Abstract
Wnt signaling is important in many aspects of cell biology and development. In the mouse female reproductive tract, Wnt4, Wnt5a, and Wnt7a show differential expression during the estrus cycle, suggesting that they participate in female reproductive physiology. Although the pituitary is a major gland regulating reproduction, the molecular mechanism of Wnt signaling here is unclear. We elucidated the subcellular distribution of Wnt4 in the pituitary of estrogen-treated ovariectomized female rats. Expression of Wnt4 mRNA increased dramatically, particularly in proestrus compared with estrus and metestrus. Wnt4 protein was observed in the cytoplasm of almost all growth hormone (GH)-producing cells and in only a few thyroid-stimulating hormone β (TSHβ)-producing cells. In rat GH-producing pituitary tumor (MtT/S) cells, estrogen-induced expression of Wnt4 mRNA was completely inhibited by estrogen receptor antagonist ICI 182,780 in vitro. Thus, rat pituitary GH cells synthesize Wnt4 and this is induced by estrogen mediated via an estrogen receptor alpha-dependent pathway.
Keywords: Wnt signaling, pituitary gland, estrogen, estrus cycle, growth hormone
I. Introduction
Estrogens play various roles in the mammalian pituitary gland, affecting prolactin (PRL) and gonadotropin gene transcription, and are involved in lactotroph cell growth, which regulates the estrus cycle [35]. The biological actions of estrogens are mediated by binding to one of two specific estrogen receptors (ERs), ERα or ERβ, belonging to the nuclear receptor superfamily [24, 31]. ERα is highly expressed in the pituitary, ovary, uterus, testis, prostate, epididymis, kidney, and adrenal gland [21]. ERβ is highly expressed in the reproductive organs such as the rat ovary and prostate, but moderate expression is expressed in numerous other tissues including the testis and uterus, some of which also express ERα [20, 21]. ERα and ERβ can be dimerized in several forms such as ERα/ERα, ERα/ERβ, and ERβ/ERβ, which are able to bind to estrogen responsive elements (EREs) in the promoter region of various genes [5]. However, the physiological relevance of this ERα/ERβ interaction remains unclear [32].
The Wnt proteins are important regulators of the Wnt signaling pathway and have diverse roles in governing cell fate, proliferation, migration, polarity, and cell death [2]. The Wnt genes encode a family of 38–45 kDa secreted cysteine-rich proteins that lack transmembrane domains and are modified by N-linked glycosylation. The secreted Wnt proteins associate with extracellular matrix proteins on or near the cell surface and thus can exert autocrine or paracrine effects [17]. Frizzled (Fzd) proteins are receptors for the Wnt ligands, which resemble typical G protein coupled receptors [28]. Wnt signaling includes three different pathways: (i) the canonical Wnt/β-catenin pathway; (ii) the noncanonical Wnt/JNK pathway, involving regulation of tissue polarity and cell movement through the activation of RhoA, c-Jun N-terminal kinase (JNK), and nemo-like kinase (NLK) signaling cascades; and (iii) the noncanonical Wnt/Ca2+ pathway, involving activation of calcium/calmodulin-dependent kinase II (CamKII) and protein kinase C (PKC) [14, 16, 17, 19].
One member of the Wnt family of genes, Wnt4, is a growth factor that is known to be involved in multiple developmental processes, such as the formation of the kidney, adrenal gland, and mammary gland [13, 18, 39, 42, 43]. In the pituitary gland Wnt4−/− mutant mice repress the differentiation of growth hormone (GH)-, thyroid stimulating hormone (TSH)β- and glycoprotein hormone alpha-subunit (αGSU)-producing cells, and pituitary-specific positive transcription factor 1 (Pit-1)-positive cells during the embryonic stage [33, 41]. In human pituitary adenomas, we have previously shown that Wnt4 is highly expressed in GHomas, PRLomas, and TSHomas, all of which belong to the Pit-1 cell lineage of anterior pituitary cells [30]. Wnt5a is expressed at all stages of mammary development except during lactation and is required for posterior growth of the female reproductive tract [9, 25]. Wnt7a directly regulates the prenatal growth of the female reproductive tract and maintains proper uterine function in the adult mouse [26]. Wnt4, Wnt5a, and Wnt7a are expressed at high levels in the mouse female reproductive tract [27]. In particular, these Wnts have been reported to show differential expression during the estrus cycle in the murine uterus and the mammary gland and have been implicated as having important roles in the female reproductive system [27, 38]. The pituitary gland is a major gland that regulates the reproductive system, but the regulatory mechanism of Wnt signaling is unclear. These observations led us to investigate the role of estrogen in modulating the expression and molecular functions of Wnts including Wnt4, Wnt5a, and Wnt7a in the rat pituitary gland.
II. Materials and Methods
Animals and vaginal smears
Female 7-week-old Sprague Dawley rats were purchased from Charles River Japan, Inc. (Kanagawa, Japan). They were housed under a 12/12 hr light/dark cycle (lights on at 08:00 hr) at 22±2°C room temperature, with access to rat chow and tap water ad libitum. Animal usage and all procedures were approved by the Tokai University Institutional Animal Care and Use Committee. The estrus cycles of the animals were monitored through daily examination of vaginal cytology [34]. Rats were selected for experiments after exhibiting at least two normal 4-day estrus cycles.
Ovariectomy
After acclimation, the rats were divided into three groups using a randomized complete block design, as follows: sham-operated and treated with sesame oil (Sham+vehicle); ovariectomized treated with sesame oil (OVX+vehicle) and ovariectomized treated with estrogen (OVX+E2). Ovaries were removed while the rats were under Nembutal anesthesia (50 mg/kg, i.p.) at random stages of the estrus cycle. All animals were housed individually after surgery. The estrus cycle was checked daily by examining vaginal smears between 09:00 and 10:00 hr. Four weeks after sham operation or OVX, the animals received an intramuscular injection of estradiol dipropionate (E2, 300 mg/kg, Aska Pharmaceutical Co., Ltd., Tokyo, Japan) or sesame oil every other day for three doses. The rats were euthanized with ether 48 hr after the last treatment.
Cell culture and treatment
Rat somatotrophic pituitary tumor (MtT/S) cell lines (kindly provided by Professor Kinji Inoue, Saitama University, Saitama, Japan) were cultured in Dulbecco’s modified Eagle’s medium (DMEM)/F-12 (4.5 g/L glucose; Gibco BRL, Grand Island, NY, USA) supplemented with 10% horse serum (Bio Whittaker Inc., Walkersville, MD, USA), 2.5% fetal bovine serum (Gibco BRL) and 100 U/L penicillin/streptomycin (Gibco BRL) under an atmosphere of 5% CO2 and 95% air at 37°C. For estrogen time course studies, when cells reached about 70% confluency they were cultured in phenol red-free DMEM/F-12 supplemented with 10% dextran-charcoal-treated fetal bovine serum for 2 d. The MtT/S cells were seeded into 6-well plates at a density of 5×105 cells/well and cultured with 10−9 M of 17β-estradiol (Wako Pure Chemicals Industries, Ltd., Osaka, Japan) at 37°C for 2, 6 or 10 hr. For estrogen receptor antagonist studies, cells were grown as described above, 10−9 M of 17β-estradiol was added alone or with 10−8 M to 10−6 M of ICI 182,780 (Tocris Bioscience, Ellisville, MO, USA) at 37°C for 2 hr. Control cultures were exposed to medium containing 0.1% dimethyl sulfoxide (DMSO).
Real-time reverse transcription polymerase chain reaction (RT-PCR)
Total cellular RNA was isolated using the SV Total RNA Isolation System (Promega, Madison, WI, USA) supplemented with RNase-free DNase I, according to the manufacturer’s recommended protocol. One milligram of total RNA was converted to double-stranded cDNA using QuantiTect Reverse Transcription Kit (Qiagen, Valencia, CA, USA). For ERα and ERβ mRNA expression levels, PCR amplifications were performed as follows: 10 min at 94°C; followed by 30 cycles consisting of 30 sec at 94°C, 30 sec at 60°C and 30 sec at 72°C and then a final 10 min extension at 72°C. The PCR products were separated by electrophoresis on 2.0% agarose gels and detected under UV illumination. Quantification of Wnt4 and Wnt5a mRNA expression levels were done using real-time RT-PCR using a reaction mixture containing SYBR Green fluorescent dye (DyNAmo SYBR Green qPCR kit; Finnzymes, Espoo, Finland). Real-time RT-PCR analysis was done using a DNA Opticon system (MJ Research, Waltham, MA, USA). Primers used for PCR are summarized in Table 1. PCR primers were designed using Primer3 software (http://primer3.sourceforge.net/). The levels of Wnt4 and Wnt5a mRNA expression were normalized relative to that of the Gapdh gene. The reaction protocol consisted of the following cycles: 95°C for 10 min, 40 cycles of 94°C for 10 sec, 60°C for 15 sec and 72°C for 15 sec, followed by 72°C for 5 min. All PCR reactions were performed in triplicates.
Table 1.
Primer sequences used for RT-PCR
| Gene | Forward (5'–3') | Reverse (5'–3') | Product size (bp) |
|---|---|---|---|
| Wnt4 | CTGGAGAAGTGTGGCTGTGA | GGACTGTGAGAAGGCTACGC | 108 |
| Wnt5a | TGGCCACATTTTTCTCCTTC | GCAGAGAGGCTGTGCTCCTA | 119 |
| Wnt7a | CCCGAACCCTCATGAACTTA | TGTGGTCCAGCACGTCTTAG | 125 |
| ERα | CATCGATAAGAACCGGAGGA | CATCTCTCTGACGCTTGTGC | 134 |
| ERβ | TTCCCGGCAGCACCAGTAACC | TCCCTCTTTGCGTTTGGACTA | 262 |
| Gapdh | ATGACTCTACCCACGGCAAG | TACTCAGCACCAGCATCACC | 136 |
Immunohistochemistry
Pituitary glands were dissected out and fixed in ice-cold 4% paraformaldehyde in phosphate buffered saline (PBS) overnight at 4°C. The fixed tissues were dehydrated and embedded in paraffin wax using standard procedures. Tissue sections approximately 5 µm thick were deparaffinized in xylene and rehydrated in ethanol. The slides were then heated in an autoclave for 15 min in a Target Retrieval Solution (DAKO, Carpinteria, CA, USA) and cooled to room temperature for 20 min. Endogenous peroxidase activity was blocked with 0.3% hydrogen peroxide in methanol for 30 min. For double immunostaining of Wnt4 and hormones, the sections were incubated with goat anti-Wnt4 antibody (×400, ab15699; Abcam, Cambridge, UK). After overnight incubation at 4°C, polyclonal antibodies were labeled with biotinylated anti-goat IgG antibody (Vector Laboratories, Burlingame, CA, USA) followed by staining with streptavidin-Alexa Fluor 488 conjugate (Invitrogen, Carlsbad, CA, USA). Dual fluorescence labeling was then tested on the same section with an rabbit anti-GH antibody (National Hormone and Pituitary Program; NIDDK, Torrance, CA, USA) (dilution ×750), rabbit anti-PRL antibody (NIDDK) (×2000), rabbit anti-TSHβ antibody (NIDDK) (×2000), rabbit anti-ACTH antibody (NIDDK) (×1600), rabbit anti-LHβ antibody (NIDDK) (×500) and rabbit anti-αGSU antibody (NIDDK) (×800) overnight at 4°C. After washing in PBS, sections were incubated overnight with Alexa Fluor 594-labeled goat anti-rabbit IgG antibodies (Invitrogen). Finally, sections were mounted with TOTO-3 (Invitrogen) in 1,4-diazabicyclo[2.2.2]octane (DABCO; Sigma-Aldrich, St Louis, MO, USA). To examine colocalization of Wnt4 positive and hormone positive cells, sections were scanned using a confocal laser scanning microscope (LSM510META; Carl Zeiss, Jena, Germany). For double immunostaining of ERα and GH or TSHβ, the sections were incubated with anti-ERα antibody (×50, 1D5; DAKO). After overnight incubation at 4°C, the signal was amplified with DAKO ENVISION+ Kit (DAKO) according to the manufacture’s recommendations. Immunoreactivity was visualized by incubation with 3,3'-diaminobenzidine tetrahydrochloride. After thorough washing in PBS, the slides were subsequently incubated with rabbit anti-GH antibody (NIDDK) (×750) or rabbit anti-TSHβ antibody (NIDDK) (×2000) for 1 hr at room temperature. After washing in PBS, the slides were incubated with goat anti-rabbit IgG antibody conjugated with alkaline phosphatase (DAKO) followed by visualization with 5-bromo-4-chloro-3-indolyl phosphate and nitroblue tetrazolium chloride (BCIP/NBT; DAKO).
Absorption testing
Absorption tests on the rat normal pituitary gland were performed to confirm the specificity for Wnt4 commercial antibody. For the absorption test, Wnt4 antibody (2.5 µg/mL) was incubated with the blocking peptide (ab23258; Abcam: 0.004, 0.02, 0.1, 0.5 µg/mL, respectively) at 4°C overnight; then the complex was centrifuged and the supernatant was passed through a 0.45-µm filter before incubation.
Statistical analysis
Statistical analysis was performed using a one-way ANOVA with Tukey’s multiple comparison test by StatMate III software (ATMS, Tokyo, Japan) and P<0.05 was assumed significant.
III. Results
Absorption testing
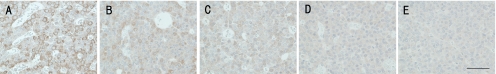
The specificity for Wnt4 was confirmed by an absorption test using a rat normal pituitary gland. Immunoreactivity was considerably decreased by the absorption (Fig. 1).
Fig. 1.
Absorption testing. One-milliliter aliquots of anti-Wnt4 antibody solution (2.5 µg/mL) were reacted with (A) 0 µg, (B) 0.004 µg, (C) 0.02 µg, (D) 0.1 µg or (E) 0.5 µg of synthetic Wnt4 peptide (Abcam) and then the reacted solution was applied to sections of rat normal pituitary gland. Original magnification ×400; Bar=100 µm. Note the dose-dependent decrease in staining by absorption.
Colocalization of Wnt4 with pituitary hormones in pituitary glands of sham-operated rats
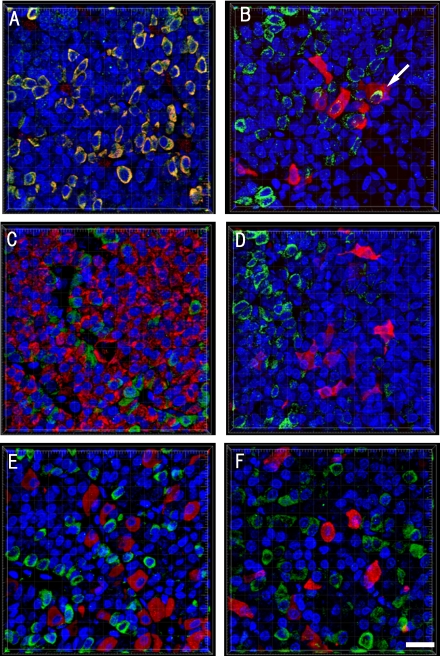
Colocalizations of Wnt4 and pituitary hormones were detected by double immunofluorescence staining in pituitary glands (Fig. 2). Wnt4 proteins were expressed in almost all GH-producing cells and in only a few of TSHβ-producing cells, whereas no other hormone-producing cells expressed Wnt4.
Fig. 2.
Colocalization of Wnt4 with pituitary hormones in pituitary glands of sham-operated rats. Expression of Wnt4 and pituitary hormones shown by double immunofluorescence staining with confocal microscopy, stained for Wnt4 (green) and (A) GH (red), (B) TSHβ, (C) PRL, (D) ACTH, (E) LHβ or (F) αGSU. Nuclei were stained with TOTO-3 (blue). Wnt4 was expressed in almost all growth hormone (GH)-producing cells, while only a few of Wnt4-positive cells were also expressed in thyroid stimulating hormone (TSH)β-producing cells (see arrow). Original magnification ×630; Bar=20 µm.
Colocalization of ERα with GH- or TSHβ-producing cells in pituitary glands of sham-operated rats
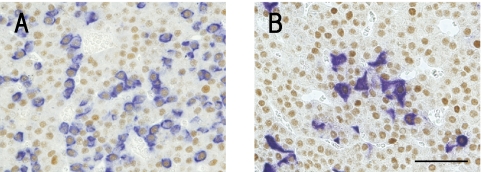
Colocalizations of ERα with GH- or TSHβ-producing cells were detected by immunohistochemical staining in pituitary glands. ERα proteins were expressed in both GH- and TSHβ-producing cells (Fig. 3).
Fig. 3.
Colocalization of estrogen receptor alpha (ERα) with GH- or TSHβ-producing cells in pituitary glands of sham-operated rats. Expression of ERα and (A) GH- and (B) TSHβ-producing cells shown by double immunohistochemical staining, stained for ERα (brown) and GH- and TSHβ-producing cells (blue).
Effects of estrogen on Wnt4 and Wnt5a mRNA expression levels in rat pituitary glands
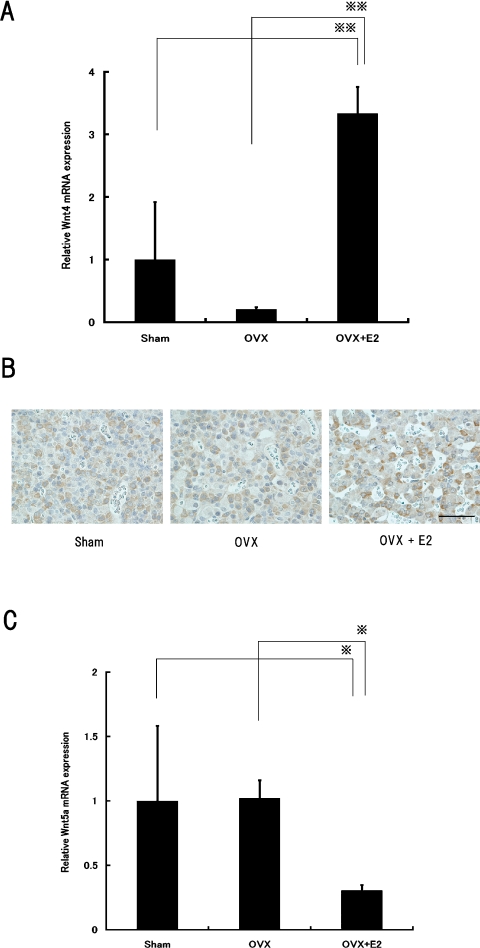
First, we analyzed the expression levels of the Wnt4, Wnt5a and Wnt7a mRNA in the anterior pituitary gland by RT-PCR. Only the expression of Wnt7a mRNA was not detected (data not shown). Therefore, we examined the effects of estrogen on the expression of Wnt4 and Wnt5a mRNA in rat pituitary by real-time PCR. Treatments of OVX rats with estrogen produced a 16-fold increase in Wnt4 mRNA expression compared with OVX+vehicle group (Fig. 4A) and these results were further supported by immunocytochemistry (Fig. 4B). Wnt5a mRNA expression levels were 3.3-fold decreased by treatment of OVX rats with estrogen in comparison with the OVX+vehicle group (Fig. 4C).
Fig. 4.
Effect of estrogen on Wnt4 and Wnt5a mRNA expression levels in rat pituitary glands. Expression levels of (A) Wnt4 and (C) Wnt5a mRNA in 4-weeks ovariectomized rats injected with estrogen for 7 d were measured by real-time reverse transcription polymerase chain reaction (RT-PCR), then normalized to Gapdh as an internal standard. Data are expressed as fold changes from the sham-operated control group. Each value represents the mean±SD of six animals per group. *P<0.01, **P<0.001. (B) Expression of Wnt4 protein was analyzed by immunohistochemical staining. Original magnification ×400. Bar=100 µm.
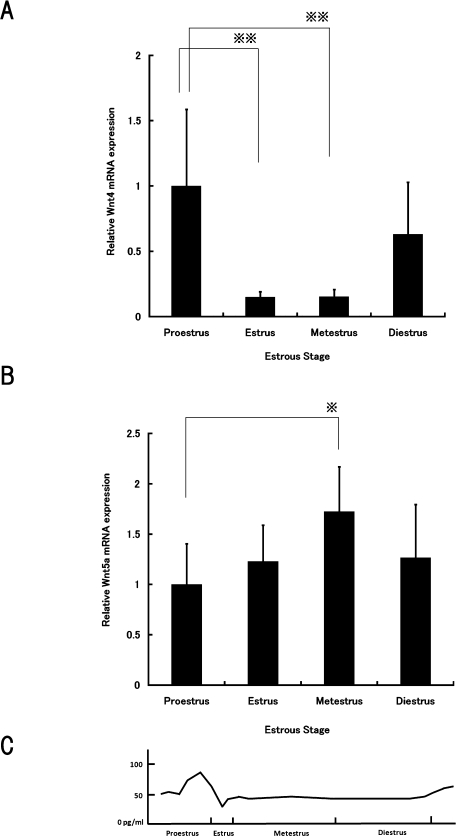
Wnt4 and Wnt5a mRNA levels during the estrus cycle
Based on the above data, we determined whether the levels of Wnt4 and Wnt5a mRNA expression differed in the rat pituitary at four stages of the estrus cycle, proestrus, estrus, metestrus and diestrus, using real-time RT-PCR. As expected, Wnt4 mRNA levels were significantly higher during proestrus than during estrus and metestrus (Fig. 5A). Wnt5a mRNA levels were significantly higher during metestrus than during proestrus (Fig. 5B). As also shown in Figure 5C, a significant elevation of plasma estrogen level in mature rats has been reported in the proestrus state [36, 37].
Fig. 5.
Wnt4 and Wnt5a mRNA levels during the estrus cycle. Expression levels of (A) Wnt4 and (B) Wnt5a mRNA in pituitary in the different stages of estrus cycle were measured by real-time RT-PCR, then normalized to Gapdh as an internal standard. Data are expressed as fold change from proestrus. Each value represents the mean±SD of eight animals per group. *P<0.01, **P<0.001. (C) The concentration of plasma estrogen in Sprague Dawley rats peaks in proestrus [36, 37].
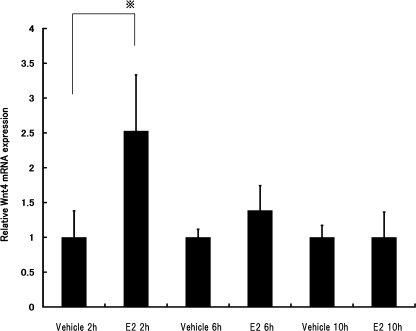
Time-dependent effect of E2 on Wnt4 mRNA expression in rat somatotrophic pituitary tumor (MtT/S) cells
To study the effects of estrogens on the expression of Wnt4 mRNA in rat pure somatotrophic MtT/S cells, they were treated with E2 at 10−9 M for 2 hr, 6 hr and 10 hr. Figure 6 shows that after E2 treatment for 2 hr, there was a significant increase in Wnt4 mRNA expression (P<0.05).
Fig. 6.
Time-dependent effect of E2 on Wnt4 mRNA expression in rat somatotrophic pituitary tumor (MtT/S) cells. Cells were treated with E2 at 10−9 M for 2, 6 and 10 hr. Expression levels of Wnt4 mRNA in MtT/S cells were measured by real-time RT-PCR, then normalized to Gapdh as an internal standard. Each value represents the mean±SD of three per group. *P<0.05.
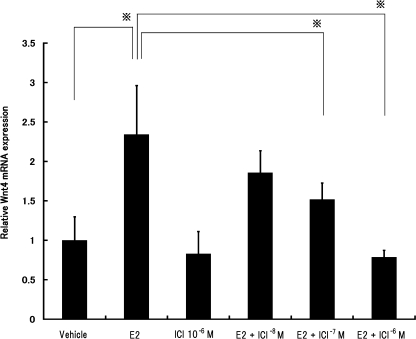
The pure ER antagonist ICI 182,780 reverses the effects of E2 on the expression of Wnt4 mRNA in MtT/S cells
If estrogen induces Wnt4 expression in an ERα- or ERβ-dependent manner, blocking these receptors should affect Wnt4 expression after treatment with ICI 182,780 (Fig. 7). MtT/S cells were treated with 10−9 M E2 alone or in the presence of 10−8 M to 10−6 M ICI 182,780 for 2 hr. E2 treatment increased Wnt4 mRNA levels compared with controls, while treatment of ICI 182,780 dose-dependently decreased Wnt4 mRNA expressions compared with E2 treatment alone (P<0.001).
Fig. 7.
The pure ER antagonist ICI 182,780 reverses the effects of E2 on the expression of Wnt4 mRNA in MtT/S cells. Cells were incubated with 10−9 M E2 and/or 10−8 M to 10−6 M ICI 182,780 for 2 hr. Expression levels of Wnt4 mRNA in MtT/S cells were measured by real-time RT-PCR, then normalized to Gapdh as an internal standard. Data are expressed as fold changes from vehicle control. Each value represents the mean±SD of seven per group. *P<0.001.
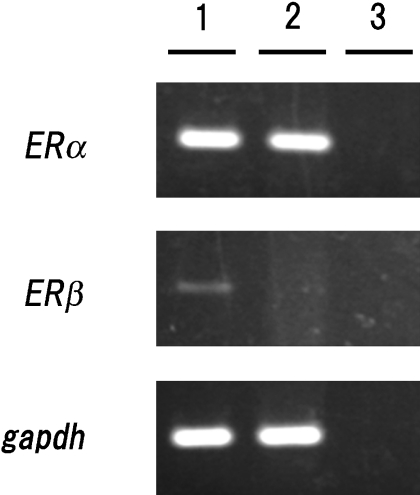
Detection of ERα and ERβ mRNA in MtT/S cells
ERα and ERβ mRNA levels in MtT/S cells were measured by RT-PCR. MtT/S cells did not express ERβ mRNA but only ERα mRNA (Fig. 8). PCR products of ERα, ERβ and Gapdh were detected at 134, 262 and 136 bp, respectively.
Fig. 8.
Detection of ERα and ERβ mRNA expression in MtT/S cells by RT-PCR analysis. Lane 1, normal rat anterior pituitary gland (positive control); lane 2, MtT/S cells; lane 3, no template cDNA (negative control). Top panel: ERα expression was detected as a 134 bp fragment. Middle panel: ERβ expression was detected as a 262 bp fragment. Bottom panel: Gapdh expression was detected as a 136 bp fragment. All products were amplified for 30 cycles.
IV. Discussion
In this study, we showed that estrogen directly upregulates Wnt4 gene expression in almost all GH-producing cells in the rat pituitary in an ER-dependent manner, but in only a few of the TSHβ-producing cells. Wnt4 mRNA expression level peaked in response to estrogen stimulation during proestrus, because the concentration of plasma estrogen in rats is highest at this stage [36, 37]. Previous reports have been shown that Wnt4 and Wnt5a gene expression levels are regulated by estrogen in the mouse uterine epithelium [12, 15]. However, we obtained different results in the anterior pituitary gland, where the expression of Wnt5a mRNA was downregulated by estrogen treatment and was not dramatically changed during the estrus cycle. Interestingly, previous studies have shown that Wnt-5a misexpression delays chondrocyte maturation, while Wnt-4 misexpression accelerates both chondrocyte and perichondrium maturation. Thus, these Wnts have opposing effects on the differentiation of chondrocytes [11].
It has been suggested that Wnt proteins are associated with the extracellular matrix and can interact with neighboring cells [17]. Therefore, this character of Wnts permits us to speculate that Wnt4 exerts autocrine/paracrine effects. Although Wnt signaling pathways are determined by the interaction between Wnt ligand and the Fzd receptor, detailed investigations are limited. Lyons et al. reported that Wnt4 showed interaction with the CRD on the N terminus of Fzd6 in MDCK cells [23]. Wnt4 activates both canonical and noncanonical Wnt signaling pathways, while FZD6 functions as a negative regulator of canonical Wnt signaling [10]. We have shown that Fzd6 in the normal human pituitary gland is expressed in gonadotrophs [30]. However, we could not analyze the presence of Fzd6 in the rat pituitary gland by immunohistochemistry because no antibodies against rat Fzd6 proteins are available for working with paraffin-embedded tissues. The signaling pathways of Wnt4 in the rat pituitary gland remain to be investigated further.
The function of Wnt4 in the mature pituitary gland is unknown. In the mouse mammary gland during pregnancy, the expression of Wnt4 is increased by progesterone, which also induces arborization and hypertrophy of the mammary ducts [1]. Wnt4 also shows differential expression patterns during the estrus cycle in the murine mammary gland and the uterus [12, 27, 38]. These results have led to speculation that the pituitary Wnt4 might be involved in the control of reproduction related to the estrus cycle as well as in the uterus and mammary gland. On the other hand, Wnt4−/− mutant mice show a dramatic decrease in the number of GH-, TSHβ-, αGSU-producing cells and Pit-1-positive cells during the embryonic stage [33, 41]. In human pituitary adenomas, Wnt4 protein is highly expressed in GHomas, PRLomas and TSHomas, all of which belong to the Pit-1 cell lineage of anterior pituitary cells [30]. Pit-1 is essential for differentiation and expansion of GH-, PRL- and TSH-producing cells [3, 7, 22, 29]. Therefore, as another function in the pituitary, Wnt4 might regulate cell proliferation and differentiation in Pit-1 cell linage.
Hou et al. have shown that estrogen rapidly induces Wnt4 expression in the murine uterus in an ER-independent manner [12]. Therefore, to analyze whether the induction of Wnt-4 by estrogen is a direct effect of ER-independent action on pituitary GH cells, we treated GH-producing MtT/S cells in culture with estrogen alone or in combination with ICI 182,780 (Fig. 7). In contrast to previous reports, Wnt4 expression was inhibited by ICI 182,780 treatment. Besides, MtT/S cells do not express ERβ but only ERα. Our results indicate that Wnt4 expression by estrogen is mediated via an ERα-dependent pathway in pituitary GH cells.
We have shown that ERα is critical for Wnt4 mRNA expression in GH-producing cells. Although ERα was expressed in GH-producing cells, PRL-producing cells and TSHβ-producing cells belonging to the Pit-1 cell lineage, Wnt4 was not expressed in PRL-producing cells. This differential expression of Wnt4 among these cells might be related to the epigenetic control mechanism of Wnt4 genes in PRL-producing cells. Recently, Elston et al. have reported that Wnt inhibitory factor 1 (WIF1) was significantly decreased in pituitary adenomas by methylation at the CpG island of WIF-1 promoter [8]. Interestingly, we have found (unpublished observations) a CpG island on the immediate promoter of Wnt4 using Methyl Primer Express® software (Applied Biosystems, Foster City, CA, USA). Thus, unexpressed Wnt4 in PRL-producing cells might result from methylation of this CpG island of the Wnt4 promoter, but further investigations are required.
Here we found that the Wnt4 protein was localized to almost all GH-producing cells. It is conceivable that estrogen induced Wnt4 secretion from these cells. Estrogen increases the percentage of GH mRNA and pituitary GH cells in female rats during diestrus and also increases GH mRNA transcription and GH release through ER-dependent pathway in rat pituitary GH3 cells [4, 6]. In contrast to increases in GH cells, estrogen exerts a rapid apoptotic response through membrane-associated ERs in lactotropes and somatotropes [44]. Thus, the expression of Wnt4 is one of the diverse effects of estrogen on GH-producing cells. Furthermore, we also found that Wnt4 was expressed in a small number of TSHβ-producing cells, primarily localized in the perinuclear region. Subcellular localization of Wnt4 protein in TSH-producing cells is obviously different from GH-producing cells. In the endoplasmic reticulum, Wnt3a is modified by the addition of the mono-unsaturated fatty acid, palmitoleic acid, at Ser209, which is required for transport to the Golgi apparatus for Wnt3a secretion [40]. Therefore, we speculated that failure of lipid modification in TSH-producing cells leads to retention of Wnt4 in the endoplasmic reticulum. Wnt4 expression in TSH-producing cells might not be important physiologically in the pituitary gland.
The precise mechanism(s) by which estrogen induces Wnt4 gene expression is not known at present. In general, estrogen is known to mediate transcription through specific sequences called EREs. Therefore, we speculate that EREs exist in the 5'-flanking region of the Wnt4 gene. The promoter sequence of Wnt4 does not contain consensus EREs; however, one putative ERE was found in the Wnt4 immediate promoter within 1.2 kb of the major transcription start site, using Match-1.0 Public website (http://www.gene-regulation.com/) (unpublished observations). Accordingly, Wnt4 upregulation by estrogen may be an ER/ERE-dependent pathway, but further investigations are required.
In conclusion, we have demonstrated that the expression of Wnt4 in pituitary GH cells is directly regulated by estrogen, which is mediated in an ERα-dependent manner. Thus, Wnt4 might play an important role in pituitary reproductive physiology related to the control of the estrus cycle.
V. Acknowledgments
The authors wish to thank Johbu Itoh and Hideo Tsukamoto from the Teaching and Research Support Center, Tokai University School of Medicine for their technical support. This work was supported by Grants-in-Aid for Scientific Research Projects (#19390105) from the Ministry of Education Culture, Sports, Science and Technology, Japan and by the Research on Measures for Intractable Diseases Project of the Hypothalamo-Pituitary Dysfunction Research Group of the Ministry of Health, Labor and Welfare, Japan.
VI. References
- 1.Brisken C., Heineman A., Chavarria T., Elenbaas B., Tan J., Dey S. K., McMahon J. A., McMahon A. P., Weinberg R. A. Essential function of Wnt-4 in mammary gland development downstream of progesterone signaling. Genes Dev. 2000;14:650–654. [PMC free article] [PubMed] [Google Scholar]
- 2.Cadigan K. M., Nusse R. Wnt signaling: A common theme in animal development. Genes Dev. 1997;11:3286–3305. doi: 10.1101/gad.11.24.3286. [DOI] [PubMed] [Google Scholar]
- 3.Camper S. A., Saunders T. L., Katz R. W., Reeves R. H. The pit-1 transcription factor gene is a candidate for the murine snell dwarf mutation. Genomics. 1990;8:586–590. doi: 10.1016/0888-7543(90)90050-5. [DOI] [PubMed] [Google Scholar]
- 4.Childs G. V., Iruthayanathan M., Akhter N., Unabia G., Whitehead-Johnson B. Bipotential effects of estrogen on growth hormone synthesis and storage in vitro. Endocrinology. 2005;146:1780–1788. doi: 10.1210/en.2004-1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cowley S. M., Hoare S., Mosselman S., Parker M. G. Estrogen receptors alpha and beta form heterodimers on DNA. J. Biol. Chem. 1997;272:19858–19862. doi: 10.1074/jbc.272.32.19858. [DOI] [PubMed] [Google Scholar]
- 6.Dang V. H., Nguyen T. H., Lee G. S., Choi K. C., Jeung E. B. In vitro exposure to xenoestrogens induces growth hormone transcription and release via estrogen receptor-dependent pathways in rat pituitary GH3 cells. Steroids. 2009;74:707–714. doi: 10.1016/j.steroids.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 7.Egashira N., Minematsu T., Miyai S., Takekoshi S., Camper S. A., Osamura R. Y. Pituitary changes in Prop1 transgenic mice: hormone producing tumors and signet-ring type gonadotropes. Acta Histochem. Cytochem. 2008;41:47–57. doi: 10.1267/ahc.08007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elston M. S., Gill A. J., Conaglen J. V., Clarkson A., Shaw J. M., Law A. J., Cook R. J., Little N. S., Clifton-Bligh R. J., Robinson B. G., McDonald K. L. Wnt pathway inhibitors are strongly down-regulated in pituitary tumors. Endocrinology. 2008;149:1235–1242. doi: 10.1210/en.2007-0542. [DOI] [PubMed] [Google Scholar]
- 9.Gavin B. J., McMahon A. P. Differential regulation of the Wnt gene family during pregnancy and lactation suggests a role in postnatal development of the mammary gland. Mol. Cell. Biol. 1992;12:2418–2423. doi: 10.1128/mcb.12.5.2418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Golan T., Yaniv A., Bafico A., Liu G., Gazit A. The human Frizzled 6 (HFz6) acts as a negative regulator of the canonical Wnt/β-catenin signaling cascade. J. Biol. Chem. 2004;279:14879–14888. doi: 10.1074/jbc.M306421200. [DOI] [PubMed] [Google Scholar]
- 11.Hartmann C., Tabin C. J. Dual roles of Wnt signaling during chondrogenesis in the chicken limb. Development. 2000;127:3141–3159. doi: 10.1242/dev.127.14.3141. [DOI] [PubMed] [Google Scholar]
- 12.Hou X., Tan Y., Li M., Dey S. K., Das S. K. Canonical Wnt signaling is critical to estrogen-mediated uterine growth. Mol. Endocrinol. 2004;18:3035–3049. doi: 10.1210/me.2004-0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jeays-Ward K., Dandonneau M., Swain A. Wnt4 is required for proper male as well as female sexual development. Dev. Biol. 2004;276:431–440. doi: 10.1016/j.ydbio.2004.08.049. [DOI] [PubMed] [Google Scholar]
- 14.Kakinuma N., Nishimura Y., Akiyama T., Senda T. APC is colocalized with β-catenin and hDLG in Henle’s loop of the mouse kidney. Acta Histochem. Cytochem. 2000;33:457–463. [Google Scholar]
- 15.Katayama S., Ashizawa K., Fukuhara T., Hiroyasu M., Tsuzuki Y., Tatemoto H., Nakada T., Nagai K. Differential expression patterns of Wnt and beta-catenin/TCF target genes in the uterus of immature female rats exposed to 17alpha-ethynyl estradiol. Toxicol. Sci. 2006;91:419–430. doi: 10.1093/toxsci/kfj167. [DOI] [PubMed] [Google Scholar]
- 16.Katoh M. WNT/PCP signaling pathway and human cancer (review) Oncol. Rep. 2005;14:1583–1588. [PubMed] [Google Scholar]
- 17.Kikuchi A., Yamamoto H., Kishida S. Multiplicity of the interactions of Wnt proteins and their receptors. Cell Signal. 2007;19:659–671. doi: 10.1016/j.cellsig.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 18.Kispert A., Vainio S., McMahon A. P. Wnt-4 is a mesenchymal signal for epithelial transformation of metanephric mesenchyme in the developing kidney. Development. 1998;125:4225–4234. doi: 10.1242/dev.125.21.4225. [DOI] [PubMed] [Google Scholar]
- 19.Kuhl M., Sheldahl L. C., Park M., Miller J. R., Moon R. T. The Wnt/Ca2+ pathway: A new vertebrate Wnt signaling pathway takes shape. Trends Genet. 2000;16:279–283. doi: 10.1016/s0168-9525(00)02028-x. [DOI] [PubMed] [Google Scholar]
- 20.Kuiper G. G., Enmark E., Pelto-Huikko M., Nilsson S., Gustafsson J. A. Cloning of a novel receptor expressed in rat prostate and ovary. Proc. Natl. Acad. Sci. U S A. 1996;93:5925–5930. doi: 10.1073/pnas.93.12.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuiper G. G., Carlsson B., Grandien K., Enmark E., Haggblad J., Nilsson S., Gustafsson J. A. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta. Endocrinology. 1997;138:863–870. doi: 10.1210/endo.138.3.4979. [DOI] [PubMed] [Google Scholar]
- 22.Li S., Crenshaw E. B., 3rd., Rawson E. J., Simmons D. M., Swanson L. W., Rosenfeld M. G. Dwarf locus mutants lacking three pituitary cell types result from mutations in the POU-domain gene pit-1. Nature. 1990;347:528–533. doi: 10.1038/347528a0. [DOI] [PubMed] [Google Scholar]
- 23.Lyons J. P., Mueller U. W., Ji H., Everett C., Fang X., Hsieh J. C., Barth A. M., McCrea P. D. Wnt-4 activates the canonical β-catenin-mediated Wnt pathway and binds Frizzled-6 CRD: functional implications of Wnt/β-catenin activity in kidney epithelial cells. Exp. Cell Res. 2004;298:369–387. doi: 10.1016/j.yexcr.2004.04.036. [DOI] [PubMed] [Google Scholar]
- 24.Matthews J., Gustafsson J. A. Estrogen signaling: A subtle balance between ER alpha and ER beta. Mol. Interv. 2003;3:281–292. doi: 10.1124/mi.3.5.281. [DOI] [PubMed] [Google Scholar]
- 25.Mericskay M., Kitajewski J., Sassoon D. Wnt5a is required for proper epithelial-mesenchymal interactions in the uterus. Development. 2004;131:2061–2072. doi: 10.1242/dev.01090. [DOI] [PubMed] [Google Scholar]
- 26.Miller C., Sassoon D. A. Wnt-7a maintains appropriate uterine patterning during the development of the mouse female reproductive tract. Development. 1998;125:3201–3211. doi: 10.1242/dev.125.16.3201. [DOI] [PubMed] [Google Scholar]
- 27.Miller C., Pavlova A., Sassoon D. A. Differential expression patterns of Wnt genes in the murine female reproductive tract during development and the estrous cycle. Mech. Dev. 1998;76:91–99. doi: 10.1016/s0925-4773(98)00112-9. [DOI] [PubMed] [Google Scholar]
- 28.Miller J. R. The Wnts. Genome Biol. 2002;3:REVIEWS3001. doi: 10.1186/gb-2001-3-1-reviews3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miyai S., Itoh J., Kajiya H., Takekoshi S., Osamura R. Y. Pit-1 gene inhibition using small interfering RNAs in rat pituitary GH secreting cell line. Acta Histochem. Cytochem. 2005;38:107–114. [Google Scholar]
- 30.Miyakoshi T., Takei M., Kajiya H., Egashira N., Takekoshi S., Teramoto A., Osamura R. Y. Expression of Wnt4 in human pituitary adenomas regulates activation of the beta-catenin-independent pathway. Endocr. Pathol. 2008;19:261–273. doi: 10.1007/s12022-008-9048-9. [DOI] [PubMed] [Google Scholar]
- 31.Miyakoshi T., Miyajima K., Takekoshi S., Osamura R. Y. The influence of endocrine disrupting chemicals on the proliferation of ERalpha knockdown-human breast cancer cell line MCF-7; new attempts by RNAi technology. Acta Histochem. Cytochem. 2009;42:23–28. doi: 10.1267/ahc.08036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mueller S. O. Xenoestrogens: Mechanisms of action and detection methods. Anal. Bioanal. Chem. 2004;378:582–587. doi: 10.1007/s00216-003-2238-x. [DOI] [PubMed] [Google Scholar]
- 33.Potok M. A., Cha K. B., Hunt A., Brinkmeier M. L., Leitges M., Kispert A., Camper S. A. WNT signaling affects gene expression in the ventral diencephalon and pituitary gland growth. Dev. Dyn. 2008;237:1006–1020. doi: 10.1002/dvdy.21511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schwartz N. B. Acute effects of ovariectomy on pituitary LH, uterine weight, and vaginal cornification. Am. J. Physiol. 1964;207:1251–1259. doi: 10.1152/ajplegacy.1964.207.6.1251. [DOI] [PubMed] [Google Scholar]
- 35.Scully K. M., Gleiberman A. S., Lindzey J., Lubahn D. B., Korach K. S., Rosenfeld M. G. Role of estrogen receptor-alpha in the anterior pituitary gland. Mol. Endocrinol. 1997;11:674–681. doi: 10.1210/mend.11.6.0019. [DOI] [PubMed] [Google Scholar]
- 36.Shaikh A. A. Estrone and estradiol levels in the ovarian venous blood from rats during the estrous cycle and pregnancy. Biol. Reprod. 1971;5:297–307. doi: 10.1093/biolreprod/5.3.297. [DOI] [PubMed] [Google Scholar]
- 37.Shaikh A. A., Shaikh S. A. Adrenal and ovarian steroid secretion in the rat estrous cycle temporally related to gonadotropins and steroid levels found in peripheral plasma. Endocrinology. 1975;96:37–44. doi: 10.1210/endo-96-1-37. [DOI] [PubMed] [Google Scholar]
- 38.Silberstein G. B., Van Horn K., Hrabeta-Robinson E., Compton J. Estrogen-triggered delays in mammary gland gene expression during the estrous cycle: Evidence for a novel timing system. J. Endocrinol. 2006;190:225–239. doi: 10.1677/joe.1.06725. [DOI] [PubMed] [Google Scholar]
- 39.Stark K., Vainio S., Vassileva G., McMahon A. P. Epithelial transformation of metanephric mesenchyme in the developing kidney regulated by Wnt-4. Nature. 1994;372:679–683. doi: 10.1038/372679a0. [DOI] [PubMed] [Google Scholar]
- 40.Takada R., Satomi Y., Kurata T., Ueno N., Norioka S., Kondoh H., Takao T., Takada S. Monounsaturated fatty acid modification of Wnt protein: its role in Wnt secretion. Dev. Cell. 2006;11:791–801. doi: 10.1016/j.devcel.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 41.Treier M., Gleiberman A. S., O’Connell S. M., Szeto D. P., McMahon J. A., McMahon A. P., Rosenfeld M. G. Multistep signaling requirements for pituitary organogenesis in vivo. Genes Dev. 1998;12:1691–1704. doi: 10.1101/gad.12.11.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vainio S., Heikkila M., Kispert A., Chin N., McMahon A. P. Female development in mammals is regulated by Wnt-4 signalling. Nature. 1999;397:405–409. doi: 10.1038/17068. [DOI] [PubMed] [Google Scholar]
- 43.Yu H., Pask A. J., Shaw G., Renfree M. B. Differential expression of WNT4 in testicular and ovarian development in a marsupial. BMC Dev. Biol. 2006;6:44. doi: 10.1186/1471-213X-6-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zarate S., Jaita G., Zaldivar V., Radl D. B., Eijo G., Ferraris J., Pisera D., Seilicovich A. Estrogens exert a rapid apoptotic action in anterior pituitary cells. Am. J. Physiol. Endocrinol. Metab. 2009;296:664–671. doi: 10.1152/ajpendo.90785.2008. [DOI] [PubMed] [Google Scholar]