Abstract
Collagenous gastritis, a counterpart of collagenous colitis, is an extremely rare disorder. The first case of collagenous gastritis in a Korean boy in his pre-teens who had been receiving treatment for refractory iron deficiency anemia has been reported. The patient had been suffering from intermittent abdominal pain, recurrent blood-tinged vomiting and poor oral intake. The gastric endoscopy revealed diffuse cobble-stone appearance of the mucosa with easy touch bleeding throughout the stomach but no abnormalities in the esophagus, duodenum, and colon. Pathologic examination of the gastric biopsies from the antrum, body and cardia showed a subepithelial collagen deposition with entrapped dilated capillaries, moderate infiltrates of lymphoplasma cells and eosinophils of the lamina propria, and marked hypertrophy of the muscularis mucosa. The collagen deposition appeared as discontinuous bands with focally irregular extension into the deeper part of the antral mucosa. It measured up to 150 µm. Helicobacter pylori infection was not detected. The biopsies from the duodenum, esophagus and colon revealed no pathologic abnormalities.
Keywords: Gastritis; Gastritis, Collagenous; Child; Endoscopy
INTRODUCTION
Collagenous gastritis and collagenous colitis are rare entities, counterparts of each other, characterized by the deposition of a subepithelial collagen band with an inflammatory infiltrate in the gastrointestinal mucosa (1). Collagenous gastritis is particularly rare, and less than 20 cases of collagenous gastritis have been reported in the literature since the first description of collagenous gastritis in 1989 by Colletti and Trainer (2). Patients with collagenous gastritis suffer from gastric pain or other accompanying symptoms such as anemia caused by gastric bleeding and watery diarrhea when colitis is present. Some authors suggest that there seems to be two distinctive clinicopathologic subtypes of collagenous gastritis: 1) collagenous gastritis occurring in children or young adults presenting with severe anemia, a nodular pattern on endoscopy, and a disease limited to the gastric mucosa without evidence of colonic involvement, and 2) collagenous gastritis associated with collagenous colitis occurring in adult patient presenting with chronic watery diarrhea (1, 3).
This report describe the first case of collagenous gastritis occurring in a Korean boy who presented with anemia caused by gastric bleeding.
CASE REPORT
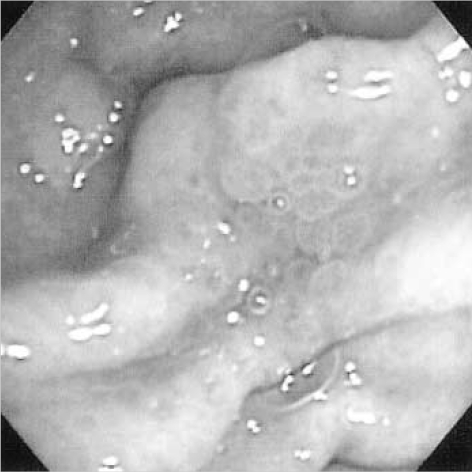
An 11-yr-old Korean boy presented with a three-year history of anemia and intermittent abdominal pain without diarrhea. The anemia (hemoglobin: 6.8 g/dL) was first detected at the age of 8 yr, when he visited a local clinic due to dizziness and pale appearance. The anemia did not improve with iron replacement, suggesting refractory iron deficiency anemia. At 9 yr of age, his hemoglobin level had increased up to 11.7 g/dL with iron replacement therapy. At that time, he underwent endoscopy that showed nodular gastritis, hemorrhagic gastritis or angiodysplasia. A screening test for Helicobacter pylori using Campylobacter-like organism (CLO) test was negative. During his follow-up, he developed intermittent blood-tinged vomiting and epigastric soreness. The vomiting occurred 1 to 4 times a day. He visited Samsung Medical Center for further evaluation and gastrointestinal endoscopic examination. Gastric endoscopy revealed a diffuse nodular pattern in the mucosa with easy touch bleeding throughout the stomach (Fig. 1). There were no remarkable changes in the esophagus, duodenum and colonic mucosa. Physical examination was unremarkable. Laboratory findings including serologic test for Helicobacter pyroli were within normal limits except mild anemia (10.6 g/dL). Endoscopic biopsy from the stomach was performed under the impression of hemorrhagic gastritis or angiodysplasia.
Fig. 1.
Endoscopy of the stomach shows polypoid mucosal nodules around the pyloric ring.
Pathologic findings
Biopsy specimens were obtained from the fundus, body, and antrum of the stomach. Sections of formalin-fixed and paraffin-embedded specimens were stained with H-E, Masson-trichrome, and Congo-red. The collagen band was measured on well-oriented Masson-trichrome sections using an ocular micrometer (4). Optimally oriented tissue from every gastrointestinal endoscopy was measured in at least five separate areas representing the thickest visually determined zones of subepithelial collagen (4). Five points were measured on every fragment of the biopsy specimens and the average size was calculated.
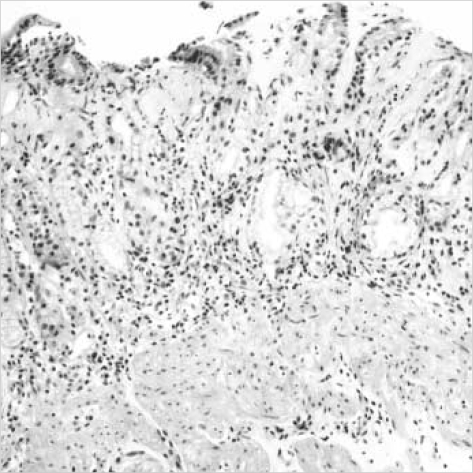
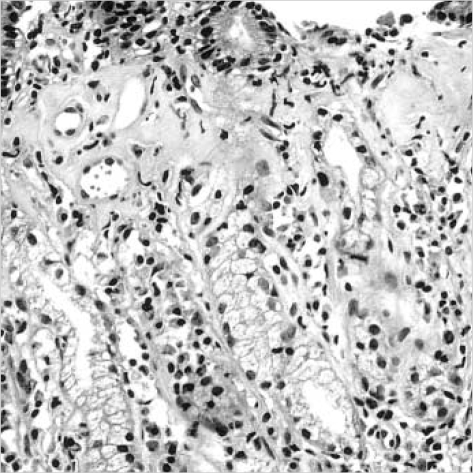
Pathologic examination revealed similar histopathologic changes in the all the gastric specimens. There was a subepithelial deposition of thick, homogenous eosinophilic collagen in the gastric mucosa. This subepithelial collagen was strongly stained with Masson-trichrome but negative for amyloid with Congo red. The collagenous deposition appeared as a band-like pattern, which involved the most subepithelial areas of the gastric mucosa. But it was often discontinuous and irregularly extended deeper into proper glands, especially in the antral mucosa (Fig. 2). The thickness of collagenous band measured up to 150 µm in the antrum of the stomach but averaged 30-50 µm in the fundus and the body. The collagen bands contained entrapped dilated capillaries and mild inflammatory infiltrates including lymphoplasma cells and eosinophils (Fig. 3). There were mild to moderate atrophy of the foveolar glands and focal surface epithelial damages in all biopsies. The surface epithelial changes included partial detachment, nuclear stratification, and rare mitosis. There was moderate inflammatory infiltrate of the lamina propria without collagen deposits, composed of lymphocytes, plasma cells and many eosinophils. The muscularis mucosa of all specimens showed marked hypertrophy with frequent smooth muscle up-growth into the lamina propria. Intraepithelial lymphocytic infiltration was rarely seen. Neither Helicobacter pylori nor endocrine cell hyperplasia were found. This histologic finding of the stomach was consistent with collagenous gastritis. The mucosal biopsies from the duodenum, esophagus and colon revealed no remarkable change. On ultrastructural examination of gastric biopsy, subepithelial collagen bands revealed haphazardly arranged collagen fibrils, and there was focal thickening of basement membrane of the proper glands.
Fig. 2.
Endoscopic biopsy from the antral mucosa shows subepithelial collagenous deposition, slough of surface epithelium, inflammatory cell infiltration, and hypertrophy of muscularis mucosa (H&E, ×100).
Fig. 3.
High power magnification of Fig. 2. The subepithelial collagenous bands contains entrapped dilated capillaries and inflammatory cells (H&E, ×200).
DISCUSSION
Collagenous gastritis is defined histologically by the presence of a thickened subepithelial collagen band greater than 10 µm thick in association with entrapping dilated capillaries and inflammatory cell infiltration of the lamina propria. Collagenous gastritis was first described in 1989 by Colletti and Trainer (2), since then, less than 20 cases have been reported to date. A summary of these cases seems to delineate two major distinct clinicopathologic patterns in patients with collagenous gastritis. The first subset is observed in children or young adults with no evidence of extragastric involvement (2, 5), the second subset is associated with collagenous colitis in adult patient (6-8). The first subset is different from adult cases by the severity of the presentation with severe anemia probably due to gastrointestinal bleeding. The gastrointestinal bleeding may be caused by entrapped dilated capillaries in the collagenous bands together with superficial epithelial damage.
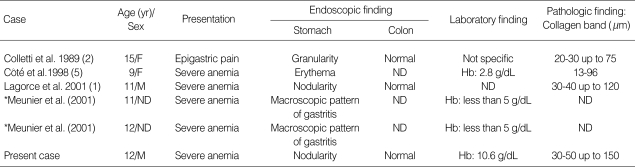
The clinicopathologic and endoscopic features of our patient are similar to the pediatric cases of collagenous gastritis reported by Lagorce-Pages et al. (1) and Côté et al. (5). There have been six pediatric cases of collagenous gastritis including this case in the literature (Table 1). The average age at detection was 11.7 yr old. The patients presented with severe anemia or epigastric pain. The gastric endoscopy revealed nodular pattern of the mucosa in three children, erythema in a child and macroscopic pattern of gastritis in two children. The lesions were limited to the gastric mucosa without evidence of colonic involvement. On the other hand, adult patients of collagenous gastritis showed a variety of clinical and endoscopic features and simultaneous occurrence of collagenous colitis, collagenous duodenitis, lymphocytic colitis, ulcerative colitis, and celiac disease (1-4, 6-10). There have been 13 adult cases of collagenous gastritis. The incidence of adult cases was more frequent in women than in men and the average age at detection was 46 yr old (1-4, 6-11). Their main clinical symptoms were chronic watery diarrhea, although there were exceptional cases that showed symptoms of the pediatric patients such as anemia, epigastric pain or abdominal distension. There were three cases of collagenous gastritis occurring in young adults aged 20 and 22 yr. The presenting symptoms were epigastric pain in 2 patients and severe anemia in one. Of these three patients, a 20 yr old woman had no pathologic findings in the colonic biopsy and no association with atopic dermatitis and bronchial asthma (11), and a 20-yr-old man showed initial presentations of features with collagenous gastritis and was subsequently found to have collagenous colitis (7). The remaining patient showed severe anemia at presentation but colonoscopic finding was not available. In young adult cases of collagenous gastritis except a case associated with collagenous colitis, the clinical manifestations were similar to those of pediatric patients.
Table 1.
Clinicopathologic features of 6 previously reported pediatric patients with collagenous gastritis (ages less than 18 yr)
ND, not described; Hb, hemoglobin. *This article was written in French and abstract was only available for review.
The cause and pathogenesis of collagenous gastritis remain unknown. Three major pathogenic hypothesis have been proposed for increased deposition of subepithelial collagen in collagenous colitis: 1) chronic inflammation (12-16) and autoimmunity (17), 2) abnormality of the pericryptal fibroblast sheath (18-21), and 3) leakage of plasma proteins and fibrinogen with subsequent replacement with collagen. The most popular theory is that the collagen deposition represents a reparative process secondary to initial injuries caused by drug, toxic or infectious agents. This hypothesis is supported by morphologic similarity of collagenous gastritis to collagenous colitis and demonstration of types I and II collagen, a repair-type collagen in collagen deposition of collagenous colitis. However, collagen deposition of collagenous gastritis could not be explained only by reparative process because other gastropathies including H. pylori or nonspecific gastritis did not show a thick band-like collagen deposition seen in collagenous gastritis. The mechanism of collagen deposition may be complex. An immune mediated process has been suggested as a mechanism for collagen deposition of collagenous gastritis because of frequent association with immune related disorders such as collagenous colitis and lymphocytic colitis, and constant signs of immune activation in gastric biopsy from the patients with collagenous gastritis. The signs of immune activation include overexpression of HLA-DR by epithelial cells and CD25 positive cells in the lamina propria. The collagen deposition may have resulted from activated immune cells producing cytokines and growth factors and thus stimulating the production or reducing the turnover of extacellular matrix. The gastric mucosa of our patient showed prominent eosinophilic infiltrate of lamina propria that may play a role in immune activation. Our patient had no history of drug or specific infection.
In summary, we described typical clinicopathologic findings of pediatric collagenous gastritis. This is the first pathologically proven case of collagenous gastritis in Korea and the sixth pediatric case in the literature. This patient is being followed up at regular intervals with oral antacid and iron supplementation and needs to be checked up for the development of lower gastrointestinal symptoms and association with immune mediated disorders. More cases will shed light on the pathogenesis of this rare disease entity.
References
- 1.Lagorce-Pages C, Fabiani B, Bouvier R, Scoazec JY, Durand L, Flejou JF. Collagenous gastritis: a report of six cases. Am J Surg Pathol. 2001;25:1174–1179. doi: 10.1097/00000478-200109000-00008. [DOI] [PubMed] [Google Scholar]
- 2.Colletti RB, Trainer TD. Collagenous gastritis. Gastroenterology. 1989;97:1552–1555. doi: 10.1016/0016-5085(89)90403-4. [DOI] [PubMed] [Google Scholar]
- 3.Stancu M, De Petris G, Palumbo TP, Lev R. Collagenous gastritis associated with lymphocytic gastritis and celiac disease. Arch Pathol Lab Med. 2001;125:1579–1584. doi: 10.5858/2001-125-1579-CGAWLG. [DOI] [PubMed] [Google Scholar]
- 4.Vesoulis Z, Lozanski G, Ravichandran P, Esber E. Collagenous gastritis: a case report, morphologic evaluation, and review. Mod Pathol. 2000;13:591–596. doi: 10.1038/modpathol.3880101. [DOI] [PubMed] [Google Scholar]
- 5.Côté JF, Hankard GF, Fauré C, Mougenot JF, Holvoet L, Cezard JP, Navarro J, Peuchmaur M. Collagenous gastritis revealed by severe anemia in a child. Hum Pathol. 1998;29:883–886. doi: 10.1016/s0046-8177(98)90461-0. [DOI] [PubMed] [Google Scholar]
- 6.Castellano VM, Munoz MT, Colina F, Nevado M, Casis B, Solis-Herruzo JA. Collagenous gastrobulbitis and collagenous colitis: case report and review of the literature. Scand J Gastroenterol. 1999;34:632–638. doi: 10.1080/003655299750026128. [DOI] [PubMed] [Google Scholar]
- 7.Pulimood AB, Ramakrishna BS, Mathan MM. Collagenous gastritis and collagenous colitis: a report with sequential histological and ultrastructural findings. Gut. 1999;44:881–885. doi: 10.1136/gut.44.6.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stolte M, Ritter M, Borchard F, Koch-Scherrer G. Collagenous gastroduodenitis on collagenous colitis. Endoscopy. 1990;22:186–187. doi: 10.1055/s-2007-1012837. [DOI] [PubMed] [Google Scholar]
- 9.Groisman GM, Meyers S, Harpaz N. Collagenous gastritis associated with lymphocytic colitis. J Clin Gastroenterol. 1996;22:134–137. doi: 10.1097/00004836-199603000-00013. [DOI] [PubMed] [Google Scholar]
- 10.Borchard F, Niederau C. Collagenous gastroduodenitis. Dtsch Med Wschr. 1989;114:1345. [PubMed] [Google Scholar]
- 11.Kajino Y, Kushima R, Koyama S, Fujiyama Y, Okabe H. Collagenous gastritis in a young Japanese woman. Pathol Int. 2003;53:174–178. doi: 10.1046/j.1440-1827.2003.01451.x. [DOI] [PubMed] [Google Scholar]
- 12.Jawhary AJ, Talbot IC. Microscopic lymphocytic and collagenous colitis. Histopathology. 1996;29:101–110. doi: 10.1046/j.1365-2559.1996.d01-498.x. [DOI] [PubMed] [Google Scholar]
- 13.Teglbjaerg PS, Thaysen EH, Jansen HH. Development of collagenous colitis in sequential biopsy specimens. Gastroenterology. 1984;87:703–709. [PubMed] [Google Scholar]
- 14.Jessurun J, Yardley JH, Lee EL, Vendrell DD, Schiller LR, Fordtran JS. Different names for the same condition? Gastroenterology. 1986;91:1583–1584. doi: 10.1016/0016-5085(86)90240-4. [DOI] [PubMed] [Google Scholar]
- 15.Rams H, Rogers AI, Ghandur-Mnaymneh L. Collagenous colitis. Ann Intern Med. 1987;106:108–113. doi: 10.7326/0003-4819-106-1-108. [DOI] [PubMed] [Google Scholar]
- 16.Lawson JM, Wolosin J, Mottet MD, Brower RA. Collagenous colitis: an association with fecal leukocytes. J Clin Gastroenterol. 1988;10:672–675. [PubMed] [Google Scholar]
- 17.Jawhari A, Talbot IC. Microscopic, lymphocytic and collagenous colitis. Histopathology. 1996;29:101–110. doi: 10.1046/j.1365-2559.1996.d01-498.x. [DOI] [PubMed] [Google Scholar]
- 18.Widgren S, Jlidi R, Cox JN. Collagenous colitis: histologic, morphometric immunohistochemical and ultrastructural studies. Report of 21 cases. Virchow Arch A Pathol Anat Histopathol. 1988;413:287–296. doi: 10.1007/BF00783020. [DOI] [PubMed] [Google Scholar]
- 19.Kingham JG, Levison DA, Morson BC, Dawson AM. Collagenous colitis. Gut. 1986;27:570–577. doi: 10.1136/gut.27.5.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Colina F, Solis-Herruzo JA, Munoz-Yague MT, Vazquez G, Perez-Barrios A. Collagenous colitis: the clinical and morphological features. Postgrad Med J. 1982;58:390–395. doi: 10.1136/pgmj.58.680.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gledhill A, Cole FM. Significance of basement membrane thickening in the human colon. Gut. 1984;25:1085–1088. doi: 10.1136/gut.25.10.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]