z., Gergely J. Zero-length crosslinking procedure with the use of active esters. Anal. Biochem. 1990;185:131–135. doi: 10.1016/0003-2697(90)90267-d. [DOI] [PubMed] [Google Scholar]
- 8.Hübke H., Garbe L.-A., Tressl R. Characterization and quantification of free and esterified 9- and 13-hydroxyoctadecadienoic acids (HODE) in barley, germinating barley, and finished malt. J. Agric. Food Chem. 2005;53:1556–1562. doi: 10.1021/jf048490s. [DOI] [PubMed] [Google Scholar]
- 9.Itabe H., Takeshima E., Iwasaki H., Kimura J., Yoshida Y., Imanaka T., Takano T. A monoclonal antibody against oxidized lipoprotein recognizes foam cells in atherosclerotic lesions. J. Biol. Chem. 1994;269:15274–15279. [PubMed] [Google Scholar]
- 10.Jostarndt K., Gellert N., Rubic T., Weber C., Kühn H., Johansen B., Hrboticky N., Neuzil J. Dissociation of apoptosis induction and CD36 upregulation by enzymatically modified low-density lipoprotein in monocytic cells. Biochem. Biophys. Res. Commun. 2002;290:988–993. doi: 10.1006/bbrc.2001.6290. [DOI] [PubMed] [Google Scholar]
- 11.Kaduce T. L., Figard P. H., Leifur R., Spector A. A. Formation of 9-hydroxyoctadecaenoic acid from linoleic acid in endothelial cells. J. Biol. Chem. 1989;264:6823–6830. [PubMed] [Google Scholar]
- 12.Lagarde M., Boutillon M. M., Guichardant M., Lellouche J. P., Beaucourt J. P., Vanhove A., Grée R. Further studies on the anti-thromboxane A2 activity of monohydroxylated fatty acids. Biochem. Pharmacol. 1989;38:1863–1864. doi: 10.1016/0006-2952(89)90422-x. [DOI] [PubMed] [Google Scholar]
- 13.Liu B., Timar J., Howlett J., Diglio C. A., Honn K. V. Lipoxygenase metabolites of arachidonic and linoleic acids modulates the adhesion of tumor cells to endothelium via regulation of protein kinase C. Cell Regul. 1991;2:1045–1055. doi: 10.1091/mbc.2.12.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marx N., Bourier T., Sulhova G. K., Plutzky J. PPARγ activation in human endothelial cells increases plasminogen activator inhibitor type-1 expression. PPARγ as a potential mediator in vascular disease. Arterioscler. Thromb. Vasc. Biol. 1999;19:546–551. doi: 10.1161/01.atv.19.3.546. [DOI] [PubMed] [Google Scholar]
- 15.Matsuura E., Kobayashi K., Tabuchi M., Lopez L. R. Oxidative modification of low density lipoprotein and immune regulation of atherosclerosis. Prog. Lipid Res. 2006;45:466–486. doi: 10.1016/j.plipres.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 16.Murthy S., Born E., Mathur S., Field F. J. 13-Hydroxyoctadecadienoic acid (13-HODE) inhibits triacylglycerol-rich lipoprotein secretion by CaCo-2 cells. J. Lipid Res. 1998;39:1254–1262. [PubMed] [Google Scholar]
- 17.Nagy L., Tontonoz P., Alvarez J. G. A., Chen H., Evans R. M. Oxidized LDL regulates macrophage gene expression through ligand activation of PPARγ. Cell. 1998;93:229–240. doi: 10.1016/s0092-8674(00)81574-3. [DOI] [PubMed] [Google Scholar]
- 18.Nixon J. B., Kim K.-S., Lamb P. W., Bottone F. G., Jr, Eling T. E. V. 15-Lipoxygenase-1 has anti-tumorigenic effects in colorectal cancer. Prostaglandins Leukotriens Essential Acids. 2004;70:7–15. doi: 10.1016/j.plefa.2003.06.001. [DOI] [PubMed] [Google Scholar]
- 19.Oliw E. H., Brodowsky I. D., Hörnsten L., Hamberg M. bis-Allylic hydroxylation of polyunsaturated fatty acids by hepatic monooxygenases and its relation to the enzymatic and nonenzymatic formation of conjugated hydroxyl fatty acids. Arch. Biochem. Biophys. 1993;300:434–439. doi: 10.1006/abbi.1993.1059. [DOI] [PubMed] [Google Scholar]
- 20.Shureiqi I., Wojno K. J., Poore J. A., Reddy R. G., Moussalli M. J., Spindler S. A., Greenson J. K., Normolle D., Hasan A. A. K., Lawrence T. S., Brenner D. E. Decreased 13-S-hydroxyoctadecadienoic acid levels and 15-lipoxygenase-1 expression in human colon cancer cells. Carcinogenesis. 1999;20:1985–1995. doi: 10.1093/carcin/20.10.1985. [DOI] [PubMed] [Google Scholar]
- 21.Staprans I., Pan X.-M., Rapp J. H., Feingold K. R. The role of dietary oxidized cholesterol and oxidized fatty acids in the development of atherosclerosis. Mol. Nutr. Food Res. 2005;49:1075–1082. doi: 10.1002/mnfr.200500063. [DOI] [PubMed] [Google Scholar]
- 22.Stoll G., Bendszus M. Inflammation and atherosclerosis: novel insights into plaque formation and destabilization. Stroke. 2006;37:1923–1932. doi: 10.1161/01.STR.0000226901.34927.10. [DOI] [PubMed] [Google Scholar]
- 23.Takayama H., Gimbrone M. A., Jr., Schafer A. I. Vascular lipoxygenase activity: synthesis of 15-hydroxyeicosatetraenoic acid from arachidonic acid by blood vessels and cultured endothelial cells. Thromb. Res. 1987;45:803–816. doi: 10.1016/0049-3848(87)90090-9. [DOI] [PubMed] [Google Scholar]
- 24.Tontonoz P., Nagy L., Alvarez J. G. A., Thomazy V. A., Evans R. M. PPARγ promotes monocyte/macrophage differentiation and uptake of oxidized LDL. Cell. 1998;93:241–252. doi: 10.1016/s0092-8674(00)81575-5. [DOI] [PubMed] [Google Scholar]
- 25.Wittwer J., Hersberger M. The two faces of the 15-lipoxygenase in atherosclerosis. Prostglandins Leukotriens Essential Fatty Acids. 2007;77:67–77. doi: 10.1016/j.plefa.2007.08.001. [DOI] [PubMed] [Google Scholar]