Abstract
We treated a 49-yr-old man with neostigmine, who had liver cirrhosis, acute hepatic encephalopathy, and acute intestinal pseudo-obstruction. He was admitted in a state of hepatic confusion. On physical examination, the abdomen was distended; and bowel sound was absent. Plain abdomen film revealed multiple air-fluid levels and distention of bowel loops. Initially, we gave him lactulose enemas every 6 hr for one day without improvement in his mental state. Furthermore, he became to a state of coma. Therefore, we gave him 0.5 mg of neostigmine subcutaneously to improve his peristaltic movement, and 2 L of polyethylene glycol electrolyte solution through a nasogastric tube for 4 hr to reduce the production and absorption of gut-derived toxins of nitrogenous compounds. After these treatments, the venous ammonia level decreased to the normal range within 12 hr, and the coma disappeared after 2 days. We suggest that neostigmine may be one of the most effective treatments to initiate peristaltic movement and bowel cleansing in cirrhotic patients with acute hepatic encephalopathy and acute intestinal pseudo-obstruction.
Keywords: Hepatic Encephalopathy, Liver Cirrhosis, Intestinal Pseudo-Obstruction, Neostigmine
INTRODUCTION
Hepatic encephalopathy may be defined as a disturbance in central nervous system function because of a hepatic insufficiency. This broad definition reflects the existence of a spectrum of neuropsychiatric manifestations related to a range of pathophysiological mechanisms. Present in both acute and chronic liver failure, these neuropsychiatric manifestations are potentially reversible (1). However, patients who are irritable or confused are more difficult to care for and require greater medical supervision, and patients in deeper stages of coma are more likely to develop severe complications, such as aspiration pneumonia (2).
Acute intestinal pseudo-obstruction is one of the most fatal conditions in cirrhotic patients (3). Especially, acute hepatic encephalopathy with acute intestinal pseudo-obstruction may be a catastrophic condition in cirrhotic patients. Gut-derived toxins of nitrogenous origin are thought to play a role in the genesis of acute hepatic encephalopathy. Thus, treatment is aimed at reducing the production and absorption of these compounds. Lactulose (orally or as an enema) is one of the cornerstones of the treatment of hepatic encephalopathy (4). And, bowel cleansing is a mainstay of therapy (1). However, bowel cleansing and lactulose enemas may be useless in patients with functional or mechanical bowel obstruction. Furthermore, oral therapy can be harmful because of a risk of aspiration.
We treated a 49-yr-old man with neostigmine, who had liver cirrhosis, acute hepatic encephalopathy, and acute intestinal pseudo-obstruction. Initially, we treated him with lactulose enemas. But, there was no improvement in his mental state. Therefore, with the consent of his family, we gave him 0.5 mg of neostigmine to improve his peristaltic movement.
CASE REPORT
A 49-yr-old man presented to the emergency room with mental confusion and vomiting in December 2003. Two days earlier he had abrupt onset of abdominal pain, vomiting, and constipation. Since then, he had exhibited slurred speech. A search of his home found no prescription medication. He had liver cirrhosis with hepatitis B. He had two episodes of hepatic encephalopathy since 2002, and had been on oral lactulose therapy.
His blood pressure was 130/90 mmHg, and pulse rate was 64 beats/min. On physical examination, he was neurologically in a disoriented and drowsy mental state. His abdomen was distended, and bowel sound was absent. Ascites were noted. Laboratory investigations revealed a white blood cell count of 3,000/µL; hemoglobin 8.1 g/dL; platelet count 22,000/µL; serum aspartate transaminase 65 U/L; alanine transaminase 32 U/L; albumin 1.9 g/dL; total bilirubin 3.5 mg/dL; ammonia 185 µg/dL; blood urea nitrogen 22.3 mg/dL; creatinine 1.2 mg/dL; sodium 143 mEq/L; potassium 4.5 mEq/L; glucose 110 mg/dL; prothrombin time 25.1 sec (2.2 in international normalized ratio); partial thromboplastin time 73.2 sec (vs. a control of 35 sec); alpha-fetoprotein 3.14 ng/mL.
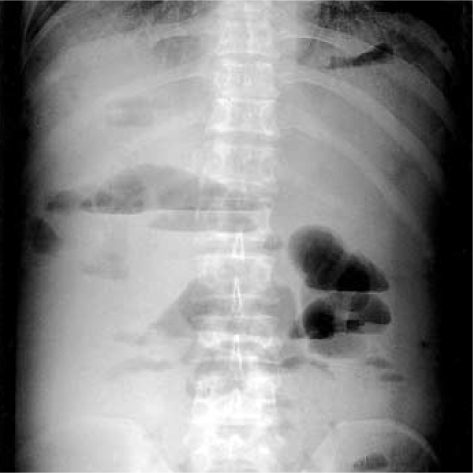
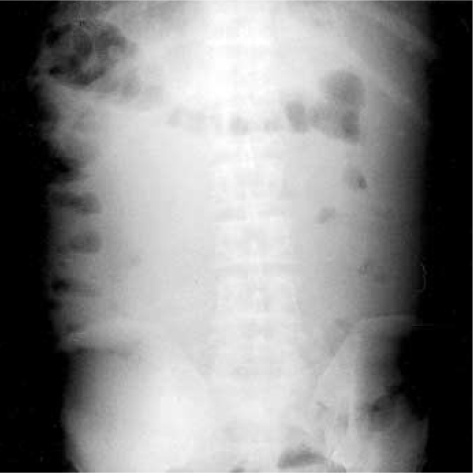
Plain abdomen (upright film) revealed multiple air-fluid levels, distention of bowel loops, and presence of bowel gas in the left lower quadrant of the abdomen (Fig. 1). Initially, we gave him lactulose enemas every 6 hr for one day. Oral lactulose was not used because of frequent vomiting and ileus. However, there was no improvement in his mental state. Furthermore, he became to a state of coma, and there was an elevation of his venous ammonia level (298 µg/dL). Computed tomography of the head revealed no evidence of intracranial hemorrhage or cerebrovascular injury. Therefore, with the consent of his family, we gave him 0.5 mg of neostigmine subcutaneously to improve his peristaltic movement. Three minutes after this treatment, there was a prompt evacuation of stool with a reduction in abdominal distention and an increase in bowel sound on physical examination. Then we gave him 2 L of polyethylene glycol electrolyte solution through a nasogastric tube for 4 hr. After these treatments, his venous ammonia level decreased to the normal range (19.0-60.0 µg/dL) within 12 hr, and the coma disappeared after 2 days. Plain abdomen (upright film) also revealed no air-fluid level (Fig. 2). Then, oral lactulose therapy was restarted, and his condition remained stable. After 5 days, he was discharged from the hospital.
Fig. 1.
Plain abdomen (upright film) reveals multiple air-fluid levels, distention of bowel loops, and presence of bowel gas in the left lower quadrant of the abdomen.
Fig. 2.
Two days after the use of neostigmine and polyethylene glycol electrolyte solution, plain abdomen (upright film) shows only colonic gas without an air-fluid level.
DISCUSSION
We present the first case of acute hepatic encephalopathy with acute intestinal pseudo-obstruction in a cirrhotic patient who was successfully treated with neostigmine. Kiba et al. (2) reported the first successful treatment with neostigmine and polyethylene glycol electrolyte solution in a cirrhotic patient with acute hepatic encephalopathy. But, the patient did not demonstrate the signs of acute intestinal pseudo-obstruction.
Nitrogenous substances derived from the gut are thought to play a role in the pathogenesis of hepatic encephalopathy. These compounds gain access to the systemic circulation as a result of decreased hepatic function or portal systemic shunts. Once in brain tissue, they produce alterations of neurotransmission that affect consciousness and behavior (1). Most manifestations of hepatic encephalopathy are reversible with medical treatment. However, some patients have progressive debilitating syndromes, such as dementia, spastic paraparesis, cerebellar degeneration, and extrapyramidal movement disorders, associated with structural abnormalities of the central nervous system (2). In addition, it is well known that mortality is higher with an acute encephalopathy that is classified as Child's C and grade III or IV hepatic encephalopathy (5). Therefore, a treatment regimen that produces a more rapid response is likely to be of greater benefit to cirrhotic patients, especially in Child's C (4).
Ileus refers to the partial or complete blockage of the small and/or large intestine either because the regular rhythmic squeezing motion is impaired or because of mechanical obstruction. Both types, functional or mechanical bowel obstruction, are associated with increased luminal pressure that might cause gut wall ischemia and may lead to increase in intra-abdominal pressure. Intra-abdominal hypertension has been found in up to 20% of critically ill patients and may lead to a broad pattern of systemic consequences with multiple organ dysfunctions, including cardiovascular, hepatic, pulmonary, renal and neurological function (6). The most serious complication is the abdominal compartment syndrome, a life-threatening condition presenting as an indication for decompressive laparotomy. Cirrhosis patients with ascites often have intestinal pseudo-obstruction (3). Consistent with this, it was reported that acute intestinal pseudo-obstruction developed after surgery or severe illness (7-9). However, there was no reported case of acute intestinal pseudo-obstruction with acute hepatic encephalopathy in cirrhotic patients according to review of English literature. We suspect that there was little possibility of survival in cirrhotic patients with acute hepatic encephalopathy and acute intestinal pseudo-obstruction, because this combined illness may be a catastrophic condition in cirrhotic patients.
Although the pathophysiology of acute intestinal pseudo-obstruction is not fully understood, it is thought to result from an imbalance in the regulation of colonic motor activity by the autonomic nervous system. Multiple pharmacologic and metabolic factors can alter the autonomic regulation of colonic function, leading to excessive parasympathetic suppression, sympathetic stimulation, or both. This imbalance results in colonic atony or pseudo-obstruction. Therefore, neostigmine has been used as a simple, safe, and effective therapy for treatment of intestinal pseudo-obstruction (7-9), because this drug enhances excitatory parasympathetic activity by competing with acetylcholine for attachment to acetylcholinesterase at sites of cholinergic transmission (10). In the present case, there was no bowel sound before the administration of neostigmine. However, after the administration of neostigmine, there was a prompt evacuation of stool with an increase in bowel sound. Therefore, we thought that neostigmine was a salvage treatment to initiate the peristaltic movement and bowel cleansing so that dietary and endogenous nitrogenous substances could be evacuated from the intestine.
The elimination half-life of neostigmine after intravenous infusion averages 80 min. Neostigmine is hydrolyzed by plasma cholinesterase and is metabolized by microsomal liver enzymes. Renal excretion accounts for the clearance of 50 percent of the drug, and the serum half-life is prolonged in patients with renal dysfunction. Therefore, patients with renal impairment may have an increased or prolonged vagomimetic response after the administration of neostigmine (8). Contraindications for neostigmine are serum creatinine concentration >3 mg/dL, heart rate <60 beats/min, systolic blood pressure <90 mmHg, active bronchospasm, and signs of bowel perforation (11). In the present case, we used 0.5 mg of neostigmine because we thought that patients with hepatic dysfunction might have an increased or prolonged vagomimetic response after the administration of neostigmine. Actually, there was a prompt bowel response after the administration of 0.5 mg of neostigmine. Therefore, a second administration of neostigmine was not used.
In the present case, we used the polyethylene glycol electrolyte solution as a whole gut irrigation solution because of several reasons. First, we initially used the lactulose enema, but there was no improvement in his mental state. Therefore, we could not use it again. Second, there was a necessity of rapid bowel cleansing, because one day was already passed over without any improvement of his mental state. Third, colonic cleansing was reported to be better with polyethylene glycol electrolyte lavage (12).
In conclusion, we suggest that neostigmine may be one of the most effective treatments to initiate peristaltic movement and bowel cleansing in cirrhotic patients with acute hepatic encephalopathy and acute intestinal pseudo-obstruction.
References
- 1.Blei AT, Cordoba J Practice Parameters Committee of the American College of Gastroenterology. Hepatic encephalopathy. Am J Gastroenterol. 2001;96:1968–1976. doi: 10.1111/j.1572-0241.2001.03964.x. [DOI] [PubMed] [Google Scholar]
- 2.Kiba T, Numata K, Saito S. Neostigmine and polyethylene glycol electrolyte solution for the therapy of acute hepatic encephalopathy with liver cirrhosis and ascites. Hepatogastroenterology. 2003;50:823–826. [PubMed] [Google Scholar]
- 3.Runyon BA. Fatal bacterial peritonitis secondary to nonobstructive colonic dilatation (Ogilvie's syndrome) in cirrhotic ascites. J Clin Gastroenterol. 1986;8:687–689. doi: 10.1097/00004836-198612000-00021. [DOI] [PubMed] [Google Scholar]
- 4.Morgan MY, Hawley KE. Lactitol vs. lactulose in the treatment of acute hepatic encephalopathy in cirrhotic patients: a double-blind, randomized trial. Hepatology. 1987;7:1278–1284. doi: 10.1002/hep.1840070617. [DOI] [PubMed] [Google Scholar]
- 5.Strauss E, da Costa MF. The importance of bacterial infections as precipiting factors of chronic hepatic encephalopathy in cirrhosis. Hepatogastroenterology. 1998;45:900–904. [PubMed] [Google Scholar]
- 6.Malbrain ML. Abdominal pressure in the critically ill: measurement and clinical relevance. Intensive Care Med. 1999;25:1453–1458. doi: 10.1007/s001340051098. [DOI] [PubMed] [Google Scholar]
- 7.Paran H, Silverberg D, Mayo A, Shwartz I, Neufeld D, Freund U. Treatment of acute colonic pseudo-obstruction with neostigmine. J Am Coll Surg. 2000;190:315–318. doi: 10.1016/s1072-7515(99)00273-2. [DOI] [PubMed] [Google Scholar]
- 8.Amaro R, Rogers AI. Neostigmine infusion: New standard of care for acute colonic pseudo-obstruction? Am J Gastroenterol. 2000;95:304–305. doi: 10.1111/j.1572-0241.2000.01737.x. [DOI] [PubMed] [Google Scholar]
- 9.Ponec RJ, Saunders MD, Kimmey MB. Neostigmine for the treatment of acute colonic pseudo-obstruction. N Engl J Med. 1999;341:137–141. doi: 10.1056/NEJM199907153410301. [DOI] [PubMed] [Google Scholar]
- 10.Trevisani GT, Hyman NH, Church JM. Neostigmine: safe and effective treatment for acute colonic pseudo-obstruction. Dis Colon Rectum. 2000;43:599–603. doi: 10.1007/BF02235569. [DOI] [PubMed] [Google Scholar]
- 11.Delgado-Aros S, Camilleri M. Pseudo-obstruction in the critically ill. Best Pract Res Clin Gastroenterol. 2003;17:427–444. doi: 10.1016/s1521-6918(03)00023-4. [DOI] [PubMed] [Google Scholar]
- 12.Beck DE, Fazio VW, Jagelman DG. Comparison of oral lavage methods for preoperative colonic cleansing. Dis Colon Rectum. 1986;29:699–703. doi: 10.1007/BF02555311. [DOI] [PubMed] [Google Scholar]