Abstract
Pharmacological blockade of GABAA receptors on CA3 pyramidal cells in hippocampal slices from immature rats (i.e. second to third postnatal weeks), compared to CA3 slices from adult rats, is known to cause prolonged burst discharges (i.e. several seconds vs. tens of milliseconds). Synaptic and intrinsic mechanisms responsible for this developmental difference in burst duration were analysed in isolated minislices of the CA3 area. The frequency and amplitude of spontaneous EPSCs in CA3 pyramidal cells were greater in slices from immature than mature rats. In the presence of GABAA- and GABAB-receptor antagonists, the burst discharges of immature CA3 pyramidal cells were still prolonged in thinner slices (350 μm, vs. 450 μm in adults, to compensate for developmental differences in neuronal density) and in NMDA- and mGlu1-receptor antagonists. The AMPA receptor antagonist DNQX blocked the remaining burst discharges, suggesting that differences in recurrent excitatory circuits contributed to the prolonged bursts of immature CA3 pyramidal cells. In slices from immature versus adult rats, the CA3 recurrent synaptic responses showed potentiation to repetitive stimulation, suggestive of a lower transmitter release probability. The intrinsic firing ability was greater in CA3 pyramidal neurons from immature than adult rats, and the medium-duration afterhyperpolarization was smaller. These data suggest that, compared to adults, the CA3 area of immature rats contains a more robust recurrent excitatory synaptic network, greater intrinsic membrane excitability, and an increased capacity for sustained transmitter release, which together may account for the more prolonged network bursts in immature versus adult CA3.
Introduction
In adult animals, the hippocampal CA3 area generates brief (i.e. tens of milliseconds) bursts when GABAA receptors are blocked pharmacologically (Schwartzkroin & Prince, 1977; Wong & Traub, 1983), but much more prolonged (i.e. ∼10 s) bursts with repetitive afterdischarges occur in the CA3 area from immature animals, particularly during the second postnatal week (Swann & Brady, 1984; Swann et al. 1991, 1993; Gomez-Di Cesare et al. 1997). The propensity for generating prolonged epileptiform bursts in immature CA3 could contribute to enhanced seizure susceptibility early in development, but the cellular mechanisms underlying the increased burst duration in the immature CA3 area are poorly understood.
Multiple synaptic and intrinsic neuronal mechanisms that may shape the bursting behaviour of the CA3 area undergo maturational changes. For example, the dendrites and axons of the CA3 neurons are more branched during the second postnatal week, and axonal pruning takes place during maturation (Gomez-Di Cesare et al. 1997). In addition, GABA is excitatory during early development (Ben-Ari et al. 1997; Ben-Ari, 2002), and may contribute to the enhanced propensity for seizure-like activity (Dzhala & Staley, 2003). NMDA receptors have unique features early in development (Ben-Ari et al. 1988; Tremblay et al. 1988; Hestrin, 1992), which may promote generation of prolonged epileptiform bursts. Activation of type 1 metabotropic glutamate (mGlu1) receptors has been reported to prolong picrotoxin-induced epileptiform bursts in CA3 slices from adult rats (Merlin & Wong, 1997; Galoyan & Merlin, 2000), and so differences in mGlu1 receptors may underlie the developmental difference in burst duration. The probability of transmitter release at the CA3 recurrent synapses may differentially regulate the duration of epileptiform bursts in the CA3 area (Staley et al. 1998; Jones et al. 2007). Also, the intrinsic membrane excitability of the CA3 pyramidal cells may change during postnatal development, like other central neurons (Viana et al. 1995; Belleau & Warren, 2000). Thus, maturational changes in some or all of these synaptic and intrinsic properties could hypothetically be responsible for the developmental differences in the duration of epileptiform bursts in the CA3 area.
To further understand the cellular mechanisms underlying the developmental difference in the synchronous bursts observed after pharmacological blockade of GABAA receptors, we (1) measured changes in excitatory synaptic activity in CA3 pyramidal neurons, (2) analysed the contribution of different receptor-mediated components of GABAergic and glutamatergic synaptic transmission (i.e. GABAA, GABAB, NMDA, mGlu1 and AMPA/kainate receptors) to synchronous bursts, (3) assessed the dynamics of transmitter release of the CA3 recurrent synapses, and (4) examined the intrinsic excitability of CA3 pyramidal neurons in immature and adult rats. Our data suggest that developmental differences in recurrent excitation, glutamate release from recurrent synapses, and intrinsic membrane properties of CA3 pyramidal neurons all contribute to the prolonged burst discharge in immature CA3, versus the relatively brief bursts in adults. Although developmental differences in GABA, NMDA and mGlu1 receptors probably also contribute to the prolonged bursts seen during the second postnatal week, they do not appear to be necessary to explain the developmental differences in the epileptiform bursts of CA3 pyramidal cells.
Methods
Animals and slice preparation
All experiments were performed in slices from Sprague–Dawley (Harlan) rats, and the procedures were approved by the Colorado State University and University of Utah Animal Care and Use Committees. In initial experiments (i.e. bicuculline-induced epileptiform bursts), the immature group contained rat pups of the second postnatal week (9–14 days (d), n= 7). Three rats aged 18, 20 and 23 d were also used for further observations beyond the second postnatal week (i.e. the third to early fourth postnatal week). Their results were similar, and so they were grouped together (n= 10). The mature group contained rats aged 90–100 d (n= 9), plus two younger animals aged 60 and 75 d. Their results were similar, and they were also grouped together.
In the initial series of experiments described above, coronal slices were cut (i.e. mostly from the dorsal hippocampus) at the same thickness for both immature and mature groups (400 μm). Because the brain volume but not the number of neurons increases significantly during this stage of development, it is possible that the slices from the immature rats may have contained more neurons and synaptic circuits than the slices of the same thickness from the mature rats, which could hypothetically explain the greater bursting activity in the slices from immature animals. To compensate for this developmental increase in brain volume, and to minimize a potential false-positive effect due to differences in neuronal density, another series of experiments were conducted in thinner slices from immature rats (i.e. cut at 350 μm) than those from adult animals (i.e. 450 μm). The rationale for and calculation of the two thicknesses is explained in detail in the Discussion. The second series of experiments were conducted in rats of 10–13 d (for immature group, n= 9) and 90–120 d (for adult group, n= 9).
Rats were deeply anaesthetized with halothane and decapitated with a guillotine. Their brains were quickly dissected out and placed in partially frozen oxygenated artificial cerebrospinal fluid (aCSF) containing (in mm): 124 NaCl, 3 KCl, 26 NaHCO3, 1.4 NaH2PO4, 2 CaCl2, 2 MgSO4 and 11 glucose. Coronal slices containing the hippocampus were cut with a vibroslicer (Lancer series 1000, Vibratome, St Louis, MO, USA). Tissues adjacent to the hippocampus were consequently removed and additional knife cuts were made between the dentate gyrus and CA3 and between CA3 and CA1/CA2 to isolate the CA3 area from the rest of the hippocampus (i.e. CA3 minislices). A large part of the CA3c (i.e. the area between the two blades of the dentate gyrus) was inevitably removed, and thus the CA3 minislices contained CA3a, CA3b and part of CA3c (Fig. 1A). Slices were allowed to recover for ≥ 2 h in a submerged storage chamber maintained at 32–34°C before they were mounted onto a ramp-type interface chamber for electrophysiological recording.
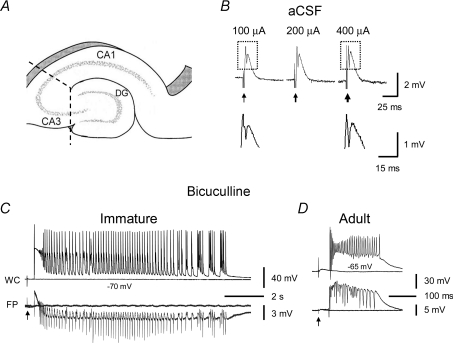
Figure 1. Prolonged population bursts after bicuculline treatment in CA3 minislices of immature versus adult rats.
A, diagram showing CA3 minislices used in this study. Dashed lines indicate knife cuts to isolate the CA3 area from CA1 and dentate gyrus. B shows that in normal aCSF, synaptic stimulation in CA3 minislices from immature rats only elicited a field EPSP and a single population spike and never evoked epileptiform bursts, regardless of the stimulus intensity (the three responses were evoked at 100 μA, 200 μA and 400 μA). Arrows indicate stimulations. Boxed parts in B are shown in expanded scales below. Note the difference in the amplitude of population spikes at different stimulus intensities (i.e. 100 μA and 400 μA). C, simultaneous whole-cell (WC, current-clamp mode) and field-potential (FP) recordings showing evoked population bursts in bicuculline (30 μm) in CA3 minislices from immature rats. These bursts typically contained an initial depolarization followed by rhythmic afterdischarges lasting for several seconds. D, by contrast, the evoked population bursts that occurred in CA3 slices of mature rats were composed of a single burst that lasted for a few hundred milliseconds.
Recording procedures and data acquisition
Simultaneous extracellular field-potential and whole-cell current-clamp recordings were performed with an Axoprobe-1A amplifier and an Axopatch-1D amplifier (Axon Instruments/Molecular Devices, Union City, CA, USA), respectively. In some experiments, only field potentials were recorded. Extracellular recordings were DC-coupled. Thick-wall glass pipettes with a tip of 1–3 μm were used for both extracellular and whole-cell recordings, and were pulled from borosilicate glass capillaries (o.d. 1.65 mm, i.d. 1.2 mm, Garner Glass, Claremont, CA, USA) with a P-87 Flaming–Brown puller (Sutter Instruments, Novato, CA, USA). For extracellular recordings, pipettes were filled with aCSF and placed in the CA3 pyramidal cell body layer. For whole-cell recordings, pipettes were filled with intracellular solution containing (in mm): 120 potassium gluconate, 1 NaCl, 5 EGTA, 10 Hepes, 1 MgCl2, 1 CaCl2, 2 ATP and 5 biocytin. The pH was adjusted to 7.2 with 5 m KOH. Patch pipettes had a resistance of 4–7 MΩ when filled with this solution. The theoretical liquid junction potential between the pipette solution and the bath solution was +12 mV at 32°C (calculated using pCLAMP 8, Molecular Devices) and was not corrected in the values presented in this study. A bipolar stimulating electrode made of two Teflon-coated platinum wires (wire diameter: 25 μm, with coating: 75 μm; separation between two wires: ∼50 μm (i.e. twice the thickness of coating)) was positioned in the stratum radiatum of CA3 to deliver synaptic stimulation. In a subset of experiments, the stimulating electrode was placed in CA1/CA2 to antidromically activate CA3 recurrent synapses; and the extracellular Mg2+ concentration ([Mg2+]o) in these experiments was elevated to 4 mm, in order to increase the membrane surface charge and block multi-synaptic responses. The intensity of antidromic stimulations was fixed (200 μA, 200 μs) to facilitate comparisons among slices and between groups. Repetitive stimulation was delivered at a varied frequency to test the dynamics of transmitter release at the CA3 recurrent synapses. In the set of experiments aimed at examining intrinsic membrane properties, synaptic transmission was eliminated by adding GABAA-, GABAB-, AMPA/kainate- and NMDA-receptor antagonists to the medium; and the experiments were performed in a submerged chamber at 34–35°C, using an upright compound microscope with infrared and differential interference contrast optics (Zeiss microscopy, Zeiss Axioskop, Germany; CCD camera, Hamamatsu, Japan) and a multiclamp 700A recording system (Molecular Devices). A 10 mV, 20 ms hyperpolarizing voltage command was used to determine the input resistance. A series of 15 s hyperpolarizing and depolarizing current pulses was injected into the CA3 pyramidal neurons to determine the input–output relationship for generating action potentials. To analyse afterhyperpolarizations (AHPs), a brief, triangular depolarizing current pulse (peak current: 200–900 pA; duration: 80 ms) was used to produce a burst of four to five action potentials, while the membrane was held at −40 mV to −50 mV in order to increase the driving force for K+ currents.
All signals were sampled at 10 kHz, low-pass filtered at 2 kHz, and recorded online with pCLAMP 8.0/10 software (Clampex, Molecular Devices) through a Digidata 1320A or 1440A digitizer (Clampex, Molecular Devices).
Data analysis and statistics
The pCLAMP 8.0/10 (Clampfit, Molecular Devices), MiniAnalysis 5.0 (Synaptosoft, Inc., Leonia, NJ, USA), SigmaPlot (SPSS Inc., Chicago, IL, USA) and Microsoft Excel programs were used for data analysis. The threshold of epileptiform bursts was defined as the lowest stimulus intensity to evoke a network burst. Because of the network nature of these bursts (see later sections), a ‘threshold’ stimulus could actually evoke no response or a full-length burst (i.e. all-or-none bursts). A network burst probability was thus calculated as the ratio of the times of occurrence of network bursts over the number of near-threshold stimulations. The latency of each epileptiform burst was measured from each stimulus to the onset of the network burst that occurred at threshold stimulation, which was variable from burst to burst in the same slices. The mean latency was averaged across slices. Spontaneous excitatory postsynaptic currents (sEPSCs) were recorded in voltage-clamp mode at −60 mV to −65 mV, detected using MiniAnalysis, and visually checked to minimize errors. A 3 min sample of whole-cell recording per cell was used for measuring sEPSC frequency and amplitude. The mean sEPSC interval and amplitude were averaged across neurons (i.e. n= cells, not events). For constructing histograms for amplitude and interval distributions, and for calculating cumulative probability, a fixed number of sEPSCs from each neuron (i.e. the first 100 sEPSCs) were pooled, in order to minimize potential sampling bias due to the difference in sEPSC frequency among neurons (i.e. if sampled on a fixed time basis (e.g. 3 min), a histogram would contain more ‘events’ from the neurons with higher frequency of sEPSCs, and thus be skewed). Values of all sEPSC intervals and amplitudes were logarithmically transformed in the histograms. The Kolmogorov–Smirnov (KS) two-sample, two-tailed test was used to compare the cumulative probability of sEPSC intervals and amplitudes between control and epileptic groups. The chi-square (χ2) test was employed to compare the ratios between groups. Student's t test was used for comparisons between two groups. Data are expressed as means ±s.e.m., and α= 0.05 in all tests.
Pharmacological agents
Bicuculline methiodide (30 μm) or SR-95531 (gabazine, 10 μm) was used to block GABAA receptors, SCH50911 (10 μm) to block GABAB receptors, dl-2-amino-5-phosphonopentanoic acid (AP-5, 50 μm) for NMDA receptors, 6,7-dinitroquinoxaline-2,3-dione (DNQX, 50 μm) for AMPA/kainate receptors, and 1-aminoindan-1,5-dicarboxylic acid (AIDA, 500 μm) to block mGlu1 receptors, respectively. SCH50911 was from Tocris (Ellisville, MO, USA); the other blockers were from Sigma (St. Louis, MO, USA).
Results
Lack of epileptiform bursts in immature and mature CA3 area in normal aCSF
It has been shown that GABAA receptor-mediated transmission is excitatory during early development and GABAergic inhibition has a delayed onset (Ben-Ari et al. 1997; Ben-Ari, 2002). This lack of GABA-mediated inhibition and an excitatory action of GABA may contribute to an enhanced propensity for epileptiform bursts during the early stages of postnatal development. The excitatory actions of GABA contribute to K+-induced seizure-like activity in slices from the developing CA3 area throughout the first three postnatal weeks (Dzhala & Staley, 2003). In the present study, we first tested synaptic responses in slices that contained only the CA3 area of the hippocampus (i.e. CA3 minislices, Fig. 1A) from immature rats in normal aCSF to determine whether abnormal or epileptiform bursts might occur during the second postnatal week, while GABAergic transmission might still be excitatory. In this condition, synaptic stimulation consistently elicited a field EPSP and a single population spike in the CA3 area (Fig. 1B). Multi-spike epileptiform bursts were never evoked regardless of the stimulus intensity, although the amplitude of the population spike increased with stimulus intensity (Fig. 1B). Similar results were seen in CA3 slices from mature rats (not shown). These data suggest the existence of a GABAergic inhibitory system during the second postnatal week (and in later developmental stages) that is sufficient to prevent synaptically activated CA3 neurons from firing repetitively and spreading to generate burst discharges.
Epileptiform bursts in CA3 minislices from immature versus mature rats
Initial experiments were performed to confirm results from earlier studies that have shown that blockade of GABAA receptors with penicillin or picrotoxin induces epileptiform bursts in the CA3 area that are much more prolonged in slices from immature compared to mature rats (Swann & Brady, 1984; Swann et al. 1993). Here, we used a different GABAA receptor antagonist, bicuculline (30 μm), to induce the epileptiform bursts. As expected, prolonged population bursts were consistently evoked (Fig. 1C, n= 12 slices) in the CA3 area from immature rats. These bursts contained an initial depolarization followed by repetitive afterdischarges, which lasted for many seconds (14.4 ± 1.7 s, n= 12 slices, Fig. 1C). In addition, these prolonged bursts were also observed in slices from rats older than 2 weeks (i.e. at postnatal day 18, 20 and 23), suggesting that the CA3 area is capable of generating prolonged population bursts during the third postnatal week. In sharp contrast, epileptiform bursts in the CA3 area from adult rats were much shorter (0.18 ± 0.03 s, n= 14 slices, P < 0.001, Fig. 1D). These data confirm the results from previous studies using other GABAA receptor blockers (i.e. penicillin or picrotoxin) that showed that the immature CA3 area generates much more prolonged epileptiform burst discharges than the mature CA3 area (Swann & Brady, 1984; Swann et al. 1993; Gomez-Di Cesare et al. 1997). However, how burst duration decreases (i.e. suddenly or gradually) during the transition period of development (i.e. after 2nd postnatal week to full mature age) is unclear.
Glutamatergic sEPSCs in CA3 pyramidal cells
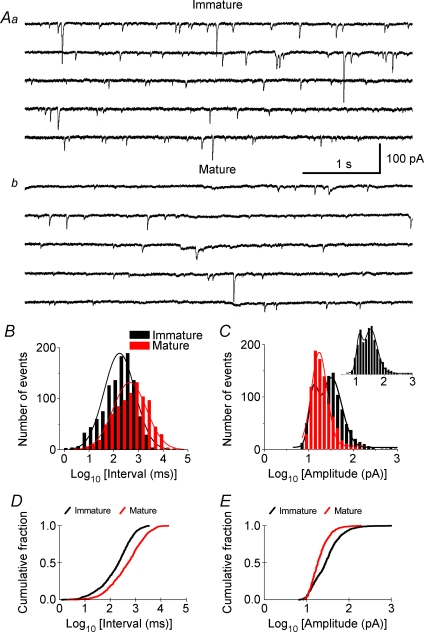
Gomez-Di Cesare and colleagues (1997) showed that the dendrites and axons of the CA3 neurons are highly branched during the second postnatal week, and axonal pruning takes place during maturation, which suggests that changes in the synaptic connectivity between CA3 pyramidal cells may occur during postnatal development. To assess the potential involvement of these synaptic changes in the changes of epileptiform bursting activity, we analysed the properties of spontaneous synaptic activity in 11 CA3 pyramidal cells. All 11 CA3 pyramidal cells (in 10 slices) from immature rats exhibited sEPSCs (Fig. 2Aa) in the presence of 30 μm bicuculline, confirming that these synaptic events were glutamatergic and not GABAergic. The amplitude of the sEPSCs ranged from tens to hundreds of picoamperes (Fig. 2Aa). Giant sEPSCs up to 1000 pA were occasionally seen in CA3 neurons from immature rats, but never seen in CA3 neurons from mature rats. The sEPSC mean amplitude in CA3 neurons from immature rats was 41 ± 9.7 pA (n= 11). The sEPSC frequency of CA3 neurons from immature rats ranged from 3 to 5 Hz with a mean inter-sEPSC interval of 376 ± 90 ms. Most CA3 neurons (10 of 13, in 10 slices) from mature rats also displayed sEPSCs (n= 10) (Fig. 2Ab); however, the amplitude of their sEPSCs was significantly smaller (22 ± 1.5 pA, n= 10, P < 0.05), and their frequency was lower (∼ 1 Hz, mean inter-event interval: 1227 ± 299 ms, n= 10, P < 0.01). Further quantitative analysis showed that the histograms for log interval of the sEPSCs in both immature and adult groups had a distribution that was nearly Gaussian (Fig. 2B), in which the peak interval of the immature group was shifted to the left (i.e. smaller, Fig. 2B, black). The histogram for log amplitude of the sEPSCs in the immature group (Fig. 2C, black) appeared to fit a two-peak distribution (Fig. 2C, inset), in which the second peak (i.e. large-amplitude sEPSCs) was largely absent in the adult group (Fig. 2C). Moreover, the cumulative distributions of sEPSC intervals and amplitudes of the immature and mature groups were significantly different (P < 0.001, KS test; Fig. 2D and E). These data show that CA3 pyramidal cells from immature rats have more frequent and larger sEPSCs than those from mature ones, suggesting an increased degree of local glutamatergic synaptic connectivity (i.e. more recurrent excitation) and/or activity in the CA3 area of immature versus adult rats, which parallels the increased duration of epileptiform bursts.
Figure 2. Spontaneous excitatory postsynaptic currents (sEPSCs) in CA3 pyramidal neurons from immature and mature rats.
A, representative sEPSCs recorded in CA3 neurons from immature (14 d, Aa) and adult (93 d, Ab) rats. Five consecutive traces (i.e. 5 s each) from continuous recordings of sEPSCs are shown in each panel. The sEPSCs in slices from immature rat (Aa) were more frequent and of larger amplitude than the sEPSCs recorded in adult slices (Ab). B, combined histograms showing the distribution of sEPSC intervals (milliseconds in log value, bin = 0.2) for the immature (black) and adult (red) groups. More sEPSCs in the immature group had short inter-event intervals (i.e. left side of histogram), and the peak of the histogram was shifted to the left (i.e. shorter interval) compared to that in the adult group. C, histograms showing the logarithmic distribution of sEPSC amplitudes for the immature (black) and adult (red) groups. The histogram of the immature group appeared to fit a two-peak distribution (inset), and the second peak (i.e. larger amplitude sEPSCs) was largely absent in the adult group. D and E, Kolmogorov–Smirnov test showing the cumulative probability of sEPSC intervals (D) and sEPSC amplitudes (E) was significantly different between immature (black) and mature (red) groups (P < 0.001). Note that 100 sEPSCs from each cell were pooled for constructing histograms and for calculating cumulative probability, except for a few cells that had fewer than (but close to) 100 sEPSCs. The peaks of histograms were fitted with a Gaussian distribution.
Effect of selective GABAergic and glutamatergic antagonists
In a second series of experiments, the dependence of the developmental difference in the duration of the epileptiform bursts on specific GABAergic and glutamatergic synaptic receptors was determined by comparing the epileptiform bursts in CA3 minislices from immature and mature rats after bath application of different receptor antagonists. In previous studies the same slice thickness was used for immature and adult slices (Swann & Brady, 1984; Gomez-Di Cesare et al. 1997). This raises the concern of a potential overestimation of the epileptiform burst propensity in immature CA3 because the immature brain slices may contain more neurons and synaptic connections than the adult ones, since the neuronal density is diluted in mature brain as brain volume increases during development while maintaining a relatively constant number of neurons. Thus, in the following experiments, slice thickness was adjusted (350 μm for immature and 450 μm for adult rats) to compensate for the brain volume increase (and thus the decrease in the density of neurons) that occurs during development.
GABAB receptors
Although the initial series of experiments in this study showed that bicuculline consistently induced prolonged epileptiform bursts in immature CA3 versus brief bursts in adult CA3, bicuculline is known to block Ca2+-activated K+ channels in addition to blocking GABAA receptors (Stocker et al. 1999), which could add another variable for analysing the developmental differences in properties of epileptiform bursting in CA3. It is also possible that maturational changes in GABAB receptors play a role in the developmental differences in the epileptiform bursts in the CA3 area. The GABAB receptors have both pre- and postsynaptic effects, and they also undergo developmental changes (Turgeon & Albin, 1994). GABAB receptor-mediated actions may impede synchronous firing (de la Prida et al. 2006), but their role in seizure generation is controversial (Veliskova et al. 1994; Snead, 1995). To eliminate possible developmental differences in GABAB receptor-mediated GABAergic transmission and to avoid the non-specific actions of bicuculline, selective GABAA- and GABAB-receptor antagonists (i.e. gabazine and SCH50911 (Ong et al. 1998), respectively) were concurrently used in all of the subsequent experiments. In the presence of gabazine and SCH50911, prolonged population bursts were consistently triggered in CA3 minislices from immature rats (Fig. 3A), and short bursts were evoked in slices from adult rats (Fig. 3B), suggesting that developmental changes in GABAB receptors and the excitatory action of GABA via GABAA receptors are not necessary for the developmental differences in burst duration in the CA3 area.
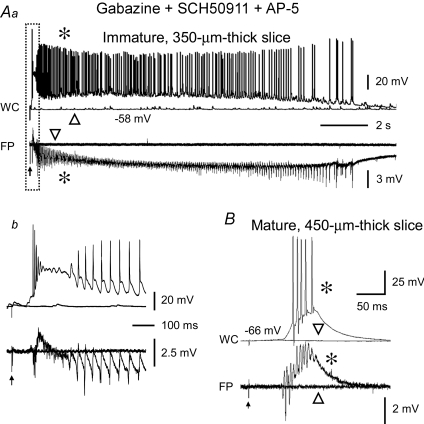
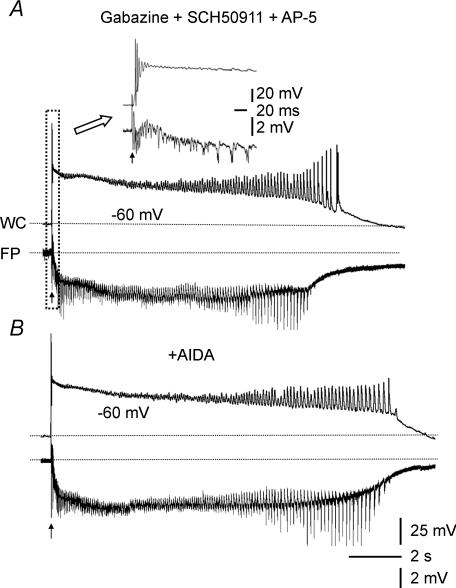
Figure 3. Prolonged population bursts were present in CA3 minislices from immature rats after pharmacological blockade of GABAB and NMDA receptors.
Simultaneous whole-cell (WC, upper traces) and field-potential (FP, lower traces) recordings showing the response of a 350 μm-thick CA3 minislice from a 10-day-old rat (Aa) and a 450 μm-thick CA3 minislice from a 99-day-old rat (B) to synaptic stimulation in the presence of a mixture of antagonists of GABAA (gabazine, 10 μm), GABAB (SCH50911, 10 μm) and NMDA (AP-5, 50 μm) receptors. The immature CA3 slice in Aa responded to synaptic stimulation at threshold intensity (arrow) with a 15 s long population burst with rhythmic afterdischarges. The initial section of the burst (i.e. boxed segment) is shown at an expanded time scale in Ab. The responses were all-or-none, such that stimuli of the same intensity evoked either an entire prolonged burst (asterisks) or no burst (triangles). Note the long latency from stimulation (arrow) to the onset of the burst in Ab (∼75 ms). In contrast, in CA3 slices from mature rats, synaptic stimulation at threshold intensity (arrow) evoked brief population bursts (B). Note the all-or-none property of the response and the long latency (∼53 ms) at threshold stimulus intensity (20 μA).
NMDA receptors
The NMDA receptors are well-developed at an early developmental stage (Ben-Ari et al. 1997), when they have several unique features. For example, NMDA receptors are less voltage dependent (Ben-Ari et al. 1988), their density is relatively high (Tremblay et al. 1988), and their kinetics are slow (Hestrin, 1992). All of these features would be expected to favour generation of robust and prolonged epileptiform bursts in the immature CA3 area. We tested the effects of NMDA receptor antagonists on evoked epileptiform bursts in the presence of gabazine and SCH50911 (see above). Prolonged bursts (>10 s) in CA3 minislices from immature rats were still present when the NMDA receptor antagonist AP-5 (50 μm) was applied to the bath medium from the beginning of the experiment (n= 13, Fig. 3A) or later in the experiments (n= 3, not shown). The bursts evoked in gabazine, SCH50911 and AP-5 (Fig. 3A) were identical to the prolonged bursts that occurred in bicuculline (13.3 ± 1.4 s, n= 16 slices vs. 14.4 ± 1.7 s, n= 12 slices, P > 0.05; compare Figs 1C and 3Aa). Specifically, the bursts were typically long duration and contained an initial depolarization followed by rhythmic afterdischarges that lasted for many seconds (Figs 1C and 3Aa).
As in bicuculline (see above), the epileptiform bursts that were present after blockade of GABAA, GABAB and NMDA receptors were elicited in an all-or-none manner; that is, threshold synaptic stimulations induced either no burst or a full-length burst with a long delay (Fig. 3A). The bursts that occurred at threshold stimulations usually had a long latency (up to 75 ms; on average: 28 ± 5 ms, n= 8 slices; Figs 3Ab and 4A, and Table 1), and the latency varied among stimulations and among slices (Fig. 4A). Once a burst was evoked by a just-suprathreshold stimulus, further increases of stimulus intensity (e.g. up to ten times threshold) no longer caused a significant increase in burst duration (Fig. 4B). The threshold to evoke a network burst was relatively low (<100 μA) and variable from slice to slice, ranging from a few microamperes to several tens of microamperes (on average, 39.7 ± 9.4 μA, n= 9 slices, Table 1). In sharp contrast, synaptic stimulation in CA3 minislices from mature rats (450 μm thick, i.e. compensated for developmental decrease in neuronal density) in the presence of gabazine (10 μm), SCH50911 (10 μm) and AP-5 (50 μm) invariably evoked short-lasting bursts (Fig. 3B). The burst duration was 0.14 ± 0.01 s (n= 10 slices) and was not different from that of bicuculline-induced bursts in CA3 slices from adult rats (0.18 ± 0.03 s, n= 14 slices, P > 0.05). While short in duration, these events also displayed the typical properties of ‘network’ bursts; that is, they had a long-and-variable latency (36.8 ± 3.8 ms, n= 10 slices; Figs 3B and 4C, and Table 1) and occurred in an all-or-none manner (Figs 3B and 4D). The mean threshold intensity of synaptic stimulation to evoke a burst and the burst probability at threshold were 15.3 ± 1.7 μA and 0.5 ± 0.05, respectively (n= 10 slices, Table 1), in CA3 minislices from adult rats. The all-or-none nature and the long-and-variable latency of the responses are both stereotypical characteristics of a neural network with recurrent excitation (Traub & Wong, 1982, 1983a,b; Wong & Traub, 1983). Thus, the short- and long-duration synaptically evoked network bursts in mature and immature CA3 minislices, respectively, appeared to be mediated by a local (i.e. within the CA3 area) pyramidal cell to pyramidal cell neuronal network. These data suggest that (1) NMDA receptor-mediated excitation alone is not necessary for the generation of prolonged epileptiform bursts in the CA3 area from immature rats or the brief bursts in CA3 area from adult rats, and (2) developmental changes in NMDA receptors alone do not account for the developmental difference in network bursting in CA3.
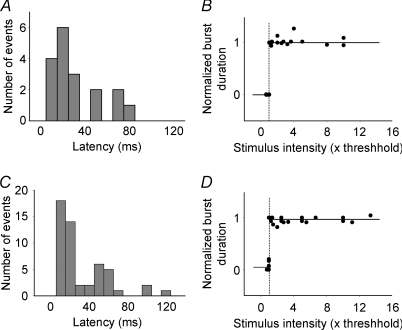
Figure 4. The long-and-variable latency and all-or-none characteristics of the CA3 population bursts from immature and mature rats.
A, histogram showing the distribution of the latency values of 18 bursts pooled from 8 CA3 minislices from immature rats. The latency values ranged from 5 to 75 ms, suggesting these bursts were multi-synaptic responses. Bin = 10 ms. B, pooled data from 9 CA3 minislices from immature rats showing the all-or-none nature of the prolonged network bursts; that is, stimulation below or at threshold intensity (≤ 1) evoked no bursts (duration = 0), while threshold or slight supra-threshold stimulations (≥1) elicited full-length bursts (duration = 1), and a further increase of stimulus intensity up to 10 times threshold no longer significantly increased burst duration. The vertical dashed line indicates the threshold (i.e. 1) and the short and long horizontal lines indicate the lack of a burst (0) and the emergence of a full length burst (1) in response to threshold stimulation, respectively. C, histogram showing the distribution of the latency values from 51 bursts pooled from 9 slices from adult rats, which ranged from 11 to 131 ms, also suggesting the poly-synaptic nature of the brief epileptiform bursts. Bin = 10 ms. D, data pooled from 9 slices illustrating that the brief population bursts in adult CA3 minislices were also all-or-none.
Table 1.
Comparison of bursting properties between the immature and adult groups
| Immature | Adult | P value | |
|---|---|---|---|
| Thickness of slices (μm) | 350 | 450 | |
| Burst threshold (μA) | 39.7 ± 9.4 (n= 9) | 15.3 ± 1.7 (n= 10) | 0.02 |
| Burst duration (s) | 13.3 ± 1.4 (n= 16) | 0.14 ± 0.01 (n= 10) | <0.001 |
| Burst latency (at threshold, ms) | 28 ± 5 (n= 8) | 36.8 ± 3.8 (n= 10) | 0.19 |
| Burst probability (at threshold) | 0.32 ± 0.05 (n= 8) | 0.5 ± 0.05 (n= 10) | 0.02 |
The data were averaged across slices and were compared using Student's unpaired t test or χ2 test.
mGlu1 receptors
Activation of mGlu1 receptors converts short (i.e. <0.5 s) picrotoxin-induced bursts in adult CA3 into prolonged (i.e. 2–4 s) bursts (Merlin & Wong, 1997; Galoyan & Merlin, 2000). Therefore, activation of mGlu1 receptors may be another possible synaptic mechanism for the generation of prolonged population bursts in the CA3 area. To test whether the prolonged epileptiform bursts in CA3 slices from immature rats involve activation of mGlu1 receptors, a selective mGlu1 receptor antagonist, AIDA (Moroni et al. 1997), was bath applied to CA3 minislices from the immature rats. The synaptically evoked bursts in gabazine, SCH50911 and AP-5 in CA3 minislices of immature rats were not reduced in duration by AIDA (500 μm) (n= 6, Fig. 5A and B). These data suggest that the effect of endogenous activation of mGlu1 receptors on the prolonged epileptiform bursts in CA3 slices from the immature rats is small and thus is highly unlikely to underlie the developmental differences in CA3 burst duration of immature vs. mature rats.
Figure 5. The prolonged epileptiform bursts of immature CA3 pyramidal cells were also present in the mGlu1-receptor antagonist, AIDA.
A, whole-cell (WC, upper trace) and field-potential (FP, lower trace) recordings showing a prolonged evoked burst in a CA3 minislice from an 11-day-old rat in gabazine, SCH50911 and AP-5. Inset at the top shows the boxed part of this burst at an expanded time scale. B, bath-application of the selective mGlu1 receptor antagonist AIDA (500 μm) did not reduce burst duration in this slice.
AMPA/kainate receptors
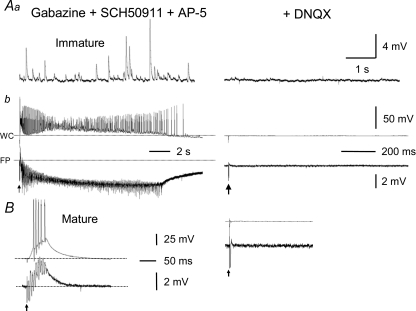
The prolonged population bursts in CA3 from immature rats that occurred in gabazine, SCH50911 and AP-5 were completely blocked by the AMPA/kainate receptor antagonist DNQX (50 μm) (Fig. 6Ab). Moreover, sEPSPs which were evident in the presence of gabazine, SCH50911 and AP-5 (Fig. 6Aa), were eliminated by DNQX, thus indicating that these events were mediated by AMPA/kainate receptors. DNQX (50 μm) also completely abolished the brief bursts in slices from adult animals (Fig. 6B). These data provide strong evidence in support of the hypotheses that (1) AMPA/kainate receptors on CA3 pyramidal cells are well-developed during the second postnatal week, (2) recurrent excitatory circuits requiring only AMPA/kainate receptors can generate the characteristic epileptiform bursts of CA3 pyramidal cells when GABA and NMDA receptors are blocked in both the immature and adult rats, and (3) the duration of these network bursts decreases sharply in adult compared to immature rats.
Figure 6. All sEPSPs and epileptiform bursts were completely abolished by the AMPA/kainate-receptor antagonist, DNQX.
A, frequent sEPSPs as large as 10 mV (Aa, left panel) were present and prolonged epileptiform bursts (Ab, left panel) were consistently elicited in an immature CA3 minislice bathed with a mixture of gabazine (10 μm), SCH50911 (10 μm) and AP-5 (50 μm). DNQX (50 μm) completely abolished the sEPSPs and prolonged population bursts (Aa and Ab, right panels), confirming that the prolonged bursts under these conditions (i.e. prior blockade of GABAA, GABAB and NMDA receptors) were solely mediated by AMPA/kainate receptors. B, DNQX similarly eliminated the brief bursts evoked in mature CA3 minislices. Arrows indicate stimulations. Dotted lines in A and B indicate baseline potentials.
Responses of CA3 recurrent synapses to repetitive stimulation in slices from immature versus adult rats
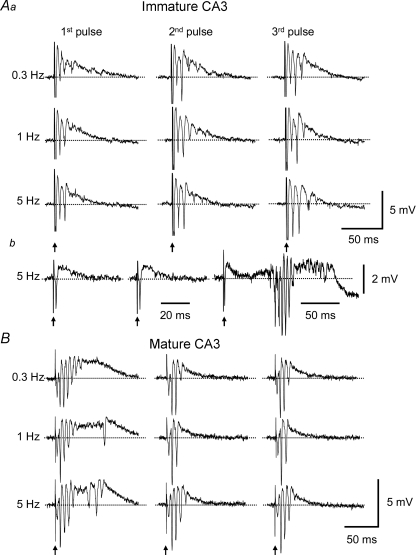
The work from Staley and colleagues (Staley et al. 1998; Jones et al. 2007) has shown that the probability of transmitter release and the amount of releasable transmitter at the CA3 recurrent synapses also contribute to the duration of epileptiform bursts. Therefore, it is reasonable to hypothesize that if the dynamics of transmitter release at the CA3 recurrent synapses change during development, it may contribute to the maturational difference in burst duration. One approach to assess this issue is to test the synaptic response at different time points after an initial stimulation (Staley et al. 1998), based on the assumption that if a large amount of transmitter at the CA3 recurrent synapses were released during the first stimulation (i.e. high-probability release), then less transmitter would be available for the next stimulation, which would result in a smaller response; and vice versa. To test this hypothesis, CA3 recurrent synapses were antidromically activated in the presence of the GABAA-, GABAB- and NMDA-receptor antagonists gabazine (10 μm), SCH50911 (10 μm) and AP-5 (50 μm). Also, [Mg2+]o was elevated to 4 mm to block multi-synaptic responses. Under these conditions, antidromic stimulation in immature CA3 evoked a field EPSP with about two population spikes (Fig. 7Aa, 1st pulses) or only a small recurrent EPSP (Fig. 7Ab, 1st pulse). The same slices generated prolonged bursts in the same solution, but with normal [Mg2+]o concentration (2 mm, data not shown). Repetitive activation of the recurrent synapses at low frequencies (0.3, 1 Hz) significantly increased the responses (i.e. facilitation) in only a small fraction of slices (1 of 7 slices at 0.3 Hz; 2 of 7 slices at 1 Hz; Fig. 7Aa); however, repetitive stimulations at higher frequency (i.e. 5 Hz) induced clear facilitation in most of the slices (6 of 7 slices from 5 rat pups, Fig. 7Aa and b). In contrast, under identical experimental conditions, single antidromic stimulations in adult CA3 evoked more robust responses with more and larger population spikes and a longer burst duration (Fig. 7B, 1st pulses, n= 6 slices from 4 rats). Repetitive activation of the mature CA3 recurrent synapses, however, led to a marked reduction in the responses at all three frequencies (3 of 6 slices at 0.3 Hz; 5 of 6 slices at 1 Hz; 4 of 6 slices at 5 Hz, Fig. 7B). These data suggest that recurrent synapses in the immature CA3 generate a relatively small initial response, but are more responsive to repetitive stimulation, presumably due to a low probability of transmitter release to single stimuli but greater transmitter release during repetitive stimulations. The opposite scenario holds for the adult CA3.
Figure 7. Responsiveness of the CA3 recurrent synapses to repetitive stimulation in slices from immature and mature rats.
CA3 recurrent synapses were antidromically activated in the presence of gabazine (10 μm), SCH50911 (10 μm), AP-5 (μm) and elevated [Mg2+]o (4 mm). Repetitive stimulations at different intervals (0.3, 1 and 5 Hz) were used to test the dynamic properties of transmitter release. Aa, field-potential recordings showed that the initial responses were comparatively small in immature CA3 slices, but they tended to increase in later responses, particularly at short intervals (i.e. 5 Hz). Ab, some slices responded to the initial stimulation with only a small field EPSP, but developed larger responses with multiple population spikes and longer duration during later stimulation. B, in contrast, in mature CA3 slices, the initial responses were on average larger than those in immature slices, and they tended to decrease during repetitive activation.
Intrinsic membrane properties in immature vs. adult CA3 pyramidal neurons
In addition to the above-mentioned synaptic mechanisms, the intrinsic excitability of individual neurons is also expected to affect network activity. A developmental change in intrinsic membrane excitability may contribute to the developmental differences in population bursts in CA3. Although it has been reported that intrinsic membrane properties of central neurons change during postnatal development (Viana et al. 1995; Belleau & Warren, 2000), it is unclear whether or how the intrinsic properties of the CA3 pyramidal neurons undergo maturational alterations.
Input–output relationship
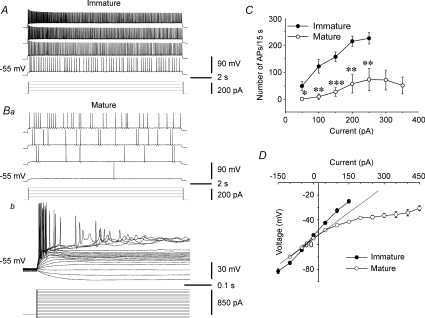
The input–output relationship of the firing properties of CA3 pyramidal neurons was assessed in the absence of synaptic transmission (i.e. blocked by GABAA-, GABAB-, AMPA/kainate- and NMDA-receptor antagonists). The resting membrane potential and the action potential threshold of the CA3 pyramidal neurons were similar for the immature and adult rats (−59.9 ± 0.8 mV, n= 9 vs.−58.5 ± 1.1 mV, n= 8, P > 0.05; and −31.1 ± 1.0 mV, n= 9 vs. 34.3 ± 0.5 mV, n= 8, P > 0.05, respectively), but input resistance was greater in neurons from immature vs. mature rats (403.8 ± 24.6 MΩ, n= 9 vs. 159.4 ± 20.7 MΩ, n= 8, P < 0.001). To test input–out relationships, the membrane potential was held at ∼–55 mV, and a series of 15 s-long hyperpolarizing and depolarizing current pulses were injected into the cells. Virtually all CA3 pyramidal neurons from immature rats were capable of firing action potentials with little adaptation over the entire 15 s period of the current pulse. The number of action potentials was graded with the intensity of the depolarizing currents (Fig. 8A and C, n= 9 cells from 7 slices, 5 rats). In contrast, most of the CA3 pyramidal neurons from adult rats (6 of 8 cells) responded to depolarizing current injections with either low-frequency firing (Fig. 8Ba) or only fired at the initial 100–200 ms and then became quiescent (Fig. 8Bb). The mean input–output relationship of the adult CA3 neurons (n= 8 cells from 7 slices, 6 rats) was significantly less steep than that of immature CA3 neurons (Fig. 8C). A marked rectification to depolarizing currents (i.e. voltage changes in response to depolarizing current injections was smaller than predicted from an ohmic relation) was observed in most of the adult CA3 pyramidal neurons (Fig. 8Bb and D), which limited their ability to fire action potentials. Altogether, these data show that CA3 pyramidal neurons have a greater intrinsic excitability in immature vs. adult rats, particularly in response to a prolonged depolarization.
Figure 8. Intrinsic firing in CA3 pyramidal neurons from immature and adult rats.
A, whole-cell recordings showing that CA3 pyramidal neurons from immature rats were able to fire action potentials over the entire duration of a depolarizing current pulse (i.e. 15 s) with little adaptation. B, in contrast, CA3 pyramidal neurons from mature rats responded to the same or larger current injections with fewer action potentials (Ba), or only generated action potentials during the first few hundred milliseconds of the current pulses (Bb). A strong rectification to depolarizing current pulses was seen in the mature CA3 neurons (Bb), which was not present in immature CA3 pyramidal cells. C, summary plot showing the difference in the input–output relations in CA3 pyramidal neurons from immature and mature rats. *P < 0.05; **P < 0.01; ***P < 0.001, Student's two-tailed unpaired t test. D, summary plot showing that the immature CA3 pyramidal neurons displayed a nearly ohmic current–voltage response, whereas adult CA3 pyramidal neurons showed a clear rectification to depolarizing current injections (i.e. deviated from the hypothetical linear response as the dotted line indicates), which limited their ability to fire repetitively. All experiments were performed in the presence of gabazine (10 μm), SCH50911 (10 μm), AP-5 (50 μm) and DNQX (50 μm), which blocked GABAergic and glutamatergic synaptic transmission.
AHPs
AHPs can regulate neuronal firing and were examined in both the immature and adult neurons. To isolate AHPs, GABAergic and glutamatergic transmission was blocked. To optimize the generation of AHPs, a burst of four to five action potentials was generated by a brief triangular current pulse, and the membrane potential was current-clamped at a depolarized level (i.e. −40 mV to −50 mV) to increase the driving force for K+ currents. A medium-duration AHP (mAHP, lasting for tens of milliseconds) was observed in half (4 of 8 cells) of the mature CA3 neurons, but only in 1 of 9 immature CA3 neurons (Fig. 9). The slow AHP (sAHP) was absent in neurons from the immature CA3, and was present but small in neurons from the adult CA3 (Fig. 9).
Figure 9. Afterhyperpolarizations (AHPs) in CA3 neurons from immature and mature rats.
A, most of the immature CA3 neurons displayed little or no discernable AHP after a burst of 4–5 action potentials (upper traces) induced by an 80 ms triangular depolarizing current injection (bottom trace). B, some of the mature CA3 neurons showed a medium-duration AHP (mAHP) and negligible slow AHP (sAHP). The membrane potential of the neurons was held at depolarized levels (−50 mV to −40 mV) to increase the driving force for AHP (K+ conductance). C, summary data showing the amplitude of mAHP and sAHP in the immature and mature groups (**P < 0.01, Student's two-tailed unpaired t test).
Discussion
The key results of the present study are: (1) the developmental difference in epileptiform burst duration in CA3 area is associated with a parallel change in local glutamatergic synaptic activity, as assessed by the increased frequency and amplitude of sEPSCs in minislices from immature rats; (2) the more prolonged epileptiform bursts in CA3 from immature rats (i.e. later second postnatal week) do not depend on the excitatory action of GABA or developmental changes in GABAB receptor activity; (3) developmental changes in neither NMDA nor mGlu1 receptors are sufficient to account for the developmental differences in the epileptiform bursts; (4) the recurrent excitatory synapses in CA3 are more responsive to repetitive stimulation in immature than mature rats (i.e. consistent with the hypothesis of a low-probability but more-sustained transmitter release in the immature CA3); and (5) the CA3 pyramidal cells from immature rats have a greater intrinsic ability to generate action potentials than those from adult rats. It is important to emphasize that the different electrophysiological properties of the network of immature (versus adult) CA3 pyramidal cells refer to the later part of the second postnatal week of development. The electrical properties at <9 days of postnatal development are likely to be quite different from the ones observed here (e.g. excitatory role of GABAA receptor activation).
Developmental difference in CA3 bursts in bicuculline and parallel changes in sEPSCs
The bicuculline-induced epileptiform bursts observed in CA3 in this study were essentially identical to the ones induced by penicillin or picrotoxin in previous studies (Swann & Brady, 1984; Swann et al. 1993). Because of the network nature of these bursts, one would expect that developmental differences in burst properties are associated with developmental changes in the local synaptic network in CA3. Indeed, we observed a significantly increased frequency and amplitude of sEPSCs in CA3 pyramidal cells from immature compared to mature rats.
The larger sEPSCs are expected to reflect primarily action potential-mediated transmission and most likely originate from local synapses (i.e. CA3 pyramidal cell connected to CA3 pyramidal cell), because the incoming axons from remote areas were cut during preparation of the CA3 minislices. Only axons with intact presynaptic neurons (i.e. neighbouring CA3 pyramidal cells) would be expected to generate spontaneous action potentials, thus producing larger sEPSCs. The largest-amplitude sEPSCs appear to be absent in the adult group (Fig. 2C), which supports the hypothesis that CA3 pyramidal neurons contain more connections from neighbouring CA3 neurons in immature than adult rats. Alternatively, the larger sEPSCs in immature CA3 neurons may originate from release sites that are electrotonically closer to the soma. This interpretation is consistent with a previous study (Gomez-Di Cesare et al. 1997) that showed that the axons of the CA3 neurons are more abundantly branched in immature than adult rats, and thus may synapse on more dendritic locations. An increase in action potential-dependent sEPSCs would suggest increased spontaneous firing of the immature CA3 neurons. Although we did not find a significant difference in the resting membrane potential or action potential threshold between the immature and mature CA3 neurons, it is still possible that the increased membrane resistance and the higher frequency of synaptic events would lead to spontaneous firing in the immature neurons.
The higher frequency of sEPSCs could have resulted from an increased degree of connectivity of the immature CA3 pyramidal neurons, an increased firing of the presynaptic neurons due to enhanced membrane excitability of the immature CA3 pyramidal neurons, or both. An increased size of a synaptic network may prolong burst duration, as suggested by an earlier modelling study (Traub et al. 1984), which showed that a sufficiently large number of interconnected neurons are required in order to keep the network burst activity ‘alive’ and to produce a series of afterdischarges (as opposed to a single burst). In a large network, a phase difference in action potential discharges exists between the neuronal populations during an initial burst, thus leaving ‘residual’ activity. Therefore, during the periods when most neurons are either below threshold or have non-conducting axons, the residual activity from one synchronized burst can spread through the population via excitatory synaptic connections to initiate a subsequent burst (Traub et al. 1984).
Neuronal density and epileptiform bursts in immature vs. adult CA3
Although our data on bicuculline-induced epileptiform bursts (i.e. Fig. 1C) and data from previous studies have demonstrated enhanced population bursting in immature CA3, it is possible that this is due to a developmental difference in neuronal density. The total number of neurons in the brain is generally believed to be relatively constant throughout the later stages of development, but brain volume increases during maturation, suggesting the density of neurons at an immature stage is higher than that in adult. It is estimated that the density of pyramidal cells in the hippocampus at the second postnatal week is roughly 1.5 times greater than that of adults (Gomez-Di Cesare et al. 1997). Therefore, brain slices cut at identical thickness from immature and mature animals (as in the initial experiments of this study and in previous studies) may generate a technical bias in that the slices from immature rats contain more neurons (and thus more local synaptic circuits) than the slices from adults; this difference in neuronal density could hypothetically be responsible for the more prolonged epileptiform bursts in the immature CA3. To compensate for this maturational decrease in neuronal density, we reduced the thickness of the CA3 minislices to 350 μm in immature versus 450 μm in adult groups. The rationale was that the neurons and synaptic circuitry in the superficial 50 μm of tissue from both sides of the slices are most likely damaged during preparation; therefore, a 450 μm-thick mature slice would actually have healthy tissue of 350 μm. To compensate for the developmental changes in neuronal density, the slices for immature rats ought to be cut at about two-thirds of the thickness of the actual viable tissue in a mature slice (i.e. 66% of 350 μm = 233 μm). Assuming 50 μm is damaged for both the upper and lower parts of the slice, the total thickness of an immature slice should be 333 μm; thus, all slices for immature rats were cut at 350 μm. The results show that the striking developmental difference in epileptiform bursting in the CA3 area was still present in the thickness-adjusted slices (Fig. 3), further supporting earlier studies reporting a fundamental difference in the network bursts between the immature and mature CA3 area.
Excitatory action of GABA and enhanced epileptiform bursting in immature CA3
GABA has been shown to be excitatory in early development (Ben-Ari et al. 1997), but it is not clear whether the excitatory action of GABA contributes to or is necessary for the generation of prolonged epileptiform bursts in the CA3 area of immature hippocampus during and after the later part of the second postnatal week. Our data show that prolonged epileptiform bursts were readily evoked in the CA3 area of immature rats during the later part of the second postnatal week, and these events were not present in normal medium but occurred when GABAA receptors were blocked by bicuculline or gabazine (Fig. 1BversusFigs 1C, 3A and 5). These observations strongly suggest that by the later part of the second postnatal week, GABAA receptor activation leads to powerful inhibition that normally and effectively prevents epileptiform bursts from occurring. These data appear to be in contrast to a recent study (Dzhala & Staley, 2003) in which bicuculline and gabazine diminished K+-induced ictal epileptiform discharges in the developing CA3. One possibility is that the excitatory GABA action may be more significant in high [K+]o, which decreases the co-transport of intracellular [Cl−]i and [K+]i to the outside of the neuron, thus resulting in an accumulation of [Cl−]i and a depolarizing shift of ECl (Staley & Proctor, 1999). Nonetheless, our results suggest that in normal [K+]o, a GABA-mediated excitatory action (if it is present in the later half of the second postnatal week) is not necessary for prolonged synaptically evoked epileptiform bursts in the immature CA3 area. The [Cl−]i may also be regulated by Na+,K+-ATPase, which determines [Na+]i and the rate of the Na+–K+–Cl− co-transport (NKCC) and thus [Cl−]i (Brumback & Staley, 2008). The expression of Na+,K+-ATPase in neurons appears to decline during maturation (Lecuona et al. 1996), suggesting an enhanced Na+,K+-ATPase function in the immature brain, which may contribute to [Cl−]i accumulation and excitatory GABA action. But again, our data argue that the excitatory GABA effect is not sufficient (or necessary) for the generation of the prolonged network bursts in developing CA3 during the later half of the second postnatal week.
NMDA and mGlu1 receptors and epileptiform bursting in CA3
Although the observed developmental difference in epileptiform bursting occurred in bicuculline and the parallel changes in sEPSCs imply an important role for glutamatergic synaptic mechanisms in the generation of network bursts, the relative importance of different components of glutamatergic transmission was still unclear. NMDA receptors have been reported to have unique features in early development, such as early emergence (Ben-Ari et al. 1997), less voltage dependence (Ben-Ari et al. 1988), higher density (Tremblay et al. 1988) and slower kinetics (Hestrin, 1992). These features are expected to favour the generation of prolonged epileptiform bursts. Our data, however, show that the prolonged epileptiform bursts in immature CA3 were still generated in AP-5 (Figs 3, 5 and 6), thus suggesting that by the late second postnatal week, NMDA receptor-mediated excitation is not required for the generation of prolonged burst discharges in CA3 pyramidal cells. Therefore, the developmental difference in burst duration between immature and mature CA3 does not seem to depend on the activation of NMDA receptors, although NMDA receptors may well be involved in and contribute to the population bursts in both immature and mature CA3.
The observation that the prolonged epileptiform bursts in the immature CA3 were not reduced by the application of the mGlu1 receptor antagonist AIDA (Fig. 5) suggests that mGlu1 receptor-mediated excitation is not significantly involved in the generation of these bursts. Although activation of mGlu1 receptors has been shown to prolong epileptiform bursts, this effect requires exogenous application of mGluR1 agonists for an extended period (i.e. a 30 min exposure prolonged bursts to ∼1 s, and the bursts lasted ∼4 s after 100–120 min; Merlin & Wong, 1997; Galoyan & Merlin, 2000). Such extensive activation of mGlu1 receptors is less likely to be reached via endogenously released glutamate; thus, endogenous activation of mGluR1s by glutamate appears to contribute little to the prolonged network bursts in CA3 slices from immature rats. Similarly, an earlier study has provided evidence that mGlu1 receptors are not involved in prolonged epileptiform bursts in the CA1 area from guinea pig (Karnup & Stelzer, 2001).
Repetitive stimulation of the recurrent synapses in immature vs. adult CA3
The work from Staley and colleagues (Staley et al. 1998; Jones et al. 2007) has suggested that the rate of transmitter release and the amount of releasable transmitter at the presynaptic terminals may determine the duration of population bursts in the CA3 area, which can be estimated by the measurement of the length of a second burst triggered at different time points after an initial burst (Staley et al. 1998). Using a similar approach, we observed that the antidromically evoked ‘monosynaptic’ responses (i.e. in 4 mm[Mg2+]o, which minimized polysynaptic responses) of CA3 recurrent synapses were shorter in duration and smaller in amplitude in slices from immature than adult rats, and they tended to be facilitated by subsequent stimulations, in a frequency-dependent manner (Fig. 7). These data support, albeit do not prove, the hypothesis that that the recurrent synapses in immature CA3 have a lower probability of transmitter release during repetitive or sustained activity than that in the adult CA3. A low release probability in the recurrent CA3 synapses may have two consequences: (1) because of the weaker synaptic strength, only a small number of neurons may be activated at once, which would require a long and variable period of time to spread through the entire network, whereas high-probability transmitter release may activate more neurons and quickly synchronize the network, and (2) an initial activation of the recurrent synapses of immature CA3 pyramidal cells would not deplete transmitter at the terminals and would leave a sufficient amount of transmitter available to be released during subsequent activation, which would occur repetitively due to recurrent excitation in the synaptic network. Therefore, in a synaptic network, a low rate of transmitter release would require more time to activate the entire network and would allow sustained transmitter release for repetitive activation, thus favouring the generation of prolonged, oscillatory population bursts. Synapses with a high release probability (i.e. in adult CA3) would tend to release most of the transmitter after an initial stimulus, which would synchronize the whole network quickly (particularly when the size of the network is small) and would potentially deplete all of the releasable transmitter if repetitively activated, thus resulting in short bursts. These data are consistent with a recent study which showed that reduction in the rate of glutamate release at adult CA3 terminals prolongs burst duration (Jones et al. 2007).
Sustained action-potential firing in immature vs. adult CA3 pyramidal cells
Prolonged population bursts are mediated by multisynaptic transmission, which relies on reliable action potential generation of the participating neurons (Traub & Wong, 1982). Therefore, the intrinsic membrane properties for action potential generation are crucial for multi-synaptic transmission and production of network bursts. The enhanced excitability in the CA3 pyramidal neurons from immature rats would be expected to boost multi-synaptic transmission and network burst generation in at least two ways: (1) weak excitatory input may be sufficient to trigger action potentials, which is particularly important given the low probability of transmitter release at these recurrent synapses; and (2) sustained depolarization resulting from summation of convergent excitatory inputs, which is likely to occur in an interconnected synaptic network, should reliably generate action potentials, as long as the excitation lasts. The intrinsic excitability in adult CA3 pyramidal neurons is observed to be compromised by three factors: (1) a lower input resistance, (2) a membrane rectification that counteracts depolarization, and (3) to a lesser extent, a larger mAHP. The presence of a lower input resistance requires larger excitatory synaptic input to reach action-potential threshold; the rectification further impedes the excitatory synaptic input from reaching action-potential threshold, and terminates repetitive firing. The mAHP may further limit repetitive firing. The membrane rectification is likely to involve the activation of one or more voltage-gated K+ conductance(s), but the responsible channels were not further investigated in this study. The contrasting differences in intrinsic excitability between the immature and adult CA3 pyramidal neurons may partly account for the developmental difference in burst duration. Interestingly, recent studies have shown that blockade of M-type K+ current transforms brief epileptiform bursts into prolonged bursts in hippocampal slices (Qiu et al. 2007), and enhancement of the M-current suppresses epileptiform bursts (Qiu et al. 2008).
Hypothetical mechanisms for the generation of differential population bursts in the CA3 area of the immature and adult rats
Synchronous epileptiform bursts arise from the activity of individual neurons that spreads along synaptic pathways (Traub & Wong, 1982, 1983a,b); this process depends on the degree of synaptic connectivity, the strength of individual synapses and the intrinsic membrane properties of the neurons (Traub et al. 1984). Our data suggest that immature CA3 pyramidal neurons (i.e. later half of the second postnatal week) have a higher intrinsic excitability and are more widely connected, but are connected with synapses that release a lower amount of transmitter per action potential. Because of the low probability of transmitter release in the immature CA3 pyramidal cells, an initial stimulus only activates a small number of neurons in the large synaptic network, and the activity of these neurons propagates to other neurons with a phase difference, which tends to generate ‘oscillatory’ activity (i.e. afterdischarges) in the neuronal network (Traub et al. 1984). The low rate of transmitter release also makes glutamate available for a prolonged period of synaptic activity. The enhanced intrinsic excitability allows immature pyramidal cells to be activated repetitively, which sustains the network activity. In adult CA3, however, where the neuronal connections are relatively sparse (MacVicar & Dudek, 1980; Miles & Wong, 1986, 1987), the intrinsic firing capacity is lower and transmitter release per action potential is greater, such that the initial synaptic excitation activates neurons more efficiently, which spreads through the CA3 network more quickly; however, depletion of transmitter occurs more easily, resulting in short bursts.
In summary, our data suggest that an enhanced connectivity and/or activity of recurrent excitatory circuits, a low probability but sustained transmitter release at the recurrent synapses, along with a greater intrinsic membrane excitability in the pyramidal neurons all contribute to the generation of prolonged population bursts in the CA3 area from the immature versus adult rats. Thus, potential contributions of an excitatory action of GABA, and differences in NMDA- and mGlu1-receptor components of glutamate synaptic transmission in the later part of the second postnatal week are not necessary for (albeit may contribute to) the generation of prolonged population bursts in immature versus adult CA3.
Acknowledgments
This work was supported by National Institute of Health (NIH) grant NS 16683.
Glossary
Abbreviations
- AHP
afterhyperpolarization
- AIDA
1-aminoindan-1,5-dicarboxylic acid
- AP-5
dl-2-amino-5-phosphonopentanoic acid
- DNQX
6,7-dinitroquinoxaline-2,3-dione
- FP
field-potential
- KS test
Kolmogorov–Smirnov test
- mGlu1 receptors
type 1 metabotropic glutamate receptors
- WC
whole-cell
Author contributions
L-R.S. designed and conducted the experiments, analysed and interpreted data, and drafted and revised the manuscript. F.E.D. contributed to the conception and design of the study and revised the manuscript. Both authors approved the final version of manuscript.
Author's present address
L-R. Shao and F. Edward Dudek: Department of Physiology, University of Utah School of Medicine, Salt Lake City, UT 84108, USA.
References
- Belleau ML, Warren RA. Postnatal development of electrophysiological properties of nucleus accumbens neurons. J Neurophysiol. 2000;84:2204–2216. doi: 10.1152/jn.2000.84.5.2204. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y, Cherubini E, Krnjevic K. Changes in voltage dependence of NMDA currents during development. Neurosci Lett. 1988;94:88–92. doi: 10.1016/0304-3940(88)90275-3. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y, Khazipov R, Leinekugel X, Caillard O, Gaiarsa JL. GABAA, NMDA and AMPA receptors: a developmentally regulated ‘menage a trois’. Trends Neurosci. 1997;20:523–529. doi: 10.1016/s0166-2236(97)01147-8. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y. Excitatory actions of GABA during development: the nature of the nurture. Nat Rev Neurosci. 2002;3:728–739. doi: 10.1038/nrn920. [DOI] [PubMed] [Google Scholar]
- Brumback AC, Staley KJ. Thermodynamic regulation of NKCC1-mediated Cl– cotransport underlies plasticity of GABAA signalling in neonatal neurons. J Neurosci. 2008;28:1301–1312. doi: 10.1523/JNEUROSCI.3378-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Prida LM, Huberfeld G, Cohen I, Miles R. Threshold behaviour in the initiation of hippocampal population bursts. Neuron. 2006;49:131–142. doi: 10.1016/j.neuron.2005.10.034. [DOI] [PubMed] [Google Scholar]
- Dzhala VI, Staley KJ. Excitatory actions of endogenously released GABA contribute to initiation of ictal epileptiform activity in the developing hippocampus. J Neurosci. 2003;23:1840–1846. doi: 10.1523/JNEUROSCI.23-05-01840.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galoyan SM, Merlin LR. Long-lasting potentiation of epileptiform bursts by group I mGluRs is NMDA receptor independent. J Neurophysiol. 2000;83:2463–2467. doi: 10.1152/jn.2000.83.4.2463. [DOI] [PubMed] [Google Scholar]
- Gomez-Di Cesare CM, Smith KL, Rice FL, Swann JW. Axonal remodelling during postnatal maturation of CA3 hippocampal pyramidal neurons. J Comp Neurol. 1997;384:165–180. [PubMed] [Google Scholar]
- Hestrin S. Developmental regulation of NMDA receptor-mediated synaptic currents at a central synapse. Nature. 1992;357:686–689. doi: 10.1038/357686a0. [DOI] [PubMed] [Google Scholar]
- Jones J, Stubblefield EA, Benke TA, Staley KJ. Desynchronization of glutamate release prolongs synchronous CA3 network activity. J Neurophysiol. 2007;97:3812–3818. doi: 10.1152/jn.01310.2006. [DOI] [PubMed] [Google Scholar]
- Karnup S, Stelzer A. Seizure-like activity in the disinhibited CA1 minislice of adult guinea-pigs. J Physiol. 2001;532:713–730. doi: 10.1111/j.1469-7793.2001.0713e.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecuona E, Luquin S, Avila J, Garcia-Segura LM, Martin-Vasallo P. Expression of the β1 and β2 (AMOG) subunits of the Na,K-ATPase in neural tissues: cellular and developmental distribution patterns. Brain Res Bull. 1996;40:167–174. doi: 10.1016/0361-9230(96)00042-1. [DOI] [PubMed] [Google Scholar]
- MacVicar BA, Dudek FE. Local synaptic circuits in rat hippocampus: interactions between pyramidal cells. Brain Res. 1980;184:220–223. doi: 10.1016/0006-8993(80)90602-2. [DOI] [PubMed] [Google Scholar]
- Merlin LR, Wong RK. Role of group I metabotropic glutamate receptors in the patterning of epileptiform activities in vitro. J Neurophysiol. 1997;78:539–544. doi: 10.1152/jn.1997.78.1.539. [DOI] [PubMed] [Google Scholar]
- Miles R, Wong RK. Excitatory synaptic interactions between CA3 neurones in the guinea-pig hippocampus. J Physiol. 1986;373:397–418. doi: 10.1113/jphysiol.1986.sp016055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles R, Wong RK. Inhibitory control of local excitatory circuits in the guinea-pig hippocampus. J Physiol. 1987;388:611–629. doi: 10.1113/jphysiol.1987.sp016634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moroni F, Lombardi G, Thomsen C, Leonardi P, Attucci S, Peruginelli F, Torregrossa SA, Pellegrini-Giampietro DE, Luneia R, Pellicciari R. Pharmacological characterization of 1-aminoindan-1,5-dicarboxylic acid, a potent mGluR1 antagonist. J Pharmacol Exp Ther. 1997;281:721–729. [PubMed] [Google Scholar]
- Ong J, Marino V, Parker DA, Kerr DI, Blythin DJ. The morpholino-acetic acid analogue Sch 50911 is a selective GABAB receptor antagonist in rat neocortical slices. Eur J Pharmacol. 1998;362:35–41. doi: 10.1016/s0014-2999(98)00723-7. [DOI] [PubMed] [Google Scholar]
- Qiu C, Johnson BN, Tallent MK. K+ M-current regulates the transition to seizures in immature and adult hippocampus. Epilepsia. 2007;48:2047–2058. doi: 10.1111/j.1528-1167.2007.01193.x. [DOI] [PubMed] [Google Scholar]
- Qiu C, Zeyda T, Johnson B, Hochgeschwender U, de Lecea L, Tallent MK. Somatostatin receptor subtype 4 couples to the M-current to regulate seizures. J Neurosci. 2008;28:3567–3576. doi: 10.1523/JNEUROSCI.4679-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartzkroin PA, Prince DA. Penicillin-induced epileptiform activity in the hippocampal in vitro preparation. Ann Neurol. 1977;1:463–469. doi: 10.1002/ana.410010510. [DOI] [PubMed] [Google Scholar]
- Snead OC., III Basic mechanisms of generalized absence seizures. Ann Neurol. 1995;37:146–157. doi: 10.1002/ana.410370204. [DOI] [PubMed] [Google Scholar]
- Staley KJ, Longacher M, Bains JS, Yee A. Presynaptic modulation of CA3 network activity. Nat Neurosci. 1998;1:201–209. doi: 10.1038/651. [DOI] [PubMed] [Google Scholar]
- Staley KJ, Proctor WR. Modulation of mammalian dendritic GABAA receptor function by the kinetics of Cl– and. J Physiol. 1999;519:693–712. doi: 10.1111/j.1469-7793.1999.0693n.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocker M, Krause M, Pedarzani P. An apamin-sensitive Ca2+-activated K+ current in hippocampal pyramidal neurons. Proc Natl Acad Sci U S A. 1999;96:4662–4667. doi: 10.1073/pnas.96.8.4662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swann JW, Brady RJ. Penicillin-induced epileptogenesis in immature rat CA3 hippocampal pyramidal cells. Brain Res. 1984;314:243–254. doi: 10.1016/0165-3806(84)90046-4. [DOI] [PubMed] [Google Scholar]
- Swann JW, Smith KL, Brady RJ. Age-dependent alterations in the operations of hippocampal neural networks. Ann N Y Acad Sci. 1991;627:264–276. doi: 10.1111/j.1749-6632.1991.tb25930.x. [DOI] [PubMed] [Google Scholar]
- Swann JW, Smith KL, Brady RJ. Localized excitatory synaptic interactions mediate the sustained depolarization of electrographic seizures in developing hippocampus. J Neurosci. 1993;13:4680–4689. doi: 10.1523/JNEUROSCI.13-11-04680.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub RD, Wong RK. Cellular mechanism of neuronal synchronization in epilepsy. Science. 1982;216:745–747. doi: 10.1126/science.7079735. [DOI] [PubMed] [Google Scholar]
- Traub RD, Wong RK. Synchronized burst discharge in disinhibited hippocampal slice. II. Model of cellular mechanism. J Neurophysiol. 1983a;49:459–471. doi: 10.1152/jn.1983.49.2.459. [DOI] [PubMed] [Google Scholar]
- Traub RD, Wong RK. Synaptic mechanisms underlying interictal spike initiation in a hippocampal network. Neurology. 1983b;33:257–266. doi: 10.1212/wnl.33.3.257. [DOI] [PubMed] [Google Scholar]
- Traub RD, Knowles WD, Miles R, Wong RK. Synchronized afterdischarges in the hippocampus: simulation studies of the cellular mechanism. Neuroscience. 1984;12:1191–1200. doi: 10.1016/0306-4522(84)90013-7. [DOI] [PubMed] [Google Scholar]
- Tremblay E, Roisin MP, Represa A, Charriaut-Marlangue C, Ben-Ari Y. Transient increased density of NMDA binding sites in the developing rat hippocampus. Brain Res. 1988;461:393–396. doi: 10.1016/0006-8993(88)90275-2. [DOI] [PubMed] [Google Scholar]
- Turgeon SM, Albin RL. Postnatal ontogeny of GABAB binding in rat brain. Neuroscience. 1994;62:601–613. doi: 10.1016/0306-4522(94)90392-1. [DOI] [PubMed] [Google Scholar]
- Veliskova J, Garant DS, Xu SG, Moshe SL. Further evidence of involvement of substantia nigra GABAB receptors in seizure suppression in developing rats. Brain Res Dev Brain Res. 1994;79:297–300. doi: 10.1016/0165-3806(94)90135-x. [DOI] [PubMed] [Google Scholar]
- Viana F, Bayliss DA, Berger AJ. Repetitive firing properties of developing rat brainstem motoneurones. J Physiol. 1995;486:745–761. doi: 10.1113/jphysiol.1995.sp020850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong RK, Traub RD. Synchronized burst discharge in disinhibited hippocampal slice. I. Initiation in CA2–CA3 region. J Neurophysiol. 1983;49:442–458. doi: 10.1152/jn.1983.49.2.442. [DOI] [PubMed] [Google Scholar]