Abstract
Isolated taste cells, taste buds and strips of lingual tissue from taste papillae secrete ATP upon taste stimulation. Taste bud receptor (Type II) cells have been identified as the source of ATP secretion. Based on studies on isolated taste buds and single taste cells, we have postulated that ATP secreted from receptor cells via pannexin 1 hemichannels acts within the taste bud to excite neighbouring presynaptic (Type III) cells. This hypothesis, however, remains to be tested in intact tissues. In this report we used confocal Ca2+ imaging and lingual slices containing intact taste buds to test the hypothesis of purinergic signalling between taste cells in a more integral preparation. Incubating lingual slices with apyrase reversibly blocked cell-to-cell communication between receptor cells and presynaptic cells, consistent with ATP being the transmitter. Inhibiting pannexin 1 gap junction hemichannels with CO2-saturated buffer or probenecid significantly reduced cell–cell signalling between receptor cells and presynaptic cells. In contrast, anandamide, a blocker of connexin gap junction channels, had no effect of cell-to-cell communication in taste buds. These findings are consistent with the model for peripheral signal processing via ATP and pannexin 1 hemichannels in mammalian taste buds.
Introduction
A fundamental question concerning signal coding within the taste system is the degree to which coding occurs within the periphery prior to signals reaching the central nervous system. Peripheral sensory organs in several modalities show a varying extent of signal processing before information is transmitted to the central nervous system (CNS), including the visual (Wehner, 1989), olfactory (Duchamp-Viret et al. 1999), nociceptive (Andrew & Greenspan, 1999) and somatosensory (Romo et al. 2002) systems. For peripheral signal processing to occur within the taste system, as has been suggested (Frank et al. 2005; Spector & Travers, 2005; Tomchik et al. 2007), there must be intercellular communication within taste buds. Such cell–cell communication in gustatory end organs is the focus of the present study.
It is now widely accepted that there are functionally distinct populations of cells within the taste bud (Sugita, 2006; Roper, 2006). Type I cells mainly perform glial functions, such as neurotransmitter clearance and potassium homeostasis within the taste bud (Lawton et al. 2000; Bartel et al. 2006; Dvoryanchikov et al. 2009). Additionally, there are indications that these cells may also participate in Na+ sensing (Vandenbeuch et al. 2008). Type II, or receptor cells possess G protein-coupled receptors for bitter, sweet and umami compounds, signalling through the phospholipase C-β2 (PLC-β2) pathway and involving the cation channel transient receptor potential melastatin 5 (TRPM5) (Perez et al. 2002; Zhang et al. 2003; DeFazio et al. 2006; Clapp et al. 2006).
Receptor cells do not possess vesicular machinery necessary for classical exocytotic release of synaptic neurotransmitters (Clapp et al. 2004; DeFazio et al. 2006), although vesicular neurotransmitter transporters are reported to be present (Iwatsuki et al. 2009). Thus, how receptor cells convey gustatory signals was unclear until recent studies revealed that they secrete the neurotransmitter ATP, mainly via gap junction hemichannels (Huang et al. 2007; Romanov et al. 2007) and possibly via vesicular exocytosis (Iwatsuki et al. 2009). There remains, however, some disagreement as to whether the hemichannels responsible for ATP release are composed of pannexins or connexins (Huang et al. 2007; Romanov et al. 2008). Presynaptic (Type III) cells, by contrast, express synapse-related proteins and contain conventional synapses (Yang et al. 2000; DeFazio et al. 2006), but do not have G protein-coupled taste receptors. Presynaptic cells, in isolation, do not respond to taste stimulation with sweet, bitter, or umami compounds (DeFazio et al. 2006). However, in situ, presynaptic cells are excited when taste buds are stimulated by taste compounds (Tomchik et al. 2007). Type IV cells are basal, or progenitor cells, yet to be fully differentiated.
The finding that despite the absence of G protein-coupled taste receptors, presynaptic cells in situ respond to taste stimulation implies that there is some form of information transfer between receptor and presynaptic cells. Based on their studies of isolated taste cells and isolated taste buds, Huang et al. (2007) reported that receptor cells communicate with presynaptic cells via pannexin 1-mediated ATP secretion. Tomchik et al. (2007) studied taste buds in a more intact preparation, the lingual slice, and showed how several receptor cells could converge onto individual presynaptic cells to generate taste responses. However, up until now the mechanisms for cell-to-cell communication in intact taste buds has only been inferred from investigations on reduced preparations. Information transfer via pannexin 1-mediated ATP secretion has not been demonstrated in intact tissues.
In this report we used confocal Ca2+ imaging to track gustatory stimulation of taste cells in lingual slices containing intact taste buds. The slice preparation ensures that taste buds and taste cells are intact and that intercellular communication between taste cells remains unperturbed. Our goal was to critically examine whether results obtained from isolated taste buds and from separate taste cells apply to intact tissue. The findings show that receptor (Type II) and presynaptic (Type III) cells have different responses to taste stimulation, and specifically that receptor cells secrete ATP via pannexin 1 hemichannels and excite adjacent presynaptic cells. These results thus provide firm support for the notion of information processing in peripheral sensory organs of taste.
Methods
Animals
All experimental procedures were approved by the University of Miami Animal Care and Use Committee. Animals were killed by exposure to CO2 followed by cervical dislocation. Three lines of mice were used in these experiments: C57BL/6 mice (wild-type); transgenic mice expressing enhanced green fluorescent protein (GFP) under control of the PLC-β2 promoter (PLCβ2-GFP mice) (Kim et al. 2006); and transgenic mice expressing GFP under the glutamate decarboxylase (GAD) 67 promoter (GAD-GFP mice) (Chattopadhyaya et al. 2004). Receptor (Type II) taste cells selectively express GFP in PLCβ2-GFP mice, thereby facilitating their identification in living tissues (Kim et al. 2006). Similarly, the majority of presynaptic (Type III) cells fluoresce green in GAD-GFP mice (Tomchik et al. 2007).
Lingual slice preparation
Lingual slices of 100 μm containing circumvallate papillae taste buds were cut from blocks of lingual tissue using a vibrating blade microtome (Leica VT1000 S). Slices were placed in a shallow recording chamber and attached to the coverslip base using Cell-Tak (BD Biosciences, San Jose, CA, USA). Lingual slices were perfused at room temperature with Tyrode buffer containing elevated Ca2+ (8 mm; see below). Elevating Ca2+ improved the stability of the recordings and improved signal-to-noise. We perfused the recording chamber at a rate of 2 ml min−1 and applied taste stimuli to the apical tips of taste buds with a focal ‘puffer’ pipette (see below). Focal taste application and buffer perfusion at 2 ml min−1 assured that tastant was entirely cleared from the bath in 15–30 s.
Confocal Ca2+ imaging
Taste cells were iontophoretically loaded with Ca2+-sensitive dye (Calcium Green Dextran, CaGD, or in mice expressing cell-specific fluorescent markers, Calcium Orange, CaO) via a 35 μm borosilicate micropipette inserted into the trench of the circumvallate papillae prior to cutting lingual slices. The iontophoresis pipette contained 2 mm CaGD or CaO in 50% DMSO (dimethylsulfoxide) to promote access to the taste bud and enhance drug penetration for agents used in the study (Pereira et al. 2008, 2009). We imaged cells confocally to record Ca2+ transients in cells within the slices while maintaining sufficient z-resolution to ensure sampling from a single cell. Taste buds at the cut surfaces of the slices were not tested to avoid recording from cells that may have been damaged during slice preparation. All stimuli were dissolved in Tyrode solution. KCl and pharmacological agents were bath-applied. We applied taste stimuli focally to the taste pore via a 2 μm ‘puffer’ micropipette, using an MSC PLI-100 pressure source (Medical Systems, Greenvale, NY, USA). Taste stimuli were ejected by applying a pressure of 3 p.s.i. for 2 s. Responses from cells were recorded using an Olympus BX50WI laser scanning confocal microscope (Olympus Optical, Thornwood, NY, USA), scanning with argon and krypton lasers, and using the Fluoview image analysis package. Images were captured at 2 s intervals. Responses are presented as changes in relative fluorescence: ΔF/F ([F−F0)]/F0). Photobleaching of the sample was corrected by plotting the gradual decline of signal over time (typically with an exponentially decreasing baseline) and subtracting this baseline from the response (Caicedo et al. 2000). Only cells that maintained consistent responses for the duration of experimentation were included in our analysis (see Data analysis, below).
We identified taste cell types either by the expression of GFP in PLCβ2-GFP or GAD-GFP mice, or physiologically by whether cells responded to focal taste stimulation but not to bath-applied KCl (receptor, Type II cells), or whether they responded to KCl depolarization (presynaptic, Type III cells) (DeFazio et al. 2006; Tomchik et al. 2007). Focal application of taste stimuli very effectively activates taste receptor cells (e.g. Tomchik et al. 2007), whereas focally applied K+ salts do not (Maruyama et al. 2006). Bath application of KCl, by contrast, effectively bathes the entire tissue, including taste buds. This procedure effectively depolarizes taste cells and elicits Ca2+ influx selectively in presynaptic cells by virtue of their expression of voltage-gated Ca2+ channels (DeFazio et al. 2006; Tomchik et al. 2007; Huang et al. 2007).
Reagents and solutions
Unless otherwise indicated, chemicals were purchased from Sigma (St Louis, MO, USA). Normal Tyrode solution contained the following (in mm): 130 NaCl, 5 KCl, 8 CaCl2, 1 MgCl2, 10 Hepes, 10 glucose, 10 sodium pyruvate and 5 NaHCO3, adjusted to pH 7.4 (318–323 mosmol l−1). Calcium was elevated to 8 mm to enhance responses and improve the signal-to-noise ratio. High-potassium Tyrode solution for depolarizing taste cells contained 50 mm KCl in an equimolar substitution for NaCl to preserve physiological osmolarity. Taste stimuli used in these experiments consisted of a mixture of an artificial sweetener, 100 μm SC45647 (2-[[[4-(aminomethyl)anilino]-[[(1R)-1-phenylethyl] amino]methyl]amino]ethane-1,1-diol) (a gift from Dr G. Dubois, Coca Cola, Atlanta, GA, USA); bitter compounds, 30 μm cycloheximide and 200 μm denatonium; and umami compounds, 200 mm monopotassium l-glutamate (MPG) with 1 mm inosine 5-monophosphate (IMP). Fluorescein at 2 μm was added to the tastant mixture to track stimulation and to ensure consistent focal application of tastants.
Data analysis
Baseline fluorescence was established in a period of 100 s before each stimulus was applied. To determine whether a trace represented a true response versus noise, we used the criteria established previously by Caicedo et al. (2000) as follows. We scored positive responses only if (1) the peak ΔF/F was at least two times the baseline fluctuation for that cell, (2) responses could be elicited at least twice in the same cell by the same stimulus and appeared approximately of the same amplitude, and (3) responses were cell specific (i.e. were not observed in all dye-loaded cells in the field of view, or recorded in control areas external to the cells). This assured that signals were not caused by tissue movement or a stimulus artifact. Data analyses were performed in Microsoft Excel and Prism v.5 (GraphPad Software, Inc., La Jolla, CA, USA).
Results
Focally applying taste stimuli to the apical tips of taste buds in a lingual slice preparation reliably and repeatedly generated Ca2+ mobilization in a subset of taste cells. We identified responses from receptor (Type II) and presynaptic (Type III) cells using a combination of two criteria. First, we used genetic markers (green fluorescent protein, GFP) expressed selectively in receptor or presynaptic cells, as described in Methods. Second, we depolarized taste buds with 50 mm KCl to activate presynaptic cells selectively (DeFazio et al. 2006; Tomchik et al. 2007). The two procedures were 100% consistent: we never observed KCl-evoked responses in cells expressing GFP in lingual slices taken from PLCβ2-GFP mice, i.e. in receptor cells. These results are concordant with findings from single cells (DeFazio et al. 2006; Huang et al. 2008) and previous data from lingual slices (Tomchik et al. 2007).
As previously reported by Tomchik et al. (2007), we found that focal taste stimulation evoked Ca2+ responses in presynaptic cells, despite the absence on these cells of cognate receptors for the taste compounds, as well as in receptor cells (Fig. 1A–C).
Figure 1. Taste stimuli focally applied to the taste pore evoke Ca2+ mobilization in lingual slice preparation of taste tissue.
A, confocal micrograph of living preparation showing Calcium Green Dextran loaded taste bud cells within lingual slice of mouse vallate papillae. Taste buds and cell–cell interactions within taste bud remain intact in the lingual slice. Tastants are focally delivered at the taste pore (marked with asterisks). B and C, calcium signals elicited from receptor (Type II, top trace), and presynaptic (Type III, bottom trace) cells upon a brief (2 s) stimulation with a bitter/sweet/umami taste mix (see Methods). These cells were identified as receptor and presynaptic cells by whether they responded to bath-applied KCl, as described in Methods and DeFazio et al. (2006) and Tomchik et al. (2007).
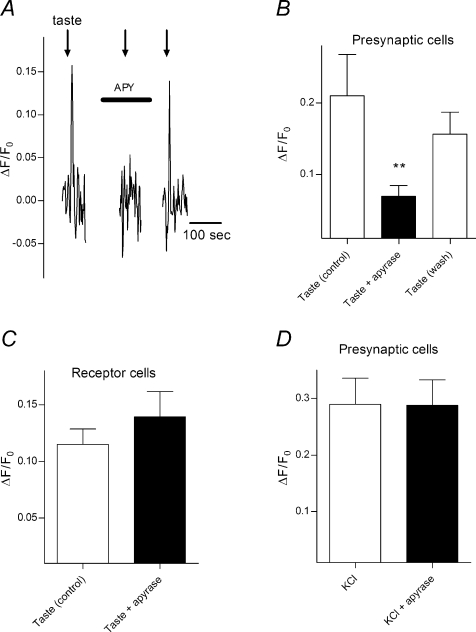
One interpretation of this result is that in the intact taste buds of the lingual slice, presynaptic cells are stimulated by ATP secreted from receptor cells during taste activation. To test this interpretation, we incubated lingual slices in an ecto-ATPase (apyrase, 30 units ml−1, 5 min). The presence of ecto-ATPase in the interstitial spaces within a taste bud should catabolize ATP if it was secreted from receptor cells and thereby reduce the effectiveness of cell–cell signalling. Indeed, treatment with apyrase significantly attenuated taste-evoked responses in presynaptic cells (Fig. 2A and B). Apyrase had no significant effect on taste-evoked responses in receptor cells or on the ability of presynaptic cells to respond to depolarization with bath-applied KCl (Fig. 2C and D). This indicates that apyrase does not cause a general degradation of taste signals.
Figure 2. Treating lingual slices with an ecto-ATPase reduces cell–cell communication in taste buds.
A, calcium signals recorded in a presynaptic cell when taste bud was stimulated with bitter/sweet/umami taste mix (arrows), i.e. a measure of cell to cell communication. Apyrase reduces extracellular ATP and blocks taste evoked response in presynaptic cell. B, summary of 10 experiments; a statistically smaller response was measured in the presence of apyrase than in control, or wash; P= 0.0075, n= 10, repeated measures ANOVA. C and D, no changes were recorded in either the receptor cell response to tastant, or the presynaptic cell ability to respond to KCl depolarization with apyrase.
Next, we tested whether blocking pannexin 1 gap junction hemichannels, the hypothesized route for taste-evoked ATP secretion from receptor cells (Huang et al. 2007), altered communication between receptor and presynaptic cells. As before, we stimulated taste buds with focal application of a taste mixture to activate receptor and presynaptic cells. Perfusing the lingual slice for 2 min with CO2–saturated buffer, a procedure that causes cytosolic acidification and blocks gap junctions (Locovei et al. 2006), reversibly attenuated taste-evoked responses recorded in presynaptic, but not receptor, cells (Fig. 3). This finding is consistent with ATP release through gap junction hemichannels. Perfusing the lingual slice with CO2-saturated buffer did not alter taste-evoked Ca2+ responses in receptor cells or KCl-evoked responses in presynaptic cells, indicating that CO2 treatment selectively affected cell-to-cell communication and not the underlying sensitivity of the cells.
Figure 3. Cytosolic acidification, which blocks gap junction hemichannels, reduces cell-to-cell signalling in taste buds.
A, acidification of cytosol of receptor cell with CO2-saturated buffer reduces taste-evoked responses. B, summary of several experiments; P < 0.0001, n= 12, repeated measures ANOVA. C and D, no significant changes were observed in taste evoked calcium signals in receptor cells in CO2-saturated buffer (C), or in presynaptic cells (D) responding to KCl depolarization in CO2 buffer.
We further tested the identification of gap junction hemichannels responsible for cell-to-cell communication in taste buds. Probenecid (1 mm), a selective blocker of pannexin 1 hemichannels (IC50= 0.15 mm; Silverman et al. 2008; Ma et al. 2008) reduced taste-evoked Ca2+ responses in presynaptic cells without altering receptor cell responses or KCl-evoked presynaptic signals (Fig. 4). (Carbenoxolone, another blocker of pannexin 1 hemichannels, could not be used because it also affects voltage-gated calcium channels; Vessey et al. 2004.)
Figure 4. Blocking pannexin 1 gap junction hemichannels reduces cell-to-cell communication in taste buds.
A, probenecid (1 mm), a blocker of pannexin 1 hemichannels, inhibits taste-evoked responses in presynaptic cells. B, summary of several experiments; P > 0.0001, n= 10, repeated measures ANOVA. C and D, probenecid treatment did not change taste-evoked responses in receptor cells (C), or in presynaptic cell responses evoked by KCl depolarization (D).
In contrast, anandamide (50 μm), a compound that selectively blocks connexin gap junction channels (IC50= 5 μm) had no significant effect on taste cell responses (Fig. 5). Taken together, our results are consistent with presynaptic cell responses being generated via ATP secreted through pannexin 1 gap junction hemichannels.
Figure 5. Blocking connexin gap junction hemichannels does not reduce cell–cell communication in taste buds.
A, treating preparation with 50 μm anandamide, a connexin gap junction inhibitor, did not reduce taste evoked responses in this presynaptic cell. B, summary of several experiments; P= 0.6332, n= 6, repeated measures ANOVA.
Discussion
The key finding in this report is that data from taste buds in a lingual slice preparation of mouse vallate papillae support the hypothesis that cell-to-cell communication between mammalian taste cells is mediated by ATP released from receptor (Type II) cells via pannexin 1 hemichannels.
Notwithstanding theoretical modelling used to argue a role for connexin hemichannels in ATP release from taste cells (Romanov et al. 2008), experimental findings tend to support pannexin 1 hemichannels as being the route for ATP secretion. These arguments are summarized as follows. Expression profiling, using both immunocytochemistry and RT-PCR (reverse transcription polymerase chain reaction) analysis shows the presence of pannexin 1 hemichannels in mouse taste cells (Huang et al. 2007). Dye uptake into isolated taste cells and ATP secretion from taste buds is blocked by low concentrations of carbenoxolone (Huang et al. 2007; Stone & Kinnamon, 2008), a gap junction channel inhibitor that has an order of magnitude higher potency for pannexin 1 versus connexins. With few exceptions (De Vuyst et al. 2006), intracellular calcium closes connexin hemichannels but opens pannexin 1 hemichannels (Li et al. 1996; Pfahnl & Dahl, 1999). This is important because taste stimulation increases intracellular Ca2+ in taste receptor cells and stimulates ATP secretion, as shown here in our report and established previously by us and others (Huang et al. 2007; Romanov et al. 2007). Finally, as shown here, probenecid, a pannexin hemichannel inhibitor, interrupts communication within the taste bud whereas anandamide, which blocks many connexin channels (Venance et al. 1995; Elisevich et al. 1997; Levin & Mercola 2000; Juszczak & Swiergiel, 2009), including Cx30 and Cx43, connexins found in taste buds that are able to form hemichannels does not (Huang et al. 2007). Although none of these findings taken individually is conclusive, collectively the data lend weight to the hypothesis that pannexin 1 hemichannels are responsible for taste-evoked ATP secretion from mouse taste receptor cells. Definitive proof awaits studies on mutant mice lacking expression of specific gap junction proteins.
The results in the present study bridge the gap between findings obtained from reduced preparations – isolated taste cells and taste buds – and more intact preparations such as taste nerve recordings in anaesthetized animals and behavioural studies in unrestrained animals. The findings at the different levels of analysis are all consistent. For instance, patch-clamp recordings from taste cells of isolated tissues containing mouse fungiform papillae indicate that some cells respond to only one taste quality and others respond to multiple qualities (Yoshida et al. 2006; Caicedo et al. 2002), consistent with signal convergence between receptor and presynaptic cells as shown here and in Tomchik et al. (2007). Similar findings have been obtained from electrophysiological recordings from sensory ganglion neurons innervating fungiform papillae in anaesthetized rats (Breza et al. 2006). Those studies also revealed ‘specialist’ (responding to only one or a very limited range of taste stimuli) and ‘generalist’ (broadly-responsive) neurons, possibly correlating with receptor and presynaptic taste cells, respectively. The inescapable conclusion is that a certain degree of information processing and signal coding takes place in the peripheral sensory organs of taste.
A recent publication reports the expression of the vesicular nucleotide transporter (VNUT) in receptor cells. The authors suggest that in addition to hemichannel-mediated release, receptor cells also secrete ATP via vesicular exocytosis (Iwatsuki et al. 2009). However, receptor cells lack morphological evidence for synapses (Clapp et al. 2004), do not express synaptic-related proteins (DeFazio et al. 2006), and do not manifest Ca2+ influx during taste excitation – these features being the sine qua non for conventional synaptic vesicular exocytosis. Whether, and to what extent vesicular exocytosis of ATP occurs during taste stimulation remains to be determined.
G protein-coupled receptors and their downstream effectors have been well-characterized for many taste qualities, although there are some notable exceptions such as salty and sour taste (Clapp et al. 2004; Herness et al. 2005; Chandrashekar et al. 2006; Ishimaru et al. 2006; Huang et al. 2006; Nelson et al. 2001; Roper, 2006). Yet, little is at present understood about the mechanisms for taste signal coding within the peripheral taste system (Sato & Beidler, 1997; Mueller et al. 2005; Simon et al. 2006; Roper, 2007, 2009). Several neurotransmitters and neuromodulators have been postulated (Nagai et al. 1996; Kaya et al. 2004; Cao et al. 2009), but to date the best evidence indicates that ATP, serotonin and noradrenaline are taste transmitters (Finger et al. 2005; Huang et al. 2005, 2007, 2008; Romanov et al. 2007). Our current understanding of taste detection is that bitter, sweet or umami compounds stimulate receptor cells within the taste bud to secrete ATP. ATP is believed to directly excite gustatory sensory afferent axons (Finger et al. 2005) as well as stimulate adjacent presynaptic cells (Huang et al. 2007). Because there are no discrete synaptic contacts between receptor and presynaptic cells but only pannexin 1 gap junction release channels, one presynaptic cell can respond to ATP secreted from any number of neighbouring receptor cells. This explains the broad range of responsiveness of presynaptic cells to several taste qualities, including those to which the cell expresses no relevant receptors (Tomchik et al. 2007). However, a further synthesis of information processing and how taste qualities are coded in the taste bud remain elusive. These are questions for further research.
Acknowledgments
This research was supported by National Institutes of Health – National Institute on Deafness and Other Communication Disorder, Grant 5R01DC000374 (SDR).
Glossary
Abbreviations
- CaGD
calcium green dextran;
- CaO
calcium orange;
- GAD
glutamate decarboxylase;
- GFP
green fluorescent protein;
- PLC-β2
phospholipase C β2
- TRPM5
transient receptor potential melastatin 5;
- VNUT
vesicular nucleotide transporter
Author contributions
R.D. and S.R. contributed equally to the concept and design of the experiments and to the intellectual content of this report. R.D. conducted the experiments, analysed the data and drafted the report. S.R. assisted in analysing and interpreting the data and in revising the report for publication.
References
- Andrew D, Greenspan JD. Peripheral coding of tonic mechanical cutaneous pain: comparison of nociceptor activity in rat and human psychophysics. J Neurophysiol. 1999;82:2641–2648. doi: 10.1152/jn.1999.82.5.2641. [DOI] [PubMed] [Google Scholar]
- Bartel DL, Sullivan SL, Lavoie EG, Sevigny J, Finger TE. Nucleoside triphosphate diphosphohydrolase-2 is the ecto-ATPase of type I cells in taste buds. J Comp Neurol. 2006;497:1–12. doi: 10.1002/cne.20954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breza JM, Curtis KS, Contreras RJ. Temperature modulates taste responsiveness and stimulates gustatory neurons in the rat geniculate ganglion. J Neurophysiol. 2006;95:674–685. doi: 10.1152/jn.00793.2005. [DOI] [PubMed] [Google Scholar]
- Caicedo A, Jafri MS, Roper SD. In situ Ca2+ imaging reveals neurotransmitter receptors for glutamate in taste receptor cells. J Neurosci. 2000;20:7978–7985. doi: 10.1523/JNEUROSCI.20-21-07978.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caicedo A, Kim KN, Roper SD. Individual mouse taste cells respond to multiple chemical stimuli. J Physiol. 2002;544:501–509. doi: 10.1113/jphysiol.2002.027862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y, Zhao FL, Kolli T, Hivley R, Herness S. GABA expression in the mammalian taste bud functions as a route of inhibitory cell-to-cell communication. Proc Natl Acad Sci U S A. 2009;106:4006–4011. doi: 10.1073/pnas.0808672106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrashekar J, Hoon MA, Ryba NJ, Zuker CS. The receptors and cells for mammalian taste. Nature. 2006;444:288–294. doi: 10.1038/nature05401. [DOI] [PubMed] [Google Scholar]
- Chattopadhyaya B, Di Cristo G, Higashiyama H, Knott GW, Kuhlman SJ, Welker E, Huang ZJ. Experience and activity-dependent maturation of perisomatic GABAergic innervation in primary visual cortex during a postnatal critical period. J Neurosci. 2004;24:9598–9611. doi: 10.1523/JNEUROSCI.1851-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapp TR, Yang R, Stoick CL, Kinnamon SC, Kinnamon JC. Morphologic characterization of rat taste receptor cells that express components of the phospholipase C signalling pathway. J Comp Neurol. 2004;468:311–321. doi: 10.1002/cne.10963. [DOI] [PubMed] [Google Scholar]
- Clapp TR, Medler KF, Damak S, Margolskee RF, Kinnamon SC. Mouse taste cells with G protein-coupled taste receptors lack voltage-gated calcium channels and SNAP-25. BMC Biol. 2006;4:7. doi: 10.1186/1741-7007-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFazio RA, Dvoryanchikov G, Maruyama Y, Kim JW, Pereira E, Roper SD, Chaudhari N. Separate populations of receptor cells and Presynaptic cells in mouse taste buds. J Neurosci. 2006;26:3971–3980. doi: 10.1523/JNEUROSCI.0515-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vuyst E, Decrock E, Cabooter L, Dubyak GR, Naus CC, Evans WH, Leybaert L. Intracellular calcium changes trigger connexin 32 hemichannel opening. EMBO J. 2006;25:34–44. doi: 10.1038/sj.emboj.7600908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchamp-Viret P, Chaput MA, Duchamp A. Odour response properties of rat olfactory receptor neurons. Science. 1999;284:2171–2174. doi: 10.1126/science.284.5423.2171. [DOI] [PubMed] [Google Scholar]
- Dvoryanchikov G, Sinclair M, Perea-Martinez I, Wang T, Chaudhari N. The inward rectifier channel, ROMK, is localized to the apical tips of glial-like cells in mouse taste buds. J Comp Neurol. 2009;517:1–14. doi: 10.1002/cne.22152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elisevich K, Rempel SA, et al. Connexin 43 mRNA expression in two experimental models of epilepsy. Mol Chem Neuropathol. 1997;32:75–88. doi: 10.1007/BF02815168. [DOI] [PubMed] [Google Scholar]
- Finger TE, Danilova V, Barrows J, Bartel DL, Vigers AJ, Stone L, Hellekant G, Kinnamon SC. ATP signalling is crucial for communication from taste buds to gustatory nerves. Science. 2005;310:1495–1499. doi: 10.1126/science.1118435. [DOI] [PubMed] [Google Scholar]
- Frank ME, Formaker BK, Hettinger TP. Peripheral gustatory processing of sweet stimuli by golden hamsters. Brain Res Bull. 2005;66:70–84. doi: 10.1016/j.brainresbull.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Herness S, Zhao FL, Kaya N, Shen T, Lu SG, Cao Y. Communication routes within the taste bud by neurotransmitters and neuropeptides. Chem Senses. 2005;30(Suppl 1):i37–i38. doi: 10.1093/chemse/bjh101. [DOI] [PubMed] [Google Scholar]
- Huang YJ, Maruyama Y, Lu KS, Pereira E, Plonsky I, Baur JE, Wu D, Roper SD. Mouse taste buds use serotonin as a neurotransmitter. J Neurosci. 2005;25:843–847. doi: 10.1523/JNEUROSCI.4446-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang AL, Chen X, et al. The cells and logic for mammalian sour taste detection. Nature. 2006;442:934–8. doi: 10.1038/nature05084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YJ, Maruyama Y, Dvoryanchikov G, Pereira E, Chaudhari N, Roper SD. The role of Pannexin 1 hemichannels in ATP release and cell-cell communication in mouse taste buds. Proc Natl Acad Sci U S A. 2007;104:6436–6441. doi: 10.1073/pnas.0611280104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YA, Maruyama Y, et al. Norepinephrine is coreleased with serotonin in mouse taste buds. J Neurosci. 2008;28:13088–93. doi: 10.1523/JNEUROSCI.4187-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishimaru Y, Inada H, Kubota M, Zhuang H, Tominaga M, Matsunami H. Transient receptor potential family members PKD1L3 and PKD2L1 form a candidate sour taste receptor. Proc Natl Acad Sci U S A. 2006;103:12569–12574. doi: 10.1073/pnas.0602702103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwatsuki K, Ichikawa R, Hiasa M, Moriyama Y, Torii K, Uneyama H. Identification of the vesicular nucleotide transporter (VNUT) in taste cells. Biochem Biophys Res Commun. 2009;388:1–5. doi: 10.1016/j.bbrc.2009.07.069. [DOI] [PubMed] [Google Scholar]
- Juszczak GR, Swiergiel AH. Properties of gap junction blockers and their behavioural, cognitive and electrophysiological effects: animal and human studies. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:181–198. doi: 10.1016/j.pnpbp.2008.12.014. [DOI] [PubMed] [Google Scholar]
- Kaya N, Shen T, Lu SG, Zhao FL, Herness S. A paracrine signalling role for serotonin in rat taste buds: expression and localization of serotonin receptor subtypes. Am J Physiol Regul Integr Comp Physiol. 2004;286:R649–658. doi: 10.1152/ajpregu.00572.2003. [DOI] [PubMed] [Google Scholar]
- Kim JW, Roberts C, Maruyama Y, Berg S, Roper S, Chaudhari N. Faithful expression of GFP from the PLCL2 promoter in a functional class of taste Receptor cells. Chem Senses. 2006;31:213–219. doi: 10.1093/chemse/bjj021. [DOI] [PubMed] [Google Scholar]
- Lawton DM, Furness DN, Lindemann B, Hackney CM. Localization of the glutamate-aspartate transporter, GLAST, in rat taste buds. Eur J Neurosci. 2000;2:3163–3171. doi: 10.1046/j.1460-9568.2000.00207.x. [DOI] [PubMed] [Google Scholar]
- Levin M, Mercola M. Expression of connexin 30 in Xenopus embryos and its involvement in hatching gland function. Dev Dyn. 2000;219:96–101. doi: 10.1002/1097-0177(200009)219:1<96::AID-DVDY1034>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Li H, Liu TF, Lazrak A, Peracchia C, Goldberg GS, Lampe PD, Johnson RG. Properties and regulation of gap junctional hemichannels in the plasma membranes of cultured cells. J Cell Biol. 1996;134:1019–1030. doi: 10.1083/jcb.134.4.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locovei S, Wang J, Dahl G. Activation of pannexin 1 channels by ATP through P2Y receptors and by cytoplasmic calcium. FEBS Lett. 2006;580:239–244. doi: 10.1016/j.febslet.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Ma W, Hui H, Pelegrin P, Surprenant A. Pharmacological characterization of pannexin-1 currents expressed in mammalian cells. J Pharmacol Exp Ther. 2008;108:146365. doi: 10.1124/jpet.108.146365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama Y, Pereira E, Margolskee RF, Chaudhari N, Roper SD. Umami responses in mouse taste cells indicate more than one receptor. J Neurosci. 2006;26:2227–2234. doi: 10.1523/JNEUROSCI.4329-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller KL, Hoon MA, Erlenbach I, Chandrashekar J, Zuker CS, Ryba NJ. The receptors and coding logic for bitter taste. Nature. 2005;10:225–229. doi: 10.1038/nature03352. [DOI] [PubMed] [Google Scholar]
- Nagai T, Kim DJ, Delay RJ, Roper SD. Neuromodulation of transduction and signal processing in the end organs of taste. Chem Senses. 1996;21:353–365. doi: 10.1093/chemse/21.3.353. [DOI] [PubMed] [Google Scholar]
- Nelson G, Hoon MA, Chandrashekar J, Zhang Y, Ryba NJ, Zuker CS. Mammalian sweet taste receptors. Cell. 2001;106:381–390. doi: 10.1016/s0092-8674(01)00451-2. [DOI] [PubMed] [Google Scholar]
- Pereira E, Tomchik S, Chaudhari N, Roper S. 2008 Annual Meeting. Minneapolis, MN, USA: Association for Chemoreception Sciences; 2008. Barriers in mouse taste buds: dye penetration studies. Abstract No. P404. [Google Scholar]
- Pereira E, Dando R, Chaudhari N, Roper S. 2009 Annual Meeting. Minneapolis, MN, USA: Association for Chemoreception Sciences; 2009. Penetrating the permeability barrier that surrounds mouse taste buds. Abstract No. P188. [Google Scholar]
- Perez CA, Huang L, Rong M, Kozak JA, Preuss AK, Zhang H, Max M, Margolskee RF. A transient receptor potential channel expressed in taste receptor cells. Nat Neurosci. 2002;5:1169–1176. doi: 10.1038/nn952. [DOI] [PubMed] [Google Scholar]
- Pfahnl A, Dahl G. Gating of cx46 gap junction hemichannels by calcium and voltage. Pflugers Arch. 1999;437:345–353. doi: 10.1007/s004240050788. [DOI] [PubMed] [Google Scholar]
- Romanov RA, Rogachevskaja OA, Bystrova MF, Jiang P, Margolskee RF, Kolesnikov SS. Afferent neurotransmission mediated by hemichannels in mammalian taste cells. EMBO J. 2007;26:657–667. doi: 10.1038/sj.emboj.7601526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanov RA, Rogachevskaja OA, Khokhlov AA, Kolesnikov SS. Voltage dependence of ATP secretion in mammalian taste cells. J Gen Physiol. 2008;132:731–744. doi: 10.1085/jgp.200810108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romo R, Hernández A, Salinas E, Brody CD, Zainos A, Lemus L, Lafuente V, Luna R. From sensation to action. Behav Brain Res. 2002;135:105–118. doi: 10.1016/s0166-4328(02)00161-4. [DOI] [PubMed] [Google Scholar]
- Roper SD. Parallel processing in mammalian taste buds? Physiol Behav. 2009;97:604–608. doi: 10.1016/j.physbeh.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roper SD. Cell communication in taste buds. Cell Mol Life Sci. 2006;63:1494–1500. doi: 10.1007/s00018-006-6112-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T, Beidler LM. Broad tuning of rat taste cells for four basic taste stimuli. Chem Senses. 1997;22:287–293. doi: 10.1093/chemse/22.3.287. [DOI] [PubMed] [Google Scholar]
- Silverman W, Locovei S, Dahl G. Probenecid, a gout remedy, inhibits pannexin 1 channels. Am J Physiol Cell Physiol. 2008;295:C761–C767. doi: 10.1152/ajpcell.00227.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon SA, de Araujo IE, Gutierrez R, Nicolelis MA. The neural mechanisms of gustation: a distributed processing code. Nat Rev Neurosci. 2006;7:890–901. doi: 10.1038/nrn2006. [DOI] [PubMed] [Google Scholar]
- Spector AC, Travers SP. The representation of taste quality in the mammalian nervous system. Behav Cogn Neurosci Rev. 2005;4:143–191. doi: 10.1177/1534582305280031. [DOI] [PubMed] [Google Scholar]
- Stone LM, Kinnamon SC. Sour taste stimuli evoke ATP release from taste buds. Chem Senses. 2008;33:S129. [Google Scholar]
- Sugita M. Taste perception and coding in the periphery. Cell Mol Life Sci. 2006;63:2000–2015. doi: 10.1007/s00018-006-6100-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomchik SM, Berg S, Kim JW, Chaudhari N, Roper SD. Breadth of tuning and taste coding in mammalian taste buds. J Neurosci. 2007;40:10840–10848. doi: 10.1523/JNEUROSCI.1863-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenbeuch A, Clapp TR, Kinnamon SC. Amiloride-sensitive channels in type I fungiform taste cells in mouse. BMC Neurosci. 2008;9:1. doi: 10.1186/1471-2202-9-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vessey JP, Lalonde MR, Mizan HA, Welch NC, Kelly ME, Barnes S. Carbenoxolone inhibition of voltage-gated Ca channels and synaptic transmission in the retina. J Neurophysiol. 2004;92:1252–1256. doi: 10.1152/jn.00148.2004. [DOI] [PubMed] [Google Scholar]
- Venance L, Piomelli D, Glowinski J, Giaume C. Inhibition by anandamide of gap junctions and intercellular calcium signalling in striatal astrocytes. Nature. 1995;376:590–594. doi: 10.1038/376590a0. [DOI] [PubMed] [Google Scholar]
- Wehner R. Neurobiology of polarization vision. Trends Neurosci. 1989;12:353–359. doi: 10.1016/0166-2236(89)90043-x. [DOI] [PubMed] [Google Scholar]
- Yang R, Crowley HH, Rock ME, Kinnamon JC. Taste cells with synapses in rat circumvallate papillae display SNAP-25-like immunoreactivity. J Comp Neurol. 2000;424:205–215. doi: 10.1002/1096-9861(20000821)424:2<205::aid-cne2>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Yoshida R, Shigemura N, Sanematsu K, Yasumatsu K, Ishizuka S, Ninomiya Y. Taste responsiveness of fungiform taste cells with action potentials. J Neurophysiol. 2006;96:3088–3095. doi: 10.1152/jn.00409.2006. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Hoon MA, Chandrashekar J, Mueller KL, Cook B, Wu D, Zuker CS, Ryba NJ. Coding of sweet, bitter, and umami tastes: different receptor cells sharing similar signalling pathways. Cell. 2003;112:293–301. doi: 10.1016/s0092-8674(03)00071-0. [DOI] [PubMed] [Google Scholar]