Abstract
Pleomorphic carcinoma of the lung (PCL) is characterized by a mixture of sarcomatoid and carcinoma components, and a poor prognosis. However, no immunophenotype of tumor markers has been characterized in PCL. To charaterize the immunophenotype for CD99 in PCL, we performed an immunohistochemical evaluation of PCLs for thyroid transcription factor-1 (TTF-1), cytokeratin (CK) 7 and 20, and for CD99. CD99 was found to be expressed in both carcinomatous (47%) and sarcomatous components such as spindle cells (92%) and giant cells (57%). In the case of spindle cells, CK7 was expressed in 6 cases (46%) and TTF-1 in 2 cases (15%), whereas for giant cells CK7 was expressed in 8 cases (57%) and TTF-1 in one case (7%). However, CK20 was not expressed in either the carcinomatous or sarcomatous components in any case. Thus, CD99 was found to be widely expressed in both sarcomatous and carcinoma component in PCL. A clinicopathological analysis showed no direct correlation between the expression of CD99 and the clinical indices (stage, survival rate, invasion) of PCL.
Keywords: Pleomorphic Carcinoma; Lung Neoplasms; CD99 Protein, Human
INTRODUCTION
Pleomorphic carcinoma of the lung (PCL) is a rare tumor, which constitutes approximately 0.1-0.4% of all lung cancers, and has a poor clinical outcome (1-3). Histologically PCLs exhibit characteristic tumor components, i.e., malignant epithelial and homologous sarcomatoid spindle/giant cells. The recently updated WHO classification defined pleomorphic carcinoma as "a poorly differentiated non-small cell carcinoma, namely, as a squamous cell carcinoma, adenocarcinoma or large cell carcinoma, containing spindle cells and/or giant cells, or as a carcinoma consisting only of spindle cells and giant cells and added that the pleomorphic component should comprise at least 10% of the neoplasm" (4). The co-expression of cytokeratin and vimentin is helpful in the diagnosis of PCL (5-9). The immunoreactivity of epithelial and mesenchymal markers in PCL suggests that PCL is a malignant neoplasm of epithelial origin (10-13). However, a variety of morphologic features in PCL suggest the possibility that PCL might be a heterogeneous group of tumors rather than an epithelial cell-derived malignant tumor.
CD99 is a transmembrane protein encoded by the MIC-2 gene and is characteristically expressed in the Ewing family of tumors/PNETs, a group of small cell tumors of childhood and adolescence with a specific EWS/ETS gene rearrangement (14-16). In addition, its expression also has been reported in lymphoblastic lymphoma/leukemia and some epithelial tumors (17-19). Recently CD99 was found to be a critical molecule that contributes to the regulation of apoptosis and the cell cycle in malignant cells (20). Nevertheless, to the best of our knowledge no study of the expression pattern and biological role of CD99 has been undertaken in PCL. Thus, the aim of this study was to evaluate the expression of CD99/MIC-2 protein in a series of PCLs and to investigate whether this expression is related to morphological differentiation or prognostic implications.
MATERIALS AND METHODS
Patients and samples
Formalin-fixed paraffin-embedded blocks of 21 cases of pleomorphic carcinomas undergoing surgical resection (14 pneumonectomies, 7 lobectomies) between January 1, 1991 and July 30, 2002 were retrieved from the histopathology files at Seoul National University Hospital and at Samsung Medical Center, Seoul, Korea. Each tumor was reevaluated with regard to staging and histologic types of tumor component. Tumor staging was performed using the TNM classification system of the International Union Against Cancer. Follow-up data were obtained from medical records.
Immunohistochemistry
Tissue samples were processed using a heat-induced antigen retrieval procedure and immunostained using the conventional streptavidin-ABC technique. Tissues were treated with mouse monoclonal anti-CD99 antibody (clone YG32, DiNonA, Seoul, Korea) at a dilution of 1:250. Other antibodies used were; anti-cytokeratin 7 (clone OV-TL 12/30, Dako, Glostrup, Denmark; dilution 1:100), anti-EMA (clone E29, Dako, Glostrup, Denmark; dilution 1:100), anti-vimentin (clone V9, Dako, Glostrup, Denmark; dilution 1:50) and anti-TTF-1 (clone 8G7G3/1, Dako, Glostrup, Denmark; dilution 1:100). As a negative control, primary antibodies were replaced by irrelevant isotype-matched antibodies. Samples were determined as immunoreactive for CD99 if cell membrane staining or granular intracytoplasmic dotting was observed, for TTF-1 if nuclear staining was present, and for the other if cytoplasmic staining was observed. Tissues were deemed as negative if staining was either completely absent or observed in less than 10% of neoplastic cells.
Statistical analysis
Correlations between immunohistochemical profiles and the patients' clinical and pathological characteristics were analyzed using the chi-square test or Fisher's exact test (2-sided) using SPSS version 10.0. p values for p<0.05 were taken to be statistically significant.
RESULTS
Clinical findings
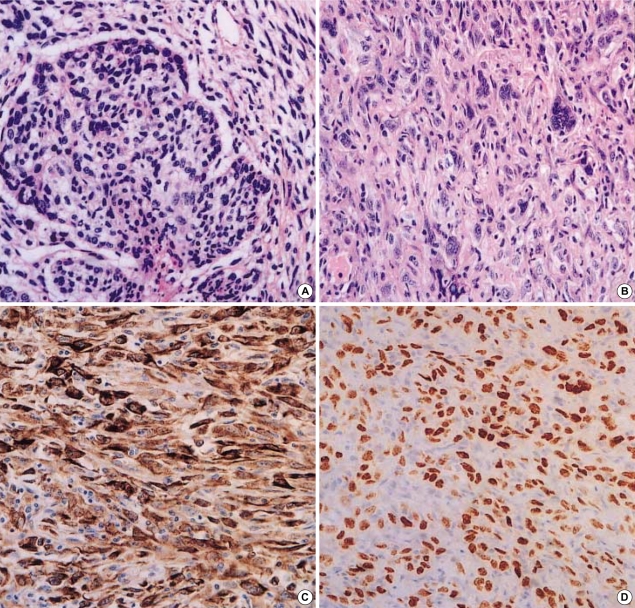
As summarized in Table 1, a total of 21 patients were included in the study. They included 20 men and 1 woman and ranged in age from 50 to 91 yr at the time of surgery (mean age 65 yr). A large number of patients had a tumor onset age of >60 yr (12 patients, 57.1%). Pathological staging was performed according to the TNM classification of the International Union Against Cancer. Of the 21 patients, 3 patients were at Stage IIA, 9 patients at Stage IIB, 6 patients at Stage IIIA, and 3 patients at Stage IIIB. Follow-up of these patients revealed that 12 patients (57.1%) died of PCL.
Table 1.
Clinicopathological details of the cases
Pathologic and immunohistochemical findings
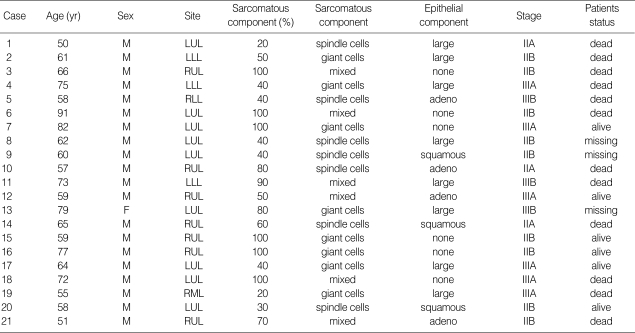
Microscopically, 15 cases contained non-small cell carcinoma combined with sarcomatous components, whereas 6 cases showed only sarcomatous areas without evidence of carcinoma. The carcinomas in these 15 cases were large cell carcinoma in 8 cases, adenocarcinoma in 4, and squamous cell carcinoma in 3. All 21 cases contained spindle cells or giant cells or a mixture of these cell types (Fig. 1A, B).
Fig. 1.
(A) Spindle cells of sarmocomatous areas whirled around carcinomatous component (H&E, ×200). (B) Giant cells are admixed with spindle cell components (H&E, ×200). (C) Spindle cells show immunoreactivity for cytokeratin 7 (×400). (D) TTF-1 nuclear stainings are detected in spindle cells in sarcomatous area (×400).
Of the 21 cases, 7 were composed exclusively of spindle cells that were arranged in long fascicles and resembled either leiomyosarcoma or fibrosarcoma. Eight cases consisted of pleomorphic cells with abundant cytoplasm and multinucleated giant cells. Six cases contained a mixture of spindle cells and giant cells. Of 6 cases with only sarcomatous components, 3 showed pure spindle cell elements, and 3 demonstrated a mixture of two sarcomatous components. Because of the morphologic difference between spindle cells and giant cells in PLC, it was speculated that the expression patterns of various proteins on these cells might differ and be correlated with the clinicopathological characteristics of PCL. Thus, we examined the expression pattern of CD99 on the spindle and giant cells of PLC, respectively.
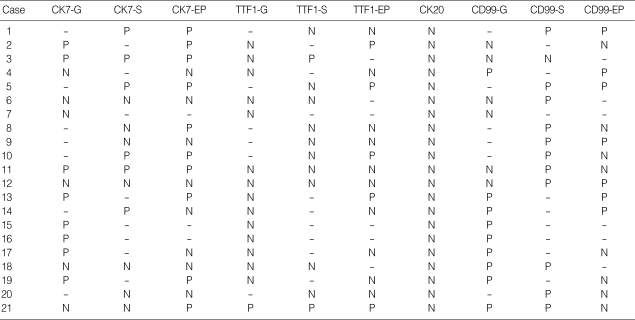
The immunohistochemistry result of the present study are summarized in Table 2. In 15 cases containing carcinomas, all carcinomatous components were immunoreactive for cytokeratin 7, and epithelial membrane antigen (EMA), whereas cytokeratin 20 was not expressed on any carcinomatous or sarcomatous component. The sarcomatous components of all 21 cases were positive for vimentin. Of 13 cases containing spindle cells, CK7 was expressed on spindle cells in 6 cases (43%), TTF-1 in 2 (15%), and CD99 in 11 (85%). Of 14 cases containing giant cells as a sarcomatous component, six cases (57%) were immunoreactive for CK7, 2 (14%) for TTF-1, and 9 (64%) for CD99. TTF-1 was expressed in 5 of 15 cases containing a carcinomatous component, 3 of which were adenocarcinomas and 2 were large cell carcinomas. However, the components of squamous cell carcinoma in three PLCs were negative for TTF-1.
Table 2.
Immunohistochemistry results
P, positive; -, absence of these component; N, negative; S, spindle cell component; G, giant cell component; EP, epithelial cell component.
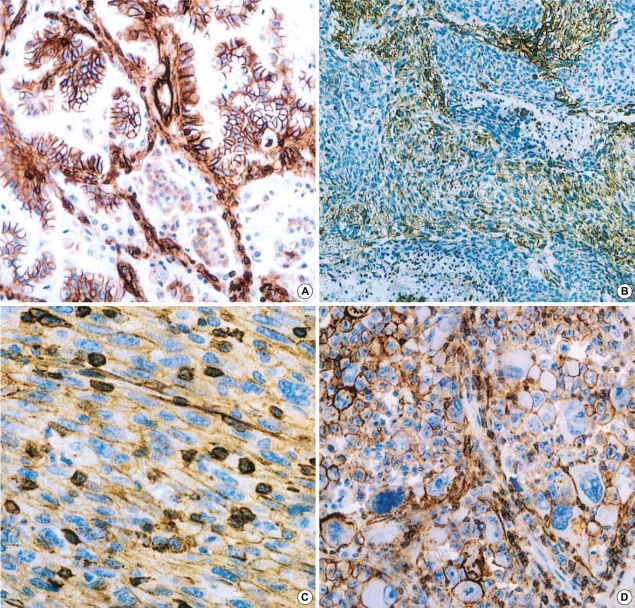
The expression of CD99 in carcinoma was observed in 7 of 15 PCLs. These were as large cell carcinomas in 3 cases, adenocarcinomas in 2, and squamous cell carcinoma in 2 (Fig. 2A). Immnoreactivity for CD99 in sarcomatoid components was intense and diffuse on the cell membrane (Fig. 2B-D). The expression patterns of TTF-1 and CD99 were not statistically related to clinical or pathological parameters, such as histological subtype and staging of PCL.
Fig. 2.
(A) Carcinoma component of PCL showing CD99 surface expression on tumor cells (×200). (B, C) CD99 immunoreacitivity was detected on spindle cells (B: ×200, C: ×400). (D) CD99 is observed on giant cells (×400).
DISCUSSION
The CD99 protein is ubiquitously expressed in a variety of human normal tissues and malignant cells (21-24). In normal tissue CD99 is highly expressed in cortical thymocytes, pancreatic islet cells, granulosa cells of the ovary, Sertoli's cells and ependymal cells. It is also expressed on fibroblasts, endothelial cells, smooth muscle cells and respiratory epithelial cells in which the level of expression is relatively low (24, 25). Even though CD99 is known to be a specific marker for Ewing/PNET, it is expressed in a variety of tumors (20-22, 24-26). With respect to neoplasms of the lung, CD99 is expressed in 25% of pulmonary neuroendocrine tumors and is preferentially confined to typical carcinoids (19). It is generally accepted that CD99 is not a specific marker for a particular subset of neoplasms. The present study demonstrates that CD99 expression is not only shown by spindle cells and giant cells in PCL, rather all cases containing a carcinomatous component showed various degrees of CD 99 positivity. In terms of sarcomatous component, the percentage of CD99-positive cells was higher in spindle cells than in giant cells. Our results support the concept that CD99/MIC-2 is not a specific marker for distinguishing a particular subset of human tumors. Moreover, it has been reported that CD99 plays critical role in the invasion, motility, and proliferation of malignant cells (27-33). Pelosi et al. reported a statistically significant relationship between the number of CD99 positive cells and the presence of local invasion and/or distant metastasis, and the Ki-67 index in gastrointestinal and pulmonary neuroendocrine tumors (19). These findings suggest that CD99 may be involved in cell-to-cell adhesion of neuroendocrine tumor cells and in downregulation of their proliferative activity. Thus, to explore the biological implication of CD99 in PCL we analyzed patient clinico-pathologic data. However, CD99 expression in PCL was not found to be correlated with patient prognostic parameters. These findings suggest that the expression of CD99 in PCL does not contribute to the anti-proliferation PCL tumor cells.
Although the histogenesis of PCL has not been clearly explored, several groups have suggested the possibility that PCL is a carcinoma with divergent sarcomatous differentiation, rather than being a collision neoplasm. Recently Rossi et al. studied 58 cases of PCL that showed immunoreactivity for CK7 and TTF-1 in the epithelial and sarcomatous elements (34). In addition, three groups have demonstrated that the epithelial and sarcomatous components of PCL show the same genetic abnormalities by microdissection analysis (35-37). These findings support the concept that the sarcomatous components of PCL might be derived from epithelial components. Like CK7, CD99 was also expressed on spindle and/or giant cells as well as PCL carcinomas in the present study. However, these findings inadequately support the concept that spindle and giant cells are derived from carcinoma because CD99 is more widely expressed on human mesenchymal components and sarcomas than carcinomas. Thus, it is more likely that the expression of CD99 on sarcomatous component of PLC reflects tumor cell mesenchymal differentiation status.
In summary, CD 99 was found to be widely expressed in both sarcomatoid and epithelial components in PCL, like other epithelial markers. Although the biological implications of its expression in PCL remain unclear, the clinicopathological analysis demonstrated no direct correlation between the expressions of CD99 in PCL and the clinical indices of PCL patients.
Footnotes
This work was supported by Korea Research Foundation Grant (KRF-2000-015-FP0039).
References
- 1.Brambilla E, Travis WD, Colby TV, Corrin B, Shimosato Y. The new World Health Organization classification of lung tumours. Eur Respir J. 2001;18:1059–1068. doi: 10.1183/09031936.01.00275301. [DOI] [PubMed] [Google Scholar]
- 2.Travis WD. Pathology of lung cancer. Clin Chest Med. 2002;23:65–81. doi: 10.1016/s0272-5231(03)00061-3. [DOI] [PubMed] [Google Scholar]
- 3.Travis WD, Travis LB, Devesa SS. Lung cancer. Cancer. 1995;75:191–202. doi: 10.1002/1097-0142(19950101)75:1+<191::aid-cncr2820751307>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 4.Travis WD, Colby TV, Corrin B. Histopathological Typing of Lung and Pleural Tumours. New York: Springer Verlag; 1999. [Google Scholar]
- 5.Addis BJ, Corrin B. Pulmonary blastoma, carcinosarcoma and spindle-cell carcinoma: an immunohistochemical study of keratin intermediate filaments. J Pathol. 1985;147:291–301. doi: 10.1002/path.1711470407. [DOI] [PubMed] [Google Scholar]
- 6.Battifora H. Spindle cell carcinoma: ultrastructural evidence of squamous origin and collagen production by the tumor cells. Cancer. 1976;37:2275–2282. doi: 10.1002/1097-0142(197605)37:5<2275::aid-cncr2820370518>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 7.Berho M, Moran CA, Suster S. Malignant mixed epithelial/mesenchymal neoplasms of the lung. Semin Diagn Pathol. 1995;12:123–139. [PubMed] [Google Scholar]
- 8.Cagle PT, Alpert LC, Carmona PA. Peripheral biphasic adenocarcinoma of the lung: light microscopic and immunohistochemical findings. Hum Pathol. 1992;23:197–200. doi: 10.1016/0046-8177(92)90245-x. [DOI] [PubMed] [Google Scholar]
- 9.Chang YL, Lee YC, Shih JY, Wu CT. Pulmonary pleomorphic (spindle) cell carcinoma: peculiar clinicopathologic manifestations different from ordinary non-small cell carcinoma. Lung Cancer. 2001;34:91–97. doi: 10.1016/s0169-5002(01)00224-0. [DOI] [PubMed] [Google Scholar]
- 10.Attanoos RL, Papagiannis A, Suttinont P, Goddard H, Papotti M, Gibbs AR. Pulmonary giant cell carcinoma: pathological entity or morphological phenotype? Histopathology. 1998;32:225–231. doi: 10.1046/j.1365-2559.1998.00378.x. [DOI] [PubMed] [Google Scholar]
- 11.Fishback NF, Travis WD, Moran CA, Guinee DG, Jr, McCarthy WF, Koss MN. Pleomorphic (spindle/giant cell) carcinoma of the lung. A clinicopathologic correlation of 78 cases. Cancer. 1994;73:2936–2945. doi: 10.1002/1097-0142(19940615)73:12<2936::aid-cncr2820731210>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 12.Nakajima M, Kasai T, Hashimoto H, Iwata Y, Manabe H. Sarcomatoid carcinoma of the lung: a clinicopathologic study of 37 cases. Cancer. 1999;86:608–616. [PubMed] [Google Scholar]
- 13.Nappi O, Glasner SD, Swanson PE, Wick MR. Biphasic and monophasic sarcomatoid carcinomas of the lung. A reappraisal of 'carcinosarcomas' and 'spindle-cell carcinomas'. Am J Clin Pathol. 1994;102:331–340. doi: 10.1093/ajcp/102.3.331. [DOI] [PubMed] [Google Scholar]
- 14.Ambros IM, Ambros PF, Strehl S, Kovar H, Gadner H, Salzer-Kuntschik M. MIC2 is a specific marker for Ewing's sarcoma and peripheral primitive neuroectodermal tumors. Evidence for a common histogenesis of Ewing's sarcoma and peripheral primitive neuroectodermal tumors from MIC2 expression and specific chromosome aberration. Cancer. 1991;67:1886–1893. doi: 10.1002/1097-0142(19910401)67:7<1886::aid-cncr2820670712>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 15.Kovar H, Dworzak M, Strehl S, Schnell E, Ambros IM, Ambros PF, Gadner H. Overexpression of the pseudoautosomal gene MIC2 in Ewing's sarcoma and peripheral primitive neuroectodermal tumor. Oncogene. 1990;5:1067–1070. [PubMed] [Google Scholar]
- 16.Delattre O, Zucman J, Melot T, Garau XS, Zucker JM, Lenoir GM, Ambros PF, Sheer D, Turc-Carel C, Triche TJ, Aurias A, Thomas G. The Ewing family of tumors--a subgroup of small-round-cell tumors defined by specific chimeric transcripts. N Engl J Med. 1994;331:294–299. doi: 10.1056/NEJM199408043310503. [DOI] [PubMed] [Google Scholar]
- 17.Ozdemirli M, Fanburg-Smith JC, Hartmann DP, Azumi N, Miettinen M. Differentiating lymphoblastic lymphoma and Ewing's sarcoma: lymphocyte markers and gene rearrangement. Mod Pathol. 2001;14:1175–1182. doi: 10.1038/modpathol.3880455. [DOI] [PubMed] [Google Scholar]
- 18.Costa MJ, Ames PF, Walls J, Roth LM. Inhibin immunohistochemistry applied to ovarian neoplasms: a novel, effective, diagnostic tool. Hum Pathol. 1997;28:1247–1254. doi: 10.1016/s0046-8177(97)90197-0. [DOI] [PubMed] [Google Scholar]
- 19.Pelosi G, Fraggetta F, Sonzogni A, Fazio N, Cavallon A, Viale G. CD99 immunoreactivity in gastrointestinal and pulmonary neuroendocrine tumours. Virchows Arch. 2000;437:270–274. doi: 10.1007/s004280000240. [DOI] [PubMed] [Google Scholar]
- 20.Jung KC, Kim NH, Park WS, Park SH, Bae Y. The CD99 signal enhances Fas-mediated apoptosis in the human leukemic cell line, Jurkat. FEBS Lett. 2003;554:478–484. doi: 10.1016/s0014-5793(03)01224-9. [DOI] [PubMed] [Google Scholar]
- 21.Fisher C. Synovial sarcoma. Ann Diagn Pathol. 1998;2:401–421. doi: 10.1016/s1092-9134(98)80042-7. [DOI] [PubMed] [Google Scholar]
- 22.Gordon MD, Corless C, Renshaw AA, Beckstead J. CD99, keratin, and vimentin staining of sex cord-stromal tumors, normal ovary, and testis. Mod Pathol. 1998;11:769–773. [PubMed] [Google Scholar]
- 23.Shin YK, Lee GK, Kook MC, Jung KC, Kim JR, Song HG, Park SH, Chi JG. Reduced expression of CD99 and functional disturbance in anencephalic cortical thymocytes. Virchows Arch. 1999;434:443–449. doi: 10.1007/s004280050364. [DOI] [PubMed] [Google Scholar]
- 24.Matias-Guiu X, Pons C, Prat J. Mullerian inhibiting substance, alpha-inhibin, and CD99 expression in sex cord-stromal tumors and endometrioid ovarian carcinomas resembling sex cord-stromal tumors. Hum Pathol. 1998;29:840–845. doi: 10.1016/s0046-8177(98)90454-3. [DOI] [PubMed] [Google Scholar]
- 25.Weidner N, Tjoe J. Immunohistochemical profile of monoclonal antibody O13: antibody that recognizes glycoprotein p30/32MIC2 and is useful in diagnosing Ewing's sarcoma and peripheral neuroepithelioma. Am J Surg Pathol. 1994;18:486–494. [PubMed] [Google Scholar]
- 26.Granter SR, Renshaw AA, Fletcher CD, Bhan AK, Rosenberg AE. CD99 reactivity in mesenchymal chondrosarcoma. Hum Pathol. 1996;27:1273–1276. doi: 10.1016/s0046-8177(96)90336-6. [DOI] [PubMed] [Google Scholar]
- 27.Jung KC, Park WS, Bae YM, Hahn JH, Hahn K, Lee H, Lee HW, Koo HJ, Shin HJ, Shin HS, Park YE, Park SH. Immunoreactivity of CD99 in stomach cancer. J Korean Med Sci. 2002;17:483–489. doi: 10.3346/jkms.2002.17.4.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee EJ, Lee HG, Park SH, Choi EY, Park SH. CD99 type II is a determining factor for the differentiation of primitive neuroectodermal cells. Exp Mol Med. 2003;35:538–547. doi: 10.1038/emm.2003.57. [DOI] [PubMed] [Google Scholar]
- 29.Lee IS, Kim SH, Song HG, Park SH. The molecular basis for the generation of Hodgkin and Reed-Sternberg cells in Hodgkin's lymphoma. Int J Hematol. 2003;77:330–335. doi: 10.1007/BF02982639. [DOI] [PubMed] [Google Scholar]
- 30.Lee HJ, Kim E, Jee B, Hahn JH, Han K, Jung KC, Park SH, Lee H. Functional involvement of src and focal adhesion kinase in a CD99 splice variant-induced motility of human breast cancer cells. Exp Mol Med. 2002;34:177–183. doi: 10.1038/emm.2002.26. [DOI] [PubMed] [Google Scholar]
- 31.Lee IS, Shin YK, Chung DH, Park SH. LMP-1 induced downregulation of CD99 molecules in Hodgkin's and Reed-Sternberg cells. Leuk Lymphoma. 2001;42:587–594. doi: 10.3109/10428190109099318. [DOI] [PubMed] [Google Scholar]
- 32.Sohn HW, Choi EY, Kim SH, Lee IS, Chung DH, Sung UA, Hwang DH, Cho SS, Jun BH, Jang JJ, Chi JG, Park SH. Engagement of CD99 induces apoptosis through calcineurin-independent pathway in Ewing's sarcoma cells. Am J Pathol. 1998;153:1937–1945. doi: 10.1016/S0002-9440(10)65707-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim SH, Choi EY, Shin YK, Kim TJ, Chung DH, Chang SI, Kim NK, Park SH. Generation of cells with Hodgkin's and Reed-Sternberg phenotype through downregulation of CD99 (MIC2) Blood. 1998;92:4287–4295. [PubMed] [Google Scholar]
- 34.Rossi G, Cavazza A, Sturm N, Migaldi M, Facciolongo N, Longo L, Maiorana A, Brambilla E. Pulmonary carcinomas with pleomorphic, sarcomatoid, or sarcomatous elements: a clinicopathologic and immunohistochemical study of 75 cases. Am J Surg Pathol. 2003;27:311–324. doi: 10.1097/00000478-200303000-00004. [DOI] [PubMed] [Google Scholar]
- 35.Dacic S, Finkelstein SD, Sasotomi E, Swalsky PA, Yousem SA. Molecular pathogenesis of pulmonary carcinosarcoma as determined by microdisection-based allelotyping. Am J Surg Pathol. 2002;26:510–516. doi: 10.1097/00000478-200204000-00015. [DOI] [PubMed] [Google Scholar]
- 36.Holst VA, Finkelstein S, Colby TV, Myers JL, Yousem SA. p53 and K-ras mutational genotyping in pulmonary carcinosarcoma, spindle cell carcinoma, and pulmonary blastoma: implication for histogenesis. Am J Surg Pathol. 1997;21:801–811. doi: 10.1097/00000478-199707000-00008. [DOI] [PubMed] [Google Scholar]
- 37.Thompson L, Chang B, Barsky SH. Mononclonal origins of malignant mixed tumors (carcinosarcomas): evidence for a divergent histogenesis. Am J Surg Pathol. 1996;20:277–285. doi: 10.1097/00000478-199603000-00003. [DOI] [PubMed] [Google Scholar]