Should whole brain radiation therapy (WBRT) be used as the sole therapy in patients with newly-diagnosed, surgically accessible, single brain metastases, compared with WBRT plus surgical resection, and in what clinical settings?
Target population
This recommendation applies to adults with newly diagnosed single brain metastases amenable to surgical resection; however, the recommendation does not apply to relatively radiosensitive tumors histologies (i.e., small cell lung cancer, leukemia, lymphoma, germ cell tumors and multiple myeloma).
Recommendation
Surgical resection plus WBRT versus WBRT alone
Level 1 Class I evidence supports the use of surgical resection plus post-operative WBRT, as compared to WBRT alone, in patients with good performance status (functionally independent and spending less than 50% of time in bed) and limited extra-cranial disease. There is insufficient evidence to make a recommendation for patients with poor performance scores, advanced systemic disease, or multiple brain metastases.
If WBRT is used, is there an optimal dosing/fractionation schedule?
Target population
This recommendation applies to adults with newly diagnosed brain metastases.
Recommendation
Level 1 Class I evidence suggests that altered dose/fractionation schedules of WBRT do not result in significant differences in median survival, local control or neurocognitive outcomes when compared with “standard” WBRT dose/fractionation. (i.e., 30 Gy in 10 fractions or a biologically effective dose (BED) of 39 Gy10).
If WBRT is used, what impact does tumor histopathology have on treatment outcomes?
Target population
This recommendation applies to adults with newly diagnosed brain metastases.
Recommendation
Given the extremely limited data available, there is insufficient evidence to support the choice of any particular dose/fractionation regimen based on histopathology.
The following question is fully addressed in the surgery guideline paper within this series by Kalkanis et al. Given that the recommendation resulting from the systematic review of the literature on this topic is also highly relevant to the discussion of the role of WBRT in the management of brain metastases, this recommendation has been included below.
Does the addition of WBRT after surgical resection improve outcomes when compared with surgical resection alone?
Target population
This recommendation applies to adults with newly diagnosed single brain metastases amenable to surgical resection.
Recommendation
Surgical resection plus WBRT versus surgical resection alone
Level 1 Surgical resection followed by WBRT represents a superior treatment modality, in terms of improving tumor control at the original site of the metastasis and in the brain overall, when compared to surgical resection alone.
Keywords: Brain metastases, Whole brain radiation therapy, Radiotherapy, Surgical resection, Fractionation, Histopathology, Systematic review, Practice guideline
Rationale
Whole-brain radiation therapy (WBRT) has long been a standard treatment for patients with brain metastases. Based on preclinical and observational data, some physicians alter dose fractionation or withhold WBRT based upon tumor histology.
This paper will systematically review the evidence available for altered WBRT dose fractionation and the impact of tumor histopathology on treatment outcomes when WBRT is used. In addition, this paper will also systematically review the evidence for the use of surgical resection plus WBRT compared with WBRT alone in patients with newly diagnosed, surgically accessible, single brain metastases. The studies identified through this process will be used to make evidence-based recommendations for the role of WBRT in the management of patients with newly diagnosed brain metastases.
As WBRT has been a mainstay of the treatment approach for patients with brain metastases, several other papers in this guideline series also include comparisons and recommendations regarding the use of WBRT for this patient population. Of particular note are the papers by Kalkanis et al. [1], (surgical resection) and Linskey et al. [2], (stereotactic radiosurgery) for patients with newly diagnosed brain metastases.
Methods
Search strategy
The following electronic databases were searched from 1990 to September 2008: MEDLINE®, Embase®, Cochrane Database of Systematic Reviews, Cochrane Controlled Trials Registry, and Cochrane Database of Abstracts of Reviews of Effects. A broad search strategy using a combination of subheadings and text words was employed. The search strategy is documented in the methodology paper for this guideline series by Robinson et al. [3] Reference lists of included studies were also reviewed.
Eligibility criteria
- For WBRT versus surgical resection plus WBRT question:
- Published in English with a publication date of 1990 forward.
- Patients with newly diagnosed single brain metastases.
- Fully published peer-reviewed primary comparative studies (all comparative study designs for primary data collection included; e.g., randomized controlled trials (RCTs), non-randomized trials, cohort studies or case–control studies)
- Study comparisons include: WBRT versus surgery + WBRT
- Number of participants with newly diagnosed brain metastases >5 per study arm
- Baseline information on study participants is provided by treatment group in studies evaluating interventions exclusively in patients with newly diagnosed brain metastases. For studies with mixed populations (i.e., includes participants with conditions other than newly diagnosed brain metastases), baseline information is provided for the intervention sub-groups of participants with newly diagnosed brain metastases.
- For optimal dosing/fractionation schedule for WBRT question:
- Published in English.
- Patients with newly diagnosed brain metastases.
- Fully published peer-reviewed primary comparative studies (all comparative study designs for primary data collection included; e.g., RCT, non-randomized trials, cohort studies or case–control studies) for studies published 1990 forward; RCTs published 1970 forward.
- Study comparisons include: WBRT dose/fractionation schedule 1 versus WBRT dose/fractionation schedule 2
- Number of participants with newly diagnosed brain metastases >5 per study arm.
- Baseline information on study participants is provided by treatment group in studies evaluating interventions exclusively in patients with newly diagnosed brain metastases. For studies with mixed populations (i.e., includes participants with conditions other than newly diagnosed brain metastases), baseline information is provided for the intervention sub-groups of participants with newly diagnosed brain metastases.
- For whether tumor histopathology has an impact on WBRT treatment outcomes?
- Published in English with a publication date of 1990 forward.
- Patients with newly diagnosed brain metastases.
- Fully published peer-reviewed primary studies (all study designs for primary data collection included; e.g., RCT, non-randomized trials, cohort studies, case–control studies or case series).
- Any study evaluating the outcome(s) of WBRT by tumor histopathology (or primary tumor type).
- Number of participants with newly diagnosed brain metastases >5 per study arm for comparative studies and >5 overall for non-comparative studies.
- For studies evaluating the outcome(s) of WBRT by histopathology (or primary tumor type) exclusively in patients with newly diagnosed brain metastases, baseline characteristics are presented and stratified by histologic/primary tumor group. For studies with mixed populations (i.e., includes participants with conditions other than newly diagnosed brain metastases), baseline characteristics are presented and stratified by histologic/primary tumor group for the sub-group of participants with newly diagnosed brain metastases.
Study selection and quality assessment
Two independent reviewers evaluated citations using a priori criteria for relevance and documented decisions in standardized forms. Cases of disagreement were resolved by a third reviewer. The same methodology was used for full text screening of potentially relevant papers. Studies which met the eligibility criteria were data extracted by one reviewer and the extracted information was checked by a second reviewer. The PEDro scale [4, 5] was used to rate the quality of randomized trials. The quality of comparative studies using non-randomized designs was evaluated using eight items selected and modified from existing scales.
Meta-analyses
Meta-analyses of RCTs were undertaken when sufficient data for pooling was available for the outcomes of interest. For the following outcomes, 6 month mortality, overall survival and neurologic function, the altered WBRT dose/fractionation schedules were compared to conventional scheduling. The pooled relative risk (RR) was estimated using a random-effects model and each RCT was weighted by the inverse of its variance. Chi-square heterogeneity tests were used to test for statistical heterogeneity amongst the RCTs. I 2 was calculated in order to quantify inconsistency across trials and assess the impact of heterogeneity on the meta-analysis. Publication bias was evaluated graphically with funnel plots. All statistical analyses were carried out using Revman 5.
Evidence classification and recommendation levels
Both the quality of the evidence and the strength of the recommendations were graded according to the American Association of Neurological Surgeons (AANS)/Congress of Neurological Surgeons (CNS) criteria. These criteria are provided in the methodology paper for this guideline series.
Guideline development process
The AANS/CNS convened a multi-disciplinary panel of clinical experts to develop a series of practice guidelines on the management of brain metastases based on a systematic review of the literature conducted in collaboration with methodologists at the McMaster University Evidence-based Practice Center.
Scientific foundation
Overall, 24 primary studies [6–29] and seven companion papers [30–36] met the eligibility criteria for this systematic review (Fig. 1).
Fig. 1.
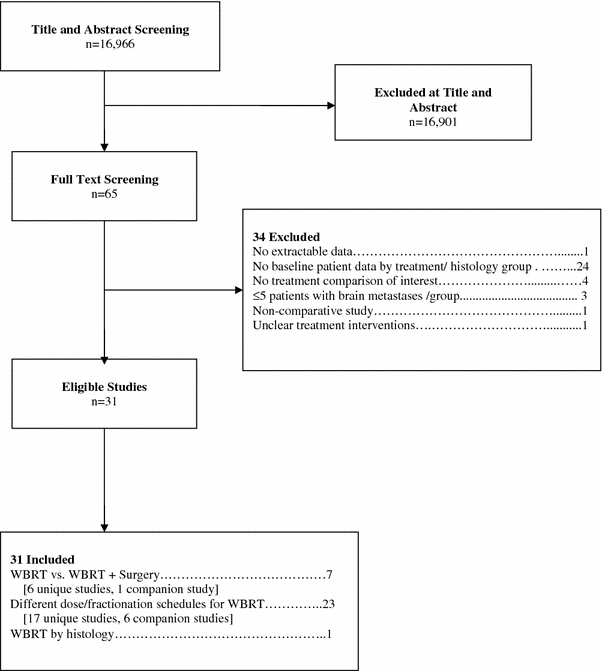
Flowchart of studies to final number of eligible studies
Surgical Resection plus WBRT versus WBRT alone
Seven studies met the eligibility criteria for this treatment comparison, and of these six were unique and one was a companion study [6–11, 30]. Three of these studies were prospective randomized trials [6–8] (Table 1). Given that the treatment modalities being compared included surgical resection in only one arm of each trial, all of the RCTs were non-blinded. In a randomized trial performed at the University of Kentucky [6], 48 patients with known systemic cancer were treated with either biopsy of the suspected brain metastasis plus WBRT or complete surgical resection of the metastasis plus WBRT. The radiation doses were the same in both groups and consisted of a total dose of 36 Gy given in 12 daily fractions of 3 Gy each. Patients had to be capable of caring for themselves independently, with a Karnofsky performance score (KPS) of at least 70. Patients were ineligible if they had a need for immediate treatment to prevent acute neurologic deterioration, or if they had tumors considered to be relatively radiosensitive [small cell lung cancer (SCLC), germ-cell tumors, lymphoma, leukemia, and multiple myeloma]. Patients were not excluded based on the extent of systemic disease. Randomization was performed by computer-generated random numbers. Information on allocation concealment was not reported. All the patients in the surgical group were considered to have had complete resection as assessed by postoperative computerized tomography (CT) scanning. Follow up brain CT or magnetic resonance imaging (MRI) scans were required every 3 months. There was a statistically significant increase in survival in the surgical group (40 vs. 15 weeks). In addition, the time to recurrence of brain metastases, freedom from death due to neurologic causes, and duration of functional independence were significantly longer in the surgical resection group. The 1 month mortality was 4% in each group, indicating that there was no extra mortality from surgery. Although surgical resection was the only variable positively associated with maintaining performance status, the extent of systemic disease and increased age were associated with poor performance status post-treatment.
Table 1.
WBRT versus surgical resection plus WBRT
| First author (year) | Study design/evidence class | Interventions | Population | Median survival | # Pts with recurrence/progressiona | Median time to recurrence/progression |
|---|---|---|---|---|---|---|
| Randomized trials | ||||||
| Patchell (1990) [6] |
RCT Evidence class I |
G1: WBRT (n = 23) G2: WBRT + surgery (n = 25) |
Single BM |
G1: 15 weeks G2: 40 weeks (Survival curves: log-rank; P < 0.01) |
At original site: G1: 12/23 (52%) G2: 5/25 (20%) (P < 0.02) |
At original site: G1: 21 weeks G2: >59 weeks (LR curves: log-rank; P < 0.0001) |
|
At distant or leptomeningeal sites: G1: 3/23 (13%) G2: 5/25 (20%) (P = NS) | ||||||
| Mintz (1996) [7] |
RCT Evidence class I |
G1: WBRT (n = 43) G2: WBRT + surgery (n = 41) |
Single BM |
G1: 6.3 months G2: 5.6 months (Survival curves: log-rank; P = NS) |
NR | NR |
| Vecht (1993) [8] |
RCT Evidence class I |
G1: WBRT (n = 31) G2: WBRT + surgery (n = 32) |
Single BM |
G1: 6 months G2: 10 months (Survival curves: log-rank; P = 0.04) |
NR | NR |
| Other study designs | ||||||
| Ampil (1996) [9] |
Retrospective cohort study Evidence class II |
G1: WBRT (n = 34) G2: WBRT + surgery (n = 11) |
Cerebellum BM |
G1: 3 months G2: 15 months (Wilcox on rank sum; P = 0.005) |
NR | NR |
| Rades (2008) [10] |
Retrospective cohort study Evidence class II |
G1: WBRT (n = 96) G2: Surgery + WBRT (n = 99) |
Single BM |
G1: 6 months G2: 11.5 months (Survival curves: log-rank; p < 0.001) |
NR | At original site: NR (LR curves: log-rank; P < 0.001) |
| At distant site: NR (DR curves: log-rank; P = NS) | ||||||
| At any site: NR (BR curves: log-rank; P < 0.001) | ||||||
| Sause (1990) [11] |
Prospective non-randomized trial Evidence class II |
G1: WBRT (n = 55) G2: WBRT + surgery (n = 25) |
Single BM |
G1: 6.7 months G2: 15.5 months (Survival curves: log-rank; P = 0.004) |
NR | NR |
BM brain metastases, BR brain recurrence (local + distant), DR distant recurrence in brain, G1 group 1, G2 group 2, LR local recurrence at original site in brain, NR not reported, NS not significant, Pts patients, RCT randomized control trial, WBRT Whole-brain radiation therapy
aNumber of pts with recurrence/progression of brain metastases, unless otherwise specified
A second randomized study [8], conducted as a multi-institutional trial in the Netherlands, contained 63 evaluable patients. Patients with single brain metastases were randomized to complete surgical resection plus WBRT or WBRT alone. Randomization was performed centrally by telephone. The WBRT schedules were the same for both treatment arms and consisted of 40 Gy given in a non-standard fractionation scheme of 2 Gy twice per day for 2 weeks (10 treatment days). Patients had to have a reasonable quality of life and neurological status, defined as spending no more than 50% of their time in bed and not requiring continuous nursing care or hospitalization. Excluded histologies were SCLC and lymphoma. Information is not given regarding the extent of resection in the surgical group or the use and frequency of imaging in follow up. Survival was significantly longer in the surgical group (10 vs. 6 months). There was also a non-significant trend toward longer duration of functional independence in the surgically treated patients. No data concerning recurrence of brain metastases were provided. The 1 month mortality rates were 9% in the surgery group and 0% in the WBRT alone group, a statistically insignificant difference. The authors concluded that the addition of surgery to WBRT provided a survival benefit except to those patients who were 60 years of age or older, or those patients with progressive systemic disease in the 3 months prior to the diagnosis of the brain metastasis.
A third randomized trial, conducted as a multi-center trial in Canada by Mintz et al. [7], failed to find a benefit from surgical treatment. In that study, 84 patients with a single brain metastasis were randomized to receive radiotherapy alone (30 Gy given in 10 daily fractions of 3 Gy) or surgery plus radiotherapy. Randomization was performed centrally by telephone. Eligible patients had to be less than 80 years of age, and they had to have a KPS of at least 50, i.e., they could be spending more than 50% of their time in bed but had to be able to care for some personal needs. Patients were not eligible if they had leukemia, lymphoma, or SCLC. A CT scan was done in the first postoperative week to assess the extent of tumor removed. Follow up CT scans were performed monthly for 6 months and every 3 months after that. A gross total resection was achieved in 38 of the 40 patients in the surgical group. No difference was found in overall survival; the median survival time was 6.3 months in the radiotherapy alone group and 5.6 months for the surgical group. There was also no difference in causes of death or quality of life.
It is unclear why the Canadian study was not in agreement with the other two trials. In all three studies, the control arms (the radiation alone arms) had median lengths of survival in the 3–6 months range—within the expected range for patients treated with radiotherapy alone. The major difference in the studies was the poor results obtained in the surgical arm of the Canadian trial. That study contained a higher proportion of patients with extensive systemic disease and lower performance scores. It is possible that these factors resulted in more patients dying of their systemic cancer before a long term benefit of surgery was seen. Additionally, MRI was not mandatory in the Canadian study, and it is theoretically possible that patients with additional lesions not detected by CT may have been included in the study.
All three of the evidence class II studies [9–11] demonstrated a survival benefit for patients who underwent surgical resection followed by WBRT as compared to WBRT alone. Ampil et al., published a single institution experience with either surgery plus WBRT (11 patients) versus WBRT alone (34 patients) in 45 patients who had a cerebellar metastasis. The majority of the patients in the WBRT alone arm had additional supratentorial brain metastases. Although there was a significant difference in survival noted between the two groups, the authors concluded that the outcome of patients with a brain metastasis within the cerebellum was improved with surgical resection if the primary was not from the lung.
WBRT dosing/fractionation schedule
Twenty-three studies met the eligibility criteria for this question, and of these, 17 were unique [12–28] and six [31–36] were companion publications (Table 2). The 17 unique studies fell into three AANS/CNS evidence class categories as follows: ten class I studies (nine RCTs [12–20] and one randomized phase I/II trial [21]), six class II studies [22–24, 26–28] (retrospective cohort studies) and one class III study [25] (prospective cohort study with historical controls).
Table 2.
Different dose/fractionation schedules of WBRT
| First author (year) | Study design/evidence class | WBRT total dose/fractionation | Population | Median survival | # Pts with recurrence/progressiona | Median time to recurrence/progression |
|---|---|---|---|---|---|---|
| Randomized trials | ||||||
| Borgelt (1981) [12] |
RCT Evidence class I |
First RCT: G1: 10 Gy/1 fraction (n = 26) G2: 30–40 Gy over 2–4 weeks (n = 112) |
BM |
First RCT: G1: 15 weeks G2: 21 weeks (Survival curves: log-rank; P = NS) |
NR |
Median time to progression (measured by deterioration in neurologic function): First RCT: Initial NF 1: G1: 9 weeks; G2: 14 weeks Initial NF 2: G1: 9 weeks; G2: 10 weeks Initial NF 3: G1: 7 weeks; G2: 12 weeks (Cox’s model; P = 0.07) |
|
Second RCT: G3: 12 Gy/2 fractions (n = 33) G4: 20 Gy over 1 week (n = 31) |
Second RCT: G3: 13 weeks G4: 12 weeks (Survival curves: log-rank; P = NS) |
Second RCT: Initial NF 1: G3: 9 weeks; G4: 10 weeks Initial NF 2: G3: 11 weeks; G4: 8 weeks Initial NF 3: G3: 3 weeks; G4: 3 weeks (Cox’s model; P = NS) |
||||
| Chantani (1994) [13] |
RCT Evidence class I |
Normal LDH: G1: 30 Gy/10 fractions (n = 46) G2: 50 Gy/20 fractions (n = 46) |
Lung cancer BM |
G1: 5.4 months G2: 4.8 months (Survival curves G1 vs. G2: log-rank; P = NS) G3: 3.4 months G4: 2.4 months (Survival curves G3 vs. G4:log-rank; P = NS) |
NR | NR |
|
High LDH: G3: 30 Gy/10 fractions (n = 35) G4: 20 Gy/5 fractions (n = 35) | ||||||
| Chantani (1985) [14] |
RCT Evidence class I |
G1: 30 Gy/10 fractions (n = 35) G2: 50 Gy in 20 fractions (n = 34) |
Lung cancer BM |
G1: 4 months G2: 3 months (Survival curves: log-rank; P = NS) |
NR | NR |
| Davey (2008) [15] |
RCT Evidence class I |
G1: 20 Gy/5 daily fractions (n = 45) G2: 40 Gy/20 fractions/twice daily (n = 45) |
BM |
G1: 19.1 weeks G2: 19.1 weeks (Survival curves: log-rank; P = NS) |
NR | NR |
| Haie-Meder (1993) [16] |
RCT Evidence class I |
G1: 18 Gy/3 fractions (n = 110) G2: 18 Gy/3 fractions; 4 weeks later a second identical course or 25 Gy/10 fractions (n = 106) |
BM from lung, breast, head and neck or unknown primaries |
G1: 4.2 months G2: 5.3 months (Survival curves: log-rank; P = NS) |
NR | NR |
| Komarnicky (1991) [17] |
RCT Evidence class I |
G1: 30 Gy/10 fractions (n = 193) G2: 30 Gy/6 fractions (n = 200) G3: 30 Gy/6 fractions + MISO (n = 196) G4: 30 Gy/10 fractions + MISO (n = 190) |
BM |
G1: 4.5 months G2: 4.1 months G3: 3.1 months G4: 3.9 months (Survival curves: log-rank; P = NS) |
# of pts retreated for BM after protocol therapy: G1: 54/179 (30%) G2: 54/180 (30%) G3: 33/173 (19%) G4: 54/163 (33%) (P = NS) |
NR |
| Kurtz [18] (1981) |
RCT Evidence class I |
G1: 30 Gy/10 fractions (n = 130) G2: 50 Gy/20 fractions (n = 125) |
BM with no extra-cranial metastases |
G1: 18.2 weeks G2: 16.9 weeks (Survival curves: log-rank; P = NS) |
Overall in brain: G1: 109/124 (88%) G2: 105/118 (89%) (P value not reported) |
Overall in brain: G1: 11 weeks G2: 10 weeks (P value not reported) |
| Murray (1997) [19] |
RCT Evidence class I |
G1: 30 Gy/10 fractions/daily (n = 213) G2: 54.4 Gy/34 fractions/twice daily (n = 216) |
BM |
G1: 4.5 months G2: 4.5 months (Survival curves: Prentice modified Wilcox on; P = NS) |
NR | NR |
| Priestman (1996) [20] |
RCT Evidence class I |
G1: 12 Gy/2 fractions (n = 270) G2: 30 Gy/10 fractions (n = 263) |
BM |
G1: 77 days G2: 84 days (Survival curves: log-rank; P = 0.04) |
NR | NR |
| Sause [21] (1993) |
Randomized phase I/II trial Evidence class I |
G1: 32 Gy in 1.6 Gy fractions + boost to 48.0 Gy] (n = 62) G2: 32 Gy in 1.6 Gy fractions + boost to 54.4 Gy] (n = 115) G3: 32 Gy in 1.6 Gy fractions + boost to 64.0 Gy] (n = 104) G4: 32 Gy in 1.6 Gy fractions + boost to 70.4 Gy] (n = 53) Fractions administered twice daily |
BM |
G1: 4.2 months G2: 5.2 months G3: 4.8 months G4: 6.4 months (Survival curves: log-rank; P = NS) |
NR | NR |
| Other study designs | ||||||
| Bach (1996) [22] |
Retrospective cohort study Evidence class II |
G1: 22 Gy/4 fractionsb (n = 57) G2: 50.4 Gy/28 fractionsb (n = 44) |
BM from SCLC |
G1: 88 days G2: 160 days (Survival curves: log-rank; P = 0.00001) |
NR | NR |
| Conill [23] (2006) |
Retrospective cohort study Evidence class II |
G1: 20 Gy/5 fractions + TMZ based chemotherapy (n = 11) G2: 30 Gy/10 fractions + TMZ based chemotherapy (n = 10) |
BM from melanoma |
G1: 4.0 months G2: 4.0 months (Survival curves: log-rank; P = NS) |
Data not reported by group; no statistically significant difference between groups (P = NS) | NR |
| Nieder (1995) [24] |
Retrospective cohort study Evidence class II |
G1: WBRT [40 Gy in 20 fractions ± local boost 10–20 Gy] (n = 39) G2: WBRT [30 Gy in 10 fractions] (n = 39) [matched to G1] |
BM |
G1: 3.5 months G2: 2.5 months (Survival curves: log-rank; P = NS) |
# Pts with local CR/PR: G1: CR 5/39 (13%); PR 25/39 (64%) G2: CR 4/39 (10%); PR 15/39 (38%) (CR or PR G1: 77% vs. G2: 48%; P = 0.037) |
Median time to BM progression: G1: 3.0 months G2: 2.5 months (Progression-free curves: log-rank; P = NS) |
| Nieder (1997) [25] |
Prospective cohort study with historical controls Evidence class III |
G1: Surgery + WBRT [30 Gy/2.5 Gy fractions/twice daily] (n = 11) G2: WBRT [50.4 Gy/1.8 Gy fractions/twice daily] (n = 15) G3: WBRT [30 Gy/2.5 Gy fractions/twice daily] (n = 36) G4: Historical group WBRT [30 Gy/3 Gy fractions/daily] (n = 246) G5: Historical group surgery + WBRT [30 Gy/3 Gy fractions/daily] (n = 37) |
BM |
G1: 3.3 months G2: 2 months G3: 2 months G4: 2.5 months G5: 7.3 months (Survival curves G2, G3, G4: log-rank; P = NS) |
# of pts with follow up CT with BM progression/recurrence anywhere in brain: G1: 3/9 (33%) G2: 4/11 (36%) G3: 11/25 (44%) G4: 29/202 (14%) G5: 5/35 (14%) |
Median time to any recurrence in brain: Not reported BR curves G3 vs. G4: log-rank; P = 0.0001 BR curves G2 vs. G3: log-rank; P = NS BR curves G2 vs. G4: log-rank; P = NS |
| Rades (2007) [26] |
Retrospective cohort study Evidence class II |
G1: 20 Gy/5 fractions (n = 140) G2: 30 Gy/10 fractions or 40 Gy/20 fractions (n = 264) |
BM from NSCLC |
Median survival: Not reported 6 month survival rate: G1: 40% G2: 30% (Survival curves: log-rank; P = NS) |
NR | NR |
| Rades (2007) [27] |
Retrospective cohort study Evidence class II |
G1: 30 Gy/10 fractions (n = 257) G2: 45 Gy/15 fractions or 40 Gy/20 fractions (n = 159) |
≥2 BM |
Median survival: NR 6 month survival rate: G1: 33% G2: 29% (Survival curves: log-rank; P = NS) |
6 month local control rate: G1: 39% G2: 41% (LR curves: log-rank; P = NS) |
NR |
| Rades (2008) [28] |
Retrospective cohort study Evidence class II |
G1: 20 Gy/5 fractions (n = 387) G2: 30 Gy/10 fractions or 40 Gy/20 fractions (n = 698) |
BM |
Median survival: Not reported 1 year survival rate: G1: 20% G2: 18% (Survival curves: log-rank; P = NS) |
NR | NR |
BM brain metastases, BR brain recurrence (local + distant), CR complete response, CT computed tomography, DR distant recurrence in brain, G1 group 1, G2 group 2, G3 group 3, G4 group 4, KPS Karnofsky performance score, LDH lactate dehydrogenase, LR local recurrence at original site in brain, MISO misonidazole, NF neurologic function, NR not reported, NS not significant, NSCLC non-small cell lung carcinoma, PR partial response, Pts patients, RCT randomized control trial, TMZ temozolomide, WBRT whole-brain radiation therapy
aNumber of pts with recurrence/progression of brain metastases, unless otherwise specified
bWBRT use similar at baseline in both groups
The radiation dosages have been expressed in terms of the tumor response biologically effective dose (BED) in order to quantitatively capture the observed biological effect between treatment arms. This was calculated from the linear quadratic equation: where n = number of treatments, d = dose per fraction; the calculation assumes α/β = 10 Gy for tumor effects of each schedule, however it is uncorrected for treatment regimes of two fractions/day. The BED units are referred to in terms of Gy10 to indicate that the BED values are single-point calculations for tumor response [37, 38] and no correction for accelerated repopulation was made.
where n = number of treatments, d = dose per fraction; the calculation assumes α/β = 10 Gy for tumor effects of each schedule, however it is uncorrected for treatment regimes of two fractions/day. The BED units are referred to in terms of Gy10 to indicate that the BED values are single-point calculations for tumor response [37, 38] and no correction for accelerated repopulation was made.
Expressing radiation dosages in terms of the BED takes into account the total dose of radiation, fraction size, and overall time to deliver the radiation, and presumed repair of irradiated tissue. The analyses are stratified by low or high dose versus control dose. The control group consists of patients treated with 30 Gy in 10 fractions for a BED = 39 Gy10 (therefore assigning the low dose regimens as a BED < 39 Gy10, and high dose regimens as a BED > 39 Gy10).
None of the trials demonstrated a meaningful improvement in any endpoint relative to dose; specifically, survival was not improved. There was considerable overlap in terms of survival even at the same dose level in different trials, underscoring the significance of host-specific variables in determining survival.
The data from the randomized trials, stratified by the BED are shown in Table 3.
Table 3.
Randomized trials stratified by the BED for different treatment schedules
| Author | Year | Total dose (Gy) | Fractions | BED (Gy; α/β = 10) | n | Median survival (weeks) |
|---|---|---|---|---|---|---|
| Borgelt | 1981 | 12 | 2 | 19.2 | 33 | 13 |
| Priestman | 1996 | 12 | 2 | 19.2 | 270 | 11 |
| Borgelt | 1981 | 10 | 1 | 20 | 26 | 15 |
| Borgelt | 1981 | 20 | 5 | 28 | 31 | 12 |
| Chatani | 1994 | 20 | 5 | 28 | 35 | 10.4 |
| Davey | 2008 | 20 | 5 | 28 | 45 | 19.1 |
| Haie-Meder | 1993 | 18 | 3 | 28.8 | 110 | 18.3 |
| Chatani | 1994 | 30 | 10 | 39 | 46 | 23.5 |
| Chatani | 1994 | 30 | 10 | 39 | 35 | 14.8 |
| Chatani | 1985 | 30 | 10 | 39 | 35 | 17.4 |
| Komarnicky | 1991 | 30 | 10 | 39 | 193 | 19.5 |
| Komarnicky | 1991 | 30 | 10 | 39 | 190 | 16.9 |
| Kurtz | 1981 | 30 | 10 | 39 | 130 | 18.2 |
| Murray | 1997 | 30 | 10 | 39 | 213 | 19.6 |
| Priestman | 1996 | 30 | 10 | 39 | 263 | 12 |
| Komarnicky | 1991 | 30 | 6 | 45 | 200 | 17.8 |
| Komarnicky | 1991 | 30 | 6 | 45 | 196 | 13.5 |
| Davey | 2008 | 40 | 20a | 48 | 45 | 19.1 |
| Sause | 1993 | 48 | 30a | 55.7 | 62 | 18.3 |
| Haie-Meder | 1993 | – | – | 50.8 | 106 | 23 |
| Chatani | 1994 | 50 | 20 | 62.5 | 46 | 20.9 |
| Chatani | 1985 | 50 | 20 | 62.5 | 34 | 13 |
| Kurtz | 1981 | 50 | 20 | 62.5 | 125 | 16.9 |
| Murray | 1997 | 54.4 | 34a | 63.1 | 216 | 19.6 |
| Sause | 1993 | 54.4 | 34a | 63.1 | 115 | 22.6 |
| Sause | 1993 | 64 | 40a | 74.2 | 104 | 20.9 |
| Sause | 1993 | 70.4 | 44a | 81.7 | 53 | 27.8 |
| Borgelt | 1981 | 30–40 | 10–20 | 34.5–56 | 112 | 21 |
aTreatment regimes of two fractions/day (BED uncorrected)
The meta-analyses were stratified by low or high dose versus control dose. Studies lacking a comparable control were excluded from meta-analyses, due to the inherent dosing variability amongst the study’s control group.
Figure 2 indicates the RR of mortality at 6 months in the low dose (BED < 39 Gy10) group compared to that in the WBRT control group (BED = 39 Gy10). Only data from two trials (Chantani et al. [13] and Priestman et al. [20]) were robust enough to be considered for this end-point. When combined, no difference (P = 0.52) was found for 6 month mortality (RR 1.05; 95% CI 0.90, 1.23). Figure 3 presents the same analysis, but for the comparison of the high dose (BED > 39 Gy10) group to the WBRT control group (BED = 39 Gy10). Data from five trials were robust enough to be considered for this end-point. Combining data for Chantani et al. [13, 14], Komarnicky et al. [17], Kurtz et al. [18], and Murray et al. [19], yielded no difference (P = 0.39) in 6 month mortality (RR 1.05; 95% CI 0.94, 1.18). A 1981 trial by Borgelt et al. [12] was excluded from the meta-analyses for lack of a comparable control. Since the administered dosage ranged from 20 to 40 Gy over 1–4 weeks, the resulting permutations and combinations of plausible radiation schedules in this group varied too substantially (22–72 Gy10) to function as a true control. The inherent dosing variability disqualified this control group thereby making the trial ineligible for comparison.
Fig. 2.
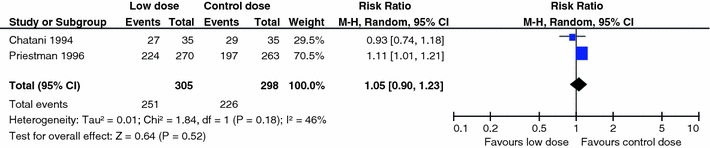
Comparison of low dose WBRT (BED < 39 Gy10) versus WBRT control dose (BED = 39 Gy10): mortality at 6 months
Fig. 3.
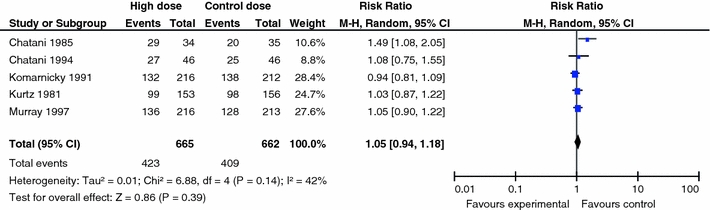
Comparison of high dose WBRT (BED > 39 Gy10) versus WBRT control dose (BED = 39 Gy10): mortality at 6 months (Note: extraction of data for Komarnicky trial is based on the K–M curve)
Similar comparisons were made for overall survival and neurologic function, and no dose–effect was identified for either end-point. Figures 4 and 5 depict the overall survival for the respective comparisons of low and high BED values versus the control BED. In Fig. 4, the pooling of two studies, Chantani et al. [13] and Priestman et al. [20], for the comparison of low dose WBRT (BED < 39 Gy10) to a control dose (BED = 39 Gy10) yielded no difference (P = 0.07) in overall survival (RR 0.86; 95% CI 0.73, 1.01). In Fig. 5, overall survival data from Chantani et al. [13, 14], Komarnicky et al. [17], Kurtz et al. [18], and Murray et al. [19], were pooled for the comparison of high dose WBRT (BED > 39Gy10) versus WBRT control dose (BED = 39 Gy10). No difference (P = 0.26) in overall survival was found (RR 1.06; 95% CI 0.96, 1.18). The hazard plots for neurologic function are not presented.
Fig. 4.
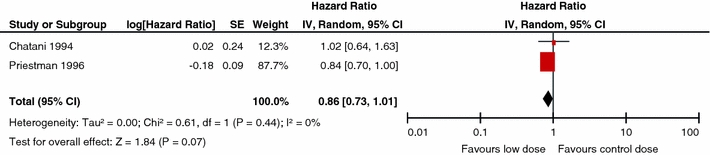
Comparison of low dose WBRT (BED < 39 Gy10) versus WBRT control dose (BED = 39 Gy10): overall survival
Fig. 5.
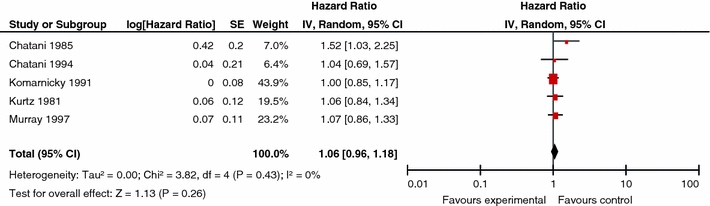
Comparison of high dose WBRT (BED > 39 Gy10) versus WBRT control dose (BED = 39 Gy): overall survival
Impact of tumor histopathology on WBRT treatment outcomes
Using the AANS/CNS evidence classification, no papers with class I or II evidence were identified by the systematic literature review that met the eligibility criteria for this question. In fact, only one paper, providing class III evidence, met the inclusion criteria (Table 4). The only data is provided by a single institution retrospective analysis with 75 cases (Sundstrom et al. [29]). In that study, there were 15 cases that were of classic “radio-resistant” histology. There were no statistically significant differences in overall survival by tumor histology. Local control by tumor type was not reported.
Table 4.
Study evaluating WBRT treatment outcome by histopathology
| First author (year): Sundström (1998) [29] | ||
|---|---|---|
| Study characteristics | Study outcomes | Study quality |
| Study design: case series | Primary outcome: survival | Quality assessment: N/A as non-comparative study |
|
Inclusion criteria: Pts treated with WBRT for BM diagnosed by CT or MRI Minimum midline dose to the whole brain of at least 25 Gy |
Median survival by primary tumor type: Breast: 7 months (range 1–62 months) Lung: 4 months (range 1–21 months) Renal cell: 4 months (range 2–34 months) Melanoma: 3 months (range 1–6 months) Other primaries: 4 months (range 1–9 months) (Survival curves: P-value not reported) |
AANS/CNS evidence classification: class III |
|
Interventions: WBRT [mean dose 30 Gy (range 25–40 Gy) in 1.8–3 Gy fractions] |
Tumor control: not reported | |
|
Median follow-up: not reported # Male: Breast: 0/19, lung: 26/35, renal cell: 4/9, melanoma: 6/6, other: 6/6 |
Median time to recurrence of brain metastases: not reported | |
|
Median age (range): Breast: 53 years (39–77 years) Lung: 64 years (43–78 years) Renal cell: 61 years (41–69 years) Melanoma: 61 years (39–62 years) Other: 61 years (50–71 years) |
Functional performance: not reported by histology | |
| Cause of death: not reported | ||
| Adverse events: not reported by tumor type | ||
|
# Of brain metastases: Breast: 1 BM 9/19, 2 BM 4/19, >2 BM 6/19 Lung: 1 BM 15/35, 2 BM 5/35, >2 BM 15/35 Renal cell: 1 BM 3/9, 2 BM 2/9, >2 BM 4/9 Melanoma: 1 BM 3/6, 2 BM 0/6, >2 BM 3/6 Other: 1 BM 3/6, 2 BM 2/6, >2 BM 1/6 |
||
|
Extra-cranial disease: Extra-cranial metastases: Breast: 17/19 Lung: 6/35 Renal cell: 5/9 Melanoma: 4/6 Other: 5/6 | ||
|
Baseline functional performance: WHO classification (0 (best) to 4 (worst) Performance status (PS): Breast: PS 0–1 9/19, PS 2 5/19, PS 3 5/19 Lung: PS 0-1 10/35, PS 2 16/35, PS 3 9/35 Renal cell: PS 0–1 2/9, PS 2 5/9, PS 3 2/9 Melanoma: PS 0–1 6/6 Other: PS 0–1 3/6, PS 2 1/6, PS 3 2/6 | ||
AANS American Association of Neurological Surgeons, BM brain metastases, CNS Congress of Neurological Surgeons, CT computed tomography, MRI magnetic resonance imaging, NA not available, Pts patients, WBRT whole brain radiation therapy, WHO World Health Organization
Conclusion
Surgical resection plus WBRT versus WBRT alone
Based on the available class I and class II evidence, surgical resection followed by WBRT is an effective treatment for patients with single, surgically accessible, brain metastases who have controlled extra-cranial disease and are in good general condition. Good general condition in the relevant studies was defined as functional independence and spending less than 50% of time in bed. Patients with disease progression in the 3 months preceding the diagnosis of the brain metastases have a relatively poor survival and poor functional status but still had a significant improvement in survival as a result of surgical resection. Due to the risk of fourth ventricle compression and the subsequent increase in intracranial pressure, surgery should be particularly considered if there is a brain metastasis situated within the posterior fossa.
Surgical resection plus WBRT versus surgical resection alone
The surgical resection guideline paper by Kalkanis et al. [1] outlines in detail the evidence supporting the addition of WBRT after surgical resection. Please refer to this paper for a further discussion of why the combined modalities of surgical resection followed by WBRT represent a superior treatment option, in terms of improving tumor control at the original site of the metastasis and in the brain overall, when compared to surgical resection alone
If WBRT is used, is there an optimal dosing/fractionation schedule?
There is class I evidence that altered dose/fractionation schedules of WBRT do not result in significant differences in median survival, local control or neurocognitive function when compared to “standard” WBRT dose/fractionation. (i.e., 30 Gy in 10 daily fractions or a BED of 39 Gy10).
If WBRT is used, what impact does tumor histopathology have on treatment outcomes?
Only one small case series (class III evidence) met the eligibility criteria for the question addressing the impact of histopathology on WBRT treatment outcomes. Further studies in this area are needed before any recommendations can be made.
Key issues for future investigation
There have been numerous studies comparing various dose/fractionation schemes for WBRT. Unless future studies incorporate more sophisticated measures of neurocognitive outcome there is little need to repeat these studies. There are, however, very few studies evaluating the effectiveness of WBRT for one histopathological type versus another. Future studies of WBRT explicitly addressing treatment outcomes for well-defined patient groups by different tumor types are needed.
The following is a list of major ongoing or recently closed randomized trials pertaining to the use of whole brain radiation therapy that evaluate treatment comparisons addressed by this guideline paper for the management of newly diagnosed brain metastases.
-
Brain metastases study: radiotherapy schemes in the treatment of brain metastases
Official Title: To determine which of two radiotherapy brain fractionation schemes is superior in the treatment of brain metastases
Status: Completed
Clinicaltrials.gov Identifier: NCT00138788
Principal Investigator: Associate Professor, Peter H Graham, Cancer Care Centre, St George Hospital
Location: Australia
Sponsors and Collaborators: St George Hospital, Australia
Acknowledgments
We would like to acknowledge the contributions of the McMaster Evidence-based Practice Center (EPC), Dr. Parminder Raina (Director). Dr. Lina Santaguida (Co-Associate Director, Senior Scientist) led the EPC staff, which was responsible for managing the systematic review process, searching for and retrieving, reviewing, data abstraction of all articles, preparation of the tables and the formatting and editing of the final manuscripts.
Disclaimer of liability
The information in these guidelines reflects the current state of knowledge at the time of completion. The presentations are designed to provide an accurate review of the subject matter covered. These guidelines are disseminated with the understanding that the recommendations by the authors and consultants who have collaborated in their development are not meant to replace the individualized care and treatment advice from a patient’s physician(s). If medical advice or assistance is required, the services of a competent physician should be sought. The proposals contained in these guidelines may not be suitable for use in all circumstances. The choice to implement any particular recommendation contained in these guidelines must be made by a managing physician in light of the situation in each particular patient and on the basis of existing resources.
Disclosures
All panel members provided full disclosure of conflicts of interest, if any, prior to establishing the recommendations contained within these guidelines.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.Kalkanis SN, Kondziolka D, Gaspar LE, Burri SH, Asher AL, Cobbs CS et al (2009) The role of surgical resection in the management of newly diagnosed brain metastases: a systematic review and evidence-based clinical practice guideline. J Neurooncol. doi:10.1007/s11060-009-0061-8 [DOI] [PMC free article] [PubMed]
- 2.Linskey ME, Andrews DW, Asher AL, Burri SH, Kondziolka DS, Robinson PD et al (2009) The role of stereotactic radiosurgery in the management of newly diagnosed brain metastases: a systematic review and evidence-based clinical practice guideline. J Neurooncol. doi:10.1007/s11060-009-0073-4 [DOI] [PMC free article] [PubMed]
- 3.Robinson PD, Kalkanis SN, Linskey ME, Santaguida PL (2009) Methodology used to develop the AANS/CNS management of brain metastases evidence-based clinical practice parameter guidelines. J Neurooncol. doi:10.1007/s11060-009-0059-2 [DOI] [PMC free article] [PubMed]
- 4.Centre for Evidence-Based Physiotherapy (2009) Physiotherapy Evidence Database (PEDro). http://www.pedro.org.au/. Accessed January 2009
- 5.Maher CG, Sherrington C, Herbert RD, Moseley AM, Elkins M. Reliability of the PEDro scale for rating quality of randomized controlled trials. Phys Ther. 2003;83(8):713–721. [PubMed] [Google Scholar]
- 6.Patchell RA, Tibbs PA, Walsh JW, Dempsey RJ, Maruyama Y, Kryscio RJ, et al. A randomized trial of surgery in the treatment of single metastases to the brain. N Engl J Med. 1990;322(8):494–500. doi: 10.1056/NEJM199002223220802. [DOI] [PubMed] [Google Scholar]
- 7.Mintz AH, Kestle J, Rathbone MP, Gaspar L, Hugenholtz H, Fisher B, et al. A randomized trial to assess the efficacy of surgery in addition to radiotherapy in patients with a single cerebral metastasis. Cancer. 1996;78(7):1470–1476. doi: 10.1002/(SICI)1097-0142(19961001)78:7<1470::AID-CNCR14>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 8.Vecht CJ, Haaxma-Reiche H, Noordijk EM, Padberg GW, Voormolen JH, Hoekstra FH, et al. Treatment of single brain metastasis: radiotherapy alone or combined with neurosurgery? Ann Neurol. 1993;33(6):583–590. doi: 10.1002/ana.410330605. [DOI] [PubMed] [Google Scholar]
- 9.Ampil FL, Nanda A, Willis BK, Nandy I, Meehan R. Metastatic disease in the cerebellum The LSU experience in 1981–1993. Am J Clin Oncol. 1996;19(5):509–511. doi: 10.1097/00000421-199610000-00016. [DOI] [PubMed] [Google Scholar]
- 10.Rades D, Kieckebusch S, Haatanen T, Lohynska R, Dunst J, Schild SE. Surgical resection followed by whole brain radiotherapy versus whole brain radiotherapy alone for single brain metastasis. Int J Radiat Oncol Biol Phys. 2008;70(5):1319–1324. doi: 10.1016/j.ijrobp.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 11.Sause WT, Crowley JJ, Morantz R, Rotman M, Mowry PA, Bouzaglou A, et al. Solitary brain metastasis: results of an RTOG/SWOG protocol evaluation surgery + RT versus RT alone. Am J Clin Oncol. 1990;13(5):427–432. doi: 10.1097/00000421-199010000-00013. [DOI] [PubMed] [Google Scholar]
- 12.Borgelt B, Gelber R, Larson M, Hendrickson F, Griffin T, Roth R. Ultra-rapid high dose irradiation schedules for the palliation of brain metastases: final results of the first two studies by the Radiation Therapy Oncology Group. Int J Radiat Oncol Biol Phys. 1981;7(12):1633–1638. doi: 10.1016/0360-3016(81)90184-x. [DOI] [PubMed] [Google Scholar]
- 13.Chatani M, Matayoshi Y, Masaki N, Inoue T. Radiation therapy for brain metastases from lung carcinoma. Prospective randomized trial according to the level of lactate dehydrogenase. Strahlenther Onkol. 1994;170(3):155–161. [PubMed] [Google Scholar]
- 14.Chatani M, Teshima T, Hata K, Inoue T, Suzuki T. Whole brain irradiation for metastases from lung carcinoma. A clinical investigation. Acta Radiol Oncol. 1985;24(4):311–314. doi: 10.3109/02841868509136057. [DOI] [PubMed] [Google Scholar]
- 15.Davey P, Hoegler D, Ennis M, Smith J. A phase III study of accelerated versus conventional hypofractionated whole brain irradiation in patients of good performance status with brain metastases not suitable for surgical excision. Radiother Oncol. 2008;88(2):173–176. doi: 10.1016/j.radonc.2008.05.020. [DOI] [PubMed] [Google Scholar]
- 16.Haie-Meder C, Pellae-Cosset B, Laplanche A, Lagrange JL, Tuchais C, Nogues C, et al. Results of a randomized clinical trial comparing two radiation schedules in the palliative treatment of brain metastases. Radiother Oncol. 1993;26(2):111–116. doi: 10.1016/0167-8140(93)90091-L. [DOI] [PubMed] [Google Scholar]
- 17.Komarnicky LT, Phillips TL, Martz K, Asbell S, Isaacson S, Urtasun R. A randomized phase III protocol for the evaluation of misonidazole combined with radiation in the treatment of patients with brain metastases (RTOG-7916) Int J Radiat Oncol Biol Phys. 1991;20(1):53–58. doi: 10.1016/0360-3016(91)90137-s. [DOI] [PubMed] [Google Scholar]
- 18.Kurtz JM, Gelber R, Brady LW, Carella RJ, Cooper JS. The palliation of brain metastases in a favorable patient population: a randomized clinical trial by the Radiation Therapy Oncology Group. Int J Radiat Oncol Biol Phys. 1981;7(7):891–895. doi: 10.1016/0360-3016(81)90005-5. [DOI] [PubMed] [Google Scholar]
- 19.Murray KJ, Scott C, Greenberg HM, Emami B, Seider M, Vora NL, et al. A randomized phase III study of accelerated hyperfractionation versus standard in patients with unresected brain metastases: a report of the Radiation Therapy Oncology Group (RTOG) 9104. Int J Radiat Oncol Biol Phys. 1997;39(3):571–574. doi: 10.1016/s0360-3016(97)00341-6. [DOI] [PubMed] [Google Scholar]
- 20.Priestman TJ, Dunn J, Brada M, Rampling R, Baker PG. Final results of the Royal College of Radiologists’ trial comparing two different radiotherapy schedules in the treatment of cerebral metastases. Clin Oncol (R Coll Radiol) 1996;8(5):308–315. doi: 10.1016/s0936-6555(05)80717-4. [DOI] [PubMed] [Google Scholar]
- 21.Sause WT, Scott C, Krisch R, Rotman M, Sneed PK, Janjan N, et al. Phase I/II trial of accelerated fractionation in brain metastases RTOG 85–28. Int J Radiat Oncol Biol Phys. 1993;26(4):653–657. doi: 10.1016/0360-3016(93)90284-3. [DOI] [PubMed] [Google Scholar]
- 22.Bach F, Sorensen JB, Adrian L, Larsen H, Langer SW, Nelausen KM, et al. Brain relapses in chemotherapy-treated small cell lung cancer: a retrospective review of two time-dose regimens of therapeutic brain irradiation. Lung Cancer. 1996;15(2):171–181. doi: 10.1016/0169-5002(95)00580-3. [DOI] [PubMed] [Google Scholar]
- 23.Conill C, Jorcano S, Domingo-Domenech J, Gallego R, Malvehy J, Puig S, et al. Whole brain irradiation and temozolomide based chemotherapy in melanoma brain metastases. Clin Transl Oncol. 2006;8(4):266–270. doi: 10.1007/BF02664937. [DOI] [PubMed] [Google Scholar]
- 24.Nieder C, Berberich W, Nestle U, Niewald M, Walter K, Schnabel K. Relation between local result and total dose of radiotherapy for brain metastases. Int J Radiat Oncol Biol Phys. 1995;33(2):349–355. doi: 10.1016/0360-3016(95)00121-E. [DOI] [PubMed] [Google Scholar]
- 25.Nieder C, Nestle U, Niewald M, Schnabel K. Accelerated radiotherapy for brain metastases. Radiother Oncol. 1997;45(1):17–22. doi: 10.1016/S0167-8140(97)00113-8. [DOI] [PubMed] [Google Scholar]
- 26.Rades D, Schild SE, Lohynska R, Veninga T, Stalpers LJ, Dunst J. Two radiation regimens and prognostic factors for brain metastases in nonsmall cell lung cancer patients. Cancer. 2007;110(5):1077–1082. doi: 10.1002/cncr.22877. [DOI] [PubMed] [Google Scholar]
- 27.Rades D, Haatanen T, Schild SE, Dunst J. Dose escalation beyond 30 grays in 10 fractions for patients with multiple brain metastases. Cancer. 2007;110(6):1345–1350. doi: 10.1002/cncr.22906. [DOI] [PubMed] [Google Scholar]
- 28.Rades D, Bohlen G, Dunst J, Lohynska R, Veninga T, Stalpers L, et al. Comparison of short-course versus long-course whole-brain radiotherapy in the treatment of brain metastases. Strahlenther Onkol. 2008;184(1):30–35. doi: 10.1007/s00066-008-1795-5. [DOI] [PubMed] [Google Scholar]
- 29.Sundstrom JT, Minn H, Lertola KK, Nordman E. Prognosis of patients treated for intracranial metastases with whole-brain irradiation. Ann Med. 1998;30(3):296–299. doi: 10.3109/07853899809005858. [DOI] [PubMed] [Google Scholar]
- 30.Noordijk EM, Vecht CJ, Haaxma-Reiche H, Padberg GW, Voormolen JH, Hoekstra FH, et al. The choice of treatment of single brain metastasis should be based on extracranial tumor activity and age. Int J Radiat Oncol Biol Phys. 1994;29(4):711–717. doi: 10.1016/0360-3016(94)90558-4. [DOI] [PubMed] [Google Scholar]
- 31.Chatani M, Teshima T, Hata K, Inoue T. Prognostic factors in patients with brain metastases from lung carcinoma. Strahlenther Onkol. 1986;162(3):157–161. [PubMed] [Google Scholar]
- 32.Regine WF, Scott C, Murray K, Curran W. Neurocognitive outcome in brain metastases patients treated with accelerated-fractionation vs. accelerated-hyperfractionated radiotherapy: an analysis from Radiation Therapy Oncology Group Study 91–04. Int J Radiat Oncol Biol Phys. 2001;51(3):711–717. doi: 10.1016/s0360-3016(01)01676-5. [DOI] [PubMed] [Google Scholar]
- 33.Epstein BE, Scott CB, Sause WT, Rotman M, Sneed PK, Janjan NA, et al. Improved survival duration in patients with unresected solitary brain metastasis using accelerated hyperfractionated radiation therapy at total doses of 54.4 gray and greater. Results of Radiation Therapy Oncology Group 85–28. Cancer. 1993;71(4):1362–1367. doi: 10.1002/1097-0142(19930215)71:4<1362::AID-CNCR2820710431>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 34.Rades D, Bohlen G, Lohynska R, Veninga T, Stalpers LJ, Schild SE, et al. Whole-brain radiotherapy with 20 Gy in 5 fractions for brain metastases in patients with cancer of unknown primary (CUP) Strahlenther Onkol. 2007;183(11):631–636. doi: 10.1007/s00066-007-1763-5. [DOI] [PubMed] [Google Scholar]
- 35.Rades D, Kieckebusch S, Lohynska R, Veninga T, Stalpers LJ, Dunst J et al (2007) Reduction of overall treatment time in patients irradiated for more than three brain metastases. Int J Radiat Oncol Biol Phys 69(5):1509–1513 (update Sept 24 2008) [DOI] [PubMed]
- 36.Rades D, Lohynska R, Veninga T, Stalpers LJ, Schild SE. Evaluation of 2 whole-brain radiotherapy schedules and prognostic factors for brain metastases in breast cancer patients. Cancer. 2007;110(11):2587–2592. doi: 10.1002/cncr.23082. [DOI] [PubMed] [Google Scholar]
- 37.Fowler JF. Practical time-dose evaluations, or how to stop worrying and learn to love linear quadratics. In: Levitt SH, Purdy JA, Perez CA, Vijayakumar S, editors. Technical basis of radiation therapy: practical clinical applications. 4. Berlin, Heidelberg: Springer; 2006. pp. 3–31. [Google Scholar]
- 38.Jones B, Dale RG, Deehan C, Hopkins KI, Morgan DA. The role of biologically effective dose (BED) in clinical oncology. Clin Oncol (R Coll Radiol) 2001;13(2):71–81. doi: 10.1053/clon.2001.9221. [DOI] [PubMed] [Google Scholar]


