Abstract
Recent evidence indicates that regular physical activity enhances brain plasticity (i.e. the ability to reorganise neural connections) and improves neurocognitive function. However, the effect of regular physical activity on human motor cortex function is unknown. The purpose of this study was to examine motor cortex plasticity for a small hand muscle in highly active and sedentary individuals. Electromyographic recordings were obtained from the left abductor pollicis brevis (APB) muscle of 14 active and 14 sedentary subjects (aged 18–38 yrs). The extent of physical activity was assessed by questionnaire, where the physically active subjects performed >150 min per day moderate-to-vigorous aerobic activity on at least 5 days per week, whereas the sedentary group performed <20 min per day of physical activity on no more than 3 days per week. Transcranial magnetic stimulation (TMS) of the right hemisphere was used to assess changes in APB motor-evoked potentials (MEPs), input–output curve (IO curve), short-interval intracortical inhibition (SICI) and cortical silent period (CSP). Neuroplastic changes were induced using paired-associative stimulation (PAS), which consisted of 90 paired stimuli (0.05 Hz for 30 min) of median nerve electrical stimulation at the wrist followed 25 ms later by TMS to the hand area of motor cortex. The IO curve slope was 35% steeper in individuals with increased physical activity (combined before and after PAS, P < 0.05), suggesting increased motor cortex excitability, although there was no difference in SICI or CSP between groups. PAS induced an increase in MEP amplitude in the physically active subjects (54% increase compared with before, P < 0.01), but no significant facilitation in the sedentary subjects. We conclude that participation in regular physical activity may offer global benefits to motor cortex function that enhances neuroplasticity, which could improve motor learning and neurorehabilitation in physically active individuals.
Introduction
There is converging evidence at the molecular, cellular, systems and behavioural levels that participation in physical activity and exercise is beneficial to brain health and function. Within the last decade, epidemiological evidence has accumulated to suggest that physical activity may confer health-protective benefits for several neurological diseases including Parkinson's disease, Alzheimer's dementia and ischaemic stroke (see Kramer & Erickson, 2007 for review), and may even slow functional decline during the neurodegeneration process (Heyn et al. 2004). Furthermore, exciting new evidence has emerged indicating that regular physical activity and exercise can increase brain plasticity (see Cotman & Berchtold, 2002; Colcombe et al. 2004), which is believed to be instrumental in the process of memory and learning. In humans, robust effects of exercise have been most clearly demonstrated in ageing populations, where sustained exercise participation enhances learning and memory, improves executive function, counteracts age-related mental decline, and protects against age-related brain atrophy (Kramer et al. 1999; see Colcombe & Kramer, 2003 for review). These studies suggest that regular physical activity and exercise provides both neuroprotective and neuroplastic benefits to the brain, and may serve to improve memory and learning in humans.
In contrast to the cognitive aspects, much less is known about how regular exercise influences plasticity in human primary motor cortex (M1), which plays a fundamental role in learning new motor skills (Sanes & Donoghue, 2000). It is well established that different types of exercise produce experience-specific alterations in cortical aspects of movement (see Adkins et al. 2006 for review), but these changes in M1 function have usually been examined only for the limbs involved in the exercise. Furthermore, it has been shown that the molecular mechanisms believed responsible for improved cognition with exercise occur in several brain regions including the hippocampus, cerebellum and M1 (Ding et al. 2004; Klintsova et al. 2004, see Vaynman & Gomez-Pinilla, 2005) suggesting that exercise may have a more general effect on brain and motor function. We were particularly interested in whether, like for neurocognitive function, the benefits of regular exercise would extend beyond the neural boundaries responsible for control of the exercising limbs, and offer more global benefits for M1 control of muscles not specifically related to the exercise. To address this issue, we have used transcranial magnetic stimulation (TMS) applied in single and paired-pulse protocols to test the excitability of corticospinal projections and extent of intracortical inhibition to a small hand muscle in physically active and sedentary subjects.
To experimentally induce neuroplasticity, the technique of paired associative stimulation (PAS) was used, as it has been deliberately adapted from similar protocols used in brain slices and neuronal cultures, which demonstrate bidirectional spike timing-dependent synaptic plasticity (Dan & Poo, 2004; Caporale & Dan, 2008). A major strength of this technique is that it shares many physiological properties of synaptic plasticity obtained at the cellular level in animal preparations, such as rapid onset, duration, specificity, associativity and NMDA-receptor dependence (see Ziemann et al. 2008 for review). PAS in humans involves a stimulus to the median nerve followed by a single TMS pulse applied over the hand area of the motor cortex (Stefan et al. 2000). When appropriately timed, PAS induces a lasting increase in corticospinal excitability which is interpreted as a marker of plasticity within M1 (Di Lazzaro et al. 2009), with LTP-like processes thought to play a major role (Stefan et al. 2002). An interaction between PAS and motor training suggests that this technique tests functionally relevant neuronal circuits (Ziemann et al. 2004; Stefan et al. 2006; Jung & Ziemann, 2009), and there is strong evidence that altered neuroplasticity (assessed with PAS) may be related to impaired motor learning in some clinical conditions, such as Parkinson's disease (Ueki et al. 2006) and schizophrenia (Frantseva et al. 2008). Taken together, these characteristics of PAS suggest that LTP-like plasticity can be tested at the systems level of the human motor cortex, and the circuits tested are functionally relevant and clinically important (Ziemann et al. 2008).
The purpose of this study was to examine M1 plasticity in highly active and sedentary young subjects. The exercise routine of the active individuals consisted largely of endurance (aerobic) exercise involving lower limb muscles such as running and cycling. Subjects reported no specialised use of their hand muscles such as playing a musical instrument, as this is known to influence hand muscle excitability and plasticity (Rosenkranz et al. 2007). Because exercise is able to improve overall brain health and function in several brain areas including M1, we hypothesize that there will be increased plasticity in the motor cortical projection to a small hand muscle in physically active compared with sedentary subjects. Such a finding might suggest that regular exercise may offer global benefits to human M1 function, which may improve the acquisition of new motor skills and be beneficial for recovery of function following brain injury.
Methods
Subjects
Experiments were performed on the left hand of 28 young subjects (13 women, 15 men; mean ±s.d., 24 ± 4 years; range 18–38 years) with no known history of peripheral or neurological impairment. All subjects were right handed (median LQ = 0.77, range 0.5–1.0) as assessed by the Edinburgh Handedness Questionnaire (Oldfield, 1971) and were free of any cognitive mental state disorders as assessed by the mini-mental state examination (MMSE) (Folstein et al. 1975). Subjects were categorised into active (5 women and 9 men) and sedentary (8 women and 6 men) classifications using the International Physical Activity Questionnaire (IPAQ). The long version of the IPAQ was used, consisting of 31 items describing the extent of leisure-time physical activity involving aerobic exercises such as running, cycling and walking (Craig et al. 2003; Fogelholm et al. 2006). This questionnaire has been shown to produce reliable and repeatable measures of physical activity, and is comparable to objective assessment by accelerometer (Craig et al. 2003). To more accurately equate the self reported IPAQ score to physical (cardiorespiratory) fitness, subjects were asked to focus on leisure-time physical activity, with an emphasis on vigorous physical activity such as running and cycling (Fogelholm et al. 2006). All experiments were performed in the afternoon or evening to minimize variations in circulating cortisol and its effect on plasticity induction (Sale et al. 2008), and subjects were asked to refrain from physical activity prior to the experiment (on that day), but could perform exercise after the experiment was completed. Furthermore, no subjects reported long term specialized use of the hands, such as playing a musical instrument (Rosenkranz et al. 2007). All subjects gave written informed consent prior to participation in the study, which was approved by the University of Adelaide Human Research Ethics Committee and was conducted in accordance to the standards established by the Declaration of Helsinki.
Experimental arrangements
Subjects were seated comfortably in an experimental chair with their left shoulder abducted approximately 45 deg to allow the hand and arm to rest on a manipulandum. The hand was positioned with the palm facing down and the proximal phalanx of the thumb was placed in a metal ring attached to a load cell (LC 1205-K100, A&D Co., Tokyo, Japan) to facilitate measurement of thumb abduction force. Thumb abduction force was displayed on an oscilloscope to provide visual feedback to the subject, and was also digitized online (2 kHz) via a CED 1401 interface (Cambridge Electronic Design, Cambridge, UK) and stored on computer for offline analysis.
Surface electromyography (EMG) was recorded from the abductor pollicis brevis (APB) and first dorsal interosseous (FDI) muscles of the left hand using Ag–AgCl electrodes placed 2 cm apart. The EMG signals were amplified (×1000), filtered (13 Hz–1000 Hz), digitized online (2 kHz/channel) via a CED 1401 interface, and stored on computer for offline analysis. The EMG signals of both muscles were displayed on an oscilloscope to assist the subject in maintaining EMG silence when required.
TMS
Transcranial magnetic stimulation (TMS) was applied using a figure-of-eight coil (outer coil diameter 70 mm) with two Magstim 200 magnetic stimulators connected with a Magstim Bistim unit (Magstim, Whitland, Dyfed, UK). The coil was held tangentially to the skull with the handle pointing backwards and laterally at an angle of 45 deg to the sagittal plane. The coil was positioned at the optimal scalp position over the right hemisphere for eliciting a motor evoked potential (MEP) in the relaxed left APB muscle. The optimal scalp position was marked for reference, and the coil position was continually checked throughout the experiment.
PAS
Paired associative stimulation (PAS) was performed as described previously by Stefan et al. (2000). The PAS protocol consisted of percutaneous electrical stimulation of the median nerve at the left wrist (300% of perceptual threshold) followed by suprathreshold TMS (130% resting motor threshold) 25 ms later over the right motor cortex. The intervention consisted of 90 paired stimuli delivered at 0.05 Hz with the procedure lasting for 30 min. Electrical stimuli were applied to the median nerve at the wrist using a constant current stimulator (DS7A stimulator, Digitimer Ltd, Welwyn Garden City, UK) with bipolar surface electrodes, separated by 30 mm, and with the cathode proximal. Stimuli were square wave pulses with a pulse width of 200 μs.
The attentional focus of the subject has been shown to be an important factor influencing PAS effectiveness (Stefan et al. 2004). Therefore, subjects were instructed to direct their attention on the stimulated (left) hand and count the peripheral stimuli they perceived during the PAS intervention (total of 90 stimuli).
Experimental parameters
At the beginning of each experiment, abduction force exerted by the left thumb during a maximum voluntary contraction (MVC) was measured. Visual feedback of thumb abduction force was displayed on an oscilloscope to aid the subject. Three MVC trials were performed, with a minimum of 30 s rest between trials, and the MVC with the largest thumb abduction force was used for the assessment of muscle strength.
Measures of motor cortical excitability using TMS included resting motor threshold (RMT), active motor threshold (AMT), MEP amplitude, input–output curve (IO curve), short-interval intracortical inhibition (SICI) and cortical silent period (CSP) duration. All measures were performed before and after PAS, with the exception of AMT, which was only recorded before PAS.
Resting motor threshold was determined as the minimum stimulus intensity required to produce a MEP in the relaxed APB of at least 50 μV in 3 out of 5 consecutive trials. Active motor threshold was defined as the minimum stimulus intensity required to produce a MEP in the APB muscle of at least 200 μV in 3 out of 5 consecutive trials during a low-level voluntary thumb abduction (10% MVC). The stimulus intensity was altered in 1% increments of maximum stimulator output (MSO) throughout this process.
The stimulus intensity that produced a MEP amplitude of approximately 1 mV in resting APB was determined before PAS. Using this stimulator intensity, 10 trials were recorded to investigate resting MEP amplitude before PAS, 5 min after PAS (After 5), and 30–40 min following PAS (After 30). The mean amplitude was calculated from each trial at each time point.
The intensities used to construct the IO curves were determined for each individual according to their RMT before PAS. Eight trials at 90, 100, 110, 120, 130 and 140% of RMT were recorded for each subject at rest. The order of presentation of the six conditions was pseudorandomised throughout the trials and stimuli were given every 5 s. Amplitudes were measured for each trial to calculate the mean MEP amplitude for each TMS intensity.
Short-interval intracortical inhibition (SICI) was investigated using a paired-pulse TMS protocol consisting of a subthreshold conditioning stimulus that preceded a suprathreshold test stimulus by 3 ms (Kujirai et al. 1993). The intensity of the conditioning stimulus was randomised as 70, 80, or 90% of AMT, and the test stimulus intensity was that used to produce a MEP amplitude of approximately 1 mV in resting APB. The test stimulus intensity in paired-pulse trials was adjusted following PAS, if required, so that test MEP amplitudes were equivalent before and after PAS (APB MEP amplitude of approximately 1 mV). Each data block consisted of 12 trials for each of two conditions: test stimulus alone and SICI (conditioning and test stimulus ISI = 3 ms). The order of presentation of the two conditions was randomised throughout the trials and stimuli were given every 5 s. The conditioned MEP amplitude was expressed as a percentage of the unconditioned test MEP amplitude to calculate the influence of the conditioning stimulus.
Measurements of CSP duration were made during a low-level voluntary contraction of APB (10% MVC) before and after PAS. Subjects were provided with visual feedback of thumb abduction force displayed on an oscilloscope. TMS intensity was 130% RMT and 10 stimuli were given at a frequency of 0.2 Hz. CSP duration was analysed using a modified cumulative sum (CUSUM) method (Brinkworth & Turker, 2003). The EMG signal was rectified and CSP duration was assessed from the point of TMS until the EMG crossed the pre-stimulus mean (pre-stimulus period of 200 ms) following the MEP. All measurements were made off-line on individual trials and averaged for the 10 trials.
Data analysis and statistics
Student's t test for unpaired data was used to compare differences in physical activity levels (IPAQ), age, handedness, cognitive mental state (MMSE), attention, RMT and 1 mV TMS intensity before PAS. MEP amplitudes were measured peak-to-peak in each individual trial. The slopes of the IO curve were quantified by a linear regression analysis for all data points between 110 and 140% RMT and a two-way analysis of variance (ANOVA) was used to compare differences between groups (sedentary, active) and time (before, after 5, after 30). These points were used as they form the linear portion of the IO curve (see Rosenkranz et al. 2007). Two-way repeated measures ANOVA (Group, Time) was also used to analyse RMT, 1 mV MEP amplitude TMS intensity and CSP duration, and three-way repeated measures ANOVA (Group, Time, Intensity) was employed for analysis of IO curve and SICI. Fisher's LSD post hoc test that performed all possible comparisons was used to analyse significant main effects and interactions. All dependent variables were tested for non-sphericity using Mauchly's test. The only dependent variable not meeting the assumption of sphericity is the MEP input–output curve data, which was adjusted using the Greenhouse–Geisser correction. The significance level was set at P < 0.05 for all comparisons and all group data are provided as means ±s.e.m.
Results
All subjects were comfortable with the TMS procedure and no side effects were reported. There was no difference for age, handedness, cognitive mental state, attention to the intervention, and TMS thresholds between the two groups (Table 1). The one separating characteristic between the two groups was the level of physical activity, as quantified by the IPAQ. The active group had leisure-time physical activity levels that were 11-fold greater than the sedentary group (Table 1). On a weekly average, individuals in the active group performed four sessions of vigorous intensity activity (predominantly running and cycling) for a period of 60 min each session, and five sessions of moderate intensity activity (such as jogging and walking) for 90 min each session. Two of the active individuals were semi-professional athletes and performed vigorous running or cycling exercise for 120 min six times a week, along with moderate intensity exercise (jogging, swimming, or walking) for 90 min sessions three times a week. In contrast, individuals in the sedentary group performed, on average, no greater than three sessions of walking for 20 min each session during their leisure time.
Table 1.
Description of subject characteristics and baseline excitability measures before PAS
| Physical activity level |
||
|---|---|---|
| Sedentary (mean ±s.d.) | Active (mean ±s.d.) | |
| Age (years) | 24 ± 4 | 24 ± 5 |
| Sex | 6 M, 8 F | 9 M, 5 F |
| Physical activity (IPAQ, MET-min) | 491 ± 308 | 5572 ± 2075* |
| Handedness (−1 to 1) | 0.81 ± 0.16 | 0.73 ± 0.23 |
| MMSE (total of 30) | 29.4 ± 0.5 | 29.4 ± 0.9 |
| Attention (total of 90) | 89.5 ± 2.1 | 87.1 ± 4.4 |
| MVC (N) | 38.0 ± 15.8 | 47.4 ± 17.8 |
| RMT (% MSO) | 50.8 ± 10.8 | 50.3 ± 10.2 |
| 1 mV TMS intensity (% MSO) | 62.1 ± 14.3 | 59.0 ± 11.7 |
| AMT (% MSO) | 40.8 ± 7.4 | 39.1 ± 7.1 |
IPAQ, International Physical Activity Questionnaire. MMSE, mini-mental state examination. MVC, thumb abduction maximum voluntary contraction. RMT, APB resting motor threshold. AMT, APB active motor threshold. MSO, maximum stimulator output.
P < 0.001 compared with sedentary subjects.
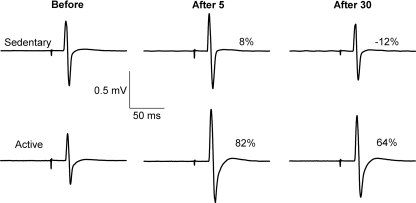
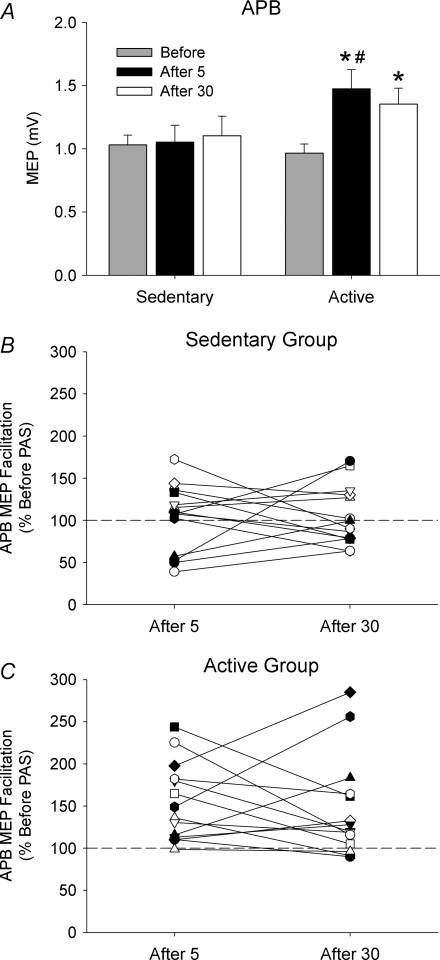
Figure 1 shows original recordings from one sedentary and one active subject before PAS, 5 min following PAS (After 5), and ∼30 min later (After 30). In the sedentary subject, there were minimal changes in the MEPs recorded in the left APB after PAS. In contrast, there was significant MEP facilitation following PAS at both time points in the active subject. The group data in Fig. 2A show the mean MEP amplitude in the target muscle (APB) before and after PAS. No difference in TMS intensity was required to evoke a ∼1 mV response in resting APB between the two groups before PAS (P= 0.54; Table 1), and this intensity was used to quantify the MEP response after PAS as a marker of neuroplasticity. A two-way repeated measures ANOVA revealed that there was no difference in MEP amplitude between physical activity groups (F= 2.1, P= 0.16), but there was a significant difference between time points (F= 5.3, P < 0.01) and a Physical Activity × Time interaction (F= 2.7, P= 0.04). Post hoc analysis indicated that APB MEP amplitude in the active group was 54% larger 5 min after PAS (P= 0.01) and 34% larger 30 min later (P= 0.03) compared with before PAS. There was also a significant difference in APB MEP amplitude in the sedentary and active groups 5 min after PAS (P= 0.02). There was no change in the control muscle FDI MEP amplitude after PAS in both groups. Despite these striking group differences in MEP facilitation after PAS, the individual subject responses revealed substantial variability within each subject group (Fig. 2B and C). Although most subjects in the active group showed marked MEP facilitation after PAS, there was only moderate facilitation in some sedentary subjects, whereas four sedentary subjects showed substantial PAS-induced MEP depression.
Figure 1. Average MEP recordings from the resting APB of one sedentary (upper panel) and one active subject (lower panel) before the onset of PAS (Before), 5 min after PAS (After 5), and 30–40 min after PAS (After 30).
At both time points after PAS, the MEP amplitudes were substantially larger in the active subject, but only small changes were observed in the sedentary subject. Both subjects participated in the experiment in the afternoon and had similar characteristics of RMT before and after PAS (Before: Sedentary = 45% MSO, Active = 52% MSO; After: Sedentary = 44% MSO, Active = 50% MSO), attention (Total of 90: Sedentary = 87, Active = 84), handedness (LQ: Sedentary = 0.7, Active = 0.9), and cognitive mental state (MMSE Total of 30: Sedentary = 29, Active = 30). The difference between the two individuals was the level of physical activity (IPAQ: Sedentary = 460 MET-min, Active = 6900 MET-min). Numbers indicate the percentage change in MEP amplitude following PAS.
Figure 2. Changes in APB MEP 1 mV amplitude before and after PAS in sedentary and active subjects.
A, mean (±s.e.m.) APB MEP amplitudes in sedentary and active subjects before, 5 min after (After 5) and 30 min after PAS (After 30). B and C, individual subject responses showing the percentage change in MEP amplitude after PAS relative to before PAS in sedentary (B) and active (C) subjects. A value of 100% represents the amplitude of the response before PAS. *P < 0.05 compared with before PAS. #P < 0.05 compared with the same time point in sedentary subjects.
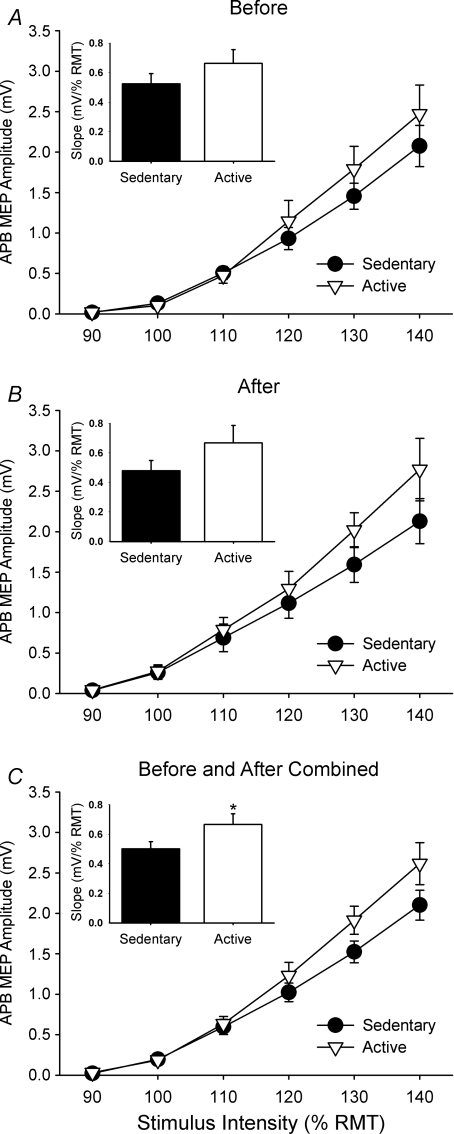
The effect of PAS on the IO curves of the relaxed APB in active and sedentary subjects is shown in Fig. 3. A three-way repeated measures ANOVA (with Greenhouse–Geisser correction for sphericity) indicated an increase in the size of the APB MEP amplitude with increasing stimulus intensity in the sedentary and active groups (Intensity effect, F= 88.5, P < 0.01) and MEP amplitude was greater after PAS (Time effect, F= 4.2, P < 0.05). There were no significant differences between Groups (F= 1.2, P= 0.28) or Interactions. To quantify the change in the IO curve, the slopes were calculated for the linear portion of the curve (between 110 and 140% RMT) and a two-way ANOVA was performed to compare the differences in slope between physical activity groups before and after PAS. This analysis revealed that the IO curve slopes were 35% steeper for the active compared with the sedentary group for both time points combined (Physical Activity effect, F= 4.1, P < 0.05) (Fig. 3C inset), but there was no change in the IO curve slopes following PAS (Time effect, F= 0.01, P= 0.92) and no significant Physical Activity × Time interactions (F= 0.18, P= 0.68). Although not statistically significant, the IO curve slopes were 27% greater in active subjects before PAS and 44% greater in active subjects after PAS compared with sedentary subjects. The RMT did not change after PAS (P= 0.18), and was 50.4 ± 10.0% MSO in sedentary subjects and 49.9 ± 9.8% MSO in active subjects after PAS (compare with before PAS in Table 1).
Figure 3. Mean (±s.e.m.) MEP amplitude data for APB IO curves in sedentary and active subjects shown before (A) and after PAS (B) and for before and after combined (C).
The slope of the curve has been calculated between 110 and 140% RMT and is shown in the inset of each graph. The slope of the IO curve was significantly steeper in the active subjects compared with the sedentary subjects when data were combined before and after PAS (*P < 0.05).
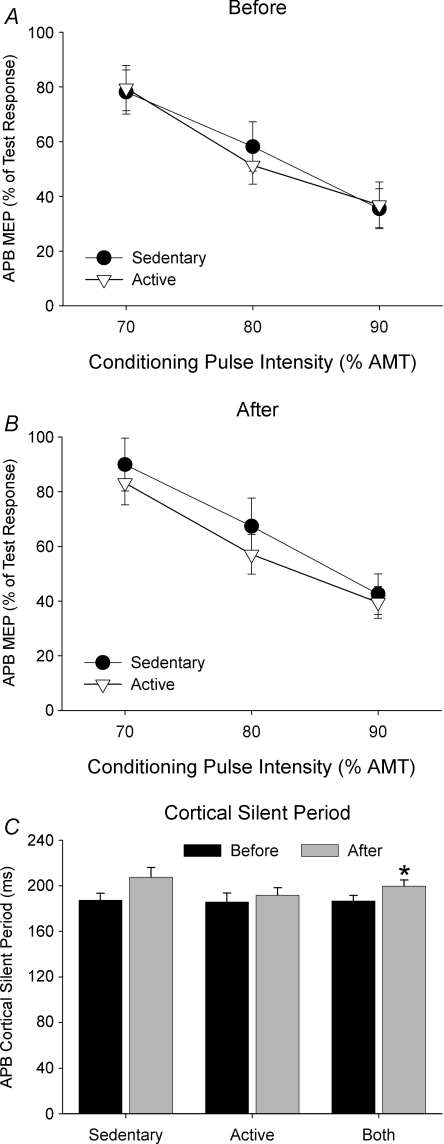
SICI was assessed using a paired-pulse protocol that utilized a ∼1 mV test pulse that was preceded by a subthreshold conditioning pulse (70, 80, or 90% AMT) at 3 ms (SICI). If necessary, the TMS intensity for the ∼1 mV test pulse was adjusted after PAS, which resulted in a significant reduction in the ∼1 mV TMS intensity after PAS (P= 0.004), where it was 61.6 ± 14.8% MSO in sedentary subjects and 57.6 ± 12.4% MSO in active subjects after PAS (compare with before PAS in Table 1). Data showing the extent of SICI in each group before and after PAS are shown in Fig. 4. Increasing the intensity of the conditioning stimulus increased the amount of SICI in both groups (F= 43.6, P < 0.001). However, SICI was not different between physical activity groups (F= 0.2, P= 0.65), and there was no difference in SICI before and after PAS (F= 3.7, P= 0.07). Furthermore, there were no significant Group × Time (F= 0.6, P= 0.45), Group × Intensity (F= 0.4, P= 0.69) or Group × Time × Intensity interactions (F= 0.1, P= 0.91).
Figure 4. Data represents mean (±s.e.m.) SICI (A and B) and CSP duration (C) shown before and after PAS in active and sedentary subjects.
SICI was obtained with a conditioning TMS intensity of 70, 80 and 90% AMT expressed as a percentage of the APB MEP amplitude evoked by the test stimulus alone (100%). The extent of SICI was influenced by conditioning TMS intensity before and after PAS, but was not different between sedentary and active subjects. There was a significant increase in CSP duration after PAS from pooled data in both groups (*P= 0.002), which was largely influenced by the sedentary subjects.
The duration of the CSP before and after PAS for the sedentary and active groups is shown in Fig. 4C. There was no difference in CSP duration between groups (Physical Activity effect, F= 0.8, P= 0.38). However, the CSP was 13 ms longer after PAS in both groups (Time effect; F= 12.3, P < 0.01). The longer CSP duration after PAS was largely due to a longer CSP in the sedentary group, although this interaction just failed to reach statistical significance (Physical Activity × Time interaction, F= 3.7, P= 0.07).
Discussion
The present study investigated specific features of the corticospinal pathway in highly active and sedentary young subjects. There were two main findings. First, the IO curve for a small hand muscle (APB) was similar in sedentary and active subjects before PAS, but was steeper in active subjects when pooled before and after PAS. Second, PAS induced significantly more facilitation of APB MEP amplitudes in the active compared with the sedentary subjects, indicating heightened synaptic plasticity in the motor system of physically active individuals. Because the main distinguishing characteristic between these subject groups was the difference in physical activity levels, we suggest that these features of corticospinal excitability and plasticity arise from enhanced regular physical activity involving endurance (aerobic) exercise.
Subjects in the sedentary and active groups were well matched for age, sex, handedness and cognitive mental state (see Table 1). However, substantial differences existed in the extent of physical activity in the two groups as reported by questionnaire (IPAQ, Craig et al. 2003). To obtain a clearly distinct group of active and sedentary subjects, individuals classified as highly active from the IPAQ had to perform at least three sessions per week of vigorous physical activity to be included in the study. This stringent selection criterion resulted in a marked (11-fold) difference in physical activity levels between active and sedentary subjects. Based on the IPAQ scores and physical fitness assessments from a large population of subjects (Fogelholm et al. 2006), we estimate that the physical activity levels of our subject groups lie within the top and bottom 20% of a healthy young population.
Corticospinal excitability in physically active and sedentary individuals
As expected, increasing TMS intensity resulted in an increase in MEP amplitude in physically active and sedentary subjects, reflecting increased activation of a population of cortico-cortical, corticospinal and spinal motor neurons activated by TMS. The TMS intensities for this IO curve were expressed relative to threshold intensity required to produce MEPs in resting APB muscle, which were similar for the sedentary and active groups, along with a similar IO curve slope between groups before PAS. However, the IO curve slope was 35% steeper in physically active subjects when the data before and after PAS were combined (Fig. 3C), suggesting an increased strength of the corticospinal connections that are activated with higher TMS intensities in physically active individuals (Ridding & Rothwell, 1997). Several studies have shown that chronic physical activity can produce functional adaptations in corticospinal and spinal motor neurons in humans. For example, there are increased cortical representations and MEP amplitudes to the involved muscles in highly skilled racquet players (Pearce et al. 2000) and steeper IO curves in musicians (Rosenkranz et al. 2007). Furthermore, examinations of spinal cord circuitry indicate that endurance-trained individuals have enhanced H and stretch reflexes (see Koceja et al. 2004 for a review), with increasing amplitude of H reflexes in more active individuals (Nielsen et al. 1993). However, these changes in excitability have all been assessed in the muscle groups involved in the training, reflecting a likely task-specific adaptation for the M1 representation of muscles in the exercising limb. For the physically active subjects in the present study, we have found increased excitability in the corticospinal pathway to muscles not directly involved in the exercise, but only when the data were combined before and after PAS. Although this adaptation could conceivably occur within the spinal cord, there is no change in spinal (H) reflexes to upper limb muscles after lower limb exercise (Motl & Dishman, 2003), suggesting that the increased slope of the IO curve in physically active individuals may occur, at least in part, through changes in M1 function.
The input–output properties of M1 can also be influenced by inhibitory interneurons that use γ-aminobutyric acid (GABA) as their transmitter (Sanes & Donoghue, 2000), which constitute approximately 25–30% of neurons in primate neocortex (Jones, 1993). These GABAergic inhibitory systems within human M1 are studied with paired-pulse TMS to assess SICI, or with suprathreshold single-pulse TMS that suppresses voluntary activation for up to 300 ms (CSP). Cortical mechanisms are believed to contribute to SICI (Di Lazzaro et al. 1998) and the later stages (>60 ms) of the silent period (Inghilleri et al. 1993). SICI is mediated by GABAA receptors while CSP is mediated by GABAB receptors, and the cortical neurons mediating these two forms of intracortical inhibition appear to be distinct (reviewed by Chen, 2004). Increasing evidence suggests that GABAergic systems responsible for the CSP (Classen et al. 1997) and SICI (Zoghi et al. 2003) play an important role in motor performance, and alterations in GABAergic inhibition have been associated with both enhanced (Rosenkranz et al. 2007) and impaired motor skills (Ridding et al. 1995; Sale & Semmler, 2005). Using these two markers of intracortical inhibition, we found no differences in SICI or CSP inhibition between physically active and sedentary subjects before or after PAS, suggesting that the threshold and/or distribution of GABAergic inhibitory interneurons in M1 is not influenced by regular physical activity.
In contrast to motor skill training, short term (<1 month) endurance exercise does not seem to result in alterations in synaptic connectivity within M1, although there can be substantial changes in cerebral vasculature and blood flow (see Adkins et al. 2006 for review). For example, Kleim et al. (2002) have shown that 30 days of exercise does not alter motor cortical representation but increases angiogenesis in rat M1. Furthermore, animals given free access to a running wheel for 30 days show increased angiogenesis that is specific to M1, but was not evident in other frontal or subcortical areas (Swain et al. 2003). These increases in regional cerebral blood flow are not only elevated during activity, but could also be enhanced in trained individuals in the resting state (Xiong et al. 2009). Several studies have shown that this increased blood flow to M1 with exercise is accompanied by increased neurotrophic factors that facilitate the survival and differentiation of neurons (Klintsova et al. 2004; Vaynman & Gomez-Pinilla, 2005), providing a more supportive neural environment (see Adkins et al. 2006). It is therefore possible that this improved cortical environment may promote neural survival and increased neural density in M1 neurons with longer-term exercise, resulting in changes in corticospinal function and excitability in individuals who have been physically active over a period of several years. This idea is supported by the finding of reduced age-related loss of brain tissue in older adults with heightened aerobic fitness (Colcombe et al. 2004).
Increased synaptic plasticity in physically active individuals
PAS is a common procedure used in neurophysiological studies to experimentally-induce neural plasticity in humans. The conventional PAS approach combines low-frequency, percutaneous electrical stimulation of the median nerve at the wrist paired with TMS over the contralateral hand area of M1 (Stefan et al. 2000). The TMS is timed to coincide with the arrival at the cortex of the afferent volley evoked 25 ms earlier by the peripheral stimulus. This protocol results in substantial increases in the amplitude of hand muscle MEPs, which is interpreted as a marker of neuroplasticity. The increase in corticospinal excitability following PAS is long-lasting, being elevated for 30–60 min in most subjects (Stefan et al. 2000). Despite some evidence of a contribution from within spinal cord circuits (Meunier et al. 2007), the increased excitability is thought largely to reflect a change in M1 function, as there is no change in spinal excitability measured with F-waves and electrical brainstem stimulation (Stefan et al. 2000). More recently, direct evidence from epidural recordings of corticospinal descending volleys have shown that PAS enhances responses of later descending (I or indirect) waves (Di Lazzaro et al. 2009), providing strong evidence of a cortical origin in the changes induced by PAS. The increased excitability is thought to occur through LTP-like effects (Stefan et al. 2002), and an interaction between PAS and motor training suggests that this technique involves functionally relevant neuronal circuits (Ziemann et al. 2004; Jung & Ziemann, 2009).
Several factors are known to influence the extent of MEP facilitation induced with a PAS intervention, including subject age (Tecchio et al. 2008), attention to the procedure (Stefan et al. 2004), and time of day the experiments were performed (Sale et al. 2008). Each of these factors was similar between the sedentary and active subjects in the present study. Despite these similarities, we found striking differences in PAS-induced neuroplasticity in a hand muscle of the physically active compared with sedentary subjects in the present study, which resulted in a 40% larger MEP in the active compared with sedentary subjects after PAS. It is likely that part of this difference can be attributed to the 27% steeper (although non-significant) IO curve slope in the active subjects before PAS, making these individuals more susceptible to PAS effects (see Rosenkranz et al. 2007). Using the theoretical observations described by Rosenkranz et al. (2007) to estimate the change in MEP amplitude based on the different IO slopes observed in the present study, an increase in the test TMS pulse by 10% (equivalent to the increase in MEP observed after PAS with the 1 mV TMS intensity) would produce an increase in MEP of 52% (1.52 mV) in the sedentary subjects and 66% (1.66 mV) in the active subjects. This represents only a 9% (relative) difference in MEP amplitude after PAS that can be explained by the baseline difference in slopes of the IO curves in each group. However, we report a 40% larger MEP (1 mV TMS intensity) in physically active compared with sedentary subjects after PAS, which suggests that a large proportion (∼75%) of the increase in MEP in physically active subjects is due to increased PAS-induced plasticity in these individuals. An additional confounding factor is that a fixed TMS intensity (130% RMT) was used in the present study (as in Stefan et al. 2000), which potentially provided a stronger activation of the corticospinal system during PAS in physically active subjects. However, there was no significant difference in MEP amplitudes at this TMS intensity between the two groups before or during PAS, suggesting that this is unlikely to be a major contributor to the increased 1 mV MEP after PAS in physically active subjects. We therefore suggest that an additional lifestyle factor that can contribute to increased neuroplasticity after PAS is the physical activity status of the subjects under investigation. However, there was still substantial variability in the extent of facilitation within subject groups, indicating that other factors are also likely to contribute to this effect.
Despite greater differences in the slope of the IO curve between physically active and sedentary subjects after PAS (27% difference before PAS, 44% difference after PAS), there was some mismatch between the effect of PAS on the 1 mV MEP compared with the IO curve. There are at least three methodological differences between these two measures that might explain this effect. First, MEP 1 mV is based on absolute MEP amplitude resulting in different TMS intensities relative to resting threshold in each subject, whereas the MEP IO curve is expressed relative to the subjects own resting threshold. Second, these two measures were obtained at different times before and after PAS. The MEP 1 mV was assessed 5–10 min following PAS whereas the IO curve was assessed an additional 10 min later (15–20 min following PAS), and it is not known how time may affect cortical excitability (MEP amplitude) following PAS in physically active individuals. Third, for logistical reasons, each method was assessed with a different number of trials, with more trials included in the analysis of MEP 1 mV (10 trials at 1 mV TMS intensity) compared with the MEP IO curve (8 trials for each TMS intensity of 90–140% RMT). The substantial trial-to-trial fluctuations in MEP amplitude could therefore influence the mean and variability of the MEP differently between the two measures (see McDonnell et al. 2004).
Several studies have shown that alterations in GABAergic inhibition play a fundamental role in cortical reorganization and plasticity. For example, studies in rat cortical slice preparations have shown that LTP is only induced in the presence of a GABA antagonist (Hess & Donoghue, 1994), which disinhibits the cortex. In humans, pharmacological studies in which levels of GABA are enhanced through the use of a GABA agonist (lorazepam) or reduced through ischaemic nerve block have clearly demonstrated that a reduction of GABA-mediated inhibition facilitates cortical plasticity (Ziemann et al. 2001). In support of this, SICI is deficient in focal task-specific dystonia (Ridding et al. 1995), whereas the response to PAS is exaggerated in these individuals (Quartarone et al. 2003). In line with previous studies (Ridding & Taylor, 2001; Stefan et al. 2002; Sale et al. 2007), we found no change in SICI before and after PAS in both subject groups, indicating that changes in the operation of GABAA inhibitory circuits cannot be responsible for the increased PAS-induced plasticity in physically active individuals. In contrast, PAS is known to produce a significant increase in CSP duration (Stefan et al. 2000; Sale et al. 2007), which was also observed in the present study, indicating that PAS increases the effectiveness of GABAB mediated inhibitory cortical circuits that are activated by TMS during voluntary contraction. However, the change in CSP after PAS was much smaller in the active subjects, indicating that these circuits are less susceptible to modulation from the PAS intervention in physically active individuals.
Factors influencing PAS-induced plasticity in physically active individuals
Despite the subject groups being well matched on a number of baseline characteristics (see Table 1), the cross-sectional design of this study allows several confounding factors to potentially contribute to the increased PAS-induced plasticity in physically active subjects. First, we cannot exclude the possibility that the increased plasticity in M1 of physically active individuals represents a genetic trait, which makes these individuals more susceptible to participation in regular physical activity. However, the only published genetic influence on PAS-induced plasticity is the presence of a BDNF polymorphism, which limits the extent of M1 plasticity in these individuals (Cheeran et al. 2008). This polymorphism is present in ∼30% of the normal population (Bath & Lee, 2006), and the probability that all 14 sedentary subjects have a BDNF polymorphism, whereas all 14 active subjects do not, is therefore extremely low. Second, we do not know what effect the recent history of (or withdrawal from) physical exercise has on PAS-induced M1 plasticity in these subjects. It is well established that prior motor learning causes a homeostatic interaction with subsequent PAS-induced plasticity when focused on the same muscle group (Ziemann et al. 2004; Stefan et al. 2006), but it is not known whether regular exercise (or withdrawal) performed with the lower limbs would produce a homeostatic interaction with PAS performed on upper limb muscles. Third, it is known that corticospinal excitability and plasticity in women is dependent on the menstrual cycle (e.g. Smith et al. 1999; Inghilleri et al. 2004). However, we found no difference between men and women in the response to PAS, and removal of the female subjects (8 sedentary, 5 active) from the analysis did not influence the main findings of the study, as there was only a 20% increase in 1 mV MEP response in the six sedentary male subjects, but a 50% increase in 1 mV MEP in the nine active male subjects. Although we cannot rule out these factors, we suggest that their contribution is likely to be minimal under the current experimental conditions, and conclude that at least some of the changes in plasticity are a consequence of regular physical activity.
In summary, we have used TMS to examine motor cortex excitability and PAS-induced plasticity in a small hand muscle of sedentary and active young subjects. We found that regular physical activity, primarily involving lower limb muscles, was accompanied by increased M1 plasticity in a small hand muscle compared with sedentary subjects. These findings indicate that regular exercise may offer benefits to M1 function that extend beyond the neural boundaries for control of the exercising limb, which has important implications for developing improved strategies for motor learning and rehabilitation following injury to the motor system.
Acknowledgments
A grant from the National Health and Medical Research Council (NHMRC) of Australia supported this work. This study forms part of the PhD of J.C., who is supported by a University of Adelaide Postgraduate Research Scholarship. M.C.R. holds a NHMRC Senior Research Fellowship.
Glossary
Abbreviations
- AMT
active motor threshold
- APB
abductor pollicis brevis
- BDNF
brain-derived neurotrophic factor
- CSP
cortical silent period
- CUSUM
cumulative sum
- FDI
first dorsal interosseous
- IO
input–output
- IPAQ
international physical activity questionnaire
- ISI
inter-stimulus interval
- LQ
laterality quotient
- LTP
long-term potentiation
- M1
primary motor cortex
- MEP
motor evoked potential
- MET-min
metabolic equivalent of task-minutes
- MMSE
mini-mental state examination
- MSO
maximum stimulator output
- MVC
maximum voluntary contraction
- PAS
paired associative stimulation
- RMT
resting motor threshold
- SICI
short-interval intracortical inhibition
- TMS
transcranial magnetic stimulation
Author contributions
J.C., M.C.R. and J.G.S. were responsible for experimental design and manuscript preparation. J.C. and A.P.L. were responsible for data collection and analysis. J.G.S. had overall responsibility for all aspects of the study, from conception to publication. All authors provided important intellectual input to the study and approved the submitted version of the manuscript. All experiments were performed at the University of Adelaide, South Australia, Australia.
References
- Adkins DL, Boychuk J, Remple MS, Kleim JA. Motor training induces experience-specific patterns of plasticity across motor cortex and spinal cord. J Appl Physiol. 2006;101:1776–1782. doi: 10.1152/japplphysiol.00515.2006. [DOI] [PubMed] [Google Scholar]
- Bath KG, Lee FS. Variant BDNF (Val66Met) impact on brain structure and function. Cogn Affect Behav Neurosci. 2006;6:79–85. doi: 10.3758/cabn.6.1.79. [DOI] [PubMed] [Google Scholar]
- Brinkworth RS, Turker KS. A method for quantifying reflex responses from intra-muscular and surface electromyogram. J Neurosci Methods. 2003;122:179–193. doi: 10.1016/s0165-0270(02)00321-7. [DOI] [PubMed] [Google Scholar]
- Caporale N, Dan Y. Spike timing-dependent plasticity: a Hebbian learning rule. Annu Rev Neurosci. 2008;31:25–46. doi: 10.1146/annurev.neuro.31.060407.125639. [DOI] [PubMed] [Google Scholar]
- Cheeran B, Talelli P, Mori F, Koch G, Suppa A, Edwards M, Houlden H, Bhatia K, Greenwood R, Rothwell JC. A common polymorphism in the brain derived neurotrophic factor gene (BDNF) modulates human cortical plasticity and the response to rTMS. J Physiol. 2008;586:5717–5725. doi: 10.1113/jphysiol.2008.159905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R. Interactions between inhibitory and excitatory circuits in the human motor cortex. Exp Brain Res. 2004;154:1–10. doi: 10.1007/s00221-003-1684-1. [DOI] [PubMed] [Google Scholar]
- Classen J, Schnitzler A, Binkofski F, Werhahn KJ, Kim YS, Kessler KR, Benecke R. The motor syndrome associated with exaggerated inhibition within the primary motor cortex of patients with hemiparetic. Brain. 1997;120:605–619. doi: 10.1093/brain/120.4.605. [DOI] [PubMed] [Google Scholar]
- Colcombe S, Kramer AF. Fitness effects on the cognitive function of older adults: a meta-analytic study. Psychol Sci. 2003;14:125–130. doi: 10.1111/1467-9280.t01-1-01430. [DOI] [PubMed] [Google Scholar]
- Colcombe SJ, Kramer AF, Erickson KI, Scalf P, McAuley E, Cohen NJ, Webb A, Jerome GJ, Marquez DX, Elavsky S. Cardiovascular fitness, cortical plasticity, and aging. Proc Natl Acad Sci U S A. 2004;101:3316–3321. doi: 10.1073/pnas.0400266101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotman CW, Berchtold NC. Exercise: a behavioral intervention to enhance brain health and plasticity. Trends Neurosci. 2002;25:295–301. doi: 10.1016/s0166-2236(02)02143-4. [DOI] [PubMed] [Google Scholar]
- Craig CL, Marshall AL, Sjostrom M, Bauman AE, Booth ML, Ainsworth BE, Pratt M, Ekelund U, Yngve A, Sallis JF, Oja P. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35:1381–1395. doi: 10.1249/01.MSS.0000078924.61453.FB. [DOI] [PubMed] [Google Scholar]
- Dan Y, Poo MM. Spike timing-dependent plasticity of neural circuits. Neuron. 2004;44:23–30. doi: 10.1016/j.neuron.2004.09.007. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Dileone M, Pilato F, Profice P, Oliviero A, Mazzone P, Insola A, Capone F, Ranieri F, Tonali PA. Associative motor cortex plasticity: direct evidence in humans. Cereb Cortex. 2009;19:2326–2330. doi: 10.1093/cercor/bhn255. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Restuccia D, Oliviero A, Profice P, Ferrara L, Insola A, Mazzone P, Tonali P, Rothwell JC. Magnetic transcranial stimulation at intensities below active motor threshold activates intracortical inhibitory circuits. Exp Brain Res. 1998;119:265–268. doi: 10.1007/s002210050341. [DOI] [PubMed] [Google Scholar]
- Ding Y, Li J, Luan X, Ding YH, Lai Q, Rafols JA, Phillis JW, Clark JC, Diaz FG. Exercise pre-conditioning reduces brain damage in ischemic rats that may be associated with regional angiogenesis and cellular overexpression of neurotrophin. Neuroscience. 2004;124:583–591. doi: 10.1016/j.neuroscience.2003.12.029. [DOI] [PubMed] [Google Scholar]
- Fogelholm M, Malmberg J, Suni J, Santtila M, Kyrolainen H, Mantysaari M, Oja P. International Physical Activity Questionnaire: Validity against fitness. Med Sci Sports Exerc. 2006;38:753–760. doi: 10.1249/01.mss.0000194075.16960.20. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. ‘Mini-mental state’A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Frantseva MV, Fitzgerald PB, Chen R, Moller B, Daigle M, Daskalakis ZJ. Evidence for impaired long-term potentiation in schizophrenia and its relationship to motor skill learning. Cereb Cortex. 2008;18:990–996. doi: 10.1093/cercor/bhm151. [DOI] [PubMed] [Google Scholar]
- Hess G, Donoghue JP. Long-term potentiation of horizontal connections provides a mechanism to reorganize cortical motor maps. J Neurophysiol. 1994;71:2543–2547. doi: 10.1152/jn.1994.71.6.2543. [DOI] [PubMed] [Google Scholar]
- Heyn P, Abreu BC, Ottenbacher KJ. The effects of exercise training on elderly persons with cognitive impairment and dementia: a meta-analysis. Arch Phys Med Rehabil. 2004;85:1694–1704. doi: 10.1016/j.apmr.2004.03.019. [DOI] [PubMed] [Google Scholar]
- Inghilleri M, Berardelli A, Cruccu G, Manfredi M. Silent period evoked by transcranial stimulation of the human cortex and cervicomedullary junction. J Physiol. 1993;466:521–534. [PMC free article] [PubMed] [Google Scholar]
- Inghilleri M, Conte A, Curra A, Frasca V, Lorenzano C, Berardelli A. Ovarian hormones and cortical excitability. An rTMS study in humans. Clin Neurophysiol. 2004;115:1063–1068. doi: 10.1016/j.clinph.2003.12.003. [DOI] [PubMed] [Google Scholar]
- Jones EG. GABAergic neurons and their role in cortical plasticity in primates. Cereb Cortex. 1993;3:361–372. doi: 10.1093/cercor/3.5.361-a. [DOI] [PubMed] [Google Scholar]
- Jung P, Ziemann U. Homeostatic and nonhomeostatic modulation of learning in human motor cortex. J Neurosci. 2009;29:5597–5604. doi: 10.1523/JNEUROSCI.0222-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleim JA, Cooper NR, VandenBerg PM. Exercise induces angiogenesis but does not alter movement representations within rat motor cortex. Brain Res. 2002;934:1–6. doi: 10.1016/s0006-8993(02)02239-4. [DOI] [PubMed] [Google Scholar]
- Klintsova AY, Dickson E, Yoshida R, Greenough WT. Altered expression of BDNF and its high-affinity receptor TrkB in response to complex motor learning and moderate exercise. Brain Res. 2004;1028:92–104. doi: 10.1016/j.brainres.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Koceja DM, Davison E, Robertson CT. Neuromuscular characteristics of endurance- and power-trained athletes. Res Q Exerc Sport. 2004;75:23–30. doi: 10.1080/02701367.2004.10609130. [DOI] [PubMed] [Google Scholar]
- Kramer AF, Erickson KI. Capitalizing on cortical plasticity: influence of physical activity on cognition and brain function. Trends Cogn Sci. 2007;11:342–348. doi: 10.1016/j.tics.2007.06.009. [DOI] [PubMed] [Google Scholar]
- Kramer AF, Hahn S, Cohen NJ, Banich MT, McAuley E, Harrison CR, Chason J, Vakil E, Bardell L, Boileau RA, Colcombe A. Ageing, fitness and neurocognitive function. Nature. 1999;400:418–419. doi: 10.1038/22682. [DOI] [PubMed] [Google Scholar]
- Kujirai T, Caramia MD, Rothwell JC, Day BL, Thompson PD, Ferbert A, Wroe S, Asselman P, Marsden CD. Corticocortical inhibition in human motor cortex. J Physiol. 1993;471:501–519. doi: 10.1113/jphysiol.1993.sp019912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonnell MN, Ridding MC, Miles TS. Do alternate methods of analysing motor evoked potentials give comparable results? J Neurosci Methods. 2004;136:63–67. doi: 10.1016/j.jneumeth.2003.12.020. [DOI] [PubMed] [Google Scholar]
- Meunier S, Russmann H, Simonetta-Moreau M, Hallett M. Changes in spinal excitability after PAS. J Neurophysiol. 2007;97:3131–3135. doi: 10.1152/jn.01086.2006. [DOI] [PubMed] [Google Scholar]
- Motl RW, Dishman RK. Acute leg-cycling exercise attenuates the H-reflex recorded in soleus but not flexor carpi radialis. Muscle Nerve. 2003;28:609–614. doi: 10.1002/mus.10479. [DOI] [PubMed] [Google Scholar]
- Nielsen J, Crone C, Hultborn H. H-reflexes are smaller in dancers from The Royal Danish Ballet than in well-trained athletes. Eur J Appl Physiol Occup Physiol. 1993;66:116–121. doi: 10.1007/BF01427051. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Pearce AJ, Thickbroom GW, Byrnes ML, Mastaglia FL. Functional reorganisation of the corticomotor projection to the hand in skilled racquet players. Exp Brain Res. 2000;130:238–243. doi: 10.1007/s002219900236. [DOI] [PubMed] [Google Scholar]
- Quartarone A, Bagnato S, Rizzo V, Siebner HR, Dattola V, Scalfari A, Morgante F, Battaglia F, Romano M, Girlanda P. Abnormal associative plasticity of the human motor cortex in writer's cramp. Brain. 2003;126:2586–2596. doi: 10.1093/brain/awg273. [DOI] [PubMed] [Google Scholar]
- Ridding MC, Rothwell JC. Stimulus/response curves as a method of measuring motor cortical excitability in man. Electroencephalogr Clin Neurophysiol. 1997;105:340–344. doi: 10.1016/s0924-980x(97)00041-6. [DOI] [PubMed] [Google Scholar]
- Ridding MC, Sheean G, Rothwell JC, Inzelberg R, Kujirai T. Changes in the balance between motor cortical excitation and inhibition in focal, task specific dystonia. J Neurol Neurosurg Psychiatry. 1995;59:493–498. doi: 10.1136/jnnp.59.5.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridding MC, Taylor JL. Mechanisms of motor-evoked potential facilitation following prolonged dual peripheral and central stimulation in humans. J Physiol. 2001;537:623–631. doi: 10.1111/j.1469-7793.2001.00623.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenkranz K, Williamon A, Rothwell JC. Motorcortical excitability and synaptic plasticity is enhanced in professional musicians. J Neurosci. 2007;27:5200–5206. doi: 10.1523/JNEUROSCI.0836-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sale MV, Ridding MC, Nordstrom MA. Factors influencing the magnitude and reproducibility of corticomotor excitability changes induced by paired associative stimulation. Exp Brain Res. 2007;181:615–626. doi: 10.1007/s00221-007-0960-x. [DOI] [PubMed] [Google Scholar]
- Sale MV, Ridding MC, Nordstrom MA. Cortisol inhibits neuroplasticity induction in human motor cortex. J Neurosci. 2008;28:8285–8293. doi: 10.1523/JNEUROSCI.1963-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sale MV, Semmler JG. Age-related differences in corticospinal control during functional isometric contractions in left and right hands. J Appl Physiol. 2005;99:1483–1493. doi: 10.1152/japplphysiol.00371.2005. [DOI] [PubMed] [Google Scholar]
- Sanes JN, Donoghue JP. Plasticity and primary motor cortex. Annu Rev Neurosci. 2000;23:393–415. doi: 10.1146/annurev.neuro.23.1.393. [DOI] [PubMed] [Google Scholar]
- Smith MJ, Keel JC, Greenberg BD, Adams LF, Schmidt PJ, Rubinow DA, Wassermann EM. Menstrual cycle effects on cortical excitability. Neurology. 1999;53:2069–2072. doi: 10.1212/wnl.53.9.2069. [DOI] [PubMed] [Google Scholar]
- Stefan K, Kunesch E, Benecke R, Cohen LG, Classen J. Mechanisms of enhancement of human motor cortex excitability induced by interventional paired associative stimulation. J Physiol. 2002;543:699–708. doi: 10.1113/jphysiol.2002.023317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefan K, Kunesch E, Cohen LG, Benecke R, Classen J. Induction of plasticity in the human motor cortex by paired associative stimulation. Brain. 2000;123:572–584. doi: 10.1093/brain/123.3.572. [DOI] [PubMed] [Google Scholar]
- Stefan K, Wycislo M, Classen J. Modulation of associative human motor cortical plasticity by attention. J Neurophysiol. 2004;92:66–72. doi: 10.1152/jn.00383.2003. [DOI] [PubMed] [Google Scholar]
- Stefan K, Wycislo M, Gentner R, Schramm A, Naumann M, Reiners K, Classen J. Temporary occlusion of associative motor cortical plasticity by prior dynamic motor training. Cereb Cortex. 2006;16:376–385. doi: 10.1093/cercor/bhi116. [DOI] [PubMed] [Google Scholar]
- Swain RA, Harris AB, Wiener EC, Dutka MV, Morris HD, Theien BE, Konda S, Engberg K, Lauterbur PC, Greenough WT. Prolonged exercise induces angiogenesis and increases cerebral blood volume in primary motor cortex of the rat. Neuroscience. 2003;117:1037–1046. doi: 10.1016/s0306-4522(02)00664-4. [DOI] [PubMed] [Google Scholar]
- Tecchio F, Zappasodi F, Pasqualetti P, De Gennaro L, Pellicciari MC, Ercolani M, Squitti R, Rossini PM. Age dependence of primary motor cortex plasticity induced by paired associative stimulation. Clin Neurophysiol. 2008;119:675–682. doi: 10.1016/j.clinph.2007.10.023. [DOI] [PubMed] [Google Scholar]
- Ueki Y, Mima T, Kotb MA, Sawada H, Saiki H, Ikeda A, Begum T, Reza F, Nagamine T, Fukuyama H. Altered plasticity of the human motor cortex in Parkinson's disease. Ann Neurol. 2006;59:60–71. doi: 10.1002/ana.20692. [DOI] [PubMed] [Google Scholar]
- Vaynman S, Gomez-Pinilla F. License to run: exercise impacts functional plasticity in the intact and injured central nervous system by using neurotrophins. Neurorehabil Neural Repair. 2005;19:283–295. doi: 10.1177/1545968305280753. [DOI] [PubMed] [Google Scholar]
- Xiong J, Ma L, Wang B, Narayana S, Duff EP, Egan GF, Fox PT. Long-term motor training induced changes in regional cerebral blood flow in both task and resting states. Neuroimage. 2009;45:75–82. doi: 10.1016/j.neuroimage.2008.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemann U, Ilic TV, Pauli C, Meintzschel F, Ruge D. Learning modifies subsequent induction of long-term potentiation-like and long-term depression-like plasticity in human motor cortex. J Neurosci. 2004;24:1666–1672. doi: 10.1523/JNEUROSCI.5016-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemann U, Muellbacher W, Hallett M, Cohen LG. Modulation of practice-dependent plasticity in human motor cortex. Brain. 2001;124:1171–1181. doi: 10.1093/brain/124.6.1171. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Paulus W, Nitsche MA, Pascual-Leone A, Byblow WD, Berardelli A, Siebner HR, Classen J, Cohen L, Rothwell JC. Consensus: Motor cortex plasticity protocols. Brain Stimulat. 2008;1:164–182. doi: 10.1016/j.brs.2008.06.006. [DOI] [PubMed] [Google Scholar]
- Zoghi M, Pearce SL, Nordstrom MA. Differential modulation of intracortical inhibition in human motor cortex during selective activation of an intrinsic hand muscle. J Physiol. 2003;550:933–946. doi: 10.1113/jphysiol.2003.042606. [DOI] [PMC free article] [PubMed] [Google Scholar]